Abstract
Unstable repeat expansions and insertions cause more than 30 neurodegenerative and neuromuscular diseases. Remarkably, bidirectional transcription of repeat expansions has been identified in at least 14 of these diseases. More remarkably, a growing number of studies has been showing that both sense and antisense repeat RNAs are able to dysregulate important cellular pathways, contributing together to the observed clinical phenotype. Notably, antisense repeat RNAs from spinocerebellar ataxia type 7, myotonic dystrophy type 1, Huntington’s disease and frontotemporal dementia/amyotrophic lateral sclerosis associated genes have been implicated in transcriptional regulation of sense gene expression, acting either at a transcriptional or posttranscriptional level. The recent evidence that antisense repeat RNAs could modulate gene expression broadens our understanding of the pathogenic pathways and adds more complexity to the development of therapeutic strategies for these disorders. In this review, we cover the amazing progress made in the understanding of the pathogenic mechanisms associated with repeat expansion neurodegenerative and neuromuscular diseases with a focus on the impact of antisense repeat transcription in the development of efficient therapies.
1. Introduction
In the last few decades, an increasing number of neurological and neuromuscular diseases has been associated with expansions of microsatellites in coding and noncoding gene regions. Microsatellites are polymorphic repeat sequences of 1 to 9 nucleotides distributed throughout the genome, composing about 3% of the human genome. These polymorphic repeats are usually pathogenic when they expand in size above a given threshold [1,2]. In 1991, the discovery of the first repeat expansions overlapped with the finding of the causative genes for fragile X syndrome (FXS) and spinal bulbar muscular atrophy [3,4]. Since then, more than 30 neurodegenerative and neuromuscular diseases originated by expansions of tri-, tetra-, penta- or hexanucleotide repeats have been molecularly identified [1,2] (Figure 1). Trinucleotide repeat expansions are located in coding gene regions or in noncoding gene regions, whereas pathogenic tetra-, penta- and hexanucleotide repeats have only been found in noncoding gene regions [5]. More recently, a new type of pathogenic repeats has been identified, consisting in the insertion of a new unstable pentanucleotide repeat within a pre-existent microsatellite. These pentanucleotide repeat insertions have been found in spinocerebellar ataxia type 31 (SCA31) [6], SCA37 [7] and six types of familial adult myoclonic epilepsy (FAME 1, 2, 3, 4, 6 and 7) [8,9,10,11] (Figure 1).
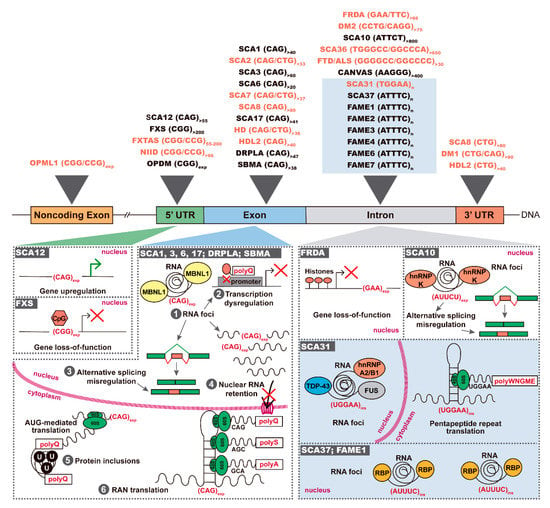
Figure 1.
Repeat expansions and insertions causing neurodegenerative and neuromuscular diseases. Top: pathogenic repeat expansions spread over 5′ and 3′ UTRs, exons and introns; novel repeat insertions are highlighted in a blue box; bidirectional transcription is known for the disorders in orange. Bottom: in SCA12, a 5′UTR (CAG)n expansion leads to gene upregulation, whereas expanded (CGG)n leads to CpG hypermethylation with silencing of FMRP expression in FXS; coding transcripts containing the expanded (CAG)n are able to (1) sequester RBPs such as MBNL1, forming nuclear RNA foci, which causes dysregulation of several cellular processes like (2) transcription, (3) mRNA splicing, (4) nucleocytoplasmic transport; in the cytoplasm, coding repeat expansions are (5) translated in proteins with expanded polyQ tracts leading to ubiquitin-positive (U) inclusions in neurons, or are (6) RAN-translated in polypeptides; in FRDA, a biallelic intronic (GAA)n expansion leads to repressive chromatin with consequent gene silencing; in SCA10, the (ATTCT)n is transcribed forming nuclear RNA foci with hnRNP K; in SCA31, nuclear RNA foci colocalize with TDP-43, FUS and hnRNP A2/B1 and pentapeptides are produced. PolyQ-polyglutamine; polyS-polyserine; polyA-polyalanine; polyWNGME-poly-tryptophan-asparagine-glycine-methionine-glutamic acid.
Depending on the repeat motif and its location in a gene, three different pathogenic mechanisms have been identified in repeat expansion diseases, consisting in protein gain-of-function, gene loss-of-function and/or RNA gain-of-function [1] (Figure 1). In coding regions, trinucleotide expansions of CAG and GCN repeats encode toxic stretches of polyglutamine (polyQ) or polyalanine (polyA), respectively. The proteins containing the expanded polyQ are misfolded and gain the aberrant ability to recruit other cellular proteins, forming toxic ubiquitin-positive aggregates in the cell cytoplasm and/or nucleus. The proteins sequestered in polyQ aggregates are then unavailable in the cell to perform their native function, dysregulating several molecular pathways [2,3]. In noncoding gene regions, the expanded repeats may trigger gene silencing or RNA-mediated toxicity. If the expanded repeat leads to modifications of the chromatin state through methylation of CpGs or histones, there is decrease or loss of gene transcription and, consequently, a reduction of gene encoded protein. In contrast, when the expanded repeat is transcribed, the RNA sequesters RNA-binding proteins (RBPs), forming nuclear RNA aggregates. Consequently, these RBPs do not play their function normally in the cell, impairing several cellular processes like splicing and nucleocytoplasmic transport [1,4]. In the cytoplasm, these expanded repeat RNAs may trigger repeat-associated non-ATG (RAN) translation because they are able to recruit ribosomal subunits initiating repeat translation across the three possible reading frames, generating homopolymeric, di-, tetra- or penta- repeat peptides [1,5,6,7] (Table 1).

Table 1.
Neurodegenerative and neuromuscular diseases with antisense repeat expansion transcription
Interestingly, in addition to being transcribed in gene orientation, many repeat expansions are also transcribed from the gene opposite DNA strand (Figure 2). Repeat expansion bidirectional transcription has first been identified in myotonic dystrophy type 1 (DM1) [8] followed by SCA8 [9]. Then, the number of neurological and neuromuscular diseases found with bidirectional transcription rapidly increased to include SCA2 [10], SCA7 [11], SCA31 [12], SCA36 [13,14], Fragile X-associated tremor/ataxia syndrome (FXTAS) [15], C9ORF72 frontotemporal dementia (FTD)/amyotrophic lateral sclerosis (ALS) [16], Huntington’s disease (HD) [17], Huntington’s disease-like 2 (HDL2) [18], DM2 [6] and Friedreich ataxia (FRDA) [19] (Figure 1). More recently, sequencing reads from RNA generated from both DNA strands have been found for neuronal intranuclear inclusion disease (NIID) [20] and oculopharyngeal myopathy and leukoencephalopathy 1 (OPML1) [21] (Figure 1). Similar to the expanded repeats expressed in gene orientation, abnormal repeats transcribed from the antisense strand also have the potential to be crucial partners in the clinical manifestation of these diseases. Thus, in this review, we will cover the astonishing progress in repeat expansion disorders with a focus on bidirectional transcription of expanded repeats and its role in disease.
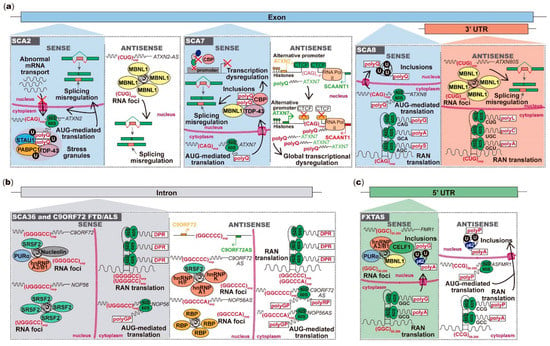
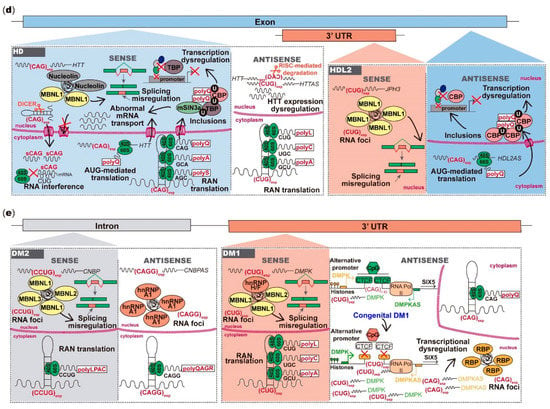
Figure 2.
Toxicity mediated by microsatellite repeats bidirectionally transcribed in neurodegenerative and neuromuscular diseases. (a) The (CAG)exp RNA from ATXN2 is translated into polyQ that abnormally interact with proteins in stress granules; the ATXN2-AS (CUG)exp forms RNA foci with splicing factors leading to misplicing of other mRNAs; from the ATXN7 strand, the (CAG)exp RNA is translated into toxic polyQ that form nuclear inclusions with RNA and DNA-binding proteins, dysregulating transcription and cellular mRNAs splicing; in ATXN7AS, the (CUG)exp reduces CTCF-binding, causing SCAANT1 downregulation and consequent derepression of ATXN7 promoter, leading to ataxin-7 overexpression and global transcriptional dysregulation; from the ATXN8 strand, the (CAG)exp RNA is translated into toxic polyQ that form ubiquitin-positive intranuclear inclusions; the ATXN8OS (CUG)exp forms nuclear RNA foci causing misplicing or is RAN-translated. (b) In SCA36 and C9ORF72 FTD/ALS, sense and antisense strand RNAs aggregate in nuclear RNA foci or are translated into DRPs. (c) The FMR1 (GGC)55–200 RNA forms nuclear foci or is RAN-translated into toxic peptides, which form intranuclear inclusions; from ASFMR1 strand, the (GCC)55–200 RNA can be translated into toxic peptides, leading to intranuclear aggregation. (d) From the HTT strand, the (CAG)exp RNA can aggregate in nuclear RNA foci or form hairpins posteriorly cleaved by DICER; the (CAG)exp RNA is also translated into polyQ proteins, forming nuclear inclusions, or it is RAN-translated; in HTTAS orientation, the (CUG)exp RNA regulates the HTT RNA on a RISC-dependent manner and can be RAN-translated into polypeptides. In HDL2, the (CAG)exp RNA forms RNA foci, leading to splicing misregulation; polyQ can be generated from the antisense (CAG)exp RNA, impairing the cellular transcriptional activity. (e) In DM2, both sense and antisense repeat RNAs form foci or are RAN-translated in polytetrapeptides; in DM1 DMPK orientation, the (CUG)exp forms RNA foci with consequent misplicing of several cellular mRNAs. In congenital DM1, the (CTG)>1000 reduces CTCF-binding and CpG methylation, downregulating antisense transcription; antisense transcription causes DMPK alternative promoter upregulation, leading to DMPK overexpression; DMPKAS CAGs also form RNA foci and are RAN-translated. DRP-dipeptide repeats; polyQ-polyglutamine; polyA-polyalanine; polyS-polyserine; polyL-polyleucine; polyC-polycysteine; polyP-polyproline; polyG-polyglycine; polyGP-poly-glycine-proline; polyPR-poly-proline-arginine; polyLPAC-poly-leucine-proline-alanine-cysteine; polyQAGR-poly-glutamine-alanine-glycine-arginine.
2. Antisense Expression of Trinucleotide and Pentanucleotide Repeats in Spinocerebellar Ataxias
Spinocerebellar ataxias are a genetically heterogeneous group of usually late-onset autosomal-dominant neurodegenerative diseases, characterized by progressive cerebellar ataxia due to Purkinje cell degeneration [22]. The estimated prevalence of SCAs varies significantly in different geographic regions from 1.6 to 5.6 per 100,000 inhabitants [23,24]. There are more than 40 types and at least 13 are caused by unstable repeats [22,25] (Figure 1). Here, we will cover the SCAs that have shown antisense transcript expression across the repeat expansion region, like SCA2 [10], SCA7 [11], SCA8 [9] and SCA31 [12] (Figure 2).
2.1. ATXN2 Sense and Antisense Trinucleotide Repeats in SCA2
SCA2 is one of the most common SCAs worldwide [24,26,27,28,29]. SCA2 is caused by a (CAG)n expansion of over 33 repeat units in exon 1 of the ATXN2 gene, which is translated into an expanded polyQ tract at the N-terminal of ataxin-2 protein [30,31,32] (Figure 1). Considering that ataxin-2 is expressed in cerebellar Purkinje cells [33,34], several mouse models have been developed to investigate the pathogenic (CAG)>33-mediated mechanism. The first mouse model with disrupted Atxn2 showed motor dysfunction in the rotarod test, but no evidence of neuronal cell loss or morphologic abnormalities in the cerebellum. This suggested that Atxn2 loss-of-function causes motor impairment, but it is not enough to cause the complete SCA2 phenotype [35]. In contrast, a transgenic mouse expressing the human full-length ATXN2 cDNA with a (CAG)58 expansion, in Purkinje cells, showed motor deficits, progressive loss of dendritic arborization, cell loss and cytoplasmic ataxin-2 aggregates [36]. To better replicate SCA2 in vivo, another mouse has been generated expressing the human full-length ATXN2 cDNA with a larger expansion, (CAG)127, in Purkinje cells, which presented earlier motor dysfunction accompanied by a significant decrease in expression of Purkinje cell genes such as calbindin 28K, indicating that an expanded ataxin-2 gain-of-function is involved in SCA2 [37].
Ataxin-2 is expressed in the endoplasmic reticulum and Golgi complex, playing a role in RNA metabolism and endocytosis [38,39]. Several studies have detected ataxin-2 protein binding to the 3′ terminal poly(A) of mRNAs through polyadenylate-binding protein 1 (PABPC1) interaction, allowing the initiation of translation by ribosomes [40,41]. The presence of an expanded polyQ tract would probably impair the ataxin-2 role in translation and consequently cause translational dysfunction in SCA2 [41]. Taking into account the observed gene expression dysregulation in mice and ataxin-2 function in translation, the generation of a BAC-transgenic mouse with the human ATXN2 containing (CAG)82 under its endogenous promoter corroborated the decreased expression of Purkinje cell-specific genes. These mice have shown decreased cerebellar mRNA and protein levels of the highly expressed regulator of G-protein signaling 8 (RGS8), supporting ataxin-2 function in mRNA stabilization and translation [42]. These SCA2 BAC-transgenic mice also presented the characteristic Purkinje cell degeneration and progressive motor dysfunction seen in SCA2 individuals [42]. Moreover, in agreement with the Sca2 knockin mouse model carrying (CAG)42 [35], a decrease in ATXN2 mRNA and protein was detected in the SCA2 BAC-transgenic mice, contrarily to BAC-transgenic mice expressing (CAG)22 [42].
Considering that ataxin-2 is key for the assembly of stress granules and P-bodies, which are important structures involved in mRNA transport, splicing and degradation in the cytoplasm, the expanded ataxin-2 interacts abnormally with several RBPs in these granules, like Staufen 1 (STAU1), Fox-1/ataxin-2-binding protein 1 (FOX/A2BP1) and DEAD-Box helicase 6 (DDX6), which suggested that these cellular processes are also dysregulated in SCA2 [43,44,45] (Table 1). In fact, in SCA2 subjects and Atxn2 (CAG)42 knockin mice Purkinje cells, the expanded ataxin-2 colocalizes with STAU1 and PABPC1 proteins, respectively, into ubiquitin-positive cytoplasm granules, further corroborating an mRNA processing dysregulation in SCA2 [40,43]. Remarkably, ataxin-2 and TAR DNA-binding protein 43 (TDP-43) interact in a complex dependent on RNA, which possibly serves as a bridge between them, and form abnormal cytoplasmic aggregates in cells [46]. Longer (CAG)n tracts in ATXN2 increase this interaction and, in conformity, as TDP-43 is a protein implicated in ALS, intermediate (CAG)31–32 ATXN2 alleles increase the risk of ALS [46]. In ALS, ataxin-2 has shown an abnormal cytoplasmic accumulation instead of the normal diffuse pattern seen in unaffected spinal cord motor neurons, supporting the hypothesis that ataxin-2 is involved in ALS pathogenesis [46].
Interestingly, the ATXN2 (CAG)n is bidirectionally transcribed. Li and colleagues have identified an antisense (CUG)n (ATXN2-AS) transcript in brain tissue of both unaffected and SCA2 affected subjects [10] (Figure 2). Notably, the (CUG)exp ATXN2-AS transcript leads to cell death of primary mouse cortical neurons and forms RNA foci in SCA2 human cerebella and BAC-transgenic mice, which likely colocalize with Muscleblind-Like Splicing Regulator 1 (MBNL1), as detected in neuroblastoma cells transfected with the expanded (CAG)n [10]. Although the ATXN2-AS with a (CUG)>37 is expressed in SCA2 human cerebella, both normal and expanded ATXN2-AS RNAs do not seem to be translated by RAN [10]. Furthermore, ATXN2-AS transcripts with intermediate (CUG)31–32 have been identified in ALS lymphoblastoid cell lines, suggesting an involvement of ATXN2-AS in ALS [10]. However, the role of ATXN2-AS in SCA2 and ALS is an important issue that needs further investigation.
2.2. ATXN7 Sense and Antisense Repeat Expression in SCA7
SCA7 is caused by a (CAG)n expansion in exon 3 of the ATXN7 [47,48]. Individuals affected with SCA7 carry one allele ranging from 37 to 400 CAG units with the larger sizes responsible for childhood disease onset [48] (Figure 1). In addition to cerebellar atrophy, SCA7 is also characterized by retinal degeneration [49]. Several transgenic mice expressing (CAG)>37 mirror the progressive ataxia and the cone-rod dystrophy seen in SCA7 individuals [50,51,52,53,54]. Ataxin-7 plays a role on the assembly and maintenance of transcriptional co-activation complexes TATA-binding protein-free TAFII/SPT3-TAF9-GCN5 acetyltransferase (TFTC/STAGA), which regulate the initiation of transcription [55,56,57]. Regarding retinal function, the polyQ region of ataxin-7 protein interacts with the transcription factor cone-rod homeobox protein (CRX), recruiting TFTC/STAGA complexes to the promoter region of photoreceptor-specific genes, regulating their transcription [51,58]. The expanded polyQ tract in ataxin-7 impairs TFTC/STAGA and CXR activities, originating transcriptional dysregulation and retinal dysfunction in transgenic mice expressing the mutant human full-length ATNX7 cDNA in rod-photoreceptors, Purkinje cells and other neurons [50,51,53,57,58]. The expanded ataxin-7 polyQ tract also interacts with other proteins like the transcriptional coactivator CAMP-response element-binding protein (CBP), forming nuclear inclusions observed in Purkinje and retinal cells in SCA7 transgenic and knockin mice, contributing to retinal dysfunction [50,51,52,53,54,59,60] (Table 1). In concordance with ataxin-7 function, zebrafish atxn7 translation inhibition also causes an early developmental impairment, leading to abnormal differentiation of photoreceptors and cerebellar neurons [61].
In contrast with transgenic mice expressing the human full-length ATXN7 coding region in neurons, transgenic mice containing the repeat flanked by its endogenous regulatory regions showed that repeat instability is related to cis-regulatory elements in the ATXN7 locus [62]. In particular, two functional CTCF-binding sites flank the (CAG)n and are involved in SCA7 instability [62]. In this region, Sopher and colleagues have also discovered a human ATXN7 alternative promoter and the spinocerebellar ataxia 7 antisense noncoding transcript 1 (SCAANT1) [11]. Notably, the SCAANT1 is responsible for ATXN7 transcriptional regulation. The authors found that, in normal size alleles, the CTCF-binding activates SCAANT1 transcription, yielding convergent transcription with ATXN7, and consequently limiting ATXN7 expression due to epigenetic modifications [11]. This transcriptional regulation mechanism is dysregulated in expanded (CAG)n alleles. The repeat expansion leads to a decrease in CTCF-binding and, consequently, a decrease in SCAANT1 transcription, which derepresses ATXN7 [11] (Figure 2). The effect in human SCA7 cerebella that different repeat sizes play in this complex regulation has not been further studied. Intriguingly, the discovery of a noncoding antisense RNA repressing the transcription of an important protein involved in global transcriptional activity highlights the importance of antisense repeat RNAs as essential regulators of crucial mechanisms like transcription and the relevance of these targets for therapeutic intervention.
2.3. Bidirectional Transcription across the Repeat Region in SCA8
SCA8 is caused by a combined polymorphic CTA with a (CTG)n expansion above 80 units in the 3′UTR of the Ataxin-8 opposite strand (ATXN8OS) [9,63,64,65,66] (Figure 1). Drosophila transgenic lines expressing normal and expanded (CUG)n RNAs in photoreceptor neurons showed retinal neurodegeneration, strongly suggesting that the overexpression of RNA containing CUG repeats is sufficient to drive neuronal degeneration [67]. To further understand the molecular pathogenic mechanism in SCA8, Moseley and colleagues have generated BAC-transgenic mice expressing the human full-length ATXN8OS with (CTG)104 under its endogenous promoter, recapitulating the motor dysfunction and cerebellar deficits seen in SCA8 [9]. Remarkably, these authors found that the CTG/CAG repeat expansion is bidirectionally transcribed in SCA8 BAC-transgenic mice, forming polyQ ubiquitin-positive intranuclear inclusions resulting from (CAG)n translation, which was also verified in human SCA8 brain tissue [9] (Figure 2). From the ATXN8OS, the expanded (CTG)n is transcribed in a noncoding transcript, suggesting that both protein and RNA gain-of-function mechanisms contribute to SCA8 [9]. The expanded (CUG)n forms ribonuclear inclusions that colocalize with MBNL1 in cerebella of SCA8 subjects and BAC-transgenic mice, leading to misplicing [68] (Table 1). Moreover, (CAG)n expanded transcripts are RAN-translated generating homopolymeric peptides of polyA and polyserine (polyS) (Table 1). Therefore, in addition to polyQ, polyA and polyS peptides also form inclusions in cerebella of SCA8 human brains and BAC-transgenic mice, further supporting a peptide gain-of-function in SCA8 [5,69].
In the human genome, the SCA8 (CAG)n is adjacent to the promoter of actin-binding protein gene Kelch-like 1 (KLHL1) [70]. This gene has an antisense RNA (KLHL1AS) that holds the ATXN8OS (CUG)n at the 3′end. Interestingly, it has been suggested that KLHL1AS regulates KLHL1 expression in the cerebellum, raising the hypothesis that the function of KLHL1AS and KLHL1 could be dysregulated in SCA8 [70,71]. In fact, a mouse with a deletion of both Klhl1 gene and Klhl1as promoter showed gait abnormalities, motor incoordination and dendritic atrophy, supporting the hypothesis that KLHL1 and/or its antisense have a role in SCA8 [72]. Although the phenotype observed in these mice suggests a KLHL1 loss-of-function in SCA8, the downregulation of human KLHL1 needs to be further investigated in human tissues of SCA8 subjects [73]. Moreover, the human KLHL1AS RNA harbors the (CUG)n, but the mouse Klhl1as RNA ends upstream the (CUG)n [71]. Therefore, this knockout mouse might not be the most suitable model to study the involvement of KLHL1AS on KLHL1 expression and the role of the (CTG)n in this regulation [73]. Altogether, both RNA and protein-mediated mechanisms are seemingly involved in SCA8, but how each of these mechanisms contributes to the disease phenotype is not clear. Further investigation of the sequestered proteins in cerebellar nuclear inclusions and RNA foci could give insight into the cellular pathways impaired in this disease.
2.4. Pentanucleotide Repeats in Introns of Genes Transcribed from Opposite Strands in SCA31
In 2009, Sato and colleagues found a (TGGAA)n insertion causing SCA31 [12] (Figure 1). This insertion is in a shared intronic region between Brain expressed associated with NEDD4-1 (BEAN1) and Thymidine kinase 2 (TK2) genes, which are transcribed from opposite DNA strands [12]. In BEAN1-orientation, the transcribed (TGGAA)n insertion forms nuclear RNA aggregates in Purkinje cells from SCA31 individuals [12,74]. Consistent with the SCA31 phenotype, Drosophila lines expressing (TGGAA)80–100 in the nervous system showed an early lethality and progressive motor abnormalities [75], suggesting that the (TGGAA)n insertion could impact neuronal development. In the Drosophila eye, (TGGAA)80–100 expression led to eye degeneration, accumulation of nuclear (UGGAA)n aggregates and poly-tryptophan-asparagine-glycine-methionine-glutamic acid (polyWNGME) pentapeptide repeat proteins (PPR) in imaginal discs [75]. These abnormal PPRs have also been detected in cell bodies and dendrites of SCA31 Purkinje cells [75]. The (UGGAA)n insertion binds in vitro to serine/arginine-rich splicing factors (SRSFs) 1 and 9, and in vivo to C9ORF72 FTD/ALS-related proteins such as Fused in sarcoma (FUS), heterogeneous nuclear ribonucleoproteins A2/B1 (hnRNP A2/B1) and TDP-43 [12,75,76] (Table 1). Remarkably, concomitant expression of (UGGAA)n and TDP-43 protein rescued eye degeneration in transgenic flies by decreasing RNA foci formation and PPR translation, suggesting that TDP-43 could function as an RNA chaperone [75,76]. Similar studies have been performed with FUS and hnRNP A2/B1 proteins and the results are identical to those obtained with TDP-43, indicating that these proteins could also function as RNA chaperones [75]. In the opposite orientation, the (TGGAA)n is transcribed within an intron of TK2 gene and though no RNA foci or PPR proteins have been reported, rescue of SCA31 phenotype could require targeting both sense and antisense repeats.
3. FXN Antisense Transcript in Friedreich Ataxia
Friedreich ataxia (FRDA), an autosomal-recessive ataxia with a high prevalence worldwide, is mainly caused by a biallelic (GAA)n expansion in intron 1 of the frataxin (FXN) gene [24]. FRDA alleles have over 66 GAA repeat units, leading to sensory neuron loss, progressive motor and cognitive deficits, diabetes and hypertrophic cardiomyopathy that culminate in an early death [77,78,79] (Figure 1). Expansion of the (GAA)n causes a decrease of FXN mRNA and protein through histone modifications, possibly associated with heterochromatin formation in the repeat flanking region, in human FRDA brain tissue [80,81,82,83]. In FRDA lymphoblasts, the repressive chromatin, with increased tri-methylation at the 27th lysine residue of histone 3 (H3K27me3) and decreased acetylation at the 5th lysine residue of histone 4 (H4K5Ac) in the FXN promoter and GAA-upstream regions, agrees with the FXN transcriptional repression and FXN protein deficiency [84]. Frataxin is a crucial protein for mitochondrial iron metabolism and its deficiency leads to oxidative stress and mitochondrial dysfunction [85]. Remarkably, transcription factors, as serum response factor (SRF) and transcription factor AP2 (TFAP2), with binding sites in exon 1 together with regulatory elements in intron 1 regulate FXN transcription. In FRDA lymphoblasts, overexpression of SRF and TFAP2 leads to an increase of FXN transcription [86], suggesting that the (GAA)n expansion could cause alterations in the binding of these transcription factors.
A CTCF-binding site in the FXN promoter region has shown an enrichment of CTCFs in fibroblast cell lines from unaffected individuals, contrarily to FRDA fibroblast cells, which correlates with heterochromatin formation [87]. Spanning this CTCF-binding site, there is an antisense transcript named as FXN Antisense Transcript-1 (FAST-1), whose expression is 2-fold increased, in FRDA fibroblast cells [87]. Intriguingly, siRNA-mediated knockdown of CTCF protein, in control fibroblasts, led to a decrease of FXN transcription concomitant with an increase of FAST-1 mRNA, recapitulating the FXN and FAST-1 mRNA levels detected in FRDA fibroblasts [87]. Accordingly, overexpression of FAST-1, in HeLa transfected cells, led to FXN mRNA and protein decrease, CTCF-binding depletion and histone methylation enrichment in the FXN promoter region [19]. In FRDA fibroblasts, shRNA-mediated knockdown of the FAST-1 mRNA originated increased FXN transcription and mitochondrial aconitase activity, restoring frataxin function [19]. The pathogenic mechanism in FRDA is not completely understood, but the FAST-1 transcript, which does not span the repeat expansion, seems to influence this mechanism. FAST-1 could play a role in FXN transcription regulation, as occurs in SCA7, and be a successful target for therapeutic intervention.
4. Antisense Expression in Hexanucleotide Repeat Expansion Diseases C9ORF72 FTD/ALS and SCA36
C9ORF72 FTD/ALS and SCA36 are autosomal-dominant neurodegenerative diseases, characterized by dementia and motor neuron degeneration or ataxia, respectively. Although these diseases present differences in clinical presentation, both are caused by similar noncoding hexanucleotide repeat expansions. A (GGGGCC)n expansion, of >30 repeat units, in intron 1 of chromosome 9 open reading frame 72 (C9ORF72) causes C9ORF72 FTD/ALS, whereas SCA36 is caused by a (TGGGCC)n expansion of 650 or more repeats in intron 1 of the nucleolar protein 56 (NOP56) [88,89,90,91] (Figure 1).
The transcription of C9ORF72 and NOP56 hexanucleotide repeats initiates a cascade of parallel pathogenic mechanisms that include the sequestration of essential neuronal RBPs in RNA foci and the production of toxic dipeptide repeat proteins (DPRs) by RAN translation [13,16,92,93] (Figure 2 and Table 1). In addition to these RNA-mediated toxic mechanisms, the decreased levels of C9ORF72 transcripts in brain tissue of C9ORF72 FTD/ALS individuals, together with the characteristic neuropathology observed in animal models for gene loss-of-function, indicate that also C9ORF72 haploinsufficiency is implicated in this disease [94,95]. In SCA36, the cerebellum of affected subjects shows an increase in NOP56 transcript and protein discarding gene haploinsufficiency [14].
The mechanism of RNA-mediated toxicity implicated in C9ORF72 FTD/ALS and SCA36 is not triggered only by transcription of expanded hexanucleotide repeats in C9ORF72 and NOP56 gene expression context, but also by antisense transcripts [13,14,96,97]. The C9ORF72 has five transcript start sites (TSSs) in sense orientation and three in antisense orientation [96,98]. The (GGGGCC)n is transcribed from two C9ORF72 TSSs, whereas the antisense (GGCCCC)n is transcribed from one TSS [96]. Notably, BAC-transgenic mice expressing mosaics of 100–1000 GGGGCCs within the human C9ORF72 gene and containing gene flanking sequences have presented both sense and antisense RNA foci and RAN DPRs with a brain distribution similar to C9ORF72 expansion carriers, but no detectable neurodegeneration or behavioral abnormalities [99]. Remarkably, another C9ORF72 BAC-transgenic mouse containing ~500 repeats, but without 3′ gene sequences have also shown RNA foci and RAN DPRs, but again no neurodegeneration or behavioral changes [100]. On the other hand, BAC-transgenic mice with transgenes of 500 or both 500 and 32 repeats, within the human full-length C9ORF72 flanked by regulatory regions, driving sense and antisense expression using the endogenous human promoters, presented neurodegeneration and motor dysfunction. In this mouse model, antisense expression was upregulated in frontal cortex, as seen in autopsy tissue from C9ORF72 expansion carriers, indicating that the expansion regulates antisense expression [101]. Notably, in this model, in contrast to sense RNA foci that are found throughout the brain, antisense RNA foci accumulate in vulnerable C9ORF72 FTD/ALS cell populations like the upper motor neurons of the motor cortex. This BAC mouse model shows decreased survival, paralysis, muscle denervation, motor neuron loss, anxiety-like behavior, and cortical and hippocampal neurodegeneration. What seems to distinguish these latter BAC-transgenic mice from the previous mice, leading to the manifestation of the expected neurological phenotype, is the large region flanking both sense and antisense promoters that could facilitate interaction of regulatory regions with promoters, originating robust expression of transcripts. The relevance of antisense transcripts in the severity of the disease is supported by the milder phenotype when only overexpression of sense (GGGGCC)66 throughout the central nervous system is achieved by means of somatic brain transgenesis mediated by adeno-associated virus [102].
The NOP56 transcriptional landscape has not been fully investigated, however, in addition to nuclear RNA foci triggered by the sense (TGGGCC)n expansion in brain tissue of SCA36 affected individuals, RNA foci containing the antisense expansion have been detected in brains of transgenic mice for the (TGGGCC)n expansion, suggesting that antisense RNA may have a role in SCA36 pathology [13,103].
Interestingly, it has been suggested a modest role for C9ORF72 hexanucleotide repeat RNA in the neurodegenerative phenotype [104]. A Drosophila model expressing ~800 GGGGCCs, interrupted with stop codons to avoid RAN translation, showed that the expanded repeat RNA sequesters RBPs, though it is not toxic in this organism. Similarly, an antisense RNA with ~100 CCCCGG repeats alone does not trigger a neurodegenerative phenotype [104].
The transcription of both sense and antisense expanded repeats leads to translation in RAN DPRs in neuronal brain tissue of C9ORF72 FTD/ALS individuals and BAC-transgenic mice [96,97,99,100,101]. This RAN translation produces five DPRs, polyGA (glycine-alanine) and polyGR (glycine-arginine) from sense, polyPR (proline-arginine) and polyPA (proline-alanine) from antisense and polyGP (glycine-proline) from both sense and antisense transcripts [16,96,97]. All these DPR species are widely found in cytoplasmic neuronal inclusions in several brain regions, including the frontal cortex, of C9ORF72 FTD/ALS subjects [16,96]. In C9ORF72 antisense orientation, the translation in polyPR and polyGP may occur from one and three putative ATG-start codons, respectively, however, in vitro studies using cell lines transfected with a plasmid containing a (GGCCCC)n expansion have confirmed that RAN translation from the antisense strand drives polyPR and polyGP production [96,97].
The similarities between C9ORF72 FTD/ALS and SCA36 go beyond neurodegeneration. The hexanucleotide repeats share five nucleotides by each repeat unit, which has set the stage for the investigation of DPRs resulting from RAN translation in SCA36 [13,14]. Remarkably, polyGP and polyPR are produced in C9ORF72 FTD/ALS and SCA36 brain tissue and both are encoded from antisense repeat strand [13,14] (Table 1). Furthermore, studies in Drosophila and zebrafish models using codons, other than the C9ORF72 hexanucleotide repeat, to encode the DPRs showed that the arginine-rich dipeptide species, including polyPR, are highly toxic in these organisms [105,106]. In fact, the toxic polyPR peptides form aggregates in the cytoplasm of cerebellar granule cells of SCA36 and C9ORF72 FTD/ALS subjects [13,14]. The polyGP is more abundant in cells and neuronal tissues of SCA36 than in C9ORF72 FTD/ALS subjects, however, contrarily to polyGP insoluble cytoplasmic aggregates found in C9ORF72 FTD/ALS, they remain soluble in the cytoplasm of neuronal cells in SCA36 [13,14].
Although several studies have given insight on how the hexanucleotide repeat expansion in C9ORF72 and NOP56 antisense strands could contribute to the neurodegenerative phenotype, the biological function of the antisense transcripts encompassing these repeats remains poorly understood. Interestingly, in C9ORF72 FTD/ALS, a decrease in C9ORF72 expression in brain and monocytes of affected individuals is accompanied by an increase in antisense transcript expression, suggesting that antisense transcripts could be involved in C9ORF72 transcription regulation [96,98]. The findings described above indicate that successful therapies for these two diseases have to take into account antisense repeat transcripts.
5. Antisense Expression in Neuronal CGG Repeat Diseases
5.1. Antisense Transcript Spanning FMR1 Repeat Region in FXTAS
FXS and FXTAS result from a (CGG)n expansion in the 5′UTR of the FMRP translational regulator 1 (FMR1) gene [107,108]. FXS subjects carry a full-mutation allele with >200 CGGs causing developmental delay and intellectual disability, often accompanied by autism spectrum disorder, whereas FXTAS-associated alleles harbor a premutation ranging from 55 to 200 CGG repeat units, developing late-onset progressive ataxia, tremor and cognitive impairment [107,108] (Figure 1). In FXS, the (CGG)>200 expansion spanning the promoter region triggers its hypermethylation, leading to FMR1 gene silencing [109,110,111,112]. In contrast to FMR1 loss-of-function in FXS, a slight increase of FMR1 mRNA levels has been detected in FXTAS lymphoblastoid cells, supporting an RNA gain-of-function leading to the disease [113]. However, the repeat premutation size leads to a decrease in translation efficiency of the FMR1 mRNA and, therefore, identical levels of FMR1 protein (FMRP) have been detected in FXTAS lymphoblastoid cells compared with controls [114,115]. Several studies have shown that FMR1 mRNA forms ubiquitin-positive intranuclear inclusions in neuronal and glial cells in FXTAS brain tissues [116,117,118] and (CGG)98-knockin mice [118,119,120], suggesting a neurotoxicity driven by RNA. The (CGG)n premutation expressed in Purkinje cells leads to neurodegeneration in mice, independent from FMR1 gene context [121], demonstrating that the premutation RNA is a key player in this disease. In fact, a Drosophila model expressing a human 5′UTR fragment with (CGG)90 evidenced FMRP reduction, presenting ubiquitin-positive intranuclear inclusions in photoreceptor neurons and progressive neurodegeneration [122]. In this fly model and in FXTAS human cortical tissues, RBPs involved in RNA processing like the hnRNP A2/B1, Purine-rich binding protein-α (Pur α), MBNL1 and CUGBP Elav-Like Family Member 1 (CELF1) are sequestered by the premutation mRNA in intranuclear inclusions, preventing them from performing their normal functions [118,123,124,125]. The premutation FMR1 RNA is also RAN-translated into polyA and polyglycine (polyG) peptides, which originate ubiquitin-positive intranuclear inclusions colocalizing with p62 that could also contribute to neurodegeneration in Drosophila and FXTAS brain tissue [126,127,128].
In FXTAS, in addition to (CGG)55–200 RNA and protein-mediated toxicity, Ladd and colleagues discovered two antisense promoters flanking the (CCG)n and one antisense transcript (ASFMR1) spanning the repeat [15] (Figure 2). Similar to FMR1 mRNA, the ASFMR1 RNA levels are increased mainly in FXTAS autopsy brain tissue, endorsing a role for ASFMR1 in FXTAS [15]. Moreover, a Drosophila model expressing a (CCG)90 has shown retinal neurodegeneration, suggesting a toxic role for the ASFMR1 premutation [129]. In agreement, the spliced and polyadenylated ASFMR1 RNA with the (CCG)n premutation is transported to the cytoplasm and translated into a polyproline (polyP) peptide from an AUG-start codon [126,130]. PolyP and polyA peptides are RAN-translated, forming ubiquitin-positive neuronal inclusions in FXTAS brain tissue [127,130]. Like in SCA7 [11], there are CTCF-binding sites flanking the CGG/CCG repeat [15], but their role in FMR1 mRNA upregulation or silencing is poorly understood. Interestingly, the co-expression of expanded (CCG)n and (GGC)n in Drosophila decreased their RNA levels through Dicer and Argonaute-2, reverting the neurodegenerative phenotype [129], providing clues for a future therapeutic strategy for FXTAS.
5.2. NOTCH2NLC Repeat Expansions in NIID and Expanded Noncoding RNA LOC642361 in OPML1
Novel noncoding CGG repeat expansions have recently been discovered causing NIID and OPML1, two neurodegenerative diseases that share with FXTAS the symptoms of ataxia and tremor, and the presence of ubiquitin-positive neuronal inclusions [20,21]. Broadly, muscle weakness, dementia and parkisonism are common clinical signs seen in NIID subjects [20,131], whereas OPML1 is characterized by oculopharyngeal myopathy, limb weakness and leukoencephalopathy [21]. In NIID, there is an expanded (CGG)n larger than 66 units in the 5′UTR of Notch 2 N-terminal like C (NOTCH2NLC), leading to sporadic and familial NIID [20] (Figure 1). The NOTCH2NLC gene, highly expressed in the brain, is thought to play a role in neuronal proliferation and differentiation [20,132,133]. Interestingly, antisense transcripts have been detected spanning the (CGG)n region, in fibroblasts of NIID individuals [134]. Surprisingly, these antisense transcripts have not been found in fibroblasts from unaffected subjects [134], suggesting that bidirectional transcription occurs only in expanded alleles. DNA methylation changes in the expanded (CGG)n allele could lead to this antisense transcription, however, the authors did not find significant differences in CpG methylation profiles between leukocytes and fibroblasts from NIID subjects and controls [20,134]. On the other hand, another study has shown a tendency for DNA hypermethylation of this repeat region in NIID brain tissue [21]. The size of the (CGG)n could account for the differences in DNA methylation seen in these two studies. Although no NOTCH2NLC expression changes have been detected, several genes with neuronal functions have shown differences in fibroblasts [134], suggesting that the expanded (CGG)n could dysregulate neuronal gene expression in NIID.
The first (CGG)n expansion in a long noncoding RNA bidirectionally transcribed has recently been found in LOC642361/NUTM2B-AS1 causing OPML1 [21] (Figure 1). It remains to be seen if both sense and antisense RNAs contribute to disease by toxic RNA and RAN-peptides.
6. Antisense Repeat Expression in HD and HDL2
HD and HDL2 are two clinically and pathologically similar neurodegenerative diseases, characterized by movement, cognitive and psychiatric disturbances [135]. The individuals affected by these diseases present a degeneration of the cerebral cortex and striatum, endorsing a common pathogenic mechanism in both pathologies [135]. HD is caused by an expanded (CAG)>36 in exon 1 of huntingtin (HTT) gene [136], whereas HDL2 is caused by a noncoding (CTG)>40 in exon 2A of junctophilin-3 (JPH3) gene [137,138] (Figure 1). HD mouse and cell models have demonstrated that truncated N-terminal HTT fragments with expanded polyQ form intranuclear ubiquitin-positive inclusions in neurons and dystrophic neurites, as observed in HD brain tissues [139,140,141,142,143,144]. These mouse models also recapitulate the typical progressive loss of balance and motor impairment, tremor and involuntary movements present in HD subjects [139,140,141]. Although the function of HTT protein is not well established [145], the expanded HTT forms nuclear aggregates in neurons of HD transgenic mouse models, contrarily to the characteristic diffuse pattern of normal HTT protein in the cytoplasm, being this mislocalization involved in HD pathogenesis [140,141,142]. Concomitantly, several studies have revealed that expanded HTT intranuclear inclusions colocalize with many cellular proteins with a role in transcription such as CBP, histone deacetylase complex subunit Sin3a (mSIN3a) and TATA-binding protein (TBP), in HD human cortex tissues [125,146,147,148,149,150] (Table 1). Furthermore, the HTT mRNA with the expanded (CAG)n gains the ability to sequester RBPs, such as MBNL1 in HD fibroblasts and nucleolin in HD transgenic mouse brains, possibly causing a splicing misregulation of MBNL1 target pre-mRNAs, nucleocytoplasmic transport impairment and nucleolar stress [151,152,153,154].
In 2011, Chung and colleagues discovered the existence of a natural antisense transcript spanning the (CTG)n across the HD locus (HTTAS1) [17]. The authors have detected a decrease in HTTAS1 transcripts and identical HTT RNA levels in HD brain tissues [17]. Unexpectedly, another report has shown that HTT protein levels are identical between post-mortem brain tissues from subjects with an adult HD onset and controls, but HTT protein is reduced in brain protein extracts of juvenile onset individuals [155]. Therefore, juvenile HD onset is characterized by larger repeat expansions that seem to influence the expression of HTT protein and potentially HTTAS1 transcript. Chung and colleagues have also shown that HTTAS1 is able to regulate HTT RNA degradation on a RISC-dependent manner [17] (Figure 2). Moreover, two predicted CTCF-binding sites and two Sp1 and Ap2 transcription factor-binding sites have been reported in the 5′region of HTTAS1 promoter [17], raising the hypothesis of a transcriptional regulation in HD similar to ATXN7.
HD has been one of the first diseases for which RAN-peptides have been investigated [5]. Both sense (polyA and polyS) and antisense (polycysteine (polyC) and polyleucine (polyL)) RAN-peptides accumulate into cytoplasmic, perinuclear and nuclear aggregates in the cerebellum and cortex, and mainly in nuclear aggregates in cerebral and cerebellar white matter of HD human tissue, likely contributing to the disease [156].
In HD mice expressing exon 1 of HTT under its endogenous promoter and in HD human autopsy tissue, small RNAs containing CAG repeats (sCAGs) have been identified [157]. In fact, expanded HTT mRNA forms hairpins that are cleaved by a Dicer-dependent mechanism and, consequently, the resultant neurotoxic sCAGs of approximately 21 nucleotides could silence other RNAs containing CUG repeats, disrupting their functions [157,158]. Independently, another study has also shown that Dicer-processed CAG/CUG repeat RNAs could interfere with the stability of other gene transcripts, leading to neurodegeneration in Drosophila [159].
Due to clinical and neuropathological similarities between HD and HDL2, the same neurodegenerative mechanisms could underlie both diseases. JPH3 protein is highly expressed in Purkinje cells and is involved in the formation of junctional membranes, therefore, the loss of JPH3 could partially explain motor dysfunction in HDL2 [18,160,161,162]. Although no evident morphologic and functional abnormalities have been detected in cerebellar neurons of Jph3 knockout mice, these animals have shown motor dysfunction, supporting a role for reduced JPH3 protein in HDL2 [18,162].
In brain tissue of HDL2 subjects, expanded JPH3 transcripts form RNA aggregates with MBNL1, with consequent misplicing of MBNL1 mRNA targets in frontal cortex [163] (Table 1). In concordance, HDL2 BAC-transgenic mice expressing the full JPH3 locus with a (CTG)116 has also shown cortical (CUG)n RNA foci colocalizing with Mbnl1 accompanied by cortex atrophy and motor impairment, mimicking the HDL2 phenotype [164]. Notably, these transgenic mice allowed the identification of a HDL2 antisense CAG transcript translated into an aberrant polyQ expanded protein, forming ubiquitin-positive intranuclear inclusions in cortex, hippocampus and striatum of HDL2 BAC-transgenic mice [164]. The authors demonstrated also that these nuclear inclusions are able to sequester the transcriptional coactivator CBP in HDL2 human cortical neurons, suggesting a transcription dysregulation in this disease [164]. In this regard, Seixas and colleagues have shown that the antisense (CAG)n is transcribed, however, the expanded (CAG)n transcript and the polyQ protein were not detected in autopsy brain tissue from HDL2 individuals [18], probably due to the lower levels in human brain tissue compared with the transgenic mice. This clearly demonstrates bidirectional transcription of the JPH3 repeat in unaffected chromosomes. Further investigation of a potential regulatory mechanism involving both JPH3 sense and antisense expression, as occurs in HD, may contribute to explain how JPH3 protein expression is regulated (Figure 2).
Notably, the knowledge gathered indicates that antisense repeat transcripts HTTAS1 and HDL2AS have the potential to be targets for efficient and successful therapies in HD and HDL2, respectively.
7. Antisense Repeat Expression in DM1 and DM2
DM1 and DM2 are two clinically similar neuromuscular diseases [165]. While a (CTG)n expansion, ranging from 50 to several thousand CTGs, in the 3′UTR of DMPK causes DM1 [166,167,168], a (CCTG)n expansion, from 75 to 11,000 repeats, in intron 1 of CNBP is responsible for DM2 [169] (Figure 1). Very large expansions of (CTG)>1000 cause a more severe type of disease, congenital DM1, characterized by pronounced motor and cognitive disability during development, which is associated with childhood onset and early death [170]. Clinical similarities between DM1 and DM2 individuals include cardiac dysfunction, cataracts formation, skeletal muscle abnormalities and neuronal involvement [165]. In DM1, several studies have shown that the (CUG)>90 RNA forms nuclear aggregates, causing DMPK mRNA and protein reduction, as seen in DM1 muscle [171,172,173]. Accordingly, an homozygous mouse model with Dmpk disruption has shown some DM1 clinical features, such as progressive weakness, abnormalities in skeletal muscle, skeletal myopathy and dysfunction in cardiac conduction [174,175]. Although this mouse presented some clinical features of DM1, the complex muscle phenotype observed in DM1 subjects was not fully recapitulated, indicating that DMPK haploinsufficiency is not sufficient to explain the DM1 phenotype [174,175]. On the other hand, a transgenic mouse expressing a human skeletal actin fragment with a 3′UTR (CTG)>250, mimicking the location of the repeat expansion in DMPK, in a muscle-specific gene, developed limb difficulties, myotonia and myopathy, indicating that the expanded (CUG)n RNA is sufficient to cause DM1 clinical features in muscle [176]. However, the typical muscle atrophy and weakness observed in DM1 individuals were not detected in these animals [176].
The hypothesis of CNBP haploinsufficiency for DM2 has also been investigated by the generation of two Cnbp knockout mice. These animals presented an increased lethality associated with homozygous Cnbp deletion, which demonstrates that CNBP expression is crucial during development [177,178]. The first mice developed several DM2 characteristics such as myotonia, cataracts, cardiac arrhythmia and abnormalities in skeletal and heart muscle tissues [177], whereas the other presented muscle abnormalities with progressive cell loss in adult, muscle wasting and mislocalization of CNBP from the cytoplasm to the membrane [178]. In fact, reduction of CNBP expression in the cytoplasm accompanied by aberrant membrane location has been detected in DM2 myoblasts and muscle biopsy tissues [179,180]. In agreement with the pathogenic mechanisms found in DM1, the (CCUG)>75 RNA in DM2 also forms nuclear RNA foci in muscle of affected individuals [169]. Nuclear RNA aggregates colocalizing with MBNL proteins have been detected in muscle tissues from both DM1 and DM2 subjects [181]. Interestingly, nuclear RNA foci also colocalize with MBNL1 and MBNL2 proteins in cortical neurons [125] (Table 1). Both DM1 and DM2 phenotypes correlate with MBNL downregulation and CELF1 upregulation, which leads to splicing misregulation of MBNL and CELF1 target pre-mRNAs in neuronal and cardiac muscle cells [182,183,184,185,186,187,188]. MBNL loss-of-function or its sequestration by expanded (CUG)n has also been observed in mouse [189], Drosophila [190] and zebrafish [191] models. Remarkably, MBNL1 loss-of-function is an important mechanism involved in the clinical phenotype of these diseases. A knockout mouse carrying Mbnl1 gene with a deletion in the CUG repeat-binding site presented misplicing of several key mRNAs, such as that encoding chloride channel and cardiac troponin T, recapitulating several DM1 and DM2 features in the muscle and eye [189]. Furthermore, Drosophila expressing (CUG)480 in the eye and muscle also mimicked the degeneration of these tissues and presented nuclear RNA foci colocalizing with MBNL proteins [190]. Intriguingly, the MBNL1 overexpression in this fly model rescued the degenerative phenotype in eye and muscle [190], hinting a possible therapy for these diseases. Zebrafish embryos injected with the 3′DMPK RNA holding a (CUG)91 also formed nuclear RNA foci, developed morphological and behavioral abnormalities and showed transcriptional dysregulation during early development [191]. Supporting an RNA-mediated mechanism, several RBPs involved in transcription and RNA metabolism colocalize with nuclear RNA foci in DM1 cortical neurons and in DM1 and DM2 human-derived cells [125,192] (Table 1).
Bidirectional transcription of a repeat expansion has first been discovered by Cho and colleagues, for DM1. These authors demonstrated that the antisense transcript spanning the repeat is converted into siRNAs, able to recruit DNA and histone methyltransferases, in particular the heterochromatin protein 1γ (HP1γ), leading to heterochromatin formation [8]. Abnormal heterochromatin formation had previously been seen in primary DM1 myoblasts and fibroblasts, dysregulating transcriptional activity of DMPK and the neighboring SIX5 gene [170,193,194,195,196]. Unexpectedly, there is an increase of DMPK expression in cells of congenital DM1 individuals that present increased CpG methylation of CTCF-binding sites flanking the (CTG)n substantially reducing binding of CTCFs [197]. Similar to ATXN7, Nakamori and colleagues discovered a DMPK alternative promoter upstream the (CTG)n, that is regulated by an antisense CAG repeat transcript [198]. These authors found that in muscle of congenital DM1, reduction in CTCF-binding suppresses antisense CAG transcription and promotes alternative DMPK transcription, leading to increase in (CUG)n RNA and enhancing RNA toxicity (Figure 2). Interestingly, transgenic mice generated by Huguet and collaborators showed that both (CUG)n and (CAG)n DM1 transcripts form distinct ribonuclear foci, both potentially contributing to the disease [199]. Moreover, the antisense CAG siRNAs are able to reduce the sense (CUG)n toxicity, decreasing the (CUG)n RNA foci formation and splicing abnormalities [198]. Altogether, the authors proposed that the hypermethylation of CTCF-binding sites leads to DM1 locus transcriptional dysregulation with a consequent decrease of antisense CAG siRNAs and increase of expanded (CUG)n-mediated toxicity, whose downstream effects lead to muscle deficiencies in congenital DM1 [198] (Figure 2).
In DM1, the expanded CAG transcript is RAN-translated into polyQ peptides in cardiac myocytes of DM1 mice [5] (Table 1). More recently, it has also been demonstrated that antisense transcription occurs across DM2 locus and the antisense transcripts containing the expanded (CAGG)n are upregulated in DM2 human brain tissue [6]. Both sense (CCUG)n and antisense (CAGG)n expanded RNAs are RAN-translated producing toxic poly-leucine-proline-alanine-cysteine (polyLPAC) and poly-glutamine-alanine-glycine-arginine (polyQAGR) tetrapeptides, respectively, able to induce cell death in human transfected cells (Table 1). In DM2 tissue, polyLPAC aggregates have been mainly detected in the cytoplasm of neurons and glia, and polyQAGR in oligodendrocyte nuclei, potentially contributing to the disease. Interestingly, the antisense (CAGG)n also binds to hnRNP A1, an RBP implicated in RNA processing [6].
Taken together, this emerging evidence in DM1 and DM2 strongly indicates that antisense repeat RNAs are key players in several molecular pathways that when dysregulated cause disease, being important targets for the development of therapeutic strategies.
8. Therapeutic Strategies Targeting Toxic RNAs
A potential therapeutic strategy to target mutant repeat RNAs is based on RNA interference (RNAi) molecules that are complementary RNAs to the mutant transcripts, leading to the formation of double-stranded RNAs and, consequently, to their RISC-mediated degradation or translation repression. These molecules can be delivered directly into the cell as miRNAs or siRNAs, or indirectly as short hairpin RNAs (shRNAs) that are delivered generally by adeno-associated viruses (AAV) [200]. Several RNAi molecules have been tested in animal and cellular models for SCA7 [201,202,203], HD [204,205,206,207] and DM1 [208,209,210], reducing the toxicity of the mutant repeat RNAs. Remarkably, a miRNA targeting the HTT mRNA delivered using an AAV5 has been tested in the striatum of HD rodents and minipig models, leading to mutant mRNA and protein reduction, with consequent reduction of abnormal protein aggregation, thereby ameliorating the HD phenotype [204,205]. This AAV5-miHTT is currently a promising therapy for HD, with clinical phase I/II studies currently ongoing [211,212]. The delivery of RNAi molecules is facilitated by the use of viral vectors, which can be considered as an advantage since a single dose may be sufficient for treatment.
Other promising therapies to target mutant repeat RNAs are based on antisense oligonucleotides (ASOs) [213]. These ASOs, composed by a DNA sequence flanked by RNA nucleotides, are complementary to the target RNA, leading to its degradation, in the nucleus or cytoplasm by RNase H [214]. The challenges in these treatments are ASOs stability and delivery to muscle and brain. Therefore, several chemical modifications and delivery carriers may improve stability and uptake of these molecules [215,216]. In recent years, ASOs targeting sense RNAs have successfully been tested on mouse and human cellular models, showing a decrease of toxic transcripts and a partial rescue of the disease phenotype, as in SCA2 [217,218], SCA7 [219], HD [220], DM1 [221] and C9ORF72 FTD/ALS [222,223,224,225]. ASOs have shown their potential as a therapeutic strategy in preclinical studies for SCA2 [217,218], and in clinical studies for DM1, HD [220] and C9ORF72 FTD/ALS. In SCA2, ASOs decrease the toxic RNA and protein, improving the motor function in mice [217]. Moreover, three different ASOs have been studied in clinical trials for HD; one of them, in phase III, consists in a nonallele-specific ASO targeting both wild-type and mutant HTT RNAs, in which no pathogenic effect caused by wild-type HTT protein deficiency was detected [220], and the remaining are in phase I/II and they specifically target mutant HTT, using single nucleotide polymorphisms associated with the disease haplotype [226]. In DM1, an ASO targeting the mutant DMPK RNA is currently in phase I/II clinical trial, due to the observed successful rescue of muscle dysfunction in mouse models [221]. More recently, a potential ASO for C9ORF72 FTD/ALS [222], which reduces RNA foci formation and dipeptide inclusions in C9ORF72 FTD/ALS transgenic mice, has been in clinical phase I studies. The presented therapeutic strategies are based on ASOs that specifically target the toxic sense transcripts. However, in several diseases, including HD, SCA2, DM1 and C9ORF72 FTD/ALS, both sense and antisense repeats contribute with toxic species, thus targeting both mutant sense and antisense RNAs could be crucial to improving the success of therapies designed to revert the disease phenotype and/or delay disease progression.
9. Conclusions and Future Perspectives
Antisense repeat RNAs have been identified in several genes associated with neurodegenerative and neuromuscular diseases caused by repeat expansions. Although enormous efforts have been made to understand the complex mechanisms underlying these diseases, the contribution of mutant antisense repeat RNAs remains elusive. This is due to challenges regarding the assessment of their expression levels that tend to be low, the recognition of their cell and tissue-specific expression that probably varies from development to aging hindering the discovery of their biological function and role in disease. In addition, the scarcity of animal models with bidirectional transcription of repeat expansions has also limited the understanding of the role of antisense repeat RNAs. It remains challenging to weight the contribution of each transcript, sense or antisense, in the disease process. So far, it is known that some mutant antisense repeat transcripts can modulate gene transcription, dysregulate the activity of sense repeat transcripts or other complementary RNAs, sequester RBPs and be translated into toxic peptides. Intriguingly, a dysregulation of gene expression has arisen associated with antisense repeat transcription in at least four neurological diseases (Figure 2). Therefore, there is an emerging need to understand the mysteries behind the role of antisense repeat RNAs. Targeting both sense and antisense repeats may be required to improve the success of therapeutic strategies for these diseases.
Author Contributions
A.F.C.—writing of original draft, review and editing; J.R.L.—writing, review and editing; J.B.—review, editing and supervision; I.S.—conceptualization, review, editing and supervision. All authors have read and agreed to the final version of this manuscript.
Funding
This work was funded by Fundo Europeu de Desenvolvimento Regional (FEDER), through the COMPETE 2020 Operational Program for Competitiveness and Internationalization (POCI) of Portugal 2020, and by the Fundacão para a Ciência e a Tecnologia (FCT) and Ministério da Ciência, Tecnologia e Ensino Superior Portugal; Grant number POCI-01-0145-FEDER-029255. This study was partially supported by the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement no. ERC-2015-StG-680156-ZPR). José Bessa acknowledges Fundação para a Ciência e a Tecnologia (FCT), for a FCT Scientific stimulus grant (Grant CEECIND/03482/2018).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Loureiro, J.R.; Oliveira, C.L.; Silveira, I. Unstable repeat expansions in neurodegenerative diseases: Nucleocytoplasmic transport emerges on the scene. Neurobiol. Aging 2016, 39, 174–183. [Google Scholar] [CrossRef]
- Lieberman, A.P.; Shakkottai, V.; Albin, R.L. Polyglutamine Repeats in Neurodegenerative Diseases. Annu. Rev. Pathol. Mech. Dis. 2019, 14, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Paulson, H.L.; Shakkottai, V.G.; Clark, H.B.; Orr, H.T. Polyglutamine spinocerebellar ataxias—From genes to potential treatments. Nat. Rev. Neurosci. 2017, 18, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Swinnen, B.; Robberecht, W.; Bosch, L.V.D. RNA toxicity in non-coding repeat expansion disorders. EMBO J. 2020, 39, e101112. [Google Scholar] [CrossRef] [PubMed]
- Zu, T.; Gibbens, B.; Doty, N.S.; Gomes-Pereira, M.; Huguet, A.; Stone, M.D.; Margolis, J.; Peterson, M.; Markowski, T.W.; Ingram, M.A.C.; et al. Non-ATG–initiated translation directed by microsatellite expansions. Proc. Natl. Acad. Sci. USA 2010, 108, 260–265. [Google Scholar] [CrossRef]
- Zu, T.; Cleary, J.D.; Liu, Y.; Bañez-Coronel, M.; Bubenik, J.L.; Ayhan, F.; Ashizawa, T.; Xia, G.; Clark, H.B.; Yachnis, A.T.; et al. RAN Translation Regulated by Muscleblind Proteins in Myotonic Dystrophy Type 2. Neuron 2017, 95, 1292–1305.e5. [Google Scholar] [CrossRef]
- Nguyen, L.; Cleary, J.D.; Ranum, L.P. Repeat-Associated Non-ATG Translation: Molecular Mechanisms and Contribution to Neurological Disease. Annu. Rev. Neurosci. 2019, 42, 227–247. [Google Scholar] [CrossRef]
- Cho, D.H.; Thienes, C.P.; Mahoney, S.E.; Analau, E.; Filippova, G.N.; Tapscott, S.J. Antisense Transcription and Heterochromatin at the DM1 CTG Repeats Are Constrained by CTCF. Mol. Cell 2005, 20, 483–489. [Google Scholar] [CrossRef]
- Moseley, M.L.; Zu, T.; Ikeda, Y.; Gao, W.; Mosemiller, A.K.; Daughters, R.S.; Chen, G.; Weatherspoon, M.R.; Clark, H.B.; Ebner, T.J.; et al. Bidirectional expression of CUG and CAG expansion transcripts and intranuclear polyglutamine inclusions in spinocerebellar ataxia type 8. Nat. Genet. 2006, 38, 758–769. [Google Scholar] [CrossRef]
- Margolis, R.L.; Sun, X.; Xia, G.; Arbez, N.; Paul, S.; Zhu, S.; Peng, H.B.; Ross, C.A.; Koeppen, A.H.; Margolis, R.L.; et al. ATXN2-AS, a gene antisense toATXN2, is associated with spinocerebellar ataxia type 2 and amyotrophic lateral sclerosis. Ann. Neurol. 2016, 80, 600–615. [Google Scholar] [CrossRef]
- Sopher, B.L.; Ladd, P.D.; Pineda, V.V.; Libby, R.T.; Sunkin, S.M.; Hurley, J.B.; Thienes, C.P.; Gaasterland, T.; Filippova, G.N.; La Spada, A.R. CTCF Regulates Ataxin-7 Expression through Promotion of a Convergently Transcribed, Antisense Noncoding RNA. Neuron 2011, 70, 1071–1084. [Google Scholar] [CrossRef] [PubMed]
- Sato, N.; Amino, T.; Kobayashi, K.; Asakawa, S.; Ishiguro, T.; Tsunemi, T.; Takahashi, M.; Matsuura, T.; Flanigan, K.M.; Iwasaki, S.; et al. Spinocerebellar Ataxia Type 31 Is Associated with “Inserted” Penta-Nucleotide Repeats Containing (TGGAA)n. Am. J. Hum. Genet. 2009, 85, 544–557. [Google Scholar] [CrossRef] [PubMed]
- Todd, T.W.; McEachin, Z.T.; Chew, J.; Burch, A.R.; Jansen-West, K.; Tong, J.; Yue, M.; Song, Y.; Castanedes-Casey, M.; Kurti, A.; et al. Hexanucleotide Repeat Expansions in c9FTD/ALS and SCA36 Confer Selective Patterns of Neurodegeneration In Vivo. Cell Rep. 2020, 31, 107616. [Google Scholar] [CrossRef] [PubMed]
- McEachin, Z.T.; Gendron, T.F.; Raj, N.; García-Murias, M.; Banerjee, A.; Purcell, R.H.; Ward, P.J.; Todd, T.W.; Merritt-Garza, M.E.; Jansen-West, K.; et al. Chimeric Peptide Species Contribute to Divergent Dipeptide Repeat Pathology in c9ALS/FTD and SCA36. Neuron 2020, 107, 292–305.e6. [Google Scholar] [CrossRef]
- Ladd, P.D.; Smith, L.E.; Rabaia, N.A.; Moore, J.M.; Georges, S.A.; Hansen, R.S.; Hagerman, R.; Tassone, F.; Tapscott, S.J.; Filippova, G.N. An antisense transcript spanning the CGG repeat region of FMR1 is upregulated in premutation carriers but silenced in full mutation individuals. Hum. Mol. Genet. 2007, 16, 3174–3187. [Google Scholar] [CrossRef]
- Mori, K.; Arzberger, T.; Grässer, F.A.; Gijselinck, I.; May, S.; Rentzsch, K.; Weng, S.-M.; Schludi, M.H.; Van Der Zee, J.; Cruts, M.; et al. Bidirectional transcripts of the expanded C9orf72 hexanucleotide repeat are translated into aggregating dipeptide repeat proteins. Acta Neuropathol. 2013, 126, 881–893. [Google Scholar] [CrossRef]
- Chung, D.W.; Rudnicki, D.D.; Yu, L.; Margolis, R.L. A natural antisense transcript at the Huntington’s disease repeat locus regulates HTT expression. Hum. Mol. Genet. 2011, 20, 3467–3477. [Google Scholar] [CrossRef]
- Seixas, A.I.; Holmes, S.E.; Takeshima, H.; Pavlovich, A.; Sachs, N.; Pruitt, J.L.; Silveira, I.; Ross, C.A.; Margolis, R.L.; Rudnicki, D.D. Loss of junctophilin-3 contributes to huntington disease-like 2 pathogenesis. Ann. Neurol. 2012, 71, 245–257. [Google Scholar] [CrossRef]
- Mikaeili, H.; Sandi, M.; Bayot, A.; Al-Mahdawi, S.; Pook, M.A. FAST-1 antisense RNA epigenetically alters FXN expression. Sci. Rep. 2018, 8, 17217. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, J.-L.; Huang, W.; Zeng, S.; Jiao, B.; Liu, Z.; Chen, Z.; Li, Y.; Wang, Y.; Min, H.-X.; et al. Expansion of Human-Specific GGC Repeat in Neuronal Intranuclear Inclusion Disease-Related Disorders. Am. J. Hum. Genet. 2019, 105, 166–176. [Google Scholar] [CrossRef]
- Ishiura, H.; Shibata, S.; Yoshimura, J.; Suzuki, Y.; Qu, W.; Doi, K.; Almansour, M.A.; Kikuchi, J.K.; Taira, M.; Mitsui, J.; et al. Noncoding CGG repeat expansions in neuronal intranuclear inclusion disease, oculopharyngodistal myopathy and an overlapping disease. Nat. Genet. 2019, 51, 1222–1232. [Google Scholar] [CrossRef] [PubMed]
- Klockgether, T.; Mariotti, C.; Paulson, H.L. Spinocerebellar ataxia. Nat. Rev. Dis. Prim. 2019, 5, 24. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, P.; Ruano, L.; Loureiro, J.L.; Cruz, V.T.; Barros, J.; Tuna, A.; Barbot, C.; Guimarães, J.; Alonso, I.; Silveira, I.; et al. Hereditary Ataxia and Spastic Paraplegia in Portugal: A population-based prevalence study. JAMA Neurol. 2013, 70, 746–755. [Google Scholar] [CrossRef] [PubMed]
- Sequeiros, J.; Martins, S.; Silveira, I. Epidemiology and population genetics of degenerative ataxias. Stroke 2012, 103, 227–251. [Google Scholar] [CrossRef]
- Seixas, A.I.; Loureiro, J.R.; Costa, C.; Ordóñez-Ugalde, A.; Marcelino, H.; Oliveira, C.L.; Loureiro, J.L.; Dhingra, A.; Brandão, E.; Cruz, V.T.; et al. A Pentanucleotide ATTTC Repeat Insertion in the Non-coding Region of DAB1, Mapping to SCA37, Causes Spinocerebellar Ataxia. Am. J. Hum. Genet. 2017, 101, 87–103. [Google Scholar] [CrossRef]
- Geschwind, D.H.; Perlman, S.; Figueroa, C.P.; Treiman, L.J.; Pulst, S.M. The prevalence and wide clinical spectrum of the spinocerebellar ataxia type 2 trinucleotide repeat in patients with autosomal dominant cerebellar ataxia. Am. J. Hum. Genet. 1997, 60, 842–850. [Google Scholar] [PubMed]
- Cancel, G.; Dürr, A.; Didierjean, O.; Imbert, G.; Bürk, K.; Lezin, A.; Belal, S.; Benomar, A.; Abada-Bendib, M.; Vial, C.; et al. Molecular and Clinical Correlations in Spinocerebellar Ataxia 2: A Study of 32 Families. Hum. Mol. Genet. 1997, 6, 709–715. [Google Scholar] [CrossRef]
- Lopes-Cendesi, I.; Teive, H.G.; Calcagnotto, M.E.; Da Costa, J.C.; Cardoso, F.; Viana, E.; Maciel, J.A.; Radvany, J.; Arruda, W.O.; Trevisol-Bittencourt, P.C.; et al. Frequency of the different mutations causing spinocerebellar ataxia (SCA1, SCA2, MJD/SCA3 and DRPLA) in a large group of Brazilian patients. Arq. Neuro Psiquiatr. 1997, 55, 519–529. [Google Scholar] [CrossRef]
- Silveira, I.; Miranda, C.; Guimarães, L.; Moreira, M.-C.; Alonso, I.; Mendonca, P.; Ferro, A.; Pinto-Basto, J.; Coelho, J.; Ferreirinha, F.; et al. Trinucleotide Repeats in 202 Families With Ataxia: A small expanded (CAG)n allele at the SCA17 locus. Arch. Neurol. 2002, 59, 623–629. [Google Scholar] [CrossRef]
- Pulst, S.M. Spinocerebellar Ataxia Type 2. In GeneReviews®; Adam, M.P., Ardinger, H.H., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Stephens, K., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993–2020. [Google Scholar]
- Pulst, S.M.; Nechiporuk, A.; Nechiporuk, T.; Gispert, S.; Chen, X.-N.; Lopes-Cendes, I.; Pearlman, S.; Starkman, S.; Orozco-Diaz, G.; Lunkes, A.; et al. Moderate expansion of a normally biallelic trinucleotide repeat in spinocerebellar ataxia type 2. Nat. Genet. 1996, 14, 269–276. [Google Scholar] [CrossRef]
- Ramos, E.M.; Martins, S.; Alonso, I.; Emmel, V.E.; Saraiva-Pereira, M.L.; Jardim, L.B.; Coutinho, P.; Sequeiros, J.; Silveira, I. Common origin of pure and interrupted repeat expansions in spinocerebellar ataxia type 2 (SCA2). Am. J. Med Genet. Part B Neuropsychiatr. Genet. 2009, 9999, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Nechiporuk, T.; Huynh, D.P.; Figueroa, K.; Sahba, S.; Nechiporuk, A.; Pulst, S.M. The mouse SCA2 gene: cDNA sequence, alternative splicing and protein expression. Hum. Mol. Genet. 1998, 7, 1301–1309. [Google Scholar] [CrossRef] [PubMed]
- Huynh, D.P.; Del Bigio, M.R.; Ho, D.H.; Pulst, S.-M. Expression of ataxin-2 in brains from normal individuals and patients with Alzheimer’s disease and spinocerebellar ataxia 2. Ann. Neurol. 1999, 45, 232–241. [Google Scholar] [CrossRef]
- Kiehl, T.-R.; Nechiporuk, A.; Figueroa, K.P.; Keating, M.T.; Huynh, D.P.; Pulst, S.-M. Generation and characterization of Sca2 (ataxin-2) knockout mice. Biochem. Biophys. Res. Commun. 2006, 339, 17–24. [Google Scholar] [CrossRef]
- Huynh, D.P.; Figueroa, K.P.; Hoang, N.; Pulst, S.-M. Nuclear localization or inclusion body formation of ataxin-2 are not necessary for SCA2 pathogenesis in mouse or human. Nat. Genet. 2000, 26, 44–50. [Google Scholar] [CrossRef]
- Hansen, S.T.; Meera, P.; Otis, T.S.; Pulst, S.M. Changes in Purkinje cell firing and gene expression precede behavioral pathology in a mouse model of SCA2. Hum. Mol. Genet. 2013, 22, 271–283. [Google Scholar] [CrossRef]
- Van De Loo, S.; Eich, F.; Nonis, D.; Auburger, G.; Nowock, J. Ataxin-2 associates with rough endoplasmic reticulum. Exp. Neurol. 2009, 215, 110–118. [Google Scholar] [CrossRef]
- Huynh, D.P.; Yang, H.-T.; Vakharia, H.; Nguyen, D.; Pulst, S.M. Expansion of the polyQ repeat in ataxin-2 alters its Golgi localization, disrupts the Golgi complex and causes cell death. Hum. Mol. Genet. 2003, 12, 1485–1496. [Google Scholar] [CrossRef]
- Damrath, E.; Heck, M.V.; Gispert, S.; Azizov, M.; Nowock, J.; Seifried, C.; Rüb, U.; Walter, M.; Auburger, G. ATXN2-CAG42 Sequesters PABPC1 into Insolubility and Induces FBXW8 in Cerebellum of Old Ataxic Knock-In Mice. PLoS Genet. 2012, 8, e1002920. [Google Scholar] [CrossRef]
- Yokoshi, M.; Li, Q.; Yamamoto, M.; Okada, H.; Suzuki, Y.; Kawahara, Y. Direct Binding of Ataxin-2 to Distinct Elements in 3′ UTRs Promotes mRNA Stability and Protein Expression. Mol. Cell 2014, 55, 186–198. [Google Scholar] [CrossRef]
- Dansithong, W.; Paul, S.; Figueroa, K.P.; Rinehart, M.D.; Wiest, S.; Pflieger, L.T.; Scoles, D.R.; Pulst, S.M. Ataxin-2 Regulates RGS8 Translation in a New BAC-SCA2 Transgenic Mouse Model. PLoS Genet. 2015, 11, e1005182. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Dansithong, W.; Figueroa, K.P.; Scoles, D.R.; Pulst, S.M. Staufen1 links RNA stress granules and autophagy in a model of neurodegeneration. Nat. Commun. 2018, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Shibata, H.; Huynh, D.P.; Pulst, S.-M. A novel protein with RNA-binding motifs interacts with ataxin-2. Hum. Mol. Genet. 2000, 9, 1303–1313. [Google Scholar] [CrossRef]
- Nonhoff, U.; Ralser, M.; Welzel, F.; Piccini, I.; Balzereit, D.; Yaspo, M.-L.; Lehrach, H.; Krobitsch, S. Ataxin-2 Interacts with the DEAD/H-Box RNA Helicase DDX6 and Interferes with P-Bodies and Stress Granules. Mol. Biol. Cell 2007, 18, 1385–1396. [Google Scholar] [CrossRef] [PubMed]
- Elden, A.C.; Kim, H.-J.; Hart, M.P.; Chen-Plotkin, A.S.; Johnson, B.S.; Fang, X.; Armakola, M.; Geser, F.; Greene, R.; Lu, M.M.; et al. Ataxin-2 intermediate-length polyglutamine expansions are associated with increased risk for ALS. Nat. Cell Biol. 2010, 466, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Lindblad, K.; Savontaus, M.L.; Stevanin, G.; Holmberg, M.; Digre, K.; Zander, C.; Ehrsson, H.; David, G.; Benomar, A.; Nikoskelainen, E.; et al. An expanded CAG repeat sequence in spinocerebellar ataxia type 7. Genome Res. 1996, 6, 965–971. [Google Scholar] [CrossRef][Green Version]
- David, G.; Abbas, N.; Stevanin, G.; Dürr, A.; Yvert, G.; Cancel, G.; Weber, C.; Imbert, G.; Saudou, F.; Antoniou, E.; et al. Cloning of the SCA7 gene reveals a highly unstable CAG repeat expansion. Nat. Genet. 1997, 17, 65–70. [Google Scholar] [CrossRef]
- Martin, J.-J.; Krols, L.; Ceuterick, C.; Van Broeckhoven, C.; Van Regemorter, N.; Hayer-Delatte, F.; Brucher, J.-M.; De Barsy, T.; Szliwowski, H.; Evrard, P.; et al. On an autosomal dominant form of retinal-cerebellar degeneration: An autopsy study of five patients in one family. Acta Neuropathol. 1994, 88, 277–286. [Google Scholar] [CrossRef]
- Yvert, G.; Lindenberg, K.S.; Picaud, S.; Landwehrmeyer, G.B.; Sahel, J.-A.; Mandel, J.-L. Expanded polyglutamines induce neurodegeneration and trans-neuronal alterations in cerebellum and retina of SCA7 transgenic mice. Hum. Mol. Genet. 2000, 9, 2491–2506. [Google Scholar] [CrossRef]
- La Spada, A.R.; Fu, Y.-H.; Sopher, B.L.; Libby, R.T.; Wang, X.; Li, L.Y.; Einum, D.D.; Huang, J.; Possin, D.E.; Smith, A.C.; et al. Polyglutamine-Expanded Ataxin-7 Antagonizes CRX Function and Induces Cone-Rod Dystrophy in a Mouse Model of SCA7. Neuron 2001, 31, 913–927. [Google Scholar] [CrossRef]
- Garden, G.A.; Libby, R.T.; Fu, Y.-H.; Kinoshita, Y.; Huang, J.; Possin, D.E.; Smith, A.C.; Martinez, R.A.; Fine, G.C.; Grote, S.K.; et al. Polyglutamine-Expanded Ataxin-7 Promotes Non-Cell-Autonomous Purkinje Cell Degeneration and Displays Proteolytic Cleavage in Ataxic Transgenic Mice. J. Neurosci. 2002, 22, 4897–4905. [Google Scholar] [CrossRef] [PubMed]
- Helmlinger, D.; Hardy, S.; Abou-Sleymane, G.; Eberlin, A.; Bowman, A.B.; Gansmuller, A.; Picaud, S.; Zoghbi, H.Y.; Trottier, Y.; Tora, L.; et al. Glutamine-Expanded Ataxin-7 Alters TFTC/STAGA Recruitment and Chromatin Structure Leading to Photoreceptor Dysfunction. PLoS Biol. 2006, 4, e67. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.-Y.; Pennesi, M.E.; Weeber, E.J.; Xu, B.; Atkinson, R.; Chen, S.; Armstrong, D.L.; Wu, S.M.; Sweatt, J.; Zoghbi, H.Y. SCA7 Knockin Mice Model Human SCA7 and Reveal Gradual Accumulation of Mutant Ataxin-7 in Neurons and Abnormalities in Short-Term Plasticity. Neuron 2003, 37, 383–401. [Google Scholar] [CrossRef]
- Helmlinger, D.; Hardy, S.; Sasorith, S.; Klein, F.; Robert, F.; Weber, C.; Miguet, L.; Potier, N.; Van-Dorsselaer, A.; Wurtz, J.M.; et al. Ataxin-7 is a subunit of GCN5 histone acetyltransferase-containing complexes. Hum. Mol. Genet. 2004, 13, 1257–1265. [Google Scholar] [CrossRef]
- Martinez, E.; Palhan, V.B.; Tjernberg, A.; Lymar, E.S.; Gamper, A.M.; Kundu, T.K.; Chait, B.T.; Roeder, R.G. Human STAGA Complex Is a Chromatin-Acetylating Transcription Coactivator That Interacts with Pre-mRNA Splicing and DNA Damage-Binding Factors In Vivo. Mol. Cell. Biol. 2001, 21, 6782–6795. [Google Scholar] [CrossRef]
- Palhan, V.B.; Chen, S.; Peng, G.-H.; Tjernberg, A.; Gamper, A.M.; Fan, Y.; Chait, B.T.; La Spada, A.R.; Roeder, R.G. Polyglutamine-expanded ataxin-7 inhibits STAGA histone acetyltransferase activity to produce retinal degeneration. Proc. Natl. Acad. Sci. USA 2005, 102, 8472–8477. [Google Scholar] [CrossRef]
- Chen, S.; Peng, G.-H.; Wang, X.; Smith, A.C.; Grote, S.K.; Sopher, B.L.; La Spada, A.R. Interference of Crx-dependent transcription by ataxin-7 involves interaction between the glutamine regions and requires the ataxin-7 carboxy-terminal region for nuclear localization. Hum. Mol. Genet. 2003, 13, 53–67. [Google Scholar] [CrossRef][Green Version]
- Alves, S.; Marais, T.; Biferi, M.-G.; Furling, D.; Marinello, M.; El Hachimi, K.; Cartier, N.; Ruberg, M.; Stevanin, G.; Brice, A.; et al. Lentiviral vector-mediated overexpression of mutant ataxin-7 recapitulates SCA7 pathology and promotes accumulation of the FUS/TLS and MBNL1 RNA-binding proteins. Mol. Neurodegener. 2016, 11, 58. [Google Scholar] [CrossRef]
- McCullough, S.D.; Xu, X.; Dent, S.Y.R.; Bekiranov, S.; Roeder, R.G.; Grant, P.A. Reelin is a target of polyglutamine expanded ataxin-7 in human spinocerebellar ataxia type 7 (SCA7) astrocytes. Proc. Natl. Acad. Sci. USA 2012, 109, 21319–21324. [Google Scholar] [CrossRef]
- Yanicostas, C.; Barbieri, E.; Hibi, M.; Brice, A.; Stevanin, G.; Soussi-Yanicostas, N. Requirement for Zebrafish Ataxin-7 in Differentiation of Photoreceptors and Cerebellar Neurons. PLoS ONE 2012, 7, e50705. [Google Scholar] [CrossRef]
- Libby, R.T.; Hagerman, K.A.; Pineda, V.V.; Lau, R.; Cho, D.H.; Baccam, S.L.; Axford, M.M.; Cleary, J.D.; Moore, J.M.; Sopher, B.L.; et al. CTCF cis-Regulates Trinucleotide Repeat Instability in an Epigenetic Manner: A Novel Basis for Mutational Hot Spot Determination. PLoS Genet. 2008, 4, e1000257. [Google Scholar] [CrossRef] [PubMed]
- Koob, M.D.; Moseley, M.L.; Schut, L.J.; Benzow, K.A.; Bird, T.D.; Day, J.W.; Ranum, L.P. An untranslated CTG expansion causes a novel form of spinocerebellar ataxia (SCA8). Nat. Genet. 1999, 21, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Shizuka, M.; Watanabe, M.; Okamoto, K.; Shoji, M. Molecular and clinical analyses of spinocerebellar ataxia type 8 in Japan. Neurology 2000, 54, 950–955. [Google Scholar] [CrossRef] [PubMed]
- Moseley, M.L.; Schut, L.J.; Bird, T.D.; Koob, M.D.; Day, J.W.; Ranum, L.P.W. SCA8 CTG repeat: En masse contractions in sperm and intergenerational sequence changes may play a role in reduced penetrance. Hum. Mol. Genet. 2000, 9, 2125–2130. [Google Scholar] [CrossRef]
- Day, J.W.; Schut, L.J.; Moseley, M.L.; Durand, A.C.; Ranum, L.P.W. Spinocerebellar ataxia type 8: Clinical features in a large family. Neurology 2000, 55, 649–657. [Google Scholar] [CrossRef]
- Mutsuddi, M.; Marshall, C.M.; Benzow, K.A.; Koob, M.D.; Rebay, I. The Spinocerebellar Ataxia 8 Noncoding RNA Causes Neurodegeneration and Associates with Staufen in Drosophila. Curr. Biol. 2004, 14, 302–308. [Google Scholar] [CrossRef]
- Daughters, R.S.; Tuttle, D.L.; Gao, W.; Ikeda, Y.; Moseley, M.L.; Ebner, T.J.; Swanson, M.S.; Ranum, L.P.W. RNA Gain-of-Function in Spinocerebellar Ataxia Type 8. PLoS Genet. 2009, 5, e1000600. [Google Scholar] [CrossRef]
- Ayhan, F.; Perez, B.A.; Shorrock, H.K.; Zu, T.; Banez-Coronel, M.; Reid, T.; Furuya, H.; Clark, H.B.; Troncoso, J.C.; Ross, C.A.; et al. SCA 8 RAN polySer protein preferentially accumulates in white matter regions and is regulated by eIF 3F. EMBO J. 2018, 37, e99023. [Google Scholar] [CrossRef]
- Nemes, J.P.; Benzow, K.A.; Koob, M.D. The SCA8 transcript is an antisense RNA to a brain-specific transcript encoding a novel actin-binding protein (KLHL1). Hum. Mol. Genet. 2000, 9, 1543–1551. [Google Scholar] [CrossRef]
- Benzow, K.A.; Koob, M.D. The transcript () is evolutionarily conserved. Mamm. Genome 2002, 13, 134–141. [Google Scholar] [CrossRef]
- He, Y.; Zu, T.; Benzow, K.A.; Orr, H.T.; Clark, H.B.; Koob, M.D. Targeted Deletion of a Single Sca8 Ataxia Locus Allele in Mice Causes Abnormal Gait, Progressive Loss of Motor Coordination, and Purkinje Cell Dendritic Deficits. J. Neurosci. 2006, 26, 9975–9982. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Daughters, R.S.; Ranum, L.P.W. Bidirectional expression of the SCA8 expansion mutation: One mutation, two genes. Cerebellum 2008, 7, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Niimi, Y.; Takahashi, M.; Sugawara, E.; Umeda, S.; Obayashi, M.; Sato, N.; Ishiguro, T.; Higashi, M.; Eishi, Y.; Mizusawa, H.; et al. Abnormal RNA structures (RNA foci) containing a penta-nucleotide repeat (UGGAA)nin the Purkinje cell nucleus is associated with spinocerebellar ataxia type 31 pathogenesis. Neuropathology 2013, 33, 600–611. [Google Scholar] [CrossRef] [PubMed]
- Ishiguro, T.; Sato, N.; Ueyama, M.; Fujikake, N.; Sellier, C.; Kanegami, A.; Tokuda, E.; Zamiri, B.; Gall-Duncan, T.; Mirceta, M.; et al. Regulatory Role of RNA Chaperone TDP-43 for RNA Misfolding and Repeat-Associated Translation in SCA31. Neuron 2017, 94, 108–124.e7. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, K.; Nagai, Y. Molecular Mechanisms and Future Therapeutics for Spinocerebellar Ataxia Type 31 (SCA31). Neurother. 2019, 16, 1106–1114. [Google Scholar] [CrossRef] [PubMed]
- Bidichandani, S.I.; Delatycki, M.B. Friedreich Ataxia. In GeneReviews®; Adam, M.P., Ardinger, H.H., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Stephens, K., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993–2020. [Google Scholar]
- Campuzano, V.; Montermini, L.; Moltò, M.D.; Pianese, L.; Cossée, M.; Cavalcanti, F.; Monros, E.; Rodius, F.; Duclos, F.; Monticelli, A.; et al. Friedreich’s Ataxia: Autosomal Recessive Disease Caused by an Intronic GAA Triplet Repeat Expansion. Science 1996, 271, 1423–1427. [Google Scholar] [CrossRef] [PubMed]
- Naeije, G.; Rai, M.; Allaerts, N.; Sjogard, M.; De Tiège, X.; Pandolfo, M. Cerebellar cognitive disorder parallels cerebellar motor symptoms in Friedreich ataxia. Ann. Clin. Transl. Neurol. 2020, 7, 1050–1054. [Google Scholar] [CrossRef]
- Saveliev, A.; Everett, C.; Sharpe, T.; Webster, Z.; Festenstein, R. DNA triplet repeats mediate heterochromatin-protein-1-sensitive variegated gene silencing. Nat. Cell Biol. 2003, 422, 909–913. [Google Scholar] [CrossRef]
- Greene, E.; Mahishi, L.; Entezam, A.; Kumari, D.; Usdin, K. Repeat-induced epigenetic changes in intron 1 of the frataxin gene and its consequences in Friedreich ataxia. Nucleic Acids Res. 2007, 35, 3383–3390. [Google Scholar] [CrossRef]
- Herman, D.; Jenssen, K.; Burnett, R.; Soragni, E.; Perlman, S.L.; Gottesfeld, J.M. Histone deacetylase inhibitors reverse gene silencing in Friedreich’s ataxia. Nat. Chem. Biol. 2006, 2, 551–558. [Google Scholar] [CrossRef]
- Al-Mahdawi, S.; Pinto, R.M.; Ismail, O.; Varshney, D.; Lymperi, S.; Sandi, C.; Trabzuni, D.; Pook, M.A. The Friedreich ataxia GAA repeat expansion mutation induces comparable epigenetic changes in human and transgenic mouse brain and heart tissues. Hum. Mol. Genet. 2007, 17, 735–746. [Google Scholar] [CrossRef] [PubMed]
- Chutake, Y.K.; Costello, W.N.; Lam, C.; Bidichandani, S.I. Altered nucleosome positioning at the transcription start site and deficient transcriptional initiation in Friedreich ataxia. J. Biol. Chem. 2014, 289, 15194–15202. [Google Scholar] [CrossRef] [PubMed]
- Rötig, A.; De Lonlay, P.; Chretien, D.; Foury, F.; Koenig, M.; Sidi, D.; Munnich, A.; Rustin, P. Aconitase and mitochondrial iron–sulphur protein deficiency in Friedreich ataxia. Nat. Genet. 1997, 17, 215–217. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Singh, A.; Crooks, D.R.; Dai, X.; Cong, Z.; Pan, L.; Ha, D.; Rouault, T.A. Expression of Human Frataxin Is Regulated by Transcription Factors SRF and TFAP2. PLoS ONE 2010, 5, e12286. [Google Scholar] [CrossRef] [PubMed]
- De Biase, I.; Chutake, Y.K.; Rindler, P.M.; Bidichandani, S.I. Epigenetic Silencing in Friedreich Ataxia Is Associated with Depletion of CTCF (CCCTC-Binding Factor) and Antisense Transcription. PLoS ONE 2009, 4, e7914. [Google Scholar] [CrossRef] [PubMed]
- Renton, A.E.; Majounie, E.; Waite, A.; Simón-Sánchez, J.; Rollinson, S.; Gibbs, J.R.; Schymick, J.C.; Laaksovirta, H.; Van Swieten, J.C.; Myllykangas, L.; et al. A Hexanucleotide Repeat Expansion in C9ORF72 Is the Cause of Chromosome 9p21-Linked ALS-FTD. Neuron 2011, 72, 257–268. [Google Scholar] [CrossRef] [PubMed]
- DeJesus-Hernandez, M.; MacKenzie, I.R.; Boeve, B.F.; Boxer, A.L.; Baker, M.; Rutherford, N.J.; Nicholson, A.M.; Finch, N.A.; Flynn, H.; Adamson, J.; et al. Expanded GGGGCC Hexanucleotide Repeat in Noncoding Region of C9ORF72 Causes Chromosome 9p-Linked FTD and ALS. Neuron 2011, 72, 245–256. [Google Scholar] [CrossRef]
- Kobayashi, H.; Abe, K.; Matsuura, T.; Ikeda, Y.; Hitomi, T.; Akechi, Y.; Habu, T.; Liu, W.; Okuda, H.; Koizumi, A. Expansion of Intronic GGCCTG Hexanucleotide Repeat in NOP56 Causes SCA36, a Type of Spinocerebellar Ataxia Accompanied by Motor Neuron Involvement. Am. J. Hum. Genet. 2011, 89, 121–130. [Google Scholar] [CrossRef]
- García-Murias, M.; Quintáns, B.; Arias, M.; Seixas, A.I.; Cacheiro, P.; Tarrío, R.; Pardo, J.; Millán, M.J.; Arias-Rivas, S.; Blanco-Arias, P.; et al. ‘Costa da Morte’ ataxia is spinocerebellar ataxia 36: Clinical and genetic characterization. Brain 2012, 135, 1423–1435. [Google Scholar] [CrossRef]
- Liu, W.; Ikeda, Y.; Hishikawa, N.; Yamashita, T.; Deguchi, K.; Abe, K. Characteristic RNA foci of the abnormal hexanucleotide GGCCUG repeat expansion in spinocerebellar ataxia type 36 (Asidan). Eur. J. Neurol. 2014, 21, 1377–1386. [Google Scholar] [CrossRef]
- Cooper-Knock, J.; Walsh, M.J.; Higginbottom, A.; Highley, J.R.; Dickman, M.J.; Edbauer, D.; Ince, P.G.; Wharton, S.B.; Wilson, S.A.; Kirby, J.; et al. Sequestration of multiple RNA recognition motif-containing proteins by C9orf72 repeat expansions. Brain 2014, 137, 2040–2051. [Google Scholar] [CrossRef] [PubMed]
- Ciura, S.; Lattante, S.; Le Ber, I.; Latouche, M.; Tostivint, H.; Brice, A.; Kabashi, E. Loss of function of C9orf72 causes motor deficits in a zebrafish model of Amyotrophic Lateral Sclerosis. Ann. Neurol. 2013, 74, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Therrien, M.; Rouleau, G.A.; Dion, P.A.; Parker, J.A. Deletion of C9ORF72 Results in Motor Neuron Degeneration and Stress Sensitivity in C. elegans. PLoS ONE 2013, 8, e83450. [Google Scholar] [CrossRef] [PubMed]
- Zu, T.; Liu, Y.; Bañez-Coronel, M.; Reid, T.; Pletnikova, O.; Lewis, J.; Miller, T.M.; Harms, M.B.; Falchook, A.E.; Subramony, S.H.; et al. RAN proteins and RNA foci from antisense transcripts in C9ORF72 ALS and frontotemporal dementia. Proc. Natl. Acad. Sci. USA 2013, 110, E4968–E4977. [Google Scholar] [CrossRef] [PubMed]
- Gendron, T.F.; Bieniek, K.F.; Zhang, Y.-J.; Jansen-West, K.; Ash, P.E.A.; Caulfield, T.; Daughrity, L.; Dunmore, J.H.; Castanedes-Casey, M.; Chew, J.; et al. Antisense transcripts of the expanded C9ORF72 hexanucleotide repeat form nuclear RNA foci and undergo repeat-associated non-ATG translation in c9FTD/ALS. Acta Neuropathol. 2013, 126, 829–844. [Google Scholar] [CrossRef] [PubMed]
- Rizzu, P.; Blauwendraat, C.; Heetveld, S.; Lynes, E.M.; Castillo-Lizardo, M.; Dhingra, A.; Pyz, E.; Hobert, M.; Synofzik, M.; Simón-Sánchez, J.; et al. C9orf72 is differentially expressed in the central nervous system and myeloid cells and consistently reduced in C9orf72, MAPT and GRN mutation carriers. Acta Neuropathol. Commun. 2016, 4, 37. [Google Scholar] [CrossRef]
- O’Rourke, J.G.; Bogdanik, L.; Muhammad, A.; Gendron, T.F.; Kim, K.J.; Austin, A.; Cady, J.; Liu, E.Y.; Zarrow, J.; Grant, S.; et al. C9orf72 BAC Transgenic Mice Display Typical Pathologic Features of ALS/FTD. Neuron 2015, 88, 892–901. [Google Scholar] [CrossRef]
- Peters, O.M.; Cabrera, G.T.; Tran, H.; Gendron, T.F.; McKeon, J.E.; Metterville, J.; Weiss, A.; Wightman, N.; Salameh, J.; Kim, J.; et al. Human C9ORF72 Hexanucleotide Expansion Reproduces RNA Foci and Dipeptide Repeat Proteins but Not Neurodegeneration in BAC Transgenic Mice. Neuron 2015, 88, 902–909. [Google Scholar] [CrossRef]
- Liu, Y.; Pattamatta, A.; Zu, T.; Reid, T.; Bardhi, O.; Borchelt, D.R.; Yachnis, A.T.; Ranum, L.P. C9orf72 BAC Mouse Model with Motor Deficits and Neurodegenerative Features of ALS/FTD. Neuron 2016, 90, 521–534. [Google Scholar] [CrossRef]
- Chew, J.; Gendron, T.F.; Prudencio, M.; Sasaguri, H.; Zhang, Y.-J.; Castanedes-Casey, M.; Lee, C.W.; Jansen-West, K.; Kurti, A.; Murray, M.E.; et al. C9ORF72 repeat expansions in mice cause TDP-43 pathology, neuronal loss, and behavioral deficits. Science 2015, 348, 1151–1154. [Google Scholar] [CrossRef]
- Matsuzono, K.; Imamura, K.; Murakami, N.; Tsukita, K.; Yamamoto, T.; Izumi, Y.; Kaji, R.; Ohta, Y.; Yamashita, T.; Abe, K.; et al. Antisense Oligonucleotides Reduce RNA Foci in Spinocerebellar Ataxia 36 Patient iPSCs. Mol. Ther. Nucleic Acids 2017, 8, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Moens, T.G.; Mizielinska, S.; Niccoli, T.; Mitchell, J.S.; Thoeng, A.; Ridler, C.E.; Grönke, S.; Esser, J.; Heslegrave, A.; Zetterberg, H.; et al. Sense and antisense RNA are not toxic in Drosophila models of C9orf72-associated ALS/FTD. Acta Neuropathol. 2018, 135, 445–457. [Google Scholar] [CrossRef] [PubMed]
- Mizielinska, S.; Grönke, S.; Niccoli, T.; Ridler, C.E.; Clayton, E.L.; Devoy, A.; Moens, T.; Norona, F.E.; Woollacott, I.O.C.; Pietrzyk, J.; et al. C9orf72 repeat expansions cause neurodegeneration in Drosophila through arginine-rich proteins. Science 2014, 345, 1192–1194. [Google Scholar] [CrossRef] [PubMed]
- Swinnen, B.; Bento-Abreu, A.; Gendron, T.F.; Boeynaems, S.; Bogaert, E.; Nuyts, R.; Timmers, M.; Scheveneels, W.; Hersmus, N.; Wang, J.; et al. A zebrafish model for C9orf72 ALS reveals RNA toxicity as a pathogenic mechanism. Acta Neuropathol. 2018, 135, 427–443. [Google Scholar] [CrossRef] [PubMed]
- Verkerk, A.J.; Pieretti, M.; Sutcliffe, J.S.; Fu, Y.-H.; Kuhl, D.P.; Pizzuti, A.; Reiner, O.; Richards, S.; Victoria, M.F.; Zhang, F.; et al. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell 1991, 65, 905–914. [Google Scholar] [CrossRef]
- Hagerman, R.J.; Leehey, M.; Heinrichs, W.; Tassone, F.; Wilson, R.; Hills, J.; Grigsby, J.; Gage, B. Intention tremor, parkinsonism, and generalized brain atrophy in male carriers of fragile X. Neurology 2001, 57, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Hansen, R.; Gartler, S.M.; Scott, C.; Chen, S.-H.; Xlaird, C.M. Methylation analysis of CGG sites in the CpG island of the human FMR1 gene. Hum. Mol. Genet. 1992, 1, 571–578. [Google Scholar] [CrossRef]
- Pieretti, M.; Zhang, F.; Fu, Y.-H.; Warren, S.T.; Oostra, B.A.; Caskey, C.; Nelson, D.L. Absence of expression of the FMR-1 gene in fragile X syndrome. Cell 1991, 66, 817–822. [Google Scholar] [CrossRef]
- Coffee, B.; Zhang, F.; Warren, S.T.; Reines, D. Acetylated histones are associated with FMR1 in normal but not fragile X-syndrome cells. Nat. Genet. 1999, 22, 98–101. [Google Scholar] [CrossRef]
- Coffee, B.; Zhang, F.; Ceman, S.; Warren, S.T.; Reines, D. Histone Modifications Depict an Aberrantly Heterochromatinized FMR1 Gene in Fragile X Syndrome. Am. J. Hum. Genet. 2002, 71, 923–932. [Google Scholar] [CrossRef]
- Tassone, F.; Beilina, A.; Carosi, C.; Albertosi, S.; Bagni, C.; Li, L.; Glover, K.; Bentley, D.; Hagerman, P.J. Elevated FMR1 mRNA in premutation carriers is due to increased transcription. RNA 2007, 13, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Tassone, F.; Hagerman, R.J.; Taylor, A.K.; Gane, L.W.; Godfrey, T.E.; Hagerman, P.J. Elevated Levels of FMR1 mRNA in Carrier Males: A New Mechanism of Involvement in the Fragile-X Syndrome. Am. J. Hum. Genet. 2000, 66, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Kenneson, A.; Zhang, F.; Hagedorn, C.H.; Warren, S.T. Reduced FMRP and increased FMR1 transcription is proportionally associated with CGG repeat number in intermediate-length and premutation carriers. Hum. Mol. Genet. 2001, 10, 1449–1454. [Google Scholar] [CrossRef] [PubMed]
- Greco, C.M.; Hagerman, R.J.; Tassone, F.; Chudley, A.E.; Del Bigio, M.R.; Jacquemont, S.; Leehey, M. Neuronal intranuclear inclusions in a new cerebellar tremor/ataxia syndrome among fragile X carriers. Brain 2002, 125, 1760–1771. [Google Scholar] [CrossRef] [PubMed]
- Tassone, F.; Iwahashi, C.; Hagerman, P.J. FMR1 RNA within the Intranuclear Inclusions of Fragile X-Associated Tremor/Ataxia Syndrome (FXTAS). RNA Biol. 2004, 1, 103–105. [Google Scholar] [CrossRef] [PubMed]
- Iwahashi, C.K.; Yasui, D.H.; An, H.-J.; Greco, C.M.; Tassone, F.; Nannen, K.; Babineau, B.; Lebrilla, C.B.; Hagerman, P. Protein composition of the intranuclear inclusions of FXTAS. Brain 2005, 129, 256–271. [Google Scholar] [CrossRef] [PubMed]
- Bontekoe, C.J.M.; Bakker, C.E.; Nieuwenhuizen, I.M.; Van Der Linde, H.; Lans, H.; De Lange, D.; Hirst, M.C.; Oostra, B.A. Instability of a (CGG)98 repeat in the Fmr1 promoter. Hum. Mol. Genet. 2001, 10, 1693–1699. [Google Scholar] [CrossRef]
- Willemsen, R.; Hoogeveen-Westerveld, M.; Reis, S.; Holstege, J.; Severijnen, L.-A.W.; Nieuwenhuizen, I.M.; Schrier, M.; Van Unen, L.; Tassone, F.; Hoogeveen, A.T.; et al. The FMR1 CGG repeat mouse displays ubiquitin-positive intranuclear neuronal inclusions; implications for the cerebellar tremor/ataxia syndrome. Hum. Mol. Genet. 2003, 12, 949–959. [Google Scholar] [CrossRef]
- Hashem, V.; Galloway, J.N.; Mori, M.; Willemsen, R.; Oostra, B.A.; Paylor, R.; Nelson, D.L. Ectopic expression of CGG containing mRNA is neurotoxic in mammals. Hum. Mol. Genet. 2009, 18, 2443–2451. [Google Scholar] [CrossRef]
- Jin, P.; Zarnescu, D.C.; Zhang, F.; Pearson, C.E.; Lucchesi, J.C.; Moses, K.; Warren, S.T. RNA-Mediated Neurodegeneration Caused by the Fragile X Premutation rCGG Repeats in Drosophila. Neuron 2003, 39, 739–747. [Google Scholar] [CrossRef]
- Sofola, O.A.; Jin, P.; Qin, Y.; Duan, R.; Liu, H.; De Haro, M.; Nelson, D.L.; Botas, J. RNA-Binding Proteins hnRNP A2/B1 and CUGBP1 Suppress Fragile X CGG Premutation Repeat-Induced Neurodegeneration in a Drosophila Model of FXTAS. Neuron 2007, 55, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.; Duan, R.; Qurashi, A.; Qin, Y.; Tian, D.; Rosser, T.C.; Liu, H.; Feng, Y.; Warren, S.T. Pur α Binds to rCGG Repeats and Modulates Repeat-Mediated Neurodegeneration in a Drosophila Model of Fragile X Tremor/Ataxia Syndrome. Neuron 2007, 55, 556–564. [Google Scholar] [CrossRef]
- Wojciechowska, M.; Krzyzosiak, W.J. Cellular toxicity of expanded RNA repeats: Focus on RNA foci. Hum. Mol. Genet. 2011, 20, 3811–3821. [Google Scholar] [CrossRef] [PubMed]
- Todd, P.K.; Oh, S.Y.; Krans, A.; He, F.; Sellier, C.; Frazer, M.; Renoux, A.J.; Chen, K.-C.; Scaglione, K.M.; Basrur, V.; et al. CGG Repeat-Associated Translation Mediates Neurodegeneration in Fragile X Tremor Ataxia Syndrome. Neuron 2013, 78, 440–455. [Google Scholar] [CrossRef] [PubMed]
- Krans, A.; Skariah, G.; Zhang, Y.; Bayly, B.; Todd, P.K. Neuropathology of RAN translation proteins in fragile X-associated tremor/ataxia syndrome. Acta Neuropathol. Commun. 2019, 7, 152. [Google Scholar] [CrossRef]
- Buijsen, R.A.M.; Sellier, C.; Severijnen, L.-A.W.F.M.; Oulad-Abdelghani, M.; Verhagen, R.F.M.; Berman, R.F.; Charlet-Berguerand, N.; Willemsen, R.; Hukema, R.K. FMRpolyG-positive inclusions in CNS and non-CNS organs of a fragile X premutation carrier with fragile X-associated tremor/ataxia syndrome. Acta Neuropathol. Commun. 2014, 2, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Sofola, O.A.; Jin, P.; Botas, J.; Nelson, D.L. Argonaute-2-dependent rescue of a Drosophila model of FXTAS by FRAXE premutation repeat. Hum. Mol. Genet. 2007, 16, 2326–2332. [Google Scholar] [CrossRef]
- Krans, A.; Kearse, M.G.; Todd, P.K. Repeat-associated non-AUG translation from antisense CCG repeats in fragile X tremor/ataxia syndrome. Ann. Neurol. 2016, 80, 871–881. [Google Scholar] [CrossRef]
- Sone, J.; Mori, K.; Inagaki, T.; Katsumata, R.; Takagi, S.; Yokoi, S.; Araki, K.; Kato, T.; Nakamura, T.; Koike, H.; et al. Clinicopathological features of adult-onset neuronal intranuclear inclusion disease. Brain 2016, 139, 3170–3186. [Google Scholar] [CrossRef]
- Fiddes, I.T.; Lodewijk, G.A.; Mooring, M.; Bosworth, C.M.; Ewing, A.D.; Mantalas, G.L.; Novak, A.M.; Bout, A.V.D.; Bishara, A.; Rosenkrantz, J.L.; et al. Human-Specific NOTCH2NL Genes Affect Notch Signaling and Cortical Neurogenesis. Cell 2018, 173, 1356–1369.e22. [Google Scholar] [CrossRef]
- Suzuki, I.K.; Gacquer, D.; Van Heurck, R.; Kumar, D.; Wojno, M.; Bilheu, A.; Herpoel, A.; Lambert, N.; Cheron, J.; Polleux, F.; et al. Human-Specific NOTCH2NL Genes Expand Cortical Neurogenesis through Delta/Notch Regulation. Cell 2018, 173, 1370–1384.e16. [Google Scholar] [CrossRef] [PubMed]
- Sone, J.; Mitsuhashi, S.; Fujita, A.; Mizuguchi, T.; Hamanaka, K.; Mori, K.; Koike, H.; Hashiguchi, A.; Takashima, H.; Sugiyama, H.; et al. Long-read sequencing identifies GGC repeat expansions in NOTCH2NLC associated with neuronal intranuclear inclusion disease. Nat. Genet. 2019, 51, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- Rudnicki, D.D.; Pletnikova, O.; Vonsattel, J.-P.G.; Ross, C.A.; Margolis, R.L. A Comparison of Huntington Disease and Huntington Disease-Like 2 Neuropathology. J. Neuropathol. Exp. Neurol. 2008, 67, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, M.E.; Ambrose, C.M.; Duyao, M.P.; Myers, R.H.; Lin, C.; Srinidhi, L.; Barnes, G.; Taylor, S.A.; James, M.; Groot, N.; et al. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell 1993, 72, 971–983. [Google Scholar] [CrossRef]
- Margolis, R.L.; O’Hearn, E.; Rosenblatt, A.; Willour, V.; Holmes, S.E.; Franz, M.L.; Callahan, C.; Hwang, H.S.; Troncoso, J.C.; Ross, C.A. A disorder similar to Huntington’s disease is associated with a novel CAG repeat expansion. Ann. Neurol. 2001, 50, 373–380. [Google Scholar] [CrossRef]
- Holmes, S.E.; O’Hearn, E.; Rosenblatt, A.; Callahan, C.; Hwang, H.S.; Ingersoll-Ashworth, R.G.; Fleisher, A.; Stevanin, G.; Brice, A.; Potter, N.T.; et al. A repeat expansion in the gene encoding junctophilin-3 is associated with Huntington disease–like 2. Nat. Genet. 2001, 29, 377–378. [Google Scholar] [CrossRef]
- Mangiarini, L.; Sathasivam, K.; Seller, M.; Cozens, B.; Harper, A.; Hetherington, C.; Lawton, M.; Trottier, Y.; Lehrach, H.; Davies, S.W.; et al. Exon 1 of the HD Gene with an Expanded CAG Repeat Is Sufficient to Cause a Progressive Neurological Phenotype in Transgenic Mice. Cell 1996, 87, 493–506. [Google Scholar] [CrossRef]
- Davies, S.W.; Turmaine, M.; Cozens, B.A.; DiFiglia, M.; Sharp, A.H.; Ross, C.A.; Scherzinger, E.; Wanker, E.E.; Mangiarini, L.; Bates, G.P. Formation of Neuronal Intranuclear Inclusions Underlies the Neurological Dysfunction in Mice Transgenic for the HD Mutation. Cell 1997, 90, 537–548. [Google Scholar] [CrossRef]
- Schilling, G.; Becher, M.W.; Sharp, A.H.; Jinnah, H.A.; Duan, K.; Kotzuk, J.A.; Slunt, H.H.; Ratovitski, T.; Cooper, J.K.; Jenkins, Z.W.N.G.C.N.A.; et al. Intranuclear inclusions and neuritic aggregates in transgenic mice expressing a mutant N-terminal fragment of huntingtin [published erratum appears in Hum Mol Genet 1999 May;8(5):943]. Hum. Mol. Genet. 1999, 8, 397–407. [Google Scholar] [CrossRef]
- Cooper, J.K.; Schilling, G.; Peters, M.F.; Herring, W.J.; Sharp, A.H.; Kaminsky, Z.; Masone, J.; Khan, F.A.; Delanoy, M.; Borchelt, D.R.; et al. Truncated N-terminal fragments of huntingtin with expanded glutamine repeats form nuclear and cytoplasmic aggregates in cell culture. Hum. Mol. Genet. 1998, 7, 783–790. [Google Scholar] [CrossRef]
- DiFiglia, M.; Sapp, E.; Chase, K.O.; Davies, S.W.; Bates, G.P.; Vonsattel, J.P.; Aronin, N. Aggregation of Huntingtin in Neuronal Intranuclear Inclusions and Dystrophic Neurites in Brain. Science 1997, 277, 1990–1993. [Google Scholar] [CrossRef] [PubMed]
- Becher, M.W.; Kotzuk, J.A.; Sharp, A.H.; Davies, S.W.; Bates, G.P.; Priceaef, D.L.; Rossbfg, C.A. Intranuclear Neuronal Inclusions in Huntington’s Disease and Dentatorubral and Pallidoluysian Atrophy: Correlation between the Density of Inclusions andIT15CAG Triplet Repeat Length. Neurobiol. Dis. 1998, 4, 387–397. [Google Scholar] [CrossRef]
- Bates, G.P.; Dorsey, R.; Gusella, J.F.; Hayden, M.R.; Kay, C.; Leavitt, B.R.; Nance, M.; Ross, C.A.; Scahill, R.I.; Wetzel, R.; et al. Huntington disease. Nat. Rev. Dis. Prim. 2015, 1, 15005. [Google Scholar] [CrossRef] [PubMed]
- Steffan, J.S.; Kazantsev, A.; Spasic-Boskovic, O.; Greenwald, M.; Zhu, Y.-Z.; Gohler, H.; Wanker, E.E.; Bates, G.P.; Housman, D.E.; Thompson, L.M. The Huntington’s disease protein interacts with p53 and CREB-binding protein and represses transcription. Proc. Natl. Acad. Sci. USA 2000, 97, 6763–6768. [Google Scholar] [CrossRef] [PubMed]
- Nucifora, N.C., Jr.; Sasaki, M.; Peters, M.F.; Huang, H.; Cooper, J.K.; Yamada, M.; Takahashi, H.; Tsuji, S.; Troncoso, J.; Dawson, V.L.; et al. Interference by Huntingtin and Atrophin-1 with CBP-Mediated Transcription Leading to Cellular Toxicity. Science 2001, 291, 2423–2428. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Nucifora, F.C.; Ross, C.A.; DeFranco, D.B. Cell death triggered by polyglutamine-expanded huntingtin in a neuronal cell line is associated with degradation of CREB-binding protein. Hum. Mol. Genet. 2003, 12, 1–12. [Google Scholar] [CrossRef]
- Boutell, J.M.; Thomas, P.; Neal, J.W.; Weston, V.J.; Duce, J.A.; Harper, P.S.; Jones, A.L. Aberrant interactions of transcriptional repressor proteins with the Huntington’s disease gene product, huntingtin. Hum. Mol. Genet. 1999, 8, 1647–1655. [Google Scholar] [CrossRef]
- Huang, C.C.; Faber, P.W.; Persichetti, F.; Mittal, V.; Vonsattel, J.-P.; Macdonald, M.E.; Gusella, J.F. Amyloid formation by mutant huntingtin: Threshold, progressivity and recruitment of normal polyglutamine proteins. Somat. Cell Mol. Genet. 1998, 24, 217–233. [Google Scholar] [CrossRef]
- De Mezer, M.; Wojciechowska, M.; Napierala, M.; Sobczak, K.; Krzyzosiak, W.J. Mutant CAG repeats of Huntingtin transcript fold into hairpins, form nuclear foci and are targets for RNA interference. Nucleic Acids Res. 2011, 39, 3852–3863. [Google Scholar] [CrossRef]
- Sun, X.; Li, P.P.; Zhu, S.; Cohen, R.E.; Marque, L.O.; Ross, C.A.; Pulst, S.M.; Chan, H.Y.E.; Margolis, R.L.; Rudnicki, D.D. Nuclear retention of full-length HTT RNA is mediated by splicing factors MBNL1 and U2AF65. Sci. Rep. 2015, 5, 12521. [Google Scholar] [CrossRef]
- Grima, J.C.; Daigle, J.G.; Arbez, N.; Cunningham, K.C.; Zhang, K.; Ochaba, J.; Geater, C.; Morozko, E.; Stocksdale, J.; Glatzer, J.C.; et al. Mutant Huntingtin Disrupts the Nuclear Pore Complex. Neuron 2017, 94, 93–107.e6. [Google Scholar] [CrossRef] [PubMed]
- Tsoi, H.; Chan, H.Y.E. Expression of Expanded CAG Transcripts Triggers Nucleolar Stress in Huntington’s Disease. Cerebellum 2013, 12, 310–312. [Google Scholar] [CrossRef] [PubMed]
- Evers, M.M.; Schut, M.H.; Pepers, B.A.; Atalar, M.; Van Belzen, M.J.; Faull, R.L.; Roos, R.A.; Van Roon-Mom, W.M.C. Making (anti-) sense out of huntingtin levels in Huntington disease. Mol. Neurodegener. 2015, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bañez-Coronel, M.; Ayhan, F.; Tarabochia, A.D.; Zu, T.; Perez, B.A.; Tusi, S.K.; Pletnikova, O.; Borchelt, D.R.; Ross, C.A.; Margolis, R.L.; et al. RAN Translation in Huntington Disease. Neuron 2015, 88, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Báñez-Coronel, M.; Porta, S.; Kagerbauer, B.; Mateu-Huertas, E.; Pantano, L.; Ferrer, I.; Guzmán, M.; Estivill, X.; Martí, E. A Pathogenic Mechanism in Huntington’s Disease Involves Small CAG-Repeated RNAs with Neurotoxic Activity. PLoS Genet. 2012, 8, e1002481. [Google Scholar] [CrossRef]
- Rudnicki, D.D.; Margolis, R.L.; Pearson, C.E.; Krzyzosiak, W.J. Diced triplets expose neurons to RISC. PLoS Genet. 2012, 8, e1002545. [Google Scholar] [CrossRef]
- Lawlor, K.T.; O’Keefe, L.V.; Samaraweera, S.E.; McLeod, C.J.; Dang, T.H.; Suter, C.M.; Van Eyk, C.L.; Maloney, C.A.; Richards, R.I. Double-stranded RNA is pathogenic in Drosophila models of expanded repeat neurodegenerative diseases. Hum. Mol. Genet. 2011, 20, 3757–3768. [Google Scholar] [CrossRef]
- Nishi, M.; Mizushima, A.; Nakagawara, K.-I.; Takeshima, H. Characterization of Human Junctophilin Subtype Genes. Biochem. Biophys. Res. Commun. 2000, 273, 920–927. [Google Scholar] [CrossRef]
- Takeshima, H. JunctophilinsA Novel Family of Junctional Membrane Complex Proteins. Mol. Cell 2000, 6, 11–22. [Google Scholar] [CrossRef]
- Nishia, M.; Hashimotobc, K.; Kuriyamad, K.; Komazakid, S.; Kanobc, M.; Shibatad, S.; Takeshima, H. Motor Discoordination in Mutant Mice Lacking Junctophilin Type 3. Biochem. Biophys. Res. Commun. 2002, 292, 318–324. [Google Scholar] [CrossRef]
- Rudnicki, B.D.; Holmes, S.E.; Lin, M.W.; Thornton, C.A.; Ross, C.A.; Margolis, R.L. Huntington’s disease-like 2 is associated with CUG repeat-containing RNA foci. Ann. Neurol. 2007, 61, 272–282. [Google Scholar] [CrossRef]
- Wilburn, B.; Rudnicki, D.D.; Zhao, J.; Weitz, T.M.; Cheng, Y.; Gu, X.; Greiner, E.; Park, C.S.; Wang, N.; Sopher, B.L.; et al. An Antisense CAG Repeat Transcript at JPH3 Locus Mediates Expanded Polyglutamine Protein Toxicity in Huntington’s Disease-like 2 Mice. Neuron 2011, 70, 427–440. [Google Scholar] [CrossRef] [PubMed]
- Thornton, C.A. Myotonic Dystrophy. Neurol. Clin. 2014, 32, 705–719. [Google Scholar] [CrossRef] [PubMed]
- Brook, J.D.; McCurrach, M.E.; Harley, H.G.; Buckler, A.J.; Church, D.; Aburatani, H.; Hunter, K.; Stanton, V.P.; Thirion, J.-P.; Hudson, T.; et al. Molecular basis of myotonic dystrophy: Expansion of a trinucleotide (CTG) repeat at the 3′ end of a transcript encoding a protein kinase family member. Cell 1992, 68, 799–808. [Google Scholar] [CrossRef]
- Mahadevan, M.; Tsilfidis, C.; Sabourin, L.; Shutler, G.; Amemiya, C.; Jansen, G.; Neville, C.; Narang, M.; Barcelo, J.; O’Hoy, K.; et al. Myotonic dystrophy mutation: An unstable CTG repeat in the 3′ untranslated region of the gene. Science 1992, 255, 1253–1255. [Google Scholar] [CrossRef] [PubMed]
- Panigrahi, G.B.; Cleary, J.D.; Pearson, C.E. In Vitro(CTG)·(CAG) Expansions and Deletions by Human Cell Extracts. J. Biol. Chem. 2002, 277, 13926–13934. [Google Scholar] [CrossRef] [PubMed]
- Liquori, C.L.; Ricker, K.; Moseley, M.L.; Jacobsen, J.F.; Kress, W.; Naylor, S.L.; Day, J.W.; Ranum, L.P.W. Myotonic Dystrophy Type 2 Caused by a CCTG Expansion in Intron 1 of ZNF9. Science 2001, 293, 864–867. [Google Scholar] [CrossRef] [PubMed]
- Steinbach, P.; Gläser, D.; Vogel, W.; Wolf, M.; Schwemmle, S. The DMPK Gene of Severely Affected Myotonic Dystrophy Patients Is Hypermethylated Proximal to the Largely Expanded CTG Repeat. Am. J. Hum. Genet. 1998, 62, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Taneja, K.L.; McCurrach, M.; Schalling, M.; Housman, D.E.; Singer, R.H. Foci of trinucleotide repeat transcripts in nuclei of myotonic dystrophy cells and tissues. J. Cell Biol. 1995, 128, 995–1002. [Google Scholar] [CrossRef]
- Davis, B.M.; McCurrach, M.E.; Taneja, K.L.; Singer, R.H.; Housman, D.E. Expansion of a CUG trinucleotide repeat in the 3′ untranslated region of myotonic dystrophy protein kinase transcripts results in nuclear retention of transcripts. Proc. Natl. Acad. Sci. USA 1997, 94, 7388–7393. [Google Scholar] [CrossRef]
- Fu, Y.; Friedman, D.; Richards, S.; Pearlman, J.; Gibbs, R.; Pizzuti, A.; Ashizawa, T.; Perryman, M.; Scarlato, G.; Fenwick, R.; et al. Decreased expression of myotonin-protein kinase messenger RNA and protein in adult form of myotonic dystrophy. Science 1993, 260, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.; Smith, D.B.J.; Rich, M.M.; Leferovich, J.M.; Reilly, P.; Davis, B.M.; Tran, K.; Rayburn, H.; Bronson, R.; Cros, D.; et al. Mice lacking the myotonic dystrophy protein kinase develop a late onset progressive myopathy. Nat. Genet. 1996, 13, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Berul, C.I.; Maguire, C.T.; Aronovitz, M.J.; Greenwood, J.; Miller, C.; Gehrmann, J.; Housman, D.; Mendelsohn, M.E.; Reddy, S. DMPK dosage alterations result in atrioventricular conduction abnormalities in a mouse myotonic dystrophy model. J. Clin. Investig. 1999, 103, R1–R7. [Google Scholar] [CrossRef]
- Mankodi, A.; Logigian, E.; Callahan, L.; McClain, C.; White, R.; Henderson, D.; Krym, M.; Thornton, C.A. Myotonic Dystrophy in Transgenic Mice Expressing an Expanded CUG Repeat. Sci. 2000, 289, 1769–1772. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wang, Y.; Abe, Y.; Cheney, L.; Udd, B.; Liu, X.X. Department of Infectious DiseasesThe Fifth Affiliated Hospital of Sun Yat-sen University 519000 Zhuhai China Haploinsuffciency for Znf9 in Znf9+/− Mice Is Associated with Multiorgan Abnormalities Resembling Myotonic Dystrophy. J. Mol. Biol. 2007, 368, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Stock, L.; Schneider-Gold, C.; Sommer, C.; Timchenko, N.A.; Timchenko, L. Reduction of Cellular Nucleic Acid Binding Protein Encoded by a Myotonic Dystrophy Type 2 Gene Causes Muscle Atrophy. Mol. Cell. Biol. 2018, 38, MCB.00649-17. [Google Scholar] [CrossRef]
- Huichalaf, C.; Schoser, B.; Schneider-Gold, C.; Jin, B.; Sarkar, P.; Timchenko, L.T. Reduction of the rate of protein translation in patients with myotonic dystrophy 2. J. Neurosci. 2009, 29, 9042–9049. [Google Scholar] [CrossRef]
- Raheem, O.; Olufemi, S.-E.; Bachinski, L.L.; Vihola, A.; Sirito, M.; Holmlund-Hampf, J.; Haapasalo, H.; Li, Y.-P.; Udd, B.; Krahe, R. Mutant (CCTG)n Expansion Causes Abnormal Expression of Zinc Finger Protein 9 (ZNF9) in Myotonic Dystrophy Type 2. Am. J. Pathol. 2010, 177, 3025–3036. [Google Scholar] [CrossRef]
- Mankodi, A.; Urbinati, C.R.; Yuan, Q.-P.; Moxley, R.T.; Sansone, V.; Krym, M.; Henderson, D.; Schalling, M.; Swanson, M.S.; Thornton, C.A. Muscleblind localizes to nuclear foci of aberrant RNA in myotonic dystrophy types 1 and 2. Hum. Mol. Genet. 2001, 10, 2165–2170. [Google Scholar] [CrossRef]
- Timchenko, L.T.; Timchenko, N.A.; Caskey, C.T.; Roberts, R. Novel Proteins with Binding Specificity for DNA CTG Repeats And RNA Cug Repeats: Implications for Myotonic Dystrophy. Hum. Mol. Genet. 1996, 5, 115–121. [Google Scholar] [CrossRef][Green Version]
- Timchenko, L.T.; Miller, J.W.; Timchenko, N.A.; Devore, D.R.; Datar, K.V.; Lin, L.; Roberts, R.; Caskey, C.T.; Swanson, M.S. Identification of a (CUG)n Triplet Repeat RNA-Binding Protein and Its Expression in Myotonic Dystrophy. Nucleic Acids Res. 1996, 24, 4407–4414. [Google Scholar] [CrossRef] [PubMed]
- Fardaei, M.; Rogers, M.T.; Thorpe, H.M.; Larkin, K.; Hamshere, M.G.; Harper, P.S.; Brook, J.D. Three proteins, MBNL, MBLL and MBXL, co-localize in vivo with nuclear foci of expanded-repeat transcripts in DM1 and DM2 cells. Hum. Mol. Genet. 2002, 11, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Ranum, L.P.W.; Day, J.W. Myotonic Dystrophy: RNA Pathogenesis Comes into Focus. Am. J. Hum. Genet. 2004, 74, 793–804. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Cooper, T.A. Pathogenic mechanisms of myotonic dystrophy. Biochem. Soc. Trans. 2009, 37, 1281–1286. [Google Scholar] [CrossRef]
- Jiang, H.; Mankodi, A.; Swanson, M.S.; Moxley, R.T.; Thornton, C.A. Myotonic dystrophy type 1 is associated with nuclear foci of mutant RNA, sequestration of muscleblind proteins and deregulated alternative splicing in neurons. Hum. Mol. Genet. 2004, 13, 3079–3088. [Google Scholar] [CrossRef]
- Mankodi, A.; Lin, X.; Blaxall, B.C.; Swanson, M.S.; Thornton, C.A. Nuclear RNA Foci in the Heart in Myotonic Dystrophy. Circ. Res. 2005, 97, 1152–1155. [Google Scholar] [CrossRef]
- Kanadia, R.N.; Johnstone, K.A.; Mankodi, A.; Lungu, C.; Thornton, C.A.; Esson, D.; Timmers, A.M.; Hauswirth, W.W.; Swanson, M.S. A Muscleblind Knockout Model for Myotonic Dystrophy. Science 2003, 302, 1978–1980. [Google Scholar] [CrossRef]
- De Haro, M.; Al-Ramahi, I.; De Gouyon, B.; Ukani, L.; Rosa, A.; Faustino, N.A.; Ashizawa, T.; Cooper, T.A.; Botas, J. MBNL1 and CUGBP1 modify expanded CUG-induced toxicity in a Drosophila model of myotonic dystrophy type 1. Hum. Mol. Genet. 2006, 15, 2138–2145. [Google Scholar] [CrossRef]
- Todd, P.K.; Ackall, F.Y.; Hur, J.; Sharma, K.; Paulson, H.L.; Dowling, J.J. Transcriptional changes and developmental abnormalities in a zebrafish model of myotonic dystrophy type 1. Dis. Model. Mech. 2013, 7, 143–155. [Google Scholar] [CrossRef]
- Sicot, G.; Gomes-Pereira, M. RNA toxicity in human disease and animal models: From the uncovering of a new mechanism to the development of promising therapies. Biochim. et Biophys. Acta (BBA) Mol. Basis Dis. 2013, 1832, 1390–1409. [Google Scholar] [CrossRef]
- Otten, A.D.; Tapscott, S.J. Triplet repeat expansion in myotonic dystrophy alters the adjacent chromatin structure. Proc. Natl. Acad. Sci. USA 1995, 92, 5465–5469. [Google Scholar] [CrossRef]
- Thornton, C.A.; Wymer, J.P.; Simmons, Z.; McClain, C.; Moxley, R.T. Expansion of the myotonic dystrophy CTG repeat reduces expression of the flanking DMAHP gene. Nat. Genet. 1997, 16, 407–409. [Google Scholar] [CrossRef] [PubMed]
- Klesert, T.R.; Otten, A.D.; Bird, T.D.; Tapscott, S.J. Trinucleotide repeat expansion at the myotonic dystrophy locus reduces expression of DMAHP. Nat. Genet. 1997, 16, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Boucher, C.; King, S.; Carey, N.; Krahe, R.; Winchester, C.L.; Creavin, T.; Mehji, P.; Bailey, M.E.S.; Chartier, F.; Brown, S.; et al. A novel homeodomain-encoding gene is associated with a large CpG island interrupted by the myotonic dystrophy unstable (CTG) n repeat. Hum. Mol. Genet. 1995, 4, 1919–1925. [Google Scholar] [CrossRef] [PubMed]
- Filippova, G.N.; Thienes, C.P.; Penn, B.H.; Cho, D.H.; Hu, Y.J.; Moore, J.M.; Klesert, T.R.; Lobanenkov, V.V.; Tapscott, S.J. CTCF-binding sites flank CTG/CAG repeats and form a methylation-sensitive insulator at the DM1 locus. Nat. Genet. 2001, 28, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Nakamori, M.; Hamanaka, K.; Thomas, J.D.; Wang, E.T.; Hayashi, Y.K.; Takahashi, M.P.; Swanson, M.S.; Nishino, I.; Mochizuki, H. Aberrant Myokine Signaling in Congenital Myotonic Dystrophy. Cell Rep. 2017, 21, 1240–1252. [Google Scholar] [CrossRef] [PubMed]
- Huguet, A.; Medja, F.; Nicole, A.; Vignaud, A.; Guiraud-Dogan, C.; Ferry, A.; Decostre, V.; Hogrel, J.-Y.; Metzger, F.; Hoeflich, A.; et al. Molecular, Physiological, and Motor Performance Defects in DMSXL Mice Carrying >1000 CTG Repeats from the Human DM1 Locus. PLoS Genet. 2012, 8, e1003043. [Google Scholar] [CrossRef]
- Scoles, D.R.; Pulst, S.M. Oligonucleotide therapeutics in neurodegenerative diseases. RNA Biol. 2018, 15, 1–8. [Google Scholar] [CrossRef]
- Ramachandran, P.S.; Boudreau, R.L.; Schaefer, K.A.; La Spada, A.R.; Davidson, B.L. Nonallele Specific Silencing of Ataxin-7 Improves Disease Phenotypes in a Mouse Model of SCA7. Mol. Ther. 2014, 22, 1635–1642. [Google Scholar] [CrossRef]
- Scholefield, J.; Greenberg, L.J.; Weinberg, M.S.; Arbuthnot, P.B.; Abdelgany, A.; Wood, M.J.A. Design of RNAi Hairpins for Mutation-Specific Silencing of Ataxin-7 and Correction of a SCA7 Phenotype. PLoS ONE 2009, 4, e7232. [Google Scholar] [CrossRef]
- Fiszer, A.; Wroblewska, J.P.; Nowak, B.M.; Krzyzosiak, W.J. Mutant CAG Repeats Effectively Targeted by RNA Interference in SCA7 Cells. Genes 2016, 7, 132. [Google Scholar] [CrossRef] [PubMed]
- Miniarikova, J.; Zimmer, V.; Martier, R.; Brouwers, C.C.; Pythoud, C.; Richetin, K.; Rey, M.; Lubelski, J.; Evers, M.M.; Van Deventer, S.J.; et al. AAV5-miHTT gene therapy demonstrates suppression of mutant huntingtin aggregation and neuronal dysfunction in a rat model of Huntington’s disease. Gene Ther. 2017, 24, 630–639. [Google Scholar] [CrossRef] [PubMed]
- Evers, M.M.; Miniarikova, J.; Juhas, S.; Vallès, A.; Bohuslavova, B.; Juhasova, J.; Skalnikova, H.K.; Vodicka, P.; Valekova, I.; Brouwers, C.; et al. AAV5-miHTT Gene Therapy Demonstrates Broad Distribution and Strong Human Mutant Huntingtin Lowering in a Huntington’s Disease Minipig Model. Mol. Ther. 2018, 26, 2163–2177. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.; Pfister, E.L.; Liu, W.; Andre, R.; Träger, U.; Kennington, L.A.; Lo, K.; Dijkstra, S.; Macdonald, U.; Ostroff, G.; et al. Allele-Selective Suppression of Mutant Huntingtin in Primary Human Blood Cells. Sci. Rep. 2017, 7, 46740. [Google Scholar] [CrossRef]
- Pfister, E.L.; Chase, K.O.; Sun, H.; Kennington, L.A.; Conroy, F.; Johnson, E.; Miller, R.; Borel, F.; Aronin, N.; Mueller, C. Safe and Efficient Silencing with a Pol II, but Not a Pol lII, Promoter Expressing an Artificial miRNA Targeting Human Huntingtin. Mol. Ther. Nucleic Acids 2017, 7, 324–334. [Google Scholar] [CrossRef]
- Sobczak, K.; Wheeler, T.M.; Wang, W.; Thornton, C.A. RNA Interference Targeting CUG Repeats in a Mouse Model of Myotonic Dystrophy. Mol. Ther. 2012, 21, 380–387. [Google Scholar] [CrossRef]
- Langlois, M.-A.; Boniface, C.; Wang, G.; Alluin, J.; Salvaterra, P.M.; Puymirat, J.; Rossi, J.J.; Lee, N.S. Cytoplasmic and Nuclear Retained DMPK mRNAs Are Targets for RNA Interference in Myotonic Dystrophy Cells. J. Biol. Chem. 2005, 280, 16949–16954. [Google Scholar] [CrossRef]
- Bisset, D.R.; Stepniak-Konieczna, E.A.; Zavaljevski, M.; Wei, J.; Carter, G.T.; Weiss, M.D.; Gabellini, D. Therapeutic impact of systemic AAV-mediated RNA interference in a mouse model of myotonic dystrophy. Hum. Mol. Genet. 2015, 24, 4971–4983. [Google Scholar] [CrossRef]
- Spronck, E.A.; Brouwers, C.C.; Vallès, A.; De Haan, M.; Petry, H.; Van Deventer, S.J.; Konstantinova, P.; Evers, M.M. AAV5-miHTT Gene Therapy Demonstrates Sustained Huntingtin Lowering and Functional Improvement in Huntington Disease Mouse Models. Mol. Ther. Methods Clin. Dev. 2019, 13, 334–343. [Google Scholar] [CrossRef]
- Keskin, S.; Brouwers, C.C.; Sogorb-Gonzalez, M.; Martier, R.; Depla, J.A.; Vallès, A.; Van Deventer, S.J.; Konstantinova, P.; Evers, M.M. AAV5-miHTT Lowers Huntingtin mRNA and Protein without Off-Target Effects in Patient-Derived Neuronal Cultures and Astrocytes. Mol. Ther. Methods Clin. Dev. 2019, 15, 275–284. [Google Scholar] [CrossRef]
- Scoles, D.R.; Minikel, E.V.; Pulst, S.M. Antisense oligonucleotides: A primer. Neurol. Genet. 2019, 5, e323. [Google Scholar] [CrossRef] [PubMed]
- Bennett, C.F.; Krainer, A.R.; Cleveland, D.W. Antisense Oligonucleotide Therapies for Neurodegenerative Diseases. Annu. Rev. Neurosci. 2019, 42, 385–406. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.; Silveira, I.; Veiga, F.J.; Ribeiro, A. Recent advances in characterization of nonviral vectors for delivery of nucleic acids: Impact on their biological performance. Expert Opin. Drug Deliv. 2014, 12, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Evers, M.M.; Toonen, L.J.; Van Roon-Mom, W.M. Antisense oligonucleotides in therapy for neurodegenerative disorders. Adv. Drug Deliv. Rev. 2015, 87, 90–103. [Google Scholar] [CrossRef]
- Scoles, D.R.; Meera, P.; Schneider, M.D.; Paul, S.; Dansithong, W.; Figueroa, K.P.; Hung, G.; Rigo, F.; Bennett, G.H.F.R.C.F.; Otis, P.M.T.S.; et al. Antisense oligonucleotide therapy for spinocerebellar ataxia type 2. Nat. Cell Biol. 2017, 544, 362–366. [Google Scholar] [CrossRef]
- Scoles, D.R.; Dansithong, W.; Pflieger, L.T.; Paul, S.; Gandelman, M.; Figueroa, K.P.; Rigo, F.; Bennett, C.F.; Pulst, S.M. ALS-associated genes in SCA2 mouse spinal cord transcriptomes. Hum. Mol. Genet. 2020, 29, 1658–1672. [Google Scholar] [CrossRef]
- Niu, C.; Prakash, T.P.; Kim, A.; Quach, J.L.; Huryn, L.A.; Yang, Y.; Lopez, E.; Jazayeri, A.; Hung, G.; Sopher, B.L.; et al. Antisense oligonucleotides targeting mutant Ataxin-7 restore visual function in a mouse model of spinocerebellar ataxia type 7. Sci. Transl. Med. 2018, 10, eaap8677. [Google Scholar] [CrossRef]
- Tabrizi, S.J.; Leavitt, B.R.; Landwehrmeyer, G.B.; Wild, E.J.; Saft, C.; Barker, R.A.; Blair, N.F.; Craufurd, D.; Priller, J.; Rickards, H.; et al. Targeting Huntingtin Expression in Patients with Huntington’s Disease. N. Engl. J. Med. 2019, 380, 2307–2316. [Google Scholar] [CrossRef]
- Jauvin, D.; Chrétien, J.; Pandey, S.K.; Martineau, L.; Revillod, L.; Bassez, G.; Lachon, A.; MacLeod, A.R.; Gourdon, G.; Wheeler, T.M.; et al. Targeting DMPK with Antisense Oligonucleotide Improves Muscle Strength in Myotonic Dystrophy Type 1 Mice. Mol. Ther. Nucleic Acids 2017, 7, 465–474. [Google Scholar] [CrossRef]
- Jiang, J.; Zhu, Q.; Gendron, T.F.; Saberi, S.; McAlonis-Downes, M.; Seelman, A.; Stauffer, J.E.; Jafar-Nejad, P.; Drenner, K.; Schulte, D.; et al. Gain of Toxicity from ALS/FTD-Linked Repeat Expansions in C9ORF72 Is Alleviated by Antisense Oligonucleotides Targeting GGGGCC-Containing RNAs. Neuron 2016, 90, 535–550. [Google Scholar] [CrossRef]
- Donnelly, C.J.; Zhang, P.-W.; Pham, J.T.; Haeusler, A.R.; Mistry, N.A.; Vidensky, S.; Daley, E.L.; Poth, E.M.; Hoover, B.; Fines, D.M.; et al. RNA Toxicity from the ALS/FTD C9ORF72 Expansion Is Mitigated by Antisense Intervention. Neuron 2013, 80, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Lagier-Tourenne, C.; Baughn, M.; Rigo, F.; Sun, S.; Liu, P.; Li, H.-R.; Jiang, J.; Watt, A.T.; Chun, S.; Katz, M.; et al. Targeted degradation of sense and antisense C9orf72 RNA foci as therapy for ALS and frontotemporal degeneration. Proc. Natl. Acad. Sci. USA 2013, 110, E4530–E4539. [Google Scholar] [CrossRef] [PubMed]
- Gendron, T.F.; Chew, J.; Stankowski, J.N.; Hayes, L.R.; Zhang, Y.-J.; Prudencio, M.; Carlomagno, Y.; Daughrity, L.M.; Jansen-West, K.; Perkerson, E.A.; et al. Poly(GP) proteins are a useful pharmacodynamic marker forC9ORF72-associated amyotrophic lateral sclerosis. Sci. Transl. Med. 2017, 9, eaai7866. [Google Scholar] [CrossRef] [PubMed]
- Southwell, A.L.; Kordasiewicz, H.; Langbehn, D.R.; Skotte, N.H.; Parsons, M.P.; Villanueva, E.B.; Caron, N.S.; Østergaard, M.E.; Anderson, L.M.; Xie, Y.; et al. Huntingtin suppression restores cognitive function in a mouse model of Huntington’s disease. Sci. Transl. Med. 2018, 10, eaar3959. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).