SWI/SNF Alterations in Squamous Bladder Cancers
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Samples and Tissue Microarrays
2.2. Immunohistochemistry
2.3. DNA Extraction
2.4. SNaPshot and Sanger Sequencing
2.5. Targeted Next Generation Sequencing (NGS) Analysis
2.6. Analysis of the TCGA Sq-BLCA Data Set
2.7. Statistical Analysis
3. Results
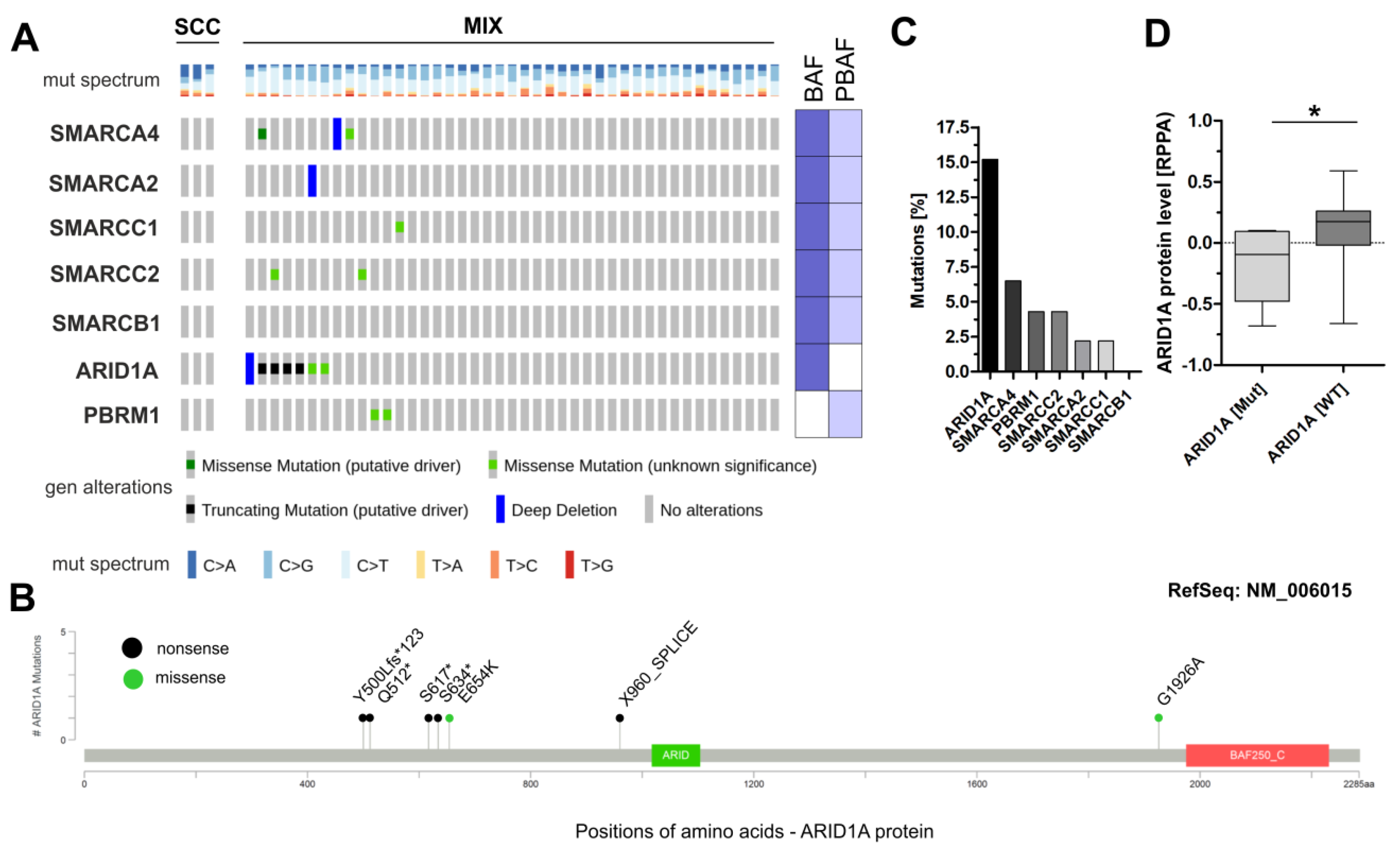
3.1. Analysis of Frequently Altered Subunits of the BAF and PBAF SWI/SNF Complexes in TCGA Sq-BLCA
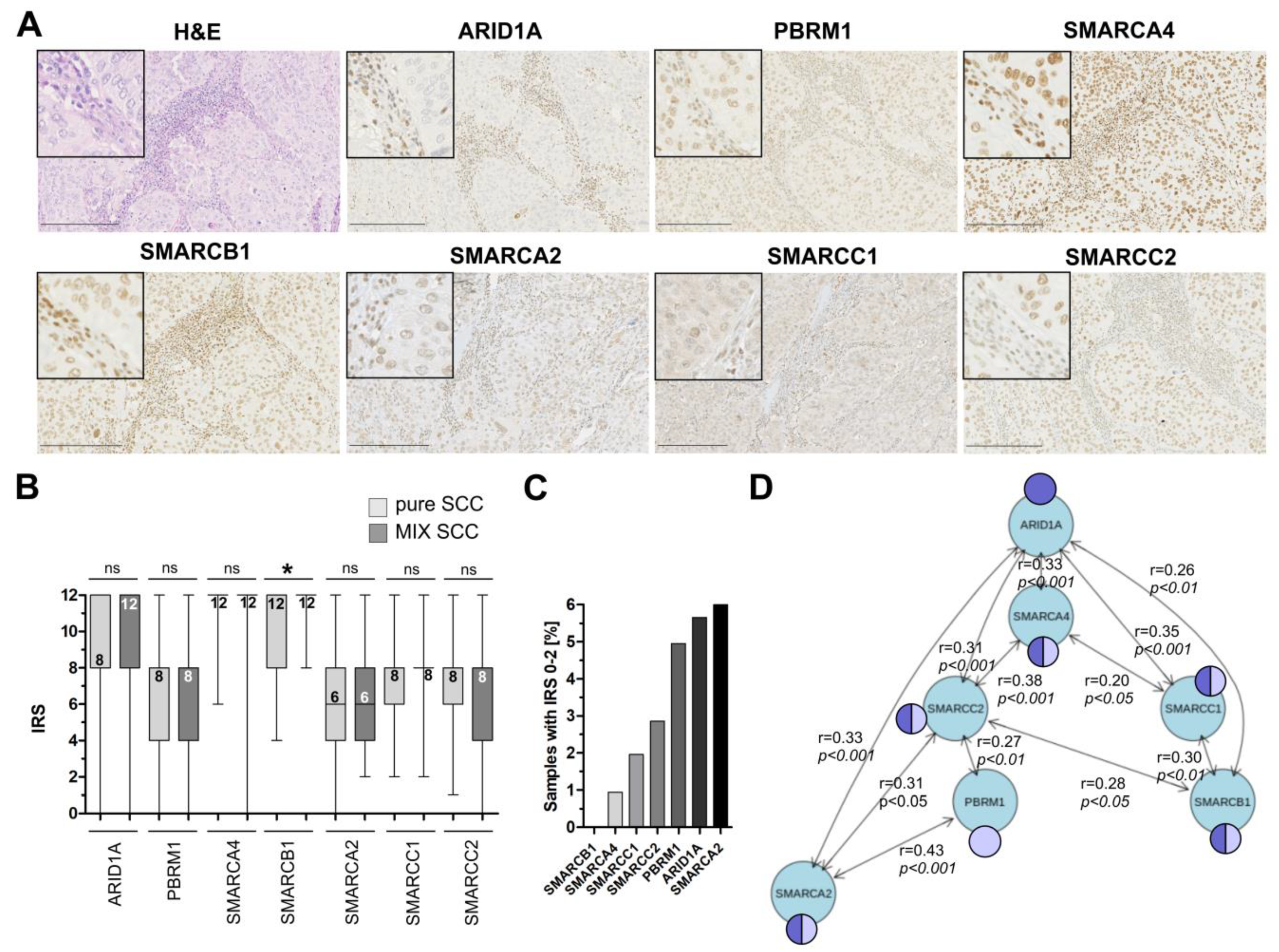
3.2. Immunohistochemical Analysis of Frequently Altered Subunits of the SWI/SNF-Complex in an Independent Squamous Bladder Cancer Cohort
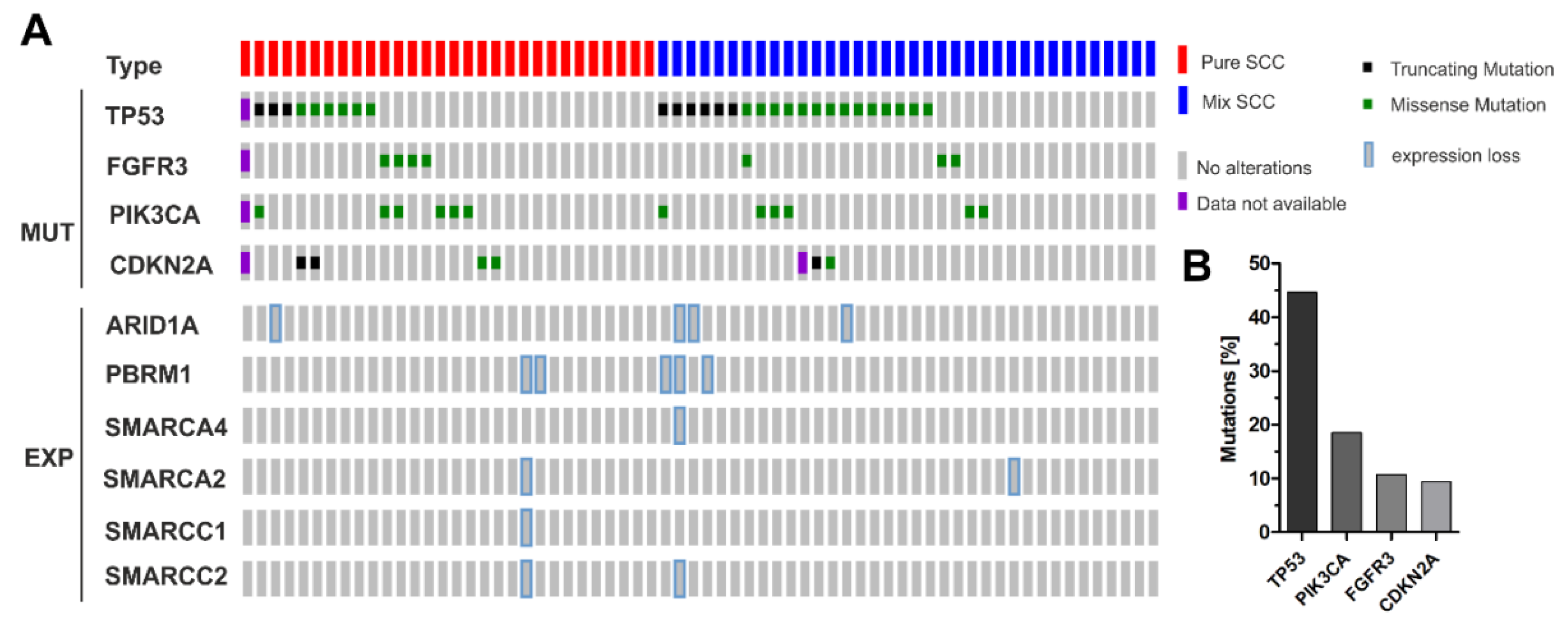
3.3. Correlation of ARID1A Expression Loss with Clinico-Pathological Parameters and Known Genetic Drivers
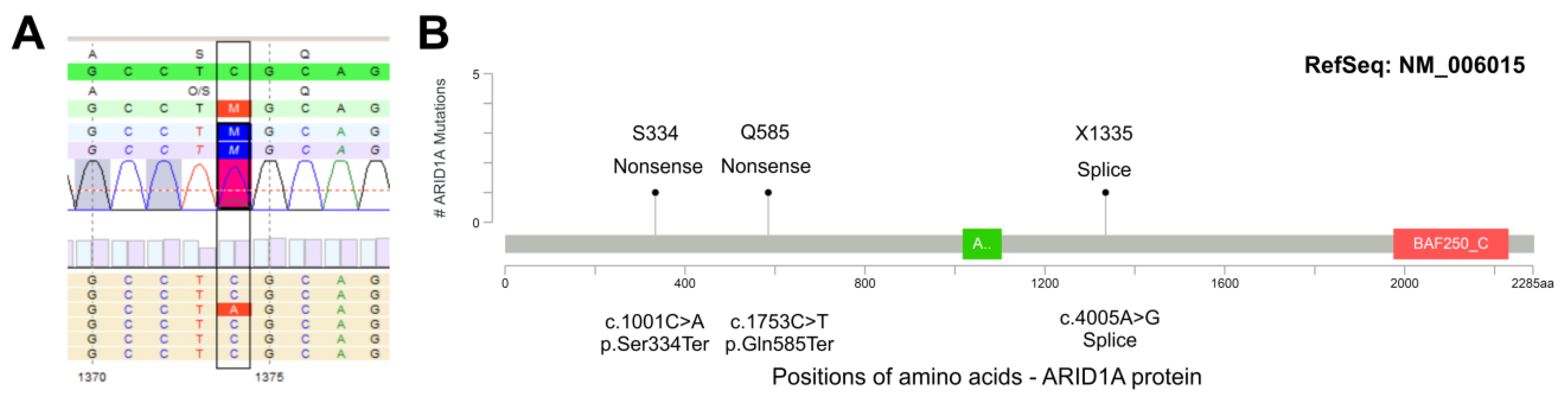
3.4. ARID1A Protein Loss Overlaps with Genetic ARID1A Alterations and PD-L1 Expression in the Independent Squamous Bladder Cancer Cohort
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Knowles, M.A.; Hurst, C.D. Molecular biology of bladder cancer: New insights into pathogenesis and clinical diversity. Nat. Rev. Cancer 2015, 15, 25–41. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, D.S.; Shipley, W.U.; Feldman, A.S. Bladder cancer. Lancet 2009, 374, 239–249. [Google Scholar] [CrossRef]
- Rose, M.; Maurer, A.; Wirtz, J.; Bleilevens, A.; Waldmann, T.; Wenz, M.; Eyll, M.; Geelvink, M.; Gereitzig, M.; Rüchel, N.; et al. EGFR activity addiction facilitates anti-ERBB based combination treatment of squamous bladder cancer. Oncogene 2020, 39, 6856–6870. [Google Scholar] [CrossRef]
- Baldia, P.H.; Maurer, A.; Heide, T.; Rose, M.; Stoehr, R.; Hartmann, A.; Williams, S.V.; Knowles, M.A.; Knuechel, R.; Gaisa, N.T. Fibroblast growth factor receptor (FGFR) alterations in squamous differentiated bladder cancer: A putative therapeutic target for a small subgroup. Oncotarget 2016, 7, 71429–71439. [Google Scholar] [CrossRef]
- Abol-Enein, H.; Kava, B.R.; Carmack, A.J. Nonurothelial cancer of the bladder. Urology 2007, 69, 93–104. [Google Scholar] [CrossRef]
- Gaisa, N.T.; Braunschweig, T.; Reimer, N.; Bornemann, J.; Eltze, E.; Siegert, S.; Toma, M.; Villa, L.; Hartmann, A.; Knuechel, R. Different immunohistochemical and ultrastructural phenotypes of squamous differentiation in bladder cancer. Virchows Arch. 2011, 458, 301–312. [Google Scholar] [CrossRef]
- Martin, J.W.; Carballido, E.M.; Ahmed, A.; Farhan, B.; Dutta, R.; Smith, C.; Youssef, R.F. Squamous cell carcinoma of the urinary bladder: Systematic review of clinical characteristics and therapeutic approaches. Arab J. Urol. 2016, 14, 183–191. [Google Scholar] [CrossRef]
- Cohen, S.M.; Shirai, T.; Steineck, G. Epidemiology and etiology of premalignant and malignant urothelial changes. Scand. J. Urol. Nephrol. Suppl. 2000, 105–115. [Google Scholar] [CrossRef]
- Michel, B.C.; D’Avino, A.R.; Cassel, S.H.; Mashtalir, N.; McKenzie, Z.M.; McBride, M.J.; Valencia, A.M.; Zhou, Q.; Bocker, M.; Soares, L.M.M.; et al. A non-canonical SWI/SNF complex is a synthetic lethal target in cancers driven by BAF complex perturbation. Nat. Cell Biol. 2018, 20, 1410–1420. [Google Scholar] [CrossRef] [PubMed]
- Mashtalir, N.; D’Avino, A.R.; Michel, B.C.; Luo, J.; Pan, J.; Otto, J.E.; Zullow, H.J.; McKenzie, Z.M.; Kubiak, R.L.; St Pierre, R.; et al. Modular Organization and Assembly of SWI/SNF Family Chromatin Remodeling Complexes. Cell 2018, 175, 1272–1288.e1220. [Google Scholar] [CrossRef] [PubMed]
- Agaimy, A.; Bertz, S.; Cheng, L.; Hes, O.; Junker, K.; Keck, B.; Lopez-Beltran, A.; Stockle, M.; Wullich, B.; Hartmann, A. Loss of expression of the SWI/SNF complex is a frequent event in undifferentiated/dedifferentiated urothelial carcinoma of the urinary tract. Virchows Arch. 2016, 469, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Schallenberg, S.; Bork, J.; Essakly, A.; Alakus, H.; Buettner, R.; Hillmer, A.M.; Bruns, C.; Schroeder, W.; Zander, T.; Loeser, H.; et al. Loss of the SWI/SNF-ATPase subunit members SMARCF1 (ARID1A), SMARCA2 (BRM), SMARCA4 (BRG1) and SMARCB1 (INI1) in oesophageal adenocarcinoma. BMC Cancer 2020, 20, 12. [Google Scholar] [CrossRef]
- Guan, B.; Wang, T.L.; Shih Ie, M. ARID1A, a factor that promotes formation of SWI/SNF-mediated chromatin remodeling, is a tumor suppressor in gynecologic cancers. Cancer Res. 2011, 71, 6718–6727. [Google Scholar] [CrossRef]
- Rodriguez-Nieto, S.; Cañada, A.; Pros, E.; Pinto, A.I.; Torres-Lanzas, J.; Lopez-Rios, F.; Sanchez-Verde, L.; Pisano, D.G.; Sanchez-Cespedes, M. Massive parallel DNA pyrosequencing analysis of the tumor suppressor BRG1/SMARCA4 in lung primary tumors. Hum. Mutat. 2011, 32, E1999–E2017. [Google Scholar] [CrossRef]
- Kadoch, C.; Hargreaves, D.C.; Hodges, C.; Elias, L.; Ho, L.; Ranish, J.; Crabtree, G.R. Proteomic and bioinformatic analysis of mammalian SWI/SNF complexes identifies extensive roles in human malignancy. Nat. Genet. 2013, 45, 592–601. [Google Scholar] [CrossRef]
- Reisman, D.; Glaros, S.; Thompson, E.A. The SWI/SNF complex and cancer. Oncogene 2009, 28, 1653–1668. [Google Scholar] [CrossRef]
- Shen, J.; Ju, Z.; Zhao, W.; Wang, L.; Peng, Y.; Ge, Z.; Nagel, Z.D.; Zou, J.; Wang, C.; Kapoor, P.; et al. ARID1A deficiency promotes mutability and potentiates therapeutic antitumor immunity unleashed by immune checkpoint blockade. Nat. Med. 2018, 24, 556–562. [Google Scholar] [CrossRef]
- Kuroda, T.; Ogiwara, H.; Sasaki, M.; Takahashi, K.; Yoshida, H.; Kiyokawa, T.; Sudo, K.; Tamura, K.; Kato, T.; Okamoto, A.; et al. Therapeutic preferability of gemcitabine for ARID1A-deficient ovarian clear cell carcinoma. Gynecol. Oncol. 2019, 155, 489–498. [Google Scholar] [CrossRef]
- Kuroda, T.; Kohno, T. Precision medicine for ovarian clear cell carcinoma based on gene alterations. Int. J. Clin. Oncol. 2020, 25, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Peng, Y.; Wei, L.; Zhang, W.; Yang, L.; Lan, L.; Kapoor, P.; Ju, Z.; Mo, Q.; Shih, I.M.; et al. ARID1A Deficiency Impairs the DNA Damage Checkpoint and Sensitizes Cells to PARP Inhibitors. Cancer Discov. 2015, 5, 752–767. [Google Scholar] [CrossRef] [PubMed]
- Williamson, C.T.; Miller, R.; Pemberton, H.N.; Jones, S.E.; Campbell, J.; Konde, A.; Badham, N.; Rafiq, R.; Brough, R.; Gulati, A.; et al. ATR inhibitors as a synthetic lethal therapy for tumours deficient in ARID1A. Nat. Commun. 2016, 7, 13837. [Google Scholar] [CrossRef] [PubMed]
- Goswami, S.; Chen, Y.; Anandhan, S.; Szabo, P.M.; Basu, S.; Blando, J.M.; Liu, W.; Zhang, J.; Natarajan, S.M.; Xiong, L.; et al. mutation plus CXCL13 expression act as combinatorial biomarkers to predict responses to immune checkpoint therapy in mUCC. Sci. Transl. Med. 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, T.; Fatkhutdinov, N.; Zundell, J.A.; Tcyganov, E.N.; Nacarelli, T.; Karakashev, S.; Wu, S.; Liu, Q.; Gabrilovich, D.I.; Zhang, R. HDAC6 Inhibition Synergizes with Anti-PD-L1 Therapy in ARID1A-Inactivated Ovarian Cancer. Cancer Res. 2019, 79, 5482–5489. [Google Scholar] [CrossRef]
- Nervi, C.; De Marinis, E.; Codacci-Pisanelli, G. Epigenetic treatment of solid tumours: A review of clinical trials. Clin. Epigenetics 2015, 7, 127. [Google Scholar] [CrossRef]
- Gupta, S.; Albertson, D.J.; Parnell, T.J.; Butterfield, A.; Weston, A.; Pappas, L.M.; Dalley, B.; O’Shea, J.M.; Lowrance, W.T.; Cairns, B.R.; et al. Histone Deacetylase Inhibition Has Targeted Clinical Benefit in. Mol. Cancer Ther. 2019, 18, 185–195. [Google Scholar] [CrossRef]
- Kaelin, W.G. The concept of synthetic lethality in the context of anticancer therapy. Nat. Rev. Cancer 2005, 5, 689–698. [Google Scholar] [CrossRef]
- O’Neil, N.J.; Bailey, M.L.; Hieter, P. Synthetic lethality and cancer. Nat. Rev. Genet. 2017, 18, 613–623. [Google Scholar] [CrossRef]
- Garczyk, S.; Schneider, U.; Lurje, I.; Becker, K.; Vögeli, T.A.; Gaisa, N.T.; Knüchel, R. ARID1A-deficiency in urothelial bladder cancer: No predictive biomarker for EZH2-inhibitor treatment response? PLoS ONE 2018, 13, e0202965. [Google Scholar] [CrossRef]
- Li, J.; Lu, S.; Lombardo, K.; Monahan, R.; Amin, A. ARID1A alteration in aggressive urothelial carcinoma and variants of urothelial carcinoma. Hum. Pathol. 2016, 55, 17–23. [Google Scholar] [CrossRef]
- Gaisa, N.T.; Graham, T.A.; McDonald, S.A.; Canadillas-Lopez, S.; Poulsom, R.; Heidenreich, A.; Jakse, G.; Tadrous, P.J.; Knuechel, R.; Wright, N.A. The human urothelium consists of multiple clonal units, each maintained by a stem cell. J. Pathol. 2011, 225, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Fischbach, A.; Rogler, A.; Erber, R.; Stoehr, R.; Poulsom, R.; Heidenreich, A.; Schneevoigt, B.S.; Hauke, S.; Hartmann, A.; Knuechel, R.; et al. Fibroblast growth factor receptor (FGFR) gene amplifications are rare events in bladder cancer. Histopathology 2015, 66, 639–649. [Google Scholar] [CrossRef]
- Molitor, M.; Junker, K.; Eltze, E.; Toma, M.; Denzinger, S.; Siegert, S.; Knuechel, R.; Gaisa, N.T. Comparison of structural genetics of non-schistosoma-associated squamous cell carcinoma of the urinary bladder. Int. J. Clin. Exp. Pathol. 2015, 8, 8143–8158. [Google Scholar] [PubMed]
- Remmele, W.; Stegner, H.E. Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologe 1987, 8, 138–140. [Google Scholar] [PubMed]
- Rose, M.; Gaisa, N.T.; Antony, P.; Fiedler, D.; Heidenreich, A.; Otto, W.; Denzinger, S.; Bertz, S.; Hartmann, A.; Karl, A.; et al. Epigenetic inactivation of ITIH5 promotes bladder cancer progression and predicts early relapse of pT1 high-grade urothelial tumours. Carcinogenesis 2014, 35, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Hurst, C.D.; Zuiverloon, T.C.; Hafner, C.; Zwarthoff, E.C.; Knowles, M.A. A SNaPshot assay for the rapid and simple detection of four common hotspot codon mutations in the PIK3CA gene. BMC Res. Notes 2009, 2, 66. [Google Scholar] [CrossRef] [PubMed]
- Keck, B.; Stoehr, R.; Wach, S.; Rogler, A.; Hofstaedter, F.; Lehmann, J.; Montironi, R.; Sibonye, M.; Fritsche, H.M.; Lopez-Beltran, A.; et al. The plasmacytoid carcinoma of the bladder--rare variant of aggressive urothelial carcinoma. Int. J. Cancer 2011, 129, 346–354. [Google Scholar] [CrossRef]
- Guricova, K.; Maurer, A.; Gaisa, N.; Garczyk, S.; Knüchel-Clarke, R.; Dahl, E.; Ortiz, B.N. AG12.P.03: Ein Robustes Tool zur Kopienzahlanalyse für Verschiedene Amplikon-Basierte NGS-Panel (ACopy). Abstracts 103. Jahrestagung der Deutschen Gesellschaft für Pathologie, Frankfurt, 13.06.2019–15.06.2019. Pathologe 2019, 40, 196. [Google Scholar]
- Robertson, A.G.; Kim, J.; Al-Ahmadie, H.; Bellmunt, J.; Guo, G.; Cherniack, A.D.; Hinoue, T.; Laird, P.W.; Hoadley, K.A.; Akbani, R.; et al. Comprehensive Molecular Characterization of Muscle-Invasive Bladder Cancer. Cell 2017, 171, 540–556. [Google Scholar] [CrossRef]
- Porter, M.P.; Voigt, L.F.; Penson, D.F.; Weiss, N.S. Racial variation in the incidence of squamous cell carcinoma of the bladder in the United States. J. Urol. 2002, 168, 1960–1963. [Google Scholar] [CrossRef]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 2013, 6, pl1. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.D.; Gentry, J.; Long, L.; Gentleman, R.; Falcon, S.; Hahne, F.; Sarkar, D. Rgraphviz: Provides Plotting Capabilities for R Graph Objects, R Package Version 2.32.0; 2020; Available online: https://www.bioconductor.org/packages/release/bioc/html/Rgraphviz.html (accessed on 17 October 2020). [CrossRef]
- Morsch, R.; Rose, M.; Maurer, A.; Cassataro, M.A.; Braunschweig, T.; Knüchel, R.; Vögeli, T.A.; Ecke, T.; Eckstein, M.; Weyerer, V.; et al. Therapeutic implications of PD-L1 expression in bladder cancer with squamous differentiation. BMC Cancer 2020, 20, 230. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Herlong, F.H.; Stroehlein, J.R.; Mishra, L. Mutations of Chromatin Structure Regulating Genes in Human Malignancies. Curr. Protein Pept. Sci. 2016, 17, 411–437. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, K.C.; Shah, S.P.; Al-Agha, O.M.; Zhao, Y.; Tse, K.; Zeng, T.; Senz, J.; McConechy, M.K.; Anglesio, M.S.; Kalloger, S.E.; et al. ARID1A mutations in endometriosis-associated ovarian carcinomas. N. Engl. J. Med. 2010, 363, 1532–1543. [Google Scholar] [CrossRef]
- Wu, J.N.; Roberts, C.W. ARID1A mutations in cancer: Another epigenetic tumor suppressor? Cancer Discov. 2013, 3, 35–43. [Google Scholar] [CrossRef]
- Luchini, C.; Veronese, N.; Solmi, M.; Cho, H.; Kim, J.H.; Chou, A.; Gill, A.J.; Faraj, S.F.; Chaux, A.; Netto, G.J.; et al. Prognostic role and implications of mutation status of tumor suppressor gene ARID1A in cancer: A systematic review and meta-analysis. Oncotarget 2015, 6, 39088–39097. [Google Scholar] [CrossRef]
- Caumanns, J.J.; Wisman, G.B.A.; Berns, K.; van der Zee, A.G.J.; De Jong, S. ARID1A mutant ovarian clear cell carcinoma: A clear target for synthetic lethal strategies. Biochim. Biophys. Acta Rev. Cancer 2018, 1870, 176–184. [Google Scholar] [CrossRef]
- Ehrenhöfer-Wölfer, K.; Puchner, T.; Schwarz, C.; Rippka, J.; Blaha-Ostermann, S.; Strobl, U.; Hörmann, A.; Bader, G.; Kornigg, S.; Zahn, S.; et al. SMARCA2-deficiency confers sensitivity to targeted inhibition of SMARCA4 in esophageal squamous cell carcinoma cell lines. Sci. Rep. 2019, 9, 11661. [Google Scholar] [CrossRef]
- Watanabe, R.; Ui, A.; Kanno, S.; Ogiwara, H.; Nagase, T.; Kohno, T.; Yasui, A. SWI/SNF factors required for cellular resistance to DNA damage include ARID1A and ARID1B and show interdependent protein stability. Cancer Res. 2014, 74, 2465–2475. [Google Scholar] [CrossRef] [PubMed]
- Allo, G.; Bernardini, M.Q.; Wu, R.C.; Shih, I.M.; Kalloger, S.; Pollett, A.; Gilks, C.B.; Clarke, B.A. ARID1A loss correlates with mismatch repair deficiency and intact p53 expression in high-grade endometrial carcinomas. Mod. Pathol. 2014, 27, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Bosse, T.; ter Haar, N.T.; Seeber, L.M.; Diest, P.J.V.; Hes, F.J.; Vasen, H.F.; Nout, R.A.; Creutzberg, C.L.; Morreau, H.; Smit, V.T. Loss of ARID1A expression and its relationship with PI3K-Akt pathway alterations, TP53 and microsatellite instability in endometrial cancer. Mod. Pathol. 2013, 26, 1525–1535. [Google Scholar] [CrossRef]
- Chandler, R.L.; Damrauer, J.S.; Raab, J.R.; Schisler, J.C.; Wilkerson, M.D.; Didion, J.P.; Starmer, J.; Serber, D.; Yee, D.; Xiong, J.; et al. Coexistent ARID1A-PIK3CA mutations promote ovarian clear-cell tumorigenesis through pro-tumorigenic inflammatory cytokine signalling. Nat. Commun. 2015, 6, 6118. [Google Scholar] [CrossRef]
- Bitler, B.G.; Fatkhutdinov, N.; Zhang, R. Potential therapeutic targets in ARID1A-mutated cancers. Expert Opin. Ther. Targets 2015, 19, 1419–1422. [Google Scholar] [CrossRef] [PubMed]
| Categorization | ∑ | n SCC | n MIX-SCC | |
|---|---|---|---|---|
| Parameter: | ||||
| Age at diagnosis | median 67.5 years | |||
| (range 33–91 years) | ||||
| ≤67.5 years | 59 | 38 | 21 | |
| >67.5 years | 57 | 30 | 27 | |
| Gender | Female | 59 | 34 | 23 |
| Male | 56 | 33 | 25 | |
| unknown | 1 | 1 | 0 | |
| Histological tumor grade | G1 | 1 | 1 | 0 |
| G2 | 34 | 25 | 9 | |
| G3 | 77 | 40 | 37 | |
| G4 | 2 | 0 | 2 | |
| unknown | 2 | 2 | 0 | |
| Tumor stage | pTx | 5 | 5 | 0 |
| pT1 | 1 | 1 | 0 | |
| pT2 | 16 | 12 | 4 | |
| pT3 | 76 | 37 | 39 | |
| pT4 | 18 | 13 | 5 | |
| Lymph node status | Negative (pN0) | 72 | 41 | 31 |
| Positive (pN1 + pN2) | 22 | 11 | 11 | |
| unknown | 22 | 16 | 6 |
| SCC | MIX | |||
|---|---|---|---|---|
| neg | pos | neg | pos | |
| ARID1A | 2 | 62 | 4 | 41 |
| PBRM1 | 2 | 54 | 3 | 42 |
| SMARCC1 | 1 | 60 | 1 | 40 |
| SMARCC2 | 2 | 60 | 1 | 42 |
| SMARCA2 | 4 | 53 | 2 | 41 |
| SMARCA4 | 0 | 62 | 1 | 43 |
| SMARCB1 | 0 | 62 | 0 | 42 |
| ARID1A Expression b | |||||
|---|---|---|---|---|---|
| na | 0–2 | 3–12 | p-Value c | ||
| Parameter: | |||||
| Age at diagnosis | |||||
| median age: 67 years | |||||
| ≤67 years | 56 | 3 | 53 | 0.954 | |
| >67 years | 53 | 3 | 50 | ||
| Gender | |||||
| female | 55 | 3 | 52 | 0.963 | |
| male | 53 | 3 | 50 | ||
| Histological tumor grade d | |||||
| G1-G2 | 30 | 2 | 28 | 0.816 | |
| G3 | 73 | 4 | 69 | ||
| Tumor stage d | |||||
| pT1-pT2 | 13 | 0 | 13 | 0.328 | |
| pT3-pT4 | 86 | 6 | 80 | ||
| Lymph node status | |||||
| neg | 65 | 4 | 61 | 0.600 | |
| neg | 21 | 2 | 19 | ||
| TP53 mut e | |||||
| neg | 30 | 0 | 30 | 0.030 | |
| neg | 27 | 4 | 23 | ||
| FGFR3 mut e | |||||
| neg | 50 | 4 | 46 | 0.442 | |
| neg | 7 | 0 | 7 | ||
| CDKNA2 mut e | |||||
| neg | 51 | 4 | 47 | 0.520 | |
| neg | 5 | 0 | 5 | ||
| PIK3CA mut e | |||||
| neg | 45 | 4 | 41 | 0.288 | |
| pos | 12 | 0 | 12 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Achenbach, F.; Rose, M.; Ortiz-Brüchle, N.; Seillier, L.; Knüchel, R.; Weyerer, V.; Hartmann, A.; Morsch, R.; Maurer, A.; Ecke, T.H.; et al. SWI/SNF Alterations in Squamous Bladder Cancers. Genes 2020, 11, 1368. https://doi.org/10.3390/genes11111368
Achenbach F, Rose M, Ortiz-Brüchle N, Seillier L, Knüchel R, Weyerer V, Hartmann A, Morsch R, Maurer A, Ecke TH, et al. SWI/SNF Alterations in Squamous Bladder Cancers. Genes. 2020; 11(11):1368. https://doi.org/10.3390/genes11111368
Chicago/Turabian StyleAchenbach, Fabian, Michael Rose, Nadina Ortiz-Brüchle, Lancelot Seillier, Ruth Knüchel, Veronika Weyerer, Arndt Hartmann, Ronja Morsch, Angela Maurer, Thorsten H. Ecke, and et al. 2020. "SWI/SNF Alterations in Squamous Bladder Cancers" Genes 11, no. 11: 1368. https://doi.org/10.3390/genes11111368
APA StyleAchenbach, F., Rose, M., Ortiz-Brüchle, N., Seillier, L., Knüchel, R., Weyerer, V., Hartmann, A., Morsch, R., Maurer, A., Ecke, T. H., Garczyk, S., & Gaisa, N. T. (2020). SWI/SNF Alterations in Squamous Bladder Cancers. Genes, 11(11), 1368. https://doi.org/10.3390/genes11111368






