Unraveling the Genome-Wide Impact of Recombinant Baculovirus Infection in Mammalian Cells for Gene Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
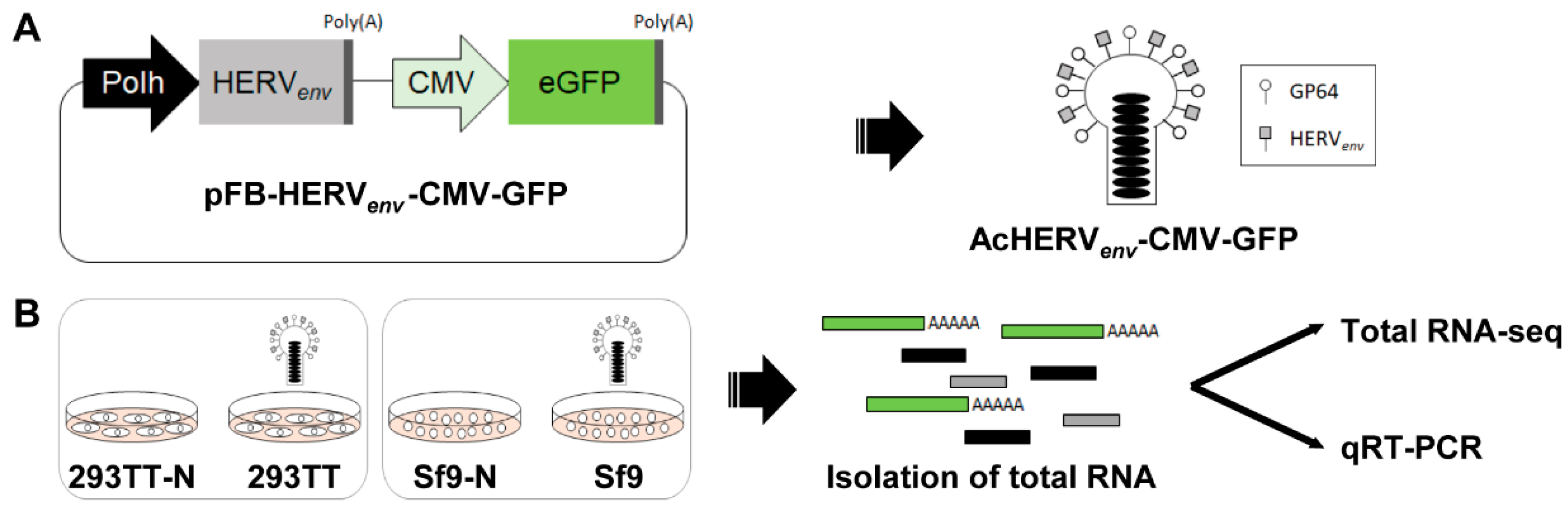
2.2. Generation of Recombinant Baculoviruses
2.3. Infection of Cells with Recombinant Baculoviruses
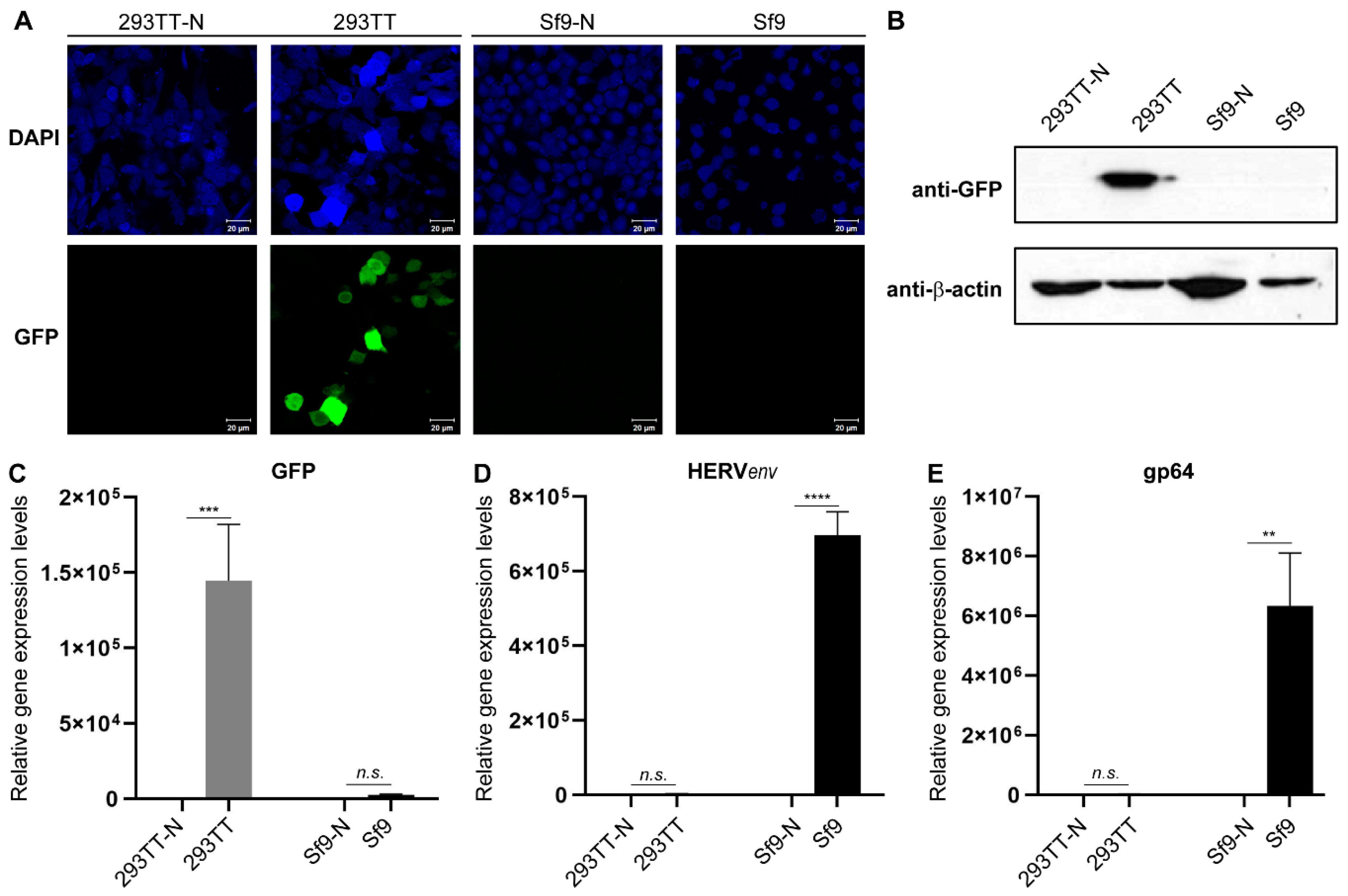
2.4. Fluorescence Microscopy
2.5. RNA Isolation
2.6. Quantitative Reverse Transcription-polymerase Chain Reaction (RT-qPCR)
2.7. Total RNA-Sequencing (RNA-seq)
2.8. Total RNA-seq Data Analysis
2.9. Statistical Analyses
3. Results
3.1. Generation of a Dual Promoter-Controlled Recombinant Baculovirus
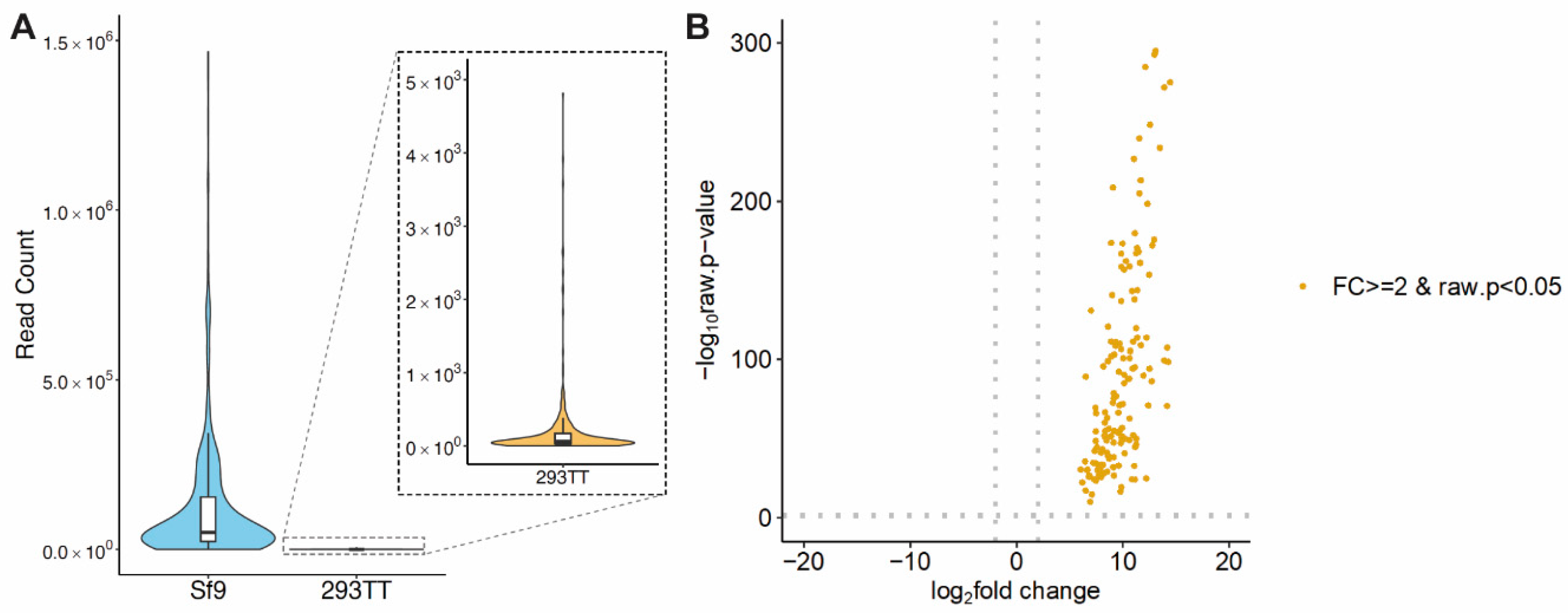
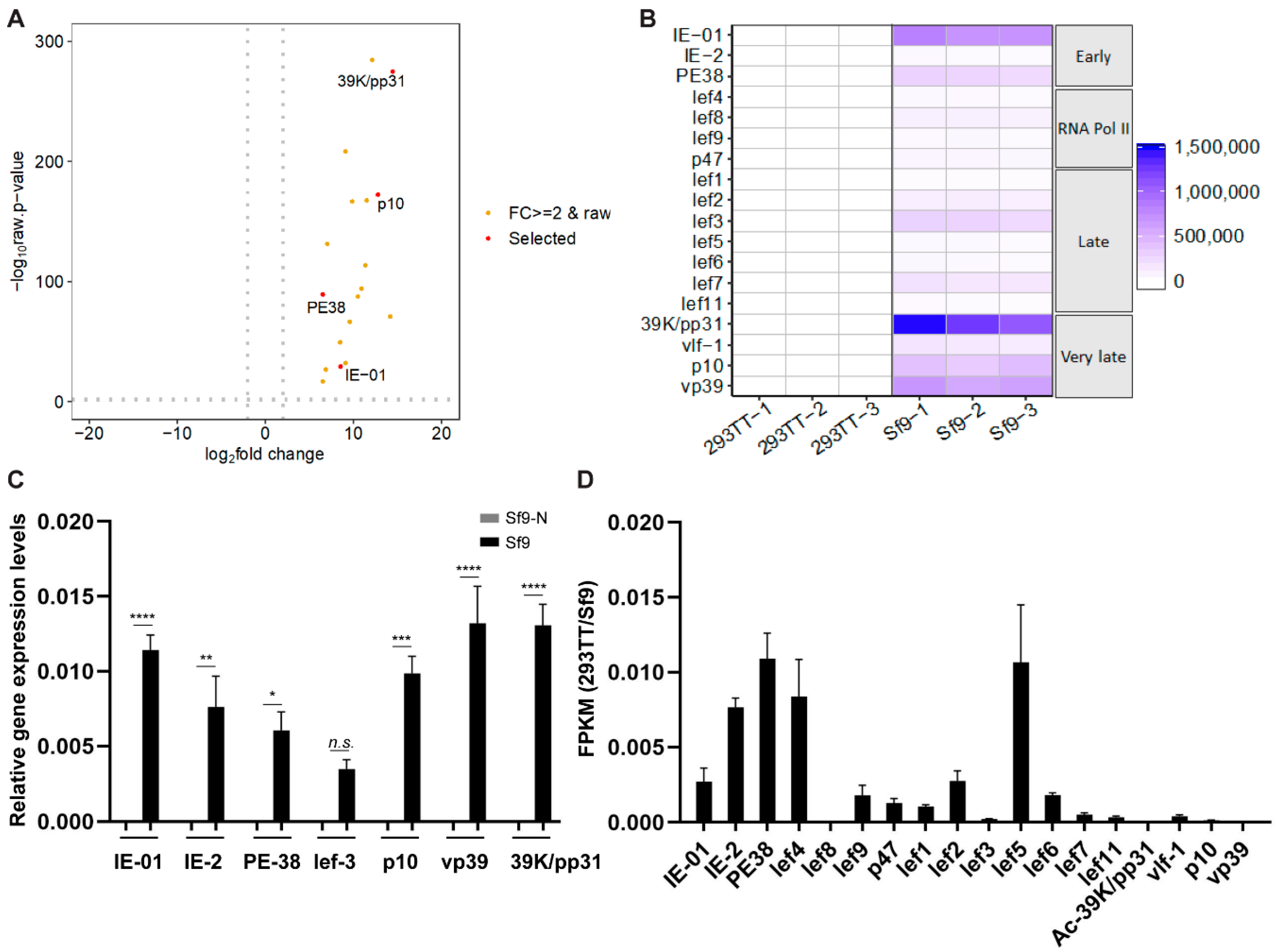
3.2. Comparative Baculovirus Transcriptome Profiles in Insect and Human Cells
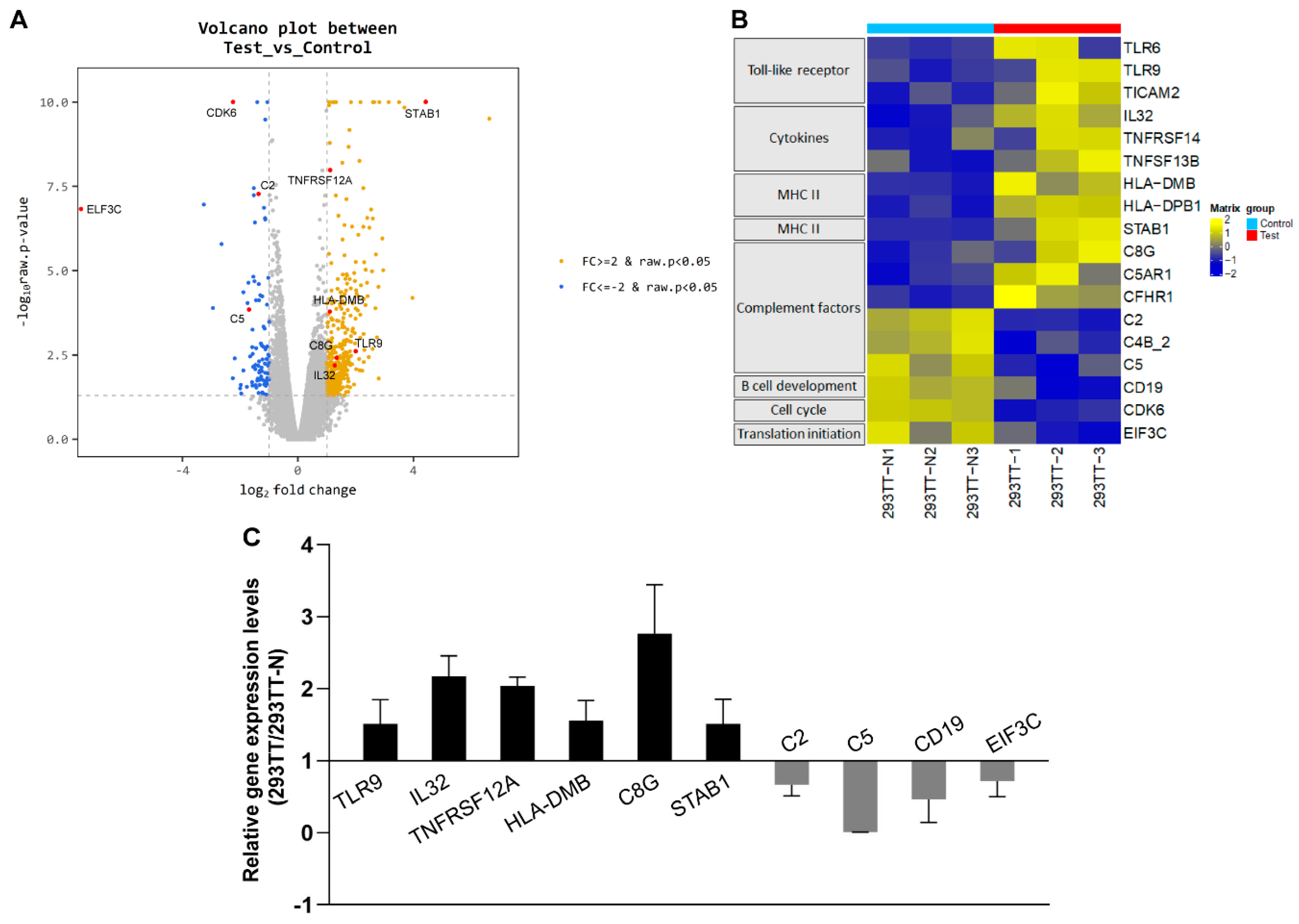
3.3. Induction of Immune-Response Genes upon Baculovirus Infection in Human Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Volkman, L.E.; Goldsmith, P.A. In Vitro Survey of Autographa californica Nuclear Polyhedrosis Virus Interaction with Nontarget Vertebrate Host Cells. Appl. Environ. Microbiol. 1983, 45, 1085–1093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, S.; Parvez, M.K.; Banerjee, K.; Sarin, S.K.; Hasnain, S.E. Baculovirus as mammalian cell expression vector for gene therapy: An emerging strategy. Mol. Ther. 2002, 6, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Ono, C.; Okamoto, T.; Abe, T.; Matsuura, Y. Baculovirus as a Tool for Gene Delivery and Gene Therapy. Viruses 2018, 10, 510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jha, P.K.; Pal, R.; Nakhai, B.; Sridhar, P.; Hasnain, S.E. Simultaneous synthesis of enzymatically active luciferase and biologically active β subunit of human chorionic gonadotropin in caterpillars infected with a recombinant baculovirus. FEBS Lett. 1992, 310, 148–152. [Google Scholar] [CrossRef] [Green Version]
- Van Oers, M.M.; Pijlman, G.P.; Vlak, J.M. Thirty years of baculovirus-insect cell protein expression: From dark horse to mainstream technology. J. Gen. Virol. 2015, 96, 6–23. [Google Scholar] [CrossRef] [PubMed]
- Blissard, G.W.; Wenz, J.R. Baculovirus gp64 envelope glycoprotein is sufficient to mediate pH-dependent membrane fusion. J. Virol. 1992, 66, 6829–6835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schultz, K.L.; Wetter, J.A.; Fiore, D.C.; Friesen, P.D. Transactivator IE1 is required for baculovirus early replication events that trigger apoptosis in permissive and nonpermissive cells. J. Virol. 2009, 83, 262–272. [Google Scholar] [CrossRef] [Green Version]
- Sokal, N.; Nie, Y.; Willis, L.G.; Yamagishi, J.; Blissard, G.W.; Rheault, M.R.; Theilmann, D.A. Defining the roles of the baculovirus regulatory proteins IE0 and IE1 in genome replication and early gene transactivation. Virology 2014, 468–470, 160–171. [Google Scholar] [CrossRef] [Green Version]
- Knebel-Morsdorf, D.; Quadt, I.; Li, Y.; Montier, L.; Guarino, L.A. Expression of baculovirus late and very late genes depends on LEF-4, a component of the viral RNA polymerase whose guanyltransferase function is essential. J. Virol. 2006, 80, 4168–4173. [Google Scholar] [CrossRef] [Green Version]
- Passarelli, A.L.; Guarino, L.A. Baculovirus late and very late gene regulation. Curr. Drug Targets 2007, 8, 1103–1115. [Google Scholar] [CrossRef]
- Beck, N.B.; Sidhu, J.S.; Omiecinski, C.J. Baculovirus vectors repress phenobarbital-mediated gene induction and stimulate cytokine expression in primary cultures of rat hepatocytes. Gene Ther. 2000, 7, 1274–1283. [Google Scholar] [CrossRef]
- Akira, S.; Takeda, K.; Kaisho, T. Toll-like receptors: Critical proteins linking innate and acquired immunity. Nat. Immunol. 2001, 2, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Takahashi, H.; Hamazaki, H.; Miyano-Kurosaki, N.; Matsuura, Y.; Takaku, H. Baculovirus induces an innate immune response and confers protection from lethal influenza virus infection in mice. J. Immunol. 2003, 171, 1133–1139. [Google Scholar] [CrossRef] [Green Version]
- Abe, T.; Hemmi, H.; Miyamoto, H.; Moriishi, K.; Tamura, S.; Takaku, H.; Akira, S.; Matsuura, Y. Involvement of the Toll-like receptor 9 signaling pathway in the induction of innate immunity by baculovirus. J. Virol. 2005, 79, 2847–2858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, W.Y.; Lin, S.Y.; Lo, K.W.; Lu, C.H.; Hung, C.L.; Chen, C.Y.; Chang, C.C.; Hu, Y.C. Adaptive immune responses elicited by baculovirus and impacts on subsequent transgene expression in vivo. J. Virol. 2013, 87, 4965–4973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hofmann, C.; Strauss, M. Baculovirus-mediated gene transfer in the presence of human serum or blood facilitated by inhibition of the complement system. Gene Ther. 1998, 5, 531–536. [Google Scholar] [CrossRef] [Green Version]
- Kaikkonen, M.U.; Maatta, A.I.; Yla-Herttuala, S.; Airenne, K.J. Screening of complement inhibitors: Shielded baculoviruses increase the safety and efficacy of gene delivery. Mol. Ther. 2010, 18, 987–992. [Google Scholar] [CrossRef] [Green Version]
- Gronowski, A.M.; Hilbert, D.M.; Sheehan, K.C.; Garotta, G.; Schreiber, R.D. Baculovirus stimulates antiviral effects in mammalian cells. J. Virol. 1999, 73, 9944–9951. [Google Scholar] [CrossRef] [Green Version]
- Laakkonen, J.P.; Kaikkonen, M.U.; Ronkainen, P.H.; Ihalainen, T.O.; Niskanen, E.A.; Hakkinen, M.; Salminen, M.; Kulomaa, M.S.; Yla-Herttuala, S.; Airenne, K.J.; et al. Baculovirus-mediated immediate-early gene expression and nuclear reorganization in human cells. Cell Microbiol. 2008, 10, 667–681. [Google Scholar] [CrossRef]
- Ono, C.; Ninomiya, A.; Yamamoto, S.; Abe, T.; Wen, X.; Fukuhara, T.; Sasai, M.; Yamamoto, M.; Saitoh, T.; Satoh, T.; et al. Innate immune response induced by baculovirus attenuates transgene expression in mammalian cells. J. Virol. 2014, 88, 2157–2167. [Google Scholar] [CrossRef] [Green Version]
- Molina, G.N.; Tavarone, E.; Taboga, O.; Molinari, P. Two Distinctive Phenotypes of AcMNPV Display Different Immune Abilities and Intracellular Destiny. PLoS ONE 2016, 11, e0168939. [Google Scholar] [CrossRef]
- Fujita, R.; Matsuyama, T.; Yamagishi, J.; Sahara, K.; Asano, S.; Bando, H. Expression of Autographa californica multiple nucleopolyhedrovirus genes in mammalian cells and upregulation of the host β-actin gene. J. Virol. 2006, 80, 2390–2395. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.J.; Park, N.; Cho, H.J.; Yoon, J.K.; Van, N.D.; Oh, Y.K.; Kim, Y.B. Development of a novel viral DNA vaccine against human papillomavirus: AcHERV-HP16L1. Vaccine 2010, 28, 1613–1619. [Google Scholar] [CrossRef]
- Lee, H.J.; Hur, Y.K.; Cho, Y.D.; Kim, M.G.; Lee, H.T.; Oh, Y.K.; Kim, Y.B. Immunogenicity of bivalent human papillomavirus DNA vaccine using human endogenous retrovirus envelope-coated baculoviral vectors in mice and pigs. PLoS ONE 2012, 7, e50296. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.J.; Yoon, J.K.; Heo, Y.; Cho, H.; Cho, Y.; Gwon, Y.; Kim, K.C.; Choi, J.; Lee, J.S.; Oh, Y.K.; et al. Therapeutic potential of an AcHERV-HPV L1 DNA vaccine. J. Microbiol. 2015, 53, 415–420. [Google Scholar] [CrossRef]
- Shrestha, A.; Bao, K.; Chen, Y.R.; Chen, W.; Wang, P.; Fei, Z.; Blissard, G.W. Global Analysis of Baculovirus Autographa californica Multiple Nucleopolyhedrovirus Gene Expression in the Midgut of the Lepidopteran Host Trichoplusia ni. J. Virol. 2018, 92. [Google Scholar] [CrossRef] [Green Version]
- Passarelli, A.L.; Miller, L.K. In vivo and in vitro analyses of recombinant baculoviruses lacking a functional cg30 gene. J. Virol. 1994, 68, 1186–1190. [Google Scholar] [CrossRef] [Green Version]
- McLachlin, J.R.; Miller, L.K. Identification and characterization of vlf-1, a baculovirus gene involved in very late gene expression. J. Virol. 1994, 68, 7746–7756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guarino, L.A.; Mistretta, T.A.; Dong, W. DNA binding activity of the baculovirus late expression factor PP31. Virus Res. 2002, 90, 187–195. [Google Scholar] [CrossRef]
- Rohel, D.Z.; Cochran, M.A.; Faulkner, P. Characterization of two abundant mRNAs of Autographa californica nuclear polyhedrosis virus present late in infection. Virology 1983, 124, 357–365. [Google Scholar] [CrossRef]
- Guarino, L.A.; Xu, B.; Jin, J.; Dong, W. A virus-encoded RNA polymerase purified from baculovirus-infected cells. J. Virol. 1998, 72, 7985–7991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Z.P.; Yin, J.; Zhang, Y.Y.; Jia, S.Y.; Chen, Z.J.; Zhong, J. Characterization of the immune responses elicited by baculovirus-based vector vaccines against influenza virus hemagglutinin. Acta Pharmacol. Sin. 2012, 33, 783–790. [Google Scholar] [CrossRef]
- Ayres, M.D.; Howard, S.C.; Kuzio, J.; Lopez-Ferber, M.; Possee, R.D. The complete DNA sequence of Autographa californica nuclear polyhedrosis virus. Virology 1994, 202, 586–605. [Google Scholar] [CrossRef]
- Chen, Y.R.; Zhong, S.; Fei, Z.; Hashimoto, Y.; Xiang, J.Z.; Zhang, S.; Blissard, G.W. The transcriptome of the baculovirus Autographa californica multiple nucleopolyhedrovirus in Trichoplusia ni cells. J. Virol. 2013, 87, 6391–6405. [Google Scholar] [CrossRef] [Green Version]
- Kenoutis, C.; Efrose, R.C.; Swevers, L.; Lavdas, A.A.; Gaitanou, M.; Matsas, R.; Iatrou, K. Baculovirus-mediated gene delivery into Mammalian cells does not alter their transcriptional and differentiating potential but is accompanied by early viral gene expression. J. Virol. 2006, 80, 4135–4146. [Google Scholar] [CrossRef] [Green Version]
- Lavdas, A.A.; Efrose, R.; Douris, V.; Gaitanou, M.; Papastefanaki, F.; Swevers, L.; Thomaidou, D.; Iatrou, K.; Matsas, R. Soluble forms of the cell adhesion molecule L1 produced by insect and baculovirus-transduced mammalian cells enhance Schwann cell motility. J. Neurochem. 2010, 115, 1137–1149. [Google Scholar] [CrossRef]
- Rogers, G.L.; Martino, A.T.; Aslanidi, G.V.; Jayandharan, G.R.; Srivastava, A.; Herzog, R.W. Innate Immune Responses to AAV Vectors. Front. Microbiol. 2011, 2, 194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rabinowitz, J.; Chan, Y.K.; Samulski, R.J. Adeno-associated Virus (AAV) versus Immune Response. Viruses 2019, 11, 102. [Google Scholar] [CrossRef] [Green Version]
- Ronzitti, G.; Gross, D.A.; Mingozzi, F. Human Immune Responses to Adeno-Associated Virus (AAV) Vectors. Front. Immunol. 2020, 11, 670. [Google Scholar] [CrossRef] [PubMed]
| Sample | Sample No. | Number of Processed Reads | Number of Mapped Reads |
|---|---|---|---|
| 293TT-N | 1 | 123,252,764 | 0 (0.00%) |
| 2 | 130,201,768 | 16 (0.00%) | |
| 3 | 126,360,742 | 0 (0.00%) | |
| 293TT | 1 | 100,237,542 | 56,316 (0.06%) |
| 2 | 98,908,778 | 107,364 (0.11%) | |
| 3 | 148,781,606 | 170,092 (0.11%) | |
| Sf9 | 1 | 136,870,040 | 63,122,142 (46.12%) |
| 2 | 127,920,774 | 56,971,000 (44.54%) | |
| 3 | 112,371,568 | 51,938,850 (46.22%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, H.Y.; Choi, H.; Kim, N.; Park, N.; Kim, H.; Kim, J.; Kim, Y.B. Unraveling the Genome-Wide Impact of Recombinant Baculovirus Infection in Mammalian Cells for Gene Delivery. Genes 2020, 11, 1306. https://doi.org/10.3390/genes11111306
Shin HY, Choi H, Kim N, Park N, Kim H, Kim J, Kim YB. Unraveling the Genome-Wide Impact of Recombinant Baculovirus Infection in Mammalian Cells for Gene Delivery. Genes. 2020; 11(11):1306. https://doi.org/10.3390/genes11111306
Chicago/Turabian StyleShin, Ha Youn, Hanul Choi, Nahyun Kim, Nayoung Park, Heesun Kim, Jaebum Kim, and Young Bong Kim. 2020. "Unraveling the Genome-Wide Impact of Recombinant Baculovirus Infection in Mammalian Cells for Gene Delivery" Genes 11, no. 11: 1306. https://doi.org/10.3390/genes11111306
APA StyleShin, H. Y., Choi, H., Kim, N., Park, N., Kim, H., Kim, J., & Kim, Y. B. (2020). Unraveling the Genome-Wide Impact of Recombinant Baculovirus Infection in Mammalian Cells for Gene Delivery. Genes, 11(11), 1306. https://doi.org/10.3390/genes11111306





