Lymphatic Metastasis of NSCLC Involves Chemotaxis Effects of Lymphatic Endothelial Cells through the CCR7–CCL21 Axis Modulated by TNF-α
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Culture
2.3. MTT Assay
2.4. Cytokine Quantification by Enzyme-Linked Immunosorbent Assay (ELISA)
2.5. Real-Time PCR Analysis
2.6. Preparation of Cytosolic and Nuclear Protein Extracts
2.7. Western Blot
2.8. Cell Apoptosis
2.9. Transient Transfection with siRNA
2.10. Cell Adhesion Assay
2.11. Cell Invasion Assay
2.12. Statistical Analysis
3. Results
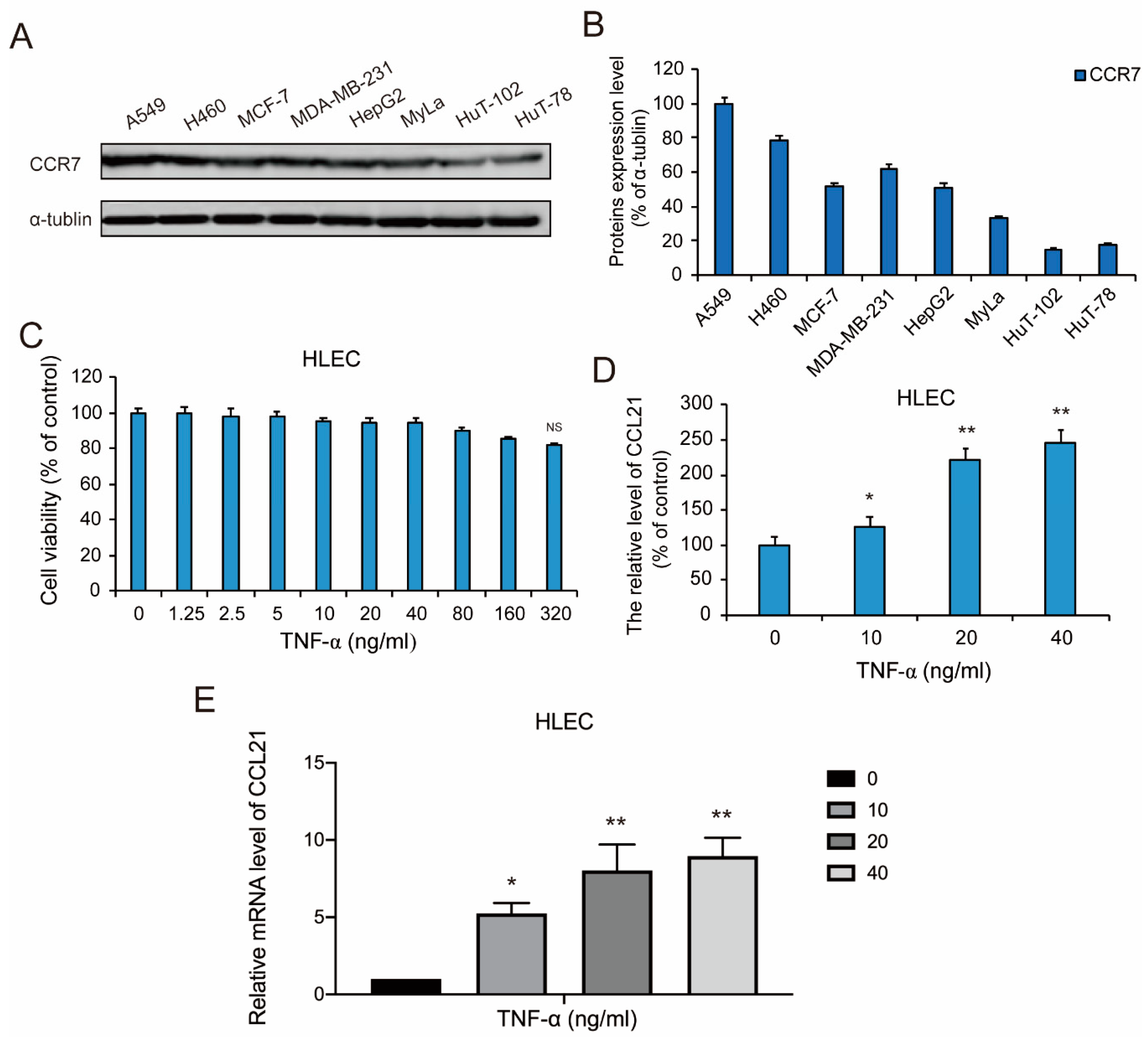
3.1. CCR7 is Overexpressed in Metastatic Lung Cancer
3.2. TNF-α Induced the Secretion of CCL21 in HLEC
3.3. TNF-α Activated NF-κB Signaling Pathway in HLEC
3.4. TNF-α-Induced Secretion of CCL21 Involving the NF-κB Signaling Pathway in HLEC
3.5. Co-Culture of HLEC and NSCLC Cells Promoted the Invasiveness and Migration of CCR7-Overexpressed NSCLC Cells
3.6. HLEC Cell Co-Culture-Induced Metastasis and Invasion of A549 Cells is CCL21 and CCR7 Dependent
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Knight, S.B.; Crosbie, P.A.; Balata, H.; Chudziak, J.; Hussell, T.; Dive, C. Progress and prospects of early detection in lung cancer. Open Biol. 2017, 7, 7. [Google Scholar] [CrossRef] [Green Version]
- Facchinetti, F.; Marabelle, A.; Rossi, G.; Soria, J.-C.; Besse, B.; Tiseo, M. Moving Immune Checkpoint Blockade in Thoracic Tumors beyond NSCLC. J. Thorac. Oncol. 2016, 11, 1819–1836. [Google Scholar] [CrossRef] [Green Version]
- Hardwicke, K. Lung cancer. In Nursing Standard (Royal College of Nursing (Great Britain): 1987; Royal College of Nursing: Sunderland, UK, 2008; Volume 22, p. 59. [Google Scholar]
- Harris, K.; Khachaturova, I.; Azab, B.; Maniatis, T.; Murukutla, S.; Chalhoub, M.; Hatoum, H.; Kilkenny, T.; Elsayegh, D.; Maroun, R. Small Cell Lung Cancer Doubling Time and its Effect on Clinical Presentation: A Concise Review. Clin. Med. Insights Oncol. 2012, 6, 199–203. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.-F.J.; Chan, D.Y.; Speicher, P.J.; Gulack, B.C.; Wang, X.; Hartwig, M.G.; Onaitis, M.W.; Tong, B.C.; D’Amico, T.A.; Berry, M.F. Role of Adjuvant Therapy in a Population-Based Cohort of Patients With Early-Stage Small-Cell Lung Cancer. J. Clin. Oncol. 2016, 34, 1057–1064. [Google Scholar] [CrossRef]
- Naylor, E.C. Adjuvant Therapy for Stage I and II Non–Small Cell Lung Cancer. Surg. Oncol. Clin. N. Am. 2016, 25, 585–599. [Google Scholar] [CrossRef]
- Wakeam, E.; Acuna, S.; Leighl, N.; Giuliani, M.; Finlayson, S.; Varghese, T.; Darling, G. Surgery Versus Chemotherapy and Radiotherapy For Early and Locally Advanced Small Cell Lung Cancer: A Propensity-Matched Analysis of Survival. Lung Cancer 2017, 109, 78–88. [Google Scholar] [CrossRef]
- Reck, M.; Heigener, D.F.; Mok, T.; Soria, J.-C.; Rabe, K.F. Management of non-small-cell lung cancer: Recent developments. Lancet 2013, 382, 709–719. [Google Scholar] [CrossRef]
- Popper, H.H. Progression and metastasis of lung cancer. Cancer Metastasis Rev. 2016, 35, 75–91. [Google Scholar] [CrossRef] [Green Version]
- Dong, M.; Gong, H.; Li, T.; Li, X.; Liu, J.; Zhang, H.; Liu, M.; Chen, G.; Liu, H.; Chen, J. Lymph node metastasis in lung squamous cell carcinoma and identification of metastasis-related genes based on the Cancer Genome Atlas. Cancer Med. 2019, 8, 6280–6294. [Google Scholar] [CrossRef]
- Mitsudomi, T.; Suda, K.; Yatabe, Y. Surgery for NSCLC in the era of personalized medicine. Nat. Rev. Clin. Oncol. 2013, 10, 235–244. [Google Scholar] [CrossRef]
- Carlsen, H.S.; Haraldsen, G.; Brandtzaeg, P.; Baekkevold, E.S. Disparate lymphoid chemokine expression in mice and men: No evidence of CCL21 synthesis by human high endothelial venules. Blood 2005, 106, 444–446. [Google Scholar] [CrossRef] [Green Version]
- Zu, G.; Luo, B.; Yang, Y.; Tan, Y.; Tang, T.; Zhang, Y.; Chen, X.; Sun, D. Meta-analysis of the prognostic value of C-C chemokine receptor type 7 in patients with solid tumors. Cancer Manag. Res. 2019, 11, 1881–1892. [Google Scholar] [CrossRef] [Green Version]
- Ji, R.-C. Lymph Nodes and Cancer Metastasis: New Perspectives on the Role of Intranodal Lymphatic Sinuses. Int. J. Mol. Sci. 2016, 18, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, H.; Gao, L.; Li, S.; Qin, J.; Chen, L.; Liu, X.; Xu, P.; Wang, F.; Xiao, H.; Zhou, S. CCR7 enhances TGF-beta1-induced epithelial-mesenchymal transition and is associated with lymph node metastasis and poor overall survival in gastric cancer. Oncotarget 2015, 6, 24348–24360. [Google Scholar] [CrossRef] [Green Version]
- Altorki, N.K.; Markowitz, G.J.; Gao, D.; Port, J.L.; Saxena, A.; Stiles, B.; McGraw, T.; Mittal, V. The lung microenvironment: An important regulator of tumour growth and metastasis. Nat. Rev. Cancer 2018, 19, 9–31. [Google Scholar] [CrossRef] [Green Version]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef] [Green Version]
- Ousterout, D.G.; Perez-pinera, P.; Thakore, P.I.; Kabadi, A.M.; Brown, M.T.; Qin, X.; Fedrigo, O.; Mouly, V.; Tremblay, J.P.; Gersbach, C.A. Corrigendum: Reading Frame Correction by Targeted Genome Editing Restores Dystrophin Expression in Cells From Duchenne Muscular Dystrophy Patients. Mol. Ther. 2013, 21, 2130. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.C. The non-canonical NF-kappaB pathway in immunity and inflammation. Nat. Rev. Immunol. 2017, 17, 545–558. [Google Scholar] [CrossRef]
- Velasquez, C.; Mansouri, S.; Mora, C.; Nassiri, F.; Suppiah, S.; Martino, J.; Zadeh, G.; Fernandez-Luna, J.L. Molecular and Clinical Insights into the Invasive Capacity of Glioblastoma Cells. J. Oncol. 2019, 2019, 1740763. [Google Scholar] [CrossRef] [Green Version]
- Gao, C.; Xiao, G.; Piersigilli, A.; Gou, J.; Ogunwobi, O.; Bargonetti, J. Context-dependent roles of MDMX (MDM4) and MDM2 in breast cancer proliferation and circulating tumor cells. Breast Cancer Res. 2019, 21, 5. [Google Scholar] [CrossRef]
- Schutze, S.; Wiegmann, K.; Machleidt, T.; Kronke, M. TNF-induced activation of NF-kappa B. Immunobiology 1995, 193, 193–203. [Google Scholar] [CrossRef]
- Li, H.; Xu, J.; Zhou, Y.; Liu, X.; Shen, L.E.; Zhu, Y.U.; Li, Z.; Wang, X.; Guo, Q.; Hui, H. PLSCR1/IP3R1/Ca(2+) axis contributes to differentiation of primary AML cells induced by wogonoside. Cell Death Dis. 2017, 8, e2768. [Google Scholar] [CrossRef]
- Gupta, G.P.; Massagué, J. Cancer Metastasis: Building a Framework. Cell 2006, 127, 679–695. [Google Scholar] [CrossRef] [Green Version]
- Liotta, L.A. An attractive force in metastasis. Nat. Cell Biol. 2001, 410, 24–25. [Google Scholar] [CrossRef]
- Tennant, D.A.; Durán, R.V.; Boulahbel, H.; Gottlieb, E. Metabolic transformation in cancer. Carcinogenesis 2009, 30, 1269–1280. [Google Scholar] [CrossRef] [Green Version]
- Balkwill, F. The chemokine system and cancer. J. Pathol. 2011, 226, 148–157. [Google Scholar] [CrossRef]
- Takanami, I. Overexpression of CCR7 mRNA in nonsmall cell lung cancer: Correlation with lymph node metastasis. Int. J. Cancer 2003, 105, 186–189. [Google Scholar] [CrossRef]
- Günther, K.; Leier, J.; Henning, G.; Dimmler, A.; Weißbach, R.; Hohenberger, W.; Förster, R. Prediction of lymph node metastasis in colorectal carcinoma by expressionof chemokine receptor CCR7. Int. J. Cancer 2005, 116, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.C.; Huang, Y.; Tang, K.; Cui, M.; Niemann, D.; Lopez, A.; Morgello, S.; Chen, S. HIV-1-infected and/or immune-activated macrophages regulate astrocyte CXCL8 production through IL-1beta and TNF-α: Involvement of mitogen-activated protein kinases and protein kinase R. J. Neuroimmunol. 2008, 200, 100–110. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; He, T.; Huang, D.; Pardo, C.A.; Ransohoff, R.M. TNF-α mediates SDF-1α–induced NF-κB activation and cytotoxic effects in primary astrocytes. J. Clin. Investig. 2001, 108, 425–435. [Google Scholar] [CrossRef]
- Sheng, W.S.; Ni, H.T.; Rowen, T.N.; Lokensgard, J.R.; Peterson, P.K.; Hu, S. TNF- -induced chemokine production and apoptosis in human neural precursor cells. J. Leukoc. Biol. 2005, 78, 1233–1241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vestergaard, C.; Johansen, C.; Otkjaer, K.; Deleuran, M.; Iversen, L. Tumor necrosis factor-α-induced CTACK/CCL27 (cutaneous T-cell-attracting chemokine) production in keratinocytes is controlled by nuclear factor kappaB. Cytokine 2005, 29, 49–55. [Google Scholar] [CrossRef]
- Cuesta-Mateos, C.; López-Giral, S.; Alfonso-Pérez, M.; De Soria, V.G.G.; Loscertales, J.; Guasch-Vidal, S.; Beltrán, A.E.; Zapata, J.M.; Muñoz-Calleja, C. Analysis of migratory and prosurvival pathways induced by the homeostatic chemokines CCL19 and CCL21 in B-cell chronic lymphocytic leukemia. Exp. Hematol. 2010, 38, 756–764e4. [Google Scholar] [CrossRef]
- Till, K.J.; Lin, K.; Zuzel, M.; Cawley, J.C. The chemokine receptor CCR7 and α4 integrin are important for migration of chronic lymphocytic leukemia cells into lymph nodes. Blood 2002, 99, 2977–2984. [Google Scholar] [CrossRef] [Green Version]
- Ishigami, S.; Natsugoe, S.; Nakajo, A.; Tokuda, K.; Uenosono, Y.; Arigami, T.; Matsumoto, M.; Okumura, H.; Hokita, S.; Aikou, T. Prognostic value of CCR7 expression in gastric cancer. Hepatogastroenterology 2007, 54, 1025–1028. [Google Scholar]
- Shuyi, Y.; Juping, D.; Zhiqun, Z.; Qiong, P.; Wuyang, J.; Ting, L.; Xiaowen, H.; Liu, X.; Yuxiang, C. A critical role of CCR7 in invasiveness and metastasis of SW620 colon cancer cell in vitro and in vivo. Cancer Biol. Ther. 2008, 7, 1037–1043. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, L.; Yin, L.; Ming, J.; Zhang, S.; Luo, W.; Qiu, X. CCL19/CCR7 upregulates heparanase via specificity protein-1 (Sp1) to promote invasion of cell in lung cancer. Tumour Biol. 2013, 34, 2703–2708. [Google Scholar] [CrossRef]
- Kuwabara, T.; Tanaka, Y.; Ishikawa, F.; Kondo, M.; Sekiya, H.; Kakiuchi, T. CCR7 ligands up-regulate IL-23 through PI3-kinase and NF-kappa B pathway in dendritic cells. J. Leukoc. Biol. 2012, 92, 309–318. [Google Scholar] [CrossRef]
- Zhao, W.; Sun, Z.; Wang, S.; Li, Z.; Zheng, L. Wnt1 Participates in Inflammation Induced by Lipopolysaccharide Through Upregulating Scavenger Receptor A and NF-kB. Inflammation 2015, 38, 1700–1706. [Google Scholar] [CrossRef] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Wang, H.; Xu, Z.; Bai, Y.; Xu, L. Lymphatic Metastasis of NSCLC Involves Chemotaxis Effects of Lymphatic Endothelial Cells through the CCR7–CCL21 Axis Modulated by TNF-α. Genes 2020, 11, 1309. https://doi.org/10.3390/genes11111309
Zhang S, Wang H, Xu Z, Bai Y, Xu L. Lymphatic Metastasis of NSCLC Involves Chemotaxis Effects of Lymphatic Endothelial Cells through the CCR7–CCL21 Axis Modulated by TNF-α. Genes. 2020; 11(11):1309. https://doi.org/10.3390/genes11111309
Chicago/Turabian StyleZhang, Shuai, Hongzheng Wang, Zhiyun Xu, Yongkang Bai, and Lin Xu. 2020. "Lymphatic Metastasis of NSCLC Involves Chemotaxis Effects of Lymphatic Endothelial Cells through the CCR7–CCL21 Axis Modulated by TNF-α" Genes 11, no. 11: 1309. https://doi.org/10.3390/genes11111309
APA StyleZhang, S., Wang, H., Xu, Z., Bai, Y., & Xu, L. (2020). Lymphatic Metastasis of NSCLC Involves Chemotaxis Effects of Lymphatic Endothelial Cells through the CCR7–CCL21 Axis Modulated by TNF-α. Genes, 11(11), 1309. https://doi.org/10.3390/genes11111309




