Thiopurine Drugs in the Treatment of Ulcerative Colitis: Identification of a Novel Deleterious Mutation in TPMT
Abstract
1. Introduction
2. Azathioprine and 6-Mercaptopurine
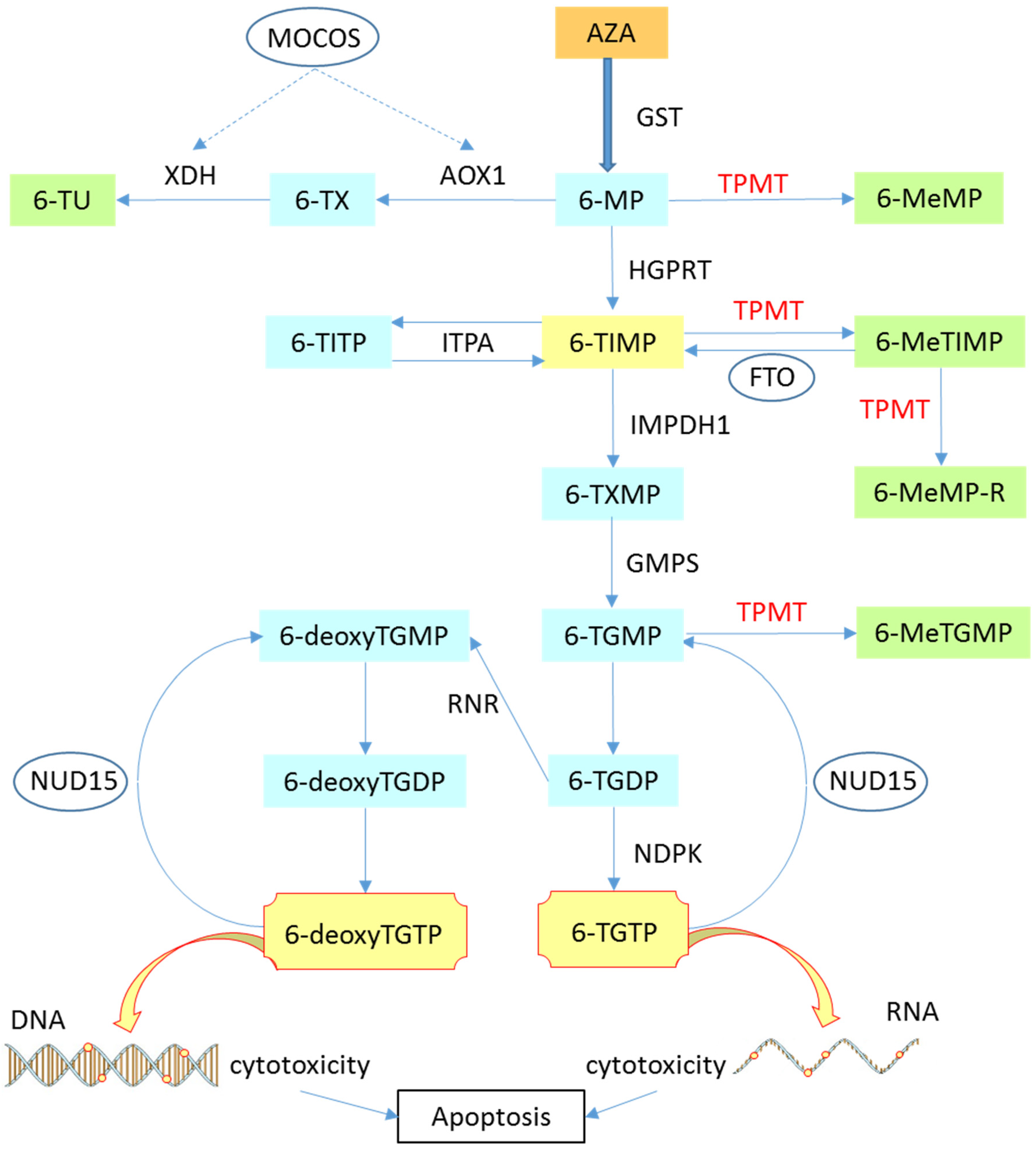
2.1. Mechanism of Action
2.2. Pharmacology
2.3. Thiopurine Side Effects
2.4. Pharmagenetics
3. TPMT
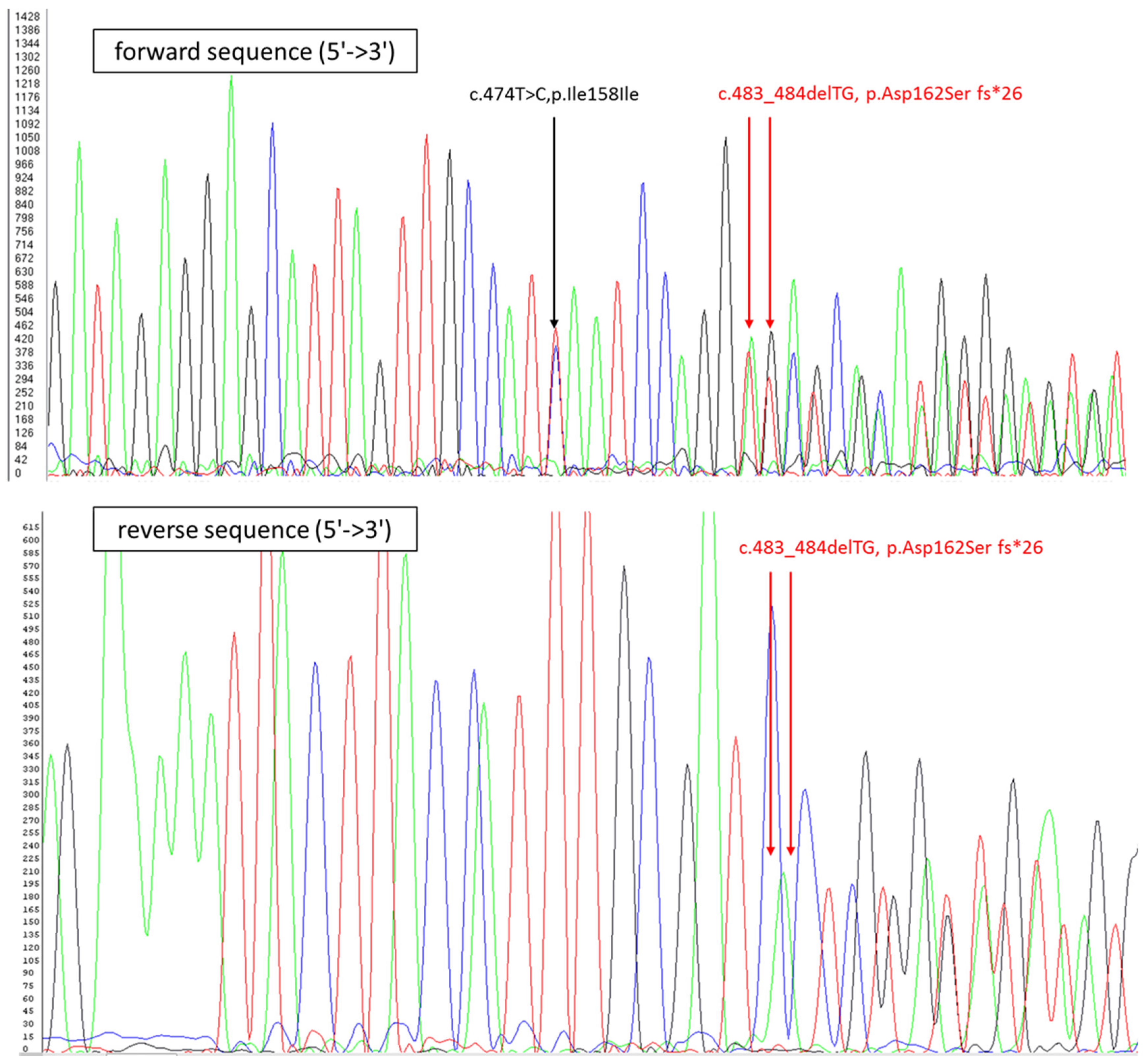
4. Case Report
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kaplan, G.G. The global burden of IBD: From 2015 to 2025. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 720–727. [Google Scholar] [CrossRef]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2018, 390, 2769–2778. [Google Scholar] [CrossRef]
- Baumgart, D.C.; Carding, S.R. Inflammatory bowel disease: Cause and immunobiology. Lancet 2007, 369, 1627–1640. [Google Scholar] [CrossRef]
- Damiao, A.; de Azevedo, M.F.C.; Carlos, A.S.; Wada, M.Y.; Silva, T.V.M.; Feitosa, F.C. Conventional therapy for moderate to severe inflammatory bowel disease: A systematic literature review. World J. Gastroenterol. 2019, 25, 1142–1157. [Google Scholar] [CrossRef] [PubMed]
- Lamb, C.A.; Kennedy, N.A.; Raine, T.; Hendy, P.A.; Smith, P.J.; Limdi, J.K.; Hayee, B.; Lomer, M.C.E.; Parkes, G.C.; Selinger, C.; et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019, 68, s1–s106. [Google Scholar] [CrossRef]
- Conrad, M.A.; Kelsen, J.R. The Treatment of Pediatric Inflammatory Bowel Disease with Biologic Therapies. Curr. Gastroenterol. Rep. 2020, 22, 36. [Google Scholar] [CrossRef]
- Soh, J.S.; Yun, W.J.; Kim, K.J.; Won, C.H.; Park, S.H.; Yang, D.H.; Ye, B.D.; Byeon, J.S.; Myung, S.J.; Yang, S.K.; et al. Concomitant use of azathioprine/6-mercaptopurine decreases the risk of anti-TNF-induced skin lesions. Inflamm. Bowel Dis. 2015, 21, 832–839. [Google Scholar] [CrossRef]
- Coenen, M.J.; de Jong, D.J.; van Marrewijk, C.J.; Derijks, L.J.; Vermeulen, S.H.; Wong, D.R.; Klungel, O.H.; Verbeek, A.L.; Hooymans, P.M.; Peters, W.H.; et al. Identification of Patients With Variants in TPMT and Dose Reduction Reduces Hematologic Events During Thiopurine Treatment of Inflammatory Bowel Disease. Gastroenterology 2015, 149, 907–917.e907. [Google Scholar] [CrossRef]
- Schwab, M.; Schaffeler, E.; Marx, C.; Fischer, C.; Lang, T.; Behrens, C.; Gregor, M.; Eichelbaum, M.; Zanger, U.M.; Kaskas, B.A. Azathioprine therapy and adverse drug reactions in patients with inflammatory bowel disease: Impact of thiopurine S-methyltransferase polymorphism. Pharmacogenetics 2002, 12, 429–436. [Google Scholar] [CrossRef]
- Dean, L.; Pratt, V.M.; McLeod, H.L.; Rubinstein, W.S.; Scott, S.A.; Dean, L.C.; Kattman, B.L.; Malheiro, A.J. Eds Azathioprine Therapy and TPMT and NUDT15 Genotype. In Medical Genetics Summaries; National Center for Biotechnology Information (US): Bethesda, MD, USA, 2012. [Google Scholar]
- Fong, W.Y.; Ho, C.C.; Poon, W.T. Comparison of Direct Sequencing, Real-Time PCR-High Resolution Melt (PCR-HRM) and PCR-Restriction Fragment Length Polymorphism (PCR-RFLP) Analysis for Genotyping of Common Thiopurine Intolerant Variant Alleles NUDT15 c.415C>T and TPMT c.719A>G (TPMT * 3C). Diagnostics 2017, 7, 27. [Google Scholar] [CrossRef]
- Pasternak, B.; Svanstrom, H.; Schmiegelow, K.; Jess, T.; Hviid, A. Use of azathioprine and the risk of cancer in inflammatory bowel disease. Am. J. Epidemiol. 2013, 177, 1296–1305. [Google Scholar] [CrossRef]
- Prefontaine, E.; Sutherland, L.R.; Macdonald, J.K.; Cepoiu, M. Azathioprine or 6-mercaptopurine for maintenance of remission in Crohn’s disease. Cochrane Database Syst. Rev. 2009, CD000067. [Google Scholar] [CrossRef]
- Amin, J.; Huang, B.; Yoon, J.; Shih, D.Q. Update 2014: Advances to optimize 6-mercaptopurine and azathioprine to reduce toxicity and improve efficacy in the management of IBD. Inflamm. Bowel Dis. 2015, 21, 445–452. [Google Scholar] [CrossRef]
- Colombel, J.F.; Sandborn, W.J.; Reinisch, W.; Mantzaris, G.J.; Kornbluth, A.; Rachmilewitz, D.; Lichtiger, S.; D’Haens, G.; Diamond, R.H.; Broussard, D.L.; et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N. Engl. J. Med. 2010, 362, 1383–1395. [Google Scholar] [CrossRef]
- Panaccione, R.; Ghosh, S.; Middleton, S.; Marquez, J.R.; Scott, B.B.; Flint, L.; van Hoogstraten, H.J.; Chen, A.C.; Zheng, H.; Danese, S.; et al. Combination therapy with infliximab and azathioprine is superior to monotherapy with either agent in ulcerative colitis. Gastroenterology 2014, 146, 392–400.e3. [Google Scholar] [CrossRef]
- Gomollon, F.; Dignass, A.; Annese, V.; Tilg, H.; Van Assche, G.; Lindsay, J.O.; Peyrin-Biroulet, L.; Cullen, G.J.; Daperno, M.; Kucharzik, T.; et al. 3rd European Evidence-based Consensus on the Diagnosis and Management of Crohn’s Disease 2016: Part 1: Diagnosis and Medical Management. J. Crohns Colitis 2017, 11, 3–25. [Google Scholar] [CrossRef]
- Harbord, M.; Eliakim, R.; Bettenworth, D.; Karmiris, K.; Katsanos, K.; Kopylov, U.; Kucharzik, T.; Molnar, T.; Raine, T.; Sebastian, S.; et al. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 2: Current Management. J. Crohns Colitis 2017, 11, 769–784. [Google Scholar] [CrossRef]
- Swann, P.F.; Waters, T.R.; Moulton, D.C.; Xu, Y.Z.; Zheng, Q.; Edwards, M.; Mace, R. Role of postreplicative DNA mismatch repair in the cytotoxic action of thioguanine. Science 1996, 273, 1109–1111. [Google Scholar] [CrossRef]
- Lennard, L.; Rees, C.A.; Lilleyman, J.S.; Maddocks, J.L. Childhood leukaemia: A relationship between intracellular 6-mercaptopurine metabolites and neutropenia. Br. J. Clin. Pharmacol. 1983, 16, 359–363. [Google Scholar] [CrossRef]
- Van Scoik, K.G.; Johnson, C.A.; Porter, W.R. The pharmacology and metabolism of the thiopurine drugs 6-mercaptopurine and azathioprine. Drug. Metab. Rev. 1985, 16, 157–174. [Google Scholar] [CrossRef]
- Hon, Y.Y.; Fessing, M.Y.; Pui, C.H.; Relling, M.V.; Krynetski, E.Y.; Evans, W.E. Polymorphism of the thiopurine S-methyltransferase gene in African-Americans. Hum. Mol. Genet. 1999, 8, 371–376. [Google Scholar] [CrossRef]
- Abaji, R.; Krajinovic, M. Thiopurine S-methyltransferase polymorphisms in acute lymphoblastic leukemia, inflammatory bowel disease and autoimmune disorders: Influence on treatment response. Pharmgenomics Pers. Med. 2017, 10, 143–156. [Google Scholar] [CrossRef]
- Chen, S.; Tan, W.Z.; Sutiman, N.; Lim, C.; Lee, S.S.; Leong, W.F.; Tjai, M.; Wang, C.; Kong, C.S.C.; Chuah, S.W.; et al. An intronic FTO variant rs16952570 confers protection against thiopurine-induced myelotoxicities in multiethnic Asian IBD patients. Pharm. J. 2020, 20, 505–515. [Google Scholar] [CrossRef]
- Coelho, T.; Andreoletti, G.; Ashton, J.J.; Batra, A.; Afzal, N.A.; Gao, Y.; Williams, A.P.; Beattie, R.M.; Ennis, S. Genes implicated in thiopurine-induced toxicity: Comparing TPMT enzyme activity with clinical phenotype and exome data in a paediatric IBD cohort. Sci. Rep. 2016, 6, 34658. [Google Scholar] [CrossRef]
- Gonzalez-Lama, Y.; Gisbert, J.P. Monitoring thiopurine metabolites in inflammatory bowel disease. Frontline Gastroenterol. 2016, 7, 301–307. [Google Scholar] [CrossRef]
- Lim, S.Z.; Chua, E.W. Revisiting the Role of Thiopurines in Inflammatory Bowel Disease Through Pharmacogenomics and Use of Novel Methods for Therapeutic Drug Monitoring. Front. Pharmacol. 2018, 9, 1107. [Google Scholar] [CrossRef]
- Choi, R.; Sohn, I.; Kim, M.J.; Woo, H.I.; Lee, J.W.; Ma, Y.; Yi, E.S.; Koo, H.H.; Lee, S.Y. Pathway genes and metabolites in thiopurine therapy in Korean children with acute lymphoblastic leukaemia. Br. J. Clin. Pharmacol. 2019, 85, 1585–1597. [Google Scholar] [CrossRef]
- Chang, J.Y.; Cheon, J.H. Thiopurine Therapy in Patients With Inflammatory Bowel Disease: A Focus on Metabolism and Pharmacogenetics. Dig. Dis. Sci. 2019, 64, 2395–2403. [Google Scholar] [CrossRef]
- Dubinsky, M.C. Azathioprine, 6-mercaptopurine in inflammatory bowel disease: Pharmacology, efficacy, and safety. Clin. Gastroenterol. Hepatol. 2004, 2, 731–743. [Google Scholar] [CrossRef]
- Lennard, L.; Van Loon, J.A.; Weinshilboum, R.M. Pharmacogenetics of acute azathioprine toxicity: Relationship to thiopurine methyltransferase genetic polymorphism. Clin. Pharm. Ther. 1989, 46, 149–154. [Google Scholar] [CrossRef]
- Van Os, E.C.; Zins, B.J.; Sandborn, W.J.; Mays, D.C.; Tremaine, W.J.; Mahoney, D.W.; Zinsmeister, A.R.; Lipsky, J.J. Azathioprine pharmacokinetics after intravenous, oral, delayed release oral and rectal foam administration. Gut 1996, 39, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Chrzanowska, M.; Hermann, T.; Gapinska, M. Kinetics of azathioprine metabolism in fresh human blood. Pol. J. Pharm. Pharm. 1985, 37, 701–708. [Google Scholar]
- Gilissen, L.P.; Derijks, L.J.; Bos, L.P.; Bus, P.J.; Hooymans, P.M.; Engels, L.G. Therapeutic drug monitoring in patients with inflammatory bowel disease and established azathioprine therapy. Clin. Drug Investig. 2004, 24, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Chande, N.; Patton, P.H.; Tsoulis, D.J.; Thomas, B.S.; MacDonald, J.K. Azathioprine or 6-mercaptopurine for maintenance of remission in Crohn’s disease. Cochrane Database Syst. Rev. 2015, Cd000067. [Google Scholar] [CrossRef] [PubMed]
- Timmer, A.; Patton, P.H.; Chande, N.; McDonald, J.W.; MacDonald, J.K. Azathioprine and 6-mercaptopurine for maintenance of remission in ulcerative colitis. Cochrane Database Syst. Rev. 2016, CD000478. [Google Scholar] [CrossRef] [PubMed]
- Broekman, M.; Coenen, M.J.H.; van Marrewijk, C.J.; Wanten, G.J.A.; Wong, D.R.; Verbeek, A.L.M.; Klungel, O.H.; Hooymans, P.M.; Guchelaar, H.J.; Scheffer, H.; et al. More Dose-dependent Side Effects with Mercaptopurine over Azathioprine in IBD Treatment Due to Relatively Higher Dosing. Inflamm. Bowel Dis. 2017, 23, 1873–1881. [Google Scholar] [CrossRef] [PubMed]
- Pruijt, J.F.; Haanen, J.B.; Hollander, A.A.; den Ottolander, G.J. Azathioprine-induced pure red-cell aplasia. Nephrol. Dial. Transpl. 1996, 11, 1371–1373. [Google Scholar] [CrossRef]
- Logan, J.K.; Yapa, S.W.S.; Harinstein, L.; Saluja, B.; Munoz, M.; Sahajwalla, C.; Neuner, R.; Seymour, S. Drug Interaction Between Febuxostat and Thiopurine Antimetabolites: A Review of the FDA Adverse Event Reporting System and Medical Literature. Pharmacotherapy 2020, 40, 125–132. [Google Scholar] [CrossRef]
- Beigel, F.; Steinborn, A.; Schnitzler, F.; Tillack, C.; Breiteneicher, S.; John, J.M.; Van Steen, K.; Laubender, R.P.; Goke, B.; Seiderer, J.; et al. Risk of malignancies in patients with inflammatory bowel disease treated with thiopurines or anti-TNF α antibodies. Pharmacoepidemiol. Drug Saf. 2014, 23, 735–744. [Google Scholar] [CrossRef]
- Fraser, A.G.; Orchard, T.R.; Robinson, E.M.; Jewell, D.P. Long-term risk of malignancy after treatment of inflammatory bowel disease with azathioprine. Aliment. Pharmacol. Ther. 2002, 16, 1225–1232. [Google Scholar] [CrossRef]
- McGovern, D.P.; Jewell, D.P. Risks and benefits of azathioprine therapy. Gut 2005, 54, 1055–1059. [Google Scholar] [CrossRef]
- Lewis, J.D.; Schwartz, J.S.; Lichtenstein, G.R. Azathioprine for maintenance of remission in Crohn’s disease: Benefits outweigh the risk of lymphoma. Gastroenterology 2000, 118, 1018–1024. [Google Scholar] [CrossRef]
- de Jong, D.J.; Goullet, M.; Naber, T.H. Side effects of azathioprine in patients with Crohn’s disease. Eur. J. Gastroenterol. Hepatol. 2004, 16, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.P.; Xu, H.Q.; Li, M.; Yang, X.; Yu, S.; Fu, W.L.; Huang, Q. Association between Thiopurine S-Methyltransferase Polymorphisms and Azathioprine-Induced Adverse Drug Reactions in Patients with Autoimmune Diseases: A Meta-Analysis. PLoS ONE 2015, 10, e0144234. [Google Scholar] [CrossRef] [PubMed]
- Kreijne, J.E.; Seinen, M.L.; Wilhelm, A.J.; Bouma, G.; Mulder, C.J.; van Bodegraven, A.A.; de Boer, N.K. Routinely Established Skewed Thiopurine Metabolism Leads to a Strikingly High Rate of Early Therapeutic Failure in Patients With Inflammatory Bowel Disease. Ther. Drug Monit. 2015, 37, 797–804. [Google Scholar] [CrossRef]
- Smith, M.A.; Blaker, P.; Marinaki, A.M.; Anderson, S.H.; Irving, P.M.; Sanderson, J.D. Optimising outcome on thiopurines in inflammatory bowel disease by co-prescription of allopurinol. J. Crohns Colitis 2012, 6, 905–912. [Google Scholar] [CrossRef]
- Moon, W.; Loftus, E.V., Jr. Review article: Recent advances in pharmacogenetics and pharmacokinetics for safe and effective thiopurine therapy in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2016, 43, 863–883. [Google Scholar] [CrossRef] [PubMed]
- Keats, B.J.B.; Sherman, S.L. Chapter 13—Population Genetics. In Emery and Rimoin’s Principles and Practice of Medical Genetics, 6th ed.; Academic Press: Oxford, UK, 2013; pp. 1–12. [Google Scholar]
- Hawwa, A.F.; Millership, J.S.; Collier, P.S.; Vandenbroeck, K.; McCarthy, A.; Dempsey, S.; Cairns, C.; Collins, J.; Rodgers, C.; McElnay, J.C. Pharmacogenomic studies of the anticancer and immunosuppressive thiopurines mercaptopurine and azathioprine. Br. J. Clin. Pharmacol. 2008, 66, 517–528. [Google Scholar] [CrossRef]
- Kham, S.K.; Soh, C.K.; Aw, D.C.; Yeoh, A.E. TPMT*26 (208F-->L), a novel mutation detected in a Chinese. Br. J. Clin. Pharmacol. 2009, 68, 120–123. [Google Scholar] [CrossRef]
- McLeod, H.L.; Siva, C. The thiopurine S-methyltransferase gene locus -- implications for clinical pharmacogenomics. Pharmacogenomics 2002, 3, 89–98. [Google Scholar] [CrossRef]
- Tamm, R.; Oselin, K.; Kallassalu, K.; Magi, R.; Anier, K.; Remm, M.; Metspalu, A. Thiopurine S-methyltransferase (TPMT) pharmacogenetics: Three new mutations and haplotype analysis in the Estonian population. Clin. Chem. Lab. Med. 2008, 46, 974–979. [Google Scholar] [CrossRef]
- Lennard, L. Implementation of TPMT testing. Br. J. Clin. Pharmacol. 2014, 77, 704–714. [Google Scholar] [CrossRef] [PubMed]
- Krynetski, E.Y.; Evans, W.E. Genetic polymorphism of thiopurine S-methyltransferase: Molecular mechanisms and clinical importance. Pharmacology 2000, 61, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Iu, Y.P.H.; Helander, S.; Kahlin, A.Z.; Cheng, C.W.; Shek, C.C.; Leung, M.H.; Wallner, B.; Martensson, L.G.; Appell, M.L. One amino acid makes a difference-Characterization of a new TPMT allele and the influence of SAM on TPMT stability. Sci. Rep. 2017, 7, 46428. [Google Scholar] [CrossRef]
- Appell, M.L.; Berg, J.; Duley, J.; Evans, W.E.; Kennedy, M.A.; Lennard, L.; Marinaki, T.; McLeod, H.L.; Relling, M.V.; Schaeffeler, E.; et al. Nomenclature for alleles of the thiopurine methyltransferase gene. Pharmacogenet. Genom. 2013, 23, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Tai, H.L.; Krynetski, E.Y.; Schuetz, E.G.; Yanishevski, Y.; Evans, W.E. Enhanced proteolysis of thiopurine S-methyltransferase (TPMT) encoded by mutant alleles in humans (TPMT*3A, TPMT*2): Mechanisms for the genetic polymorphism of TPMT activity. Proc. Natl. Acad. Sci. USA 1997, 94, 6444–6449. [Google Scholar] [CrossRef]
- Szumlanski, C.; Otterness, D.; Her, C.; Lee, D.; Brandriff, B.; Kelsell, D.; Spurr, N.; Lennard, L.; Wieben, E.; Weinshilboum, R. Thiopurine methyltransferase pharmacogenetics: Human gene cloning and characterization of a common polymorphism. DNA Cell Biol. 1996, 15, 17–30. [Google Scholar] [CrossRef]
- Wang, L.; Sullivan, W.; Toft, D.; Weinshilboum, R. Thiopurine S-methyltransferase pharmacogenetics: Chaperone protein association and allozyme degradation. Pharmacogenetics 2003, 13, 555–564. [Google Scholar] [CrossRef]
- Loennechen, T.; Utsi, E.; Hartz, I.; Lysaa, R.; Kildalsen, H.; Aarbakke, J. Detection of one single mutation predicts thiopurine S-methyltransferase activity in a population of Saami in northern Norway. Clin. Pharmacol. Ther. 2001, 70, 183–188. [Google Scholar] [CrossRef]
- Adam, L.; Phulukdaree, A.; Soma, P. Effective long-term solution to therapeutic remission in Inflammatory Bowel Disease: Role of Azathioprine. Biomed. Pharmacother. 2018, 100, 8–14. [Google Scholar] [CrossRef]
- Ichida, K.; Matsumura, T.; Sakuma, R.; Hosoya, T.; Nishino, T. Mutation of human molybdenum cofactor sulfurase gene is responsible for classical xanthinuria type II. Biochem. Biophys. Res. Commun. 2001, 282, 1194–1200. [Google Scholar] [CrossRef]

| Common Allele Name | Exon | Coding DNA | Protein |
|---|---|---|---|
| TPMT * 2 | 5 | c.238G > C | p.Ala80Pro |
| TPMT * 3A | 7 | c.460G > A | p.Ala154Thr |
| TPMT * 3A | 10 | c.719A > G | p.Tyr240Cys |
| TPMT * 3B | 7 | c.460G > A | p.Ala154Thr |
| TPMT * 3C | 10 | c.719A > G | p.Tyr240Cys |
| TPMT * 8 | 10 | c.644G > A | p.Arg215His |
| EXON 5 : | EXON 7 : | EXON 10 : | ||||||
|---|---|---|---|---|---|---|---|---|
| Variant | Nucleotide | Protein | Variant | Nucleotide | Protein | Variant | Nucleotide | Protein |
| TPMT * 2 | c.238G > C | p.Ala80Pro | TPMT * 10 | c.430G > C | p.Gly144Gln | TPMT * 8 | c.644G > A | p.Arg215His |
| TPMT * 3D | c.292G > T | p.Glu98* | TPMT * 3A | c.460G > A | p.Ala154Arg | TPMT * 7 | c.681T > G | p.His227Gln |
| TPMT * 9 | c.356A > C | p.Lys119Thr | TPMT * 3B | c.460G > A | p.Ala154Arg | TPMT * 20 | c.712A > G | p.Lys238Glu |
| TPMT * 19 | c.365A > C | p.Lys122Thr | TPMT * 22 | c.474T > C | p.Ile158Ile | TPMT * 3A | c.719A > G | p.Tyr240Cys |
| TPMT * 34 | c.319T > G | p.Tyr107Asp | TPMT * 16 | c.488G > A | p.Arg163His | TPMT * 3C | c.719A > G | p.tyr240Cys |
| TPMT * 28 | c.349G > C | p.Gly117Arg | TPMT * 22 | c.488G > C | p.Arg158Pro | TPMT * 41 | c.719A > C | p.Tyr240Ser |
| TPMT * 32 | c.340G > A | p.Glu114Lys | TPMT * 33 | c.487C > T | p.Arg158Cys | TPMT * 25 | c.634T > C | p.Cys212Ser |
| TPMT * 37 | c.648T > A | p.Cys216* | ||||||
| TPMT * 40 | c.677G > A | p.Arg226Gln | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harmand, P.-O.; Solassol, J. Thiopurine Drugs in the Treatment of Ulcerative Colitis: Identification of a Novel Deleterious Mutation in TPMT. Genes 2020, 11, 1212. https://doi.org/10.3390/genes11101212
Harmand P-O, Solassol J. Thiopurine Drugs in the Treatment of Ulcerative Colitis: Identification of a Novel Deleterious Mutation in TPMT. Genes. 2020; 11(10):1212. https://doi.org/10.3390/genes11101212
Chicago/Turabian StyleHarmand, Pierre-Olivier, and Jérôme Solassol. 2020. "Thiopurine Drugs in the Treatment of Ulcerative Colitis: Identification of a Novel Deleterious Mutation in TPMT" Genes 11, no. 10: 1212. https://doi.org/10.3390/genes11101212
APA StyleHarmand, P.-O., & Solassol, J. (2020). Thiopurine Drugs in the Treatment of Ulcerative Colitis: Identification of a Novel Deleterious Mutation in TPMT. Genes, 11(10), 1212. https://doi.org/10.3390/genes11101212






