The Effect of Light Intensity on the Expression of Leucoanthocyanidin Reductase in Grapevine Calluses and Analysis of Its Promoter Activity
Abstract
1. Introduction

2. Materials and Methods
2.1. Material and Growth Conditions
2.2. Light Intensity Treatment in Grapevine Callus
2.3. Extraction and HPLC–MS Analysis of Flavonoids in Grapevine Callus System
2.4. RNA Extraction, cDNA Library Construction and RNA-Seq
2.5. RNA-Seq Data Analysis and Quantitative Real-Time PCR (qRT-PCR) Confirmation
2.6. Cloning and Sequence Analysis of VviLAR1 Promoter (pVviLAR1) and VviLAR2 Promoter (pVviLAR2)
2.7. Transient Expression in Tobacco Leaves and Stable Expression in Arabidopsis
2.8. Light Intensity Treatment on Transgenic Arabidopsis
2.9. Statistical Analysis of Data
3. Results
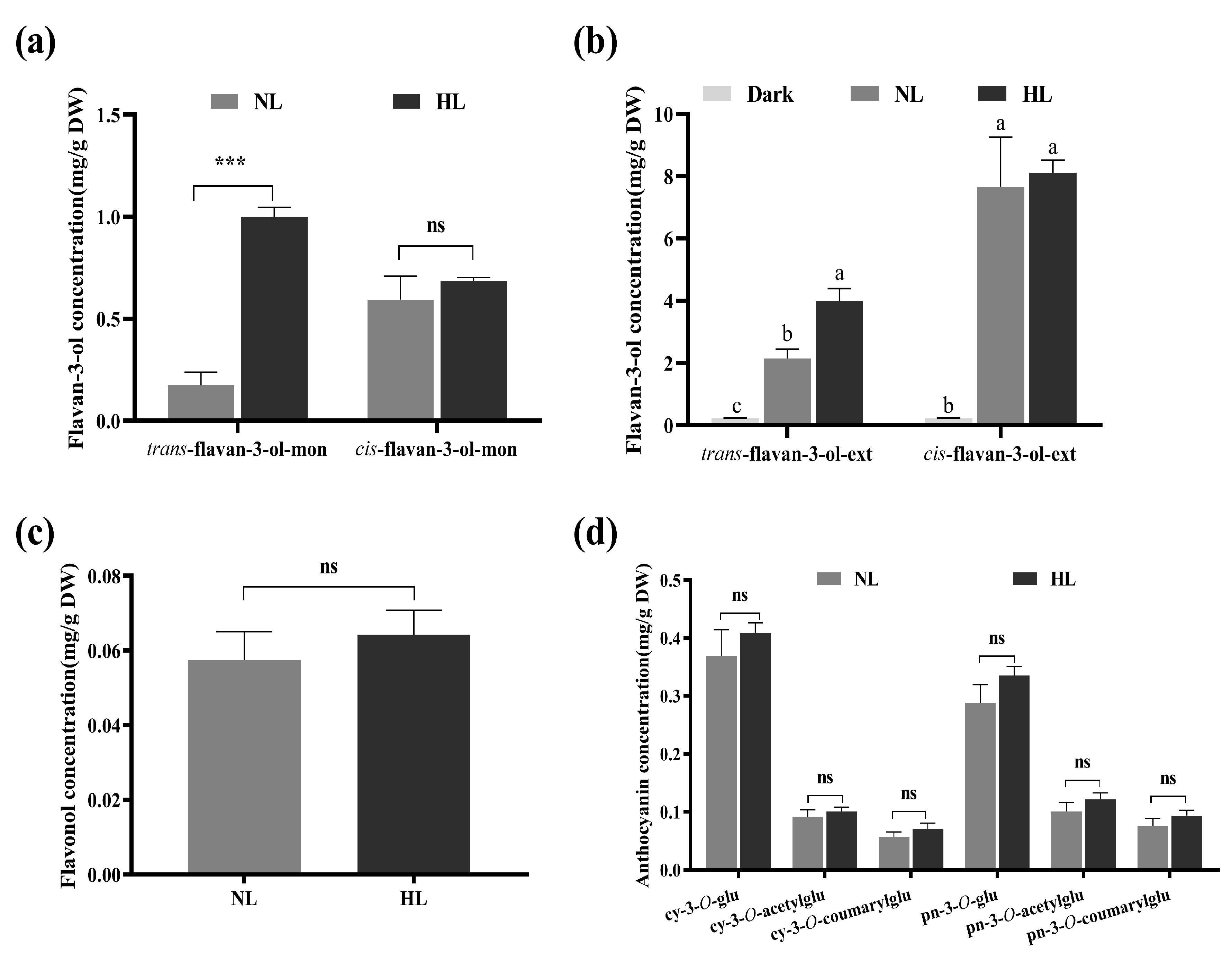
3.1. Flavonoid Compositions in Grapevine Calluses under Different Light Intensity Treatments
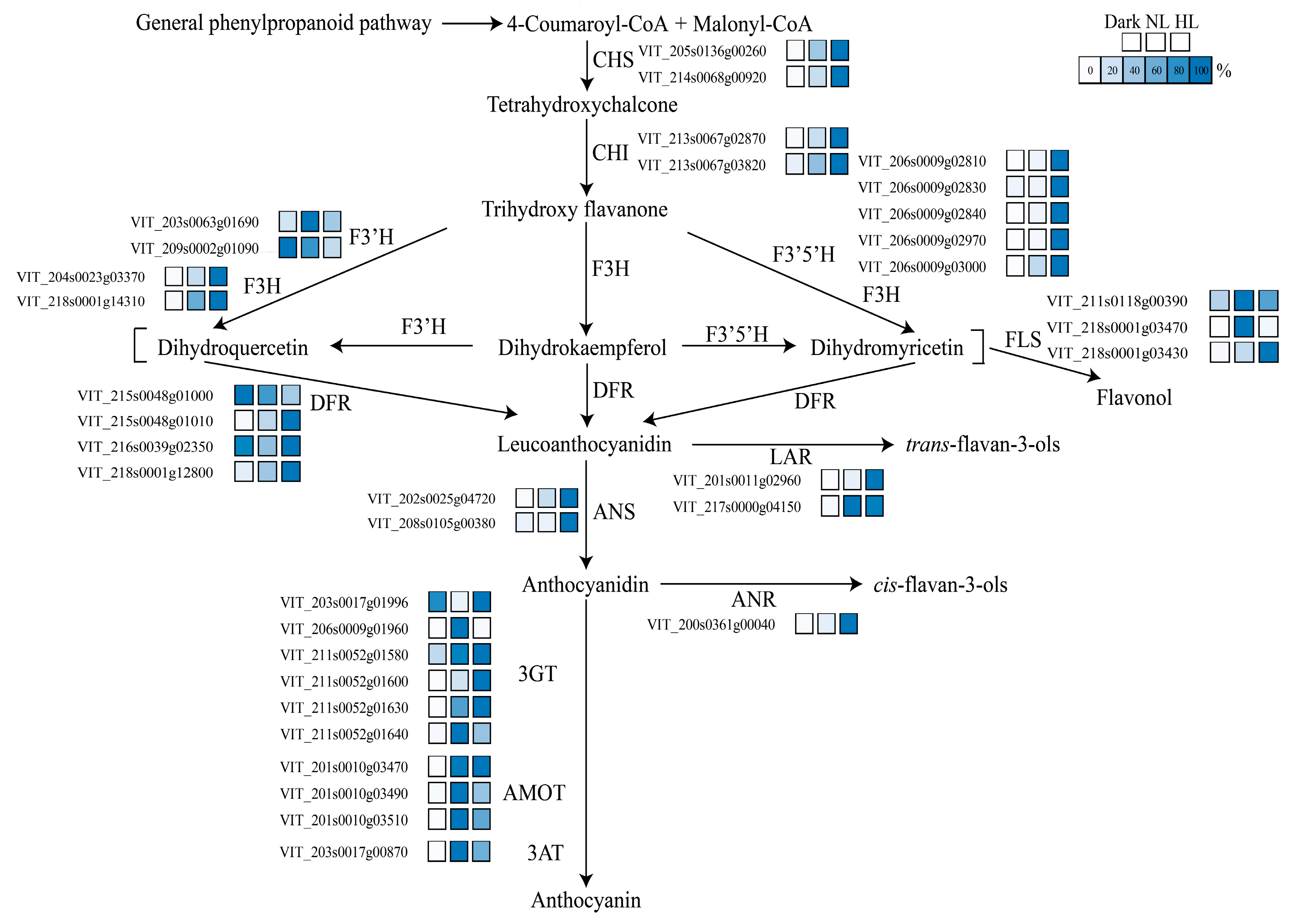
3.2. Effect of Light Intensity on Flavonoid Pathway Gene Expression
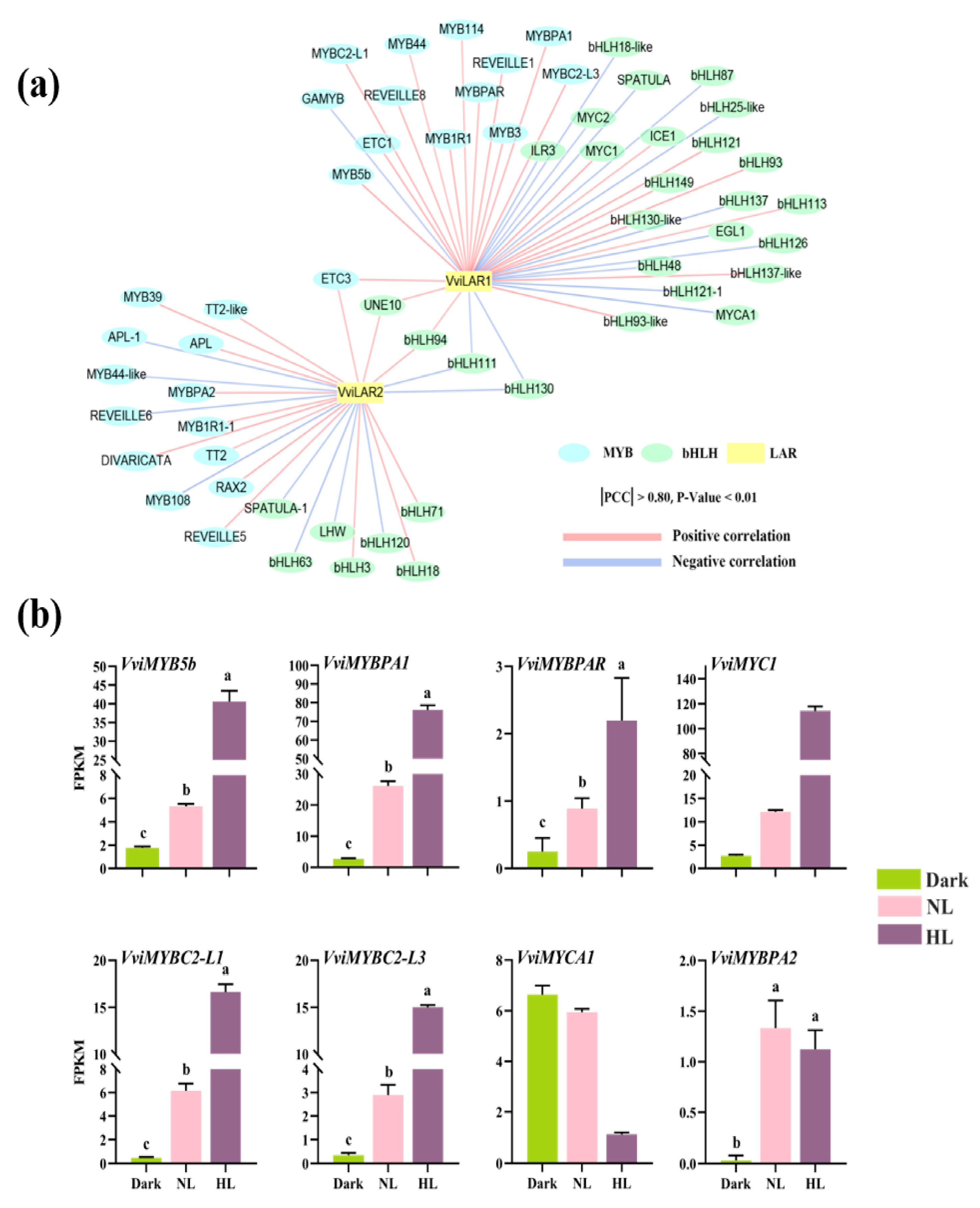
3.3. Screening for Potential TFs to Regulate the Expressions of VviLAR1 and VviLAR2
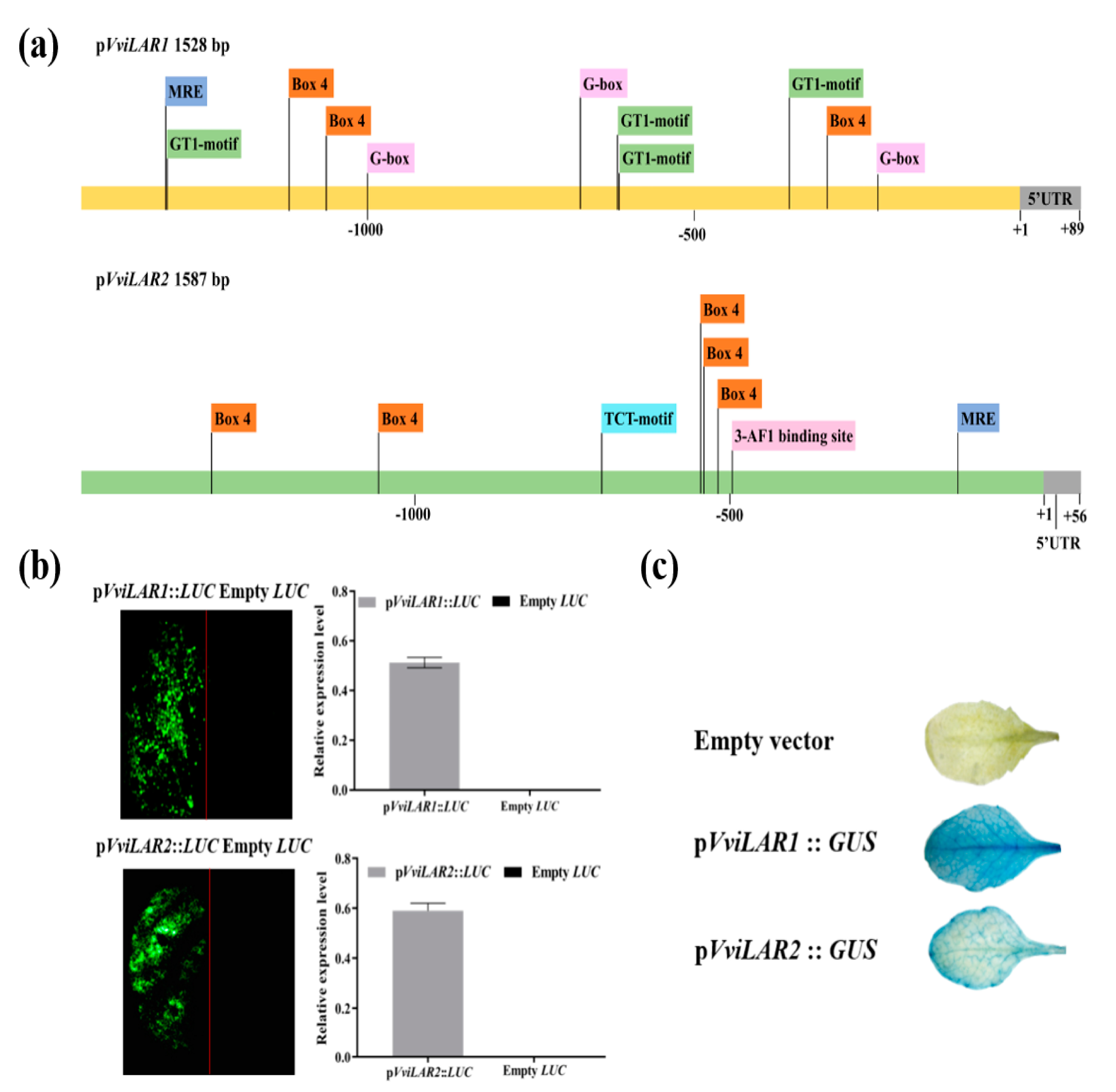
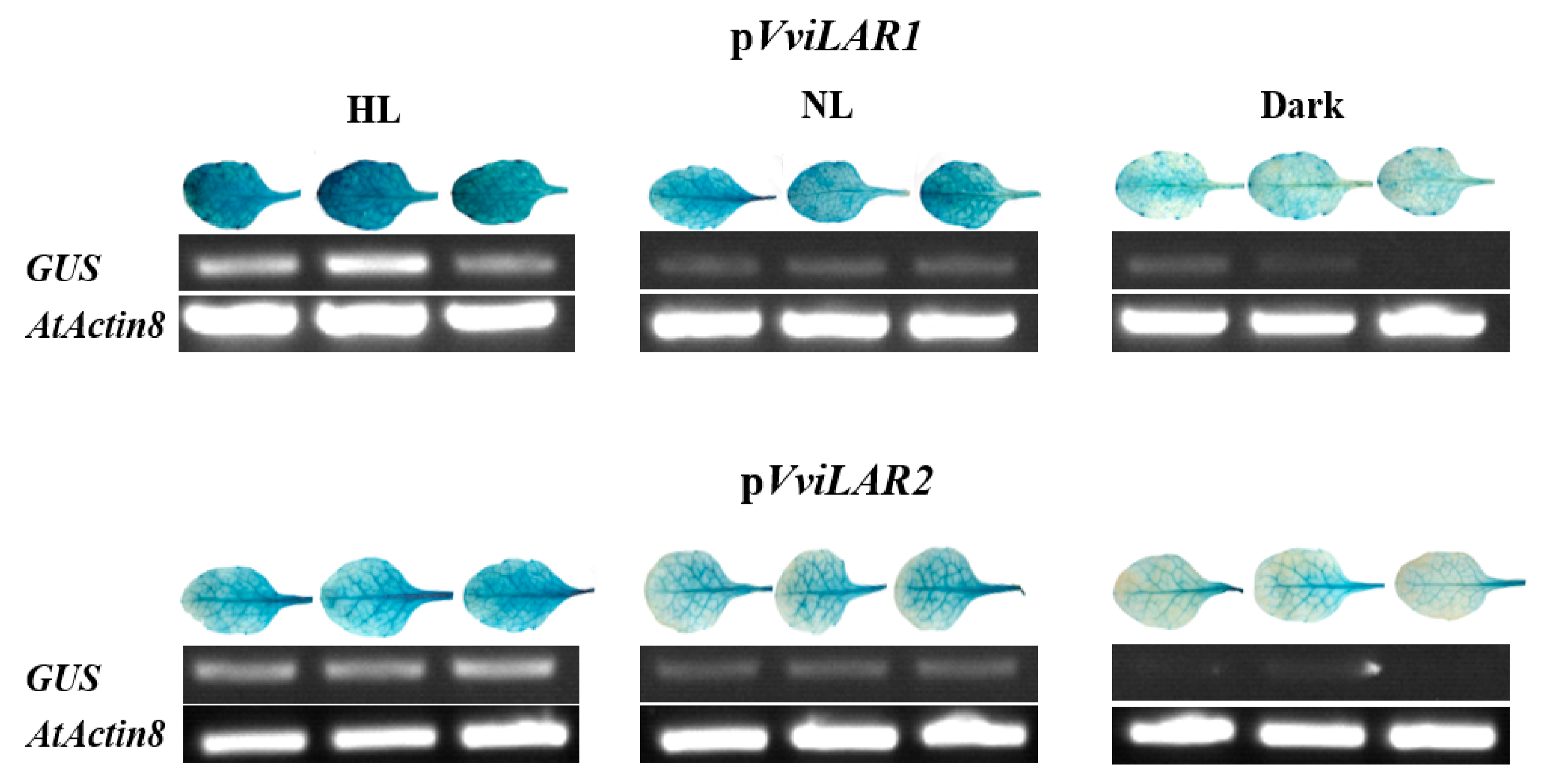
3.4. pVviLAR1 Activity Was More Sensitive than pVviLAR2 Activity in Response to Light Intensity Changes
4. Discussion
4.1. Light Intensity Mainly Regulated trans-Flavan-3-ol Biosynthesis in Grapevine Calluses
4.2. Two LAR Genes Processed Different Response Patterns to Light Intensity in Grapevine Calluses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Adams, D.O. Phenolics and ripening in grape berries. Am. J. Enol. Vitic. 2006, 57, 249–256. [Google Scholar]
- Downey, M.O.; Harvey, J.S.; Robinson, S.P. The effect of bunch shading on berry development and flavonoid accumulation in Shiraz grapes. Aust. J. Grape Wine Res. 2008, 10, 55–73. [Google Scholar] [CrossRef]
- Dixon, R.A.; Xie, D.Y.; Sharma, S.B. Proanthocyanidins—A final frontier in flavonoid research? New Phytol. 2005, 165, 9–28. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.D.; Dixon, R.A. Proanthocyanidin biosynthesis—Still more questions than answers? Phytochemistry 2005, 66, 2127–2144. [Google Scholar] [CrossRef]
- Ferrer, J.L.; Austin, M.B.; Stewart, C., Jr.; Noe, J.P. Structure and function of enzymes involved in the biosynthesis of phenylpropanoids. Plant Physiol. Biochem. 2008, 46, 356–370. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.Y.; Sharma, S.B.; Paiva, N.L.; Ferreira, D.; Dixon, R.A. Role of anthocyanidin reductase, encoded by BANYULS in plant flavonoid biosynthesis. Science 2003, 299, 396–399. [Google Scholar] [CrossRef]
- Bogs, J.; Downey, M.O.; Harvey, J.S.; Ashton, A.R.; Tanner, G.J.; Robinson, S.P. Proanthocyanidin synthesis and expression of genes encoding leucoanthocyanidin reductase and anthocyanidin reductase in developing grape berries and grapevine leaves. Plant Physiol. 2005, 139, 652–663. [Google Scholar] [CrossRef]
- Yu, K.; Jun, J.H.; Duan, C.; Dixon, R.A. VvLAR1 and VvLAR2 are bifunctional enzymes for proanthocyanidin biosynthesis in grapevine. Plant Physiol. 2019, 180, 1362–1374. [Google Scholar] [CrossRef]
- Huang, Y.F.; Vialet, S.; Guiraud, J.L.; Torregrosa, L.; Bertrand, Y.; Cheynier, V.; This, P.; Terrier, N. A negative MYB regulator of proanthocyanidin accumulation, identified through expression quantitative locus mapping in the grape berry. New Phytol. 2014, 201, 795–809. [Google Scholar] [CrossRef]
- Cavallini, E.; Matus, J.T.; Finezzo, L.; Zenoni, S.; Loyola, R.; Guzzo, F.; Schlechter, R.; Ageorges, A.; Arce-Johnson, P.; Tornielli, G.B. The phenylpropanoid pathway is controlled at different branches by a set of R2R3-MYB C2 repressors in grapevine. Plant Physiol. 2015, 167, 1448–1470. [Google Scholar] [CrossRef]
- Hichri, I.; Barrieu, F.; Bogs, J.; Kappel, C.; Delrot, S.; Lauvergeat, V. Recent advances in the transcriptional regulation of the flavonoid biosynthetic pathway. J. Exp. Bot. 2011, 62, 2465–2483. [Google Scholar] [CrossRef] [PubMed]
- Bogs, J.; Jaffé, F.W.; Takos, A.M.; Walker, A.R.; Robinson, S.P. The grapevine transcription factor VvMYBPA1 regulates proanthocyanidin synthesis during fruit development. Plant Physiol. 2007, 143, 1347–1361. [Google Scholar] [CrossRef] [PubMed]
- Terrier, N.; Torregrosa, L.; Ageorges, A.; Vialet, S.; Verries, C.; Cheynier, V.; Romieu, C. Ectopic expression of VvMYBPA2 promotes proanthocyanidin biosynthesis in grapevine and suggests additional targets in the pathway. Plant Physiol. 2009, 149, 1028–1041. [Google Scholar] [CrossRef] [PubMed]
- Koyama, K.; Numata, M.; Nakajima, I.; Gotoyamamoto, N.; Matsumura, H.; Tanaka, N. Functional characterization of a new grapevine MYB transcription factor and regulation of proanthocyanidin biosynthesis in grapes. J. Exp. Bot. 2014, 65, 4433. [Google Scholar] [CrossRef]
- Deluc, L.; Barrieu, F.; Marchive, C.; Lauvergeat, V.; Decendit, A.; Richard, T.; Carde, J.P.; Merillon, J.M.; Hamdi, S. Characterization of a grapevine R2R3-MYB transcription factor that regulates the phenylpropanoid pathway. Plant Physiol. 2006, 140, 499–511. [Google Scholar] [CrossRef]
- Deluc, L.; Bogs, J.; Walker, A.R.; Ferrier, T.; Decendit, A.; Merillon, J.M.; Robinson, S.P.; Barrieu, F. The transcription factor VvMYB5b contributes to the regulation of anthocyanin and proanthocyanidin biosynthesis in developing grape berries. Plant Physiol. 2008, 147, 2041–2053. [Google Scholar] [CrossRef]
- Hichri, I.; Heppel, S.C.; Pillet, J.; Léon, C.; Czemmel, S.; Delrot, S.; Lauvergeat, V.; Bogs, J. The basic helix-loop-helix transcription factor MYC1 is involved in the regulation of the flavonoid biosynthesis pathway in grapevine. Mol. Plant 2010, 3, 509–523. [Google Scholar] [CrossRef]
- Liu, Z.; Luo, Q.H.; Wang, J.M.; Li, X.F.; Yang, Y. Functional characterization and analysis of the Arabidopsis UGT71C5 promoter region. Genet. Mol. Res. 2015, 14, 19173–19183. [Google Scholar] [CrossRef]
- Matus, J.T.; Loyola, R.; Vega, A.; Pena-Neira, A.; Bordeu, E.; Arce-Johnson, P.; Alcalde, J.A. Post-veraison sunlight exposure induces myb-mediated transcriptional regulation of anthocyanin and flavonol synthesis in berry skins of Vitis vinifera. J. Exp. Bot. 2009, 60, 853–867. [Google Scholar] [CrossRef]
- Sun, R.Z.; Cheng, G.; Li, Q.; He, Y.N.; Wang, Y.; Lan, Y.B.; Li, S.Y.; Zhu, Y.R.; Song, W.F.; Zhang, X.; et al. Light-induced variation in phenolic compounds in Cabernet Sauvignon grapes (Vitis vinifera L.) involves extensive transcriptome reprogramming of biosynthetic enzymes, transcription factors, and phytohormonal regulators. Front. Plant Sci. 2017, 8, 547. [Google Scholar] [CrossRef]
- Sun, R.Z.; He, F.; Lan, Y.B.; Xing, R.R.; Liu, R.; Pan, Q.H.; Wang, J.; Duan, C.Q. Transcriptome comparison of Cabernet Sauvignon grape berries from two regions with distinct climate. J. Plant Physiol. 2015, 178, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Koyama, K.; Ikeda, H.; Poudel, P.R.; Goto-Yamamoto, N. Light quality affects flavonoid biosynthesis in young berries of Cabernet Sauvignon grape. Phytochemistry 2012, 78, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Zoratti, L.; Karppinen, K.; Escobar, A.L.; Häggman, H.; Jaakola, L. Light-controlled flavonoid biosynthesis in fruits. Front. Plant. Sci. 2014, 5, 534. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.L.; Gregan, S.; Winefield, C.; Jordan, B. From UVR8 to flavonol synthase: UV-B-induced gene expression in Sauvignon blanc grape berry. Plant Cell Environ. 2015, 38, 905–919. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Luescher, J.; Torres, N.; Hilbert, G.; Richard, T.; Sanchez-Diaz, M.; Delrot, S.; Aguirreolea, J.; Pascual, I.; Gomes, E. Ultraviolet-B radiation modifies the quantitative and qualitative profile of flavonoids and amino acids in grape berries. Phytochemistry 2014, 102, 106–114. [Google Scholar] [CrossRef]
- Cetin, E. Induction of secondary metabolite production by UV-C radiation in Vitis vinifera L. Öküzgözü callus cultures. Biol. Res. 2014, 47, 37. [Google Scholar] [CrossRef]
- Sun, J.; Wang, Y.; Chen, X.; Gong, X.; Wang, N.; Ma, L.; Qiu, Y.; Wang, Y.; Feng, S. Effects of methyl jasmonate and abscisic acid on anthocyanin biosynthesis in callus cultures of red-fleshed apple (Malus sieversii F. niedzwetzkyana). Plant Cell Tiss. Org. 2017, 130, 227–237. [Google Scholar] [CrossRef]
- Chen, Y.M.; Huang, J.Z.; Hou, T.W.; Pan, I.C. Effects of light intensity and plant growth regulators on callus proliferation and shoot regeneration in the ornamental succulent Haworthia. Bot. Stud. 2019, 60. [Google Scholar] [CrossRef]
- Jordao, A.M.; Ricardo-da-Silva, J.M.; Laureano, O. Evolution of catechins and oligomeric procyanidins during grape maturation of Castelao Frances and Touriga Francesa. Am. J. Enol. Vitic. 2001, 52, 230–234. [Google Scholar]
- Souquet, J.M.; Labarbe, B.; Le Guerneve, C.; Cheynier, V.; Moutounet, M. Phenolic composition of grape stems. J. Agric. Food Chem. 2000, 48, 1076–1080. [Google Scholar] [CrossRef]
- Downey, M.O.; Mazza, M.; Krstic, M.P. Development of a stable extract for anthocyanins and flavonols from grape skin. Am. J. Enol. Vitic. 2007, 58, 358–364. [Google Scholar]
- Liang, N.N.; He, F.; Pan, Q.H.; Wang, J.; Reeves, M.J.; Duan, C.Q. Optimization of sample preparation and phloroglucinol analysis of Marselan grape skin proanthocyanidins using HPLC-DAD-ESI- MS/MS. S. Afr. J. Enol. Vitic. 2012, 22, 122. [Google Scholar] [CrossRef]
- He, J.J.; Liu, Y.X.; Pan, Q.H.; Cui, X.Y.; Duan, C.Q. Different anthocyanin profiles of the skin and the pulp of Yan73 (Muscat Hamburg × Alicante Bouschet) grape berries. Molecules 2010, 15, 1141–1153. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; He, F.; Zhu, B.Q.; Liu, B.; Sun, R.Z.; Duan, C.Q.; Reeves, M.J.; Wang, J. Comparison of distinct transcriptional expression patterns of flavonoid biosynthesis in Cabernet Sauvignon grapes from east and west China. Plant Physiol. Biochem. 2014, 84, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, W.K.; Gao, X.T.; He, L.; Yang, X.H.; He, F.; Duan, C.Q.; Wang, J. Rootstock-mediated effects on Cabernet Sauvignon performance: Vine growth, berry ripening, flavonoids, and aromatic profiles. Int. J. Mol. Sci. 2019, 20, 401. [Google Scholar] [CrossRef] [PubMed]
- Siren, J.; Valimaki, N.; Makinen, V. Indexing graphs for path queries with applications in genome research. IEEE ACM Trans. Comput. Biol. Bioinform. 2014, 11, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; van Baren, M.J.; Salzberg, S.L.; Wold, B.J.; Pachter, L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef]
- Sun, R.Z.; Pan, Q.H.; Duan, C.Q.; Wang, J. Light response and potential interacting proteins of a grape flavonoid 3′-hydroxylase gene promoter. Plant Physiol. Biochem. 2015, 97, 70–81. [Google Scholar] [CrossRef]
- Ruijter, J.M.; Ramakers, C.; Hoogaars, W.M.H.; Karlen, Y.; Bakker, O.; van den Hoff, M.J.B.; Moorman, A.F.M. Amplification efficiency: Linking baseline and bias in the analysis of quantitative PCR data. Nucleic Acids Res. 2009, 37, e45. [Google Scholar] [CrossRef]
- Clough, S.J. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 2010, 16, 735–743. [Google Scholar] [CrossRef]
- Jefferson, R.A.; Kavanagh, T.A.; Bevan, M.W. GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher-plants. Embo J. 1987, 6, 3901–3907. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, N.; Tamoi, M.; Shigeoka, S. The sweet potato RbcS gene (IbRbcS1) promoter confers high-level and green tissue-specific expression of the GUS reporter gene in transgenic Arabidopsis. Gene 2015, 567, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.S.; Jacques, D.; Haslam, E. Plant proanthocyanidins—Part 1. Introduction; the isolation, structure, and distribution in nature of plant procyanidins. J. Chem. Soc. Perk. Trans. 1972, 1, 1387–1399. [Google Scholar] [CrossRef]
- He, F.; Pan, Q.H.; Shi, Y.; Duan, C.Q. Biosynthesis and genetic regulation of proanthocyanidins in plants. Molecules 2008, 13, 2674–2703. [Google Scholar] [CrossRef]
- Bogs, J.; Ebadi, A.; McDavid, D.; Robinson, S.P. Identification of the flavonoid hydroxylases from grapevine and their regulation during fruit development. Plant Physiol. 2006, 140, 279–291. [Google Scholar] [CrossRef]
- Fujino, N.; Tenma, N.; Waki, T.; Ito, K.; Komatsuzaki, Y.; Sugiyama, K.; Yamazaki, T.; Yoshida, S.; Hatayama, M.; Yamashita, S.; et al. Physical interactions among flavonoid enzymes in snapdragon and torenia reveal the diversity in the flavonoid metabolon organization of different plant species. Plant J. 2018, 94, 372–392. [Google Scholar] [CrossRef]
- Winkel-Shirley, B. Evidence for enzyme complexes in the phenylpropanoid and flavonoid pathways. Physiol. Plant 1999, 107, 142–149. [Google Scholar] [CrossRef]
- Jaakola, L. New insights into the regulation of anthocyanin biosynthesis in fruits. Trends Plant Sci. 2013, 18, 477–483. [Google Scholar] [CrossRef]
- Matus, J.T.; Poupin, M.J.; Canon, P.; Bordeu, E.; Alcalde, J.A.; Arce-Johnson, P. Isolation of WDR and bHLH genes related to flavonoid synthesis in grapevine (Vitis vinifera L.). Plant Mol. Biol. 2010, 72, 607–620. [Google Scholar] [CrossRef]
- Fujita, A.; Soma, N.; Goto-Yamamoto, N.; Mizuno, A.; Kiso, K.; Hashizume, K. Effect of shading on proanthocyanidin biosynthesis in the grape berry. J. Jpn. Soc. Hortic. Sci. 2007, 76, 112–119. [Google Scholar] [CrossRef]
- Fujita, A.; Goto-Yamamoto, N.; Aramaki, I.; Hashizume, K. Organ-specific transcription of putative flavonol synthase genes of grapevine and effects of plant hormones and shading on flavonol biosynthesis in grape berry skins. Biosci. Biotechnol. Biochem. 2006, 70, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Martens, S.; Teeri, T.; Forkmann, G. Heterologous expression of dihydroflavonol 4-reductases from various plants. Febs Lett. 2002, 531, 453–458. [Google Scholar] [CrossRef]
- Kolb, C.A.; Kopecky, J.; Riederer, M.; Pfundel, E.E. UV screening by phenolics in berries of grapevine (Vitis vinifera). Funct. Plant Biol. 2003, 30, 1177–1186. [Google Scholar] [CrossRef] [PubMed]
- Bakowska, A.; Kucharska, A.Z.; Oszmianski, J. The effects of heating, UV irradiation, and storage on stability of the anthocyanin-polyphenol copigment complex. Food Chem. 2003, 81, 349–355. [Google Scholar] [CrossRef]
- Calderon, A.A.; Garciaflorenciano, E.; Munoz, R.; Barcelo, A.R. Gamay grapevine peroxidase—Its role in vacuolar anthocyani(di)n degradation. Vitis 1992, 31, 139–147. [Google Scholar]
- Biruk, A.; Asfaw, D.; Manela, N.; Perl, A.; Oren-Shamir, M.; Fait, A. Metabolite profiling and transcript analysis reveal specificities in the response of a berry derived cell culture to abiotic stresses. Front. Plant. Sci. 2015, 6, 728. [Google Scholar] [CrossRef]
- Koyama, K.; Goto-Yamamoto, N. Bunch shading during different developmental stages affects the phenolic biosynthesis in berry skins of ‘Cabernet Sauvignon’ grapes. J. Am. Soc. Hortic. Sci. 2008, 133, 743–753. [Google Scholar] [CrossRef]
- Diharce, J.; Golebiowski, J.; Fiorucci, S.; Antonczak, S. Fine-tuning of microsolvation and hydrogen bond interaction regulates substrate channelling in the course of flavonoid biosynthesis. Phys. Chem. Chem. Phys. 2016, 18, 10337–10345. [Google Scholar] [CrossRef]
- Terzaghi, W.B.; Cashmore, A.R. Light-regulated transcription. Annu. Rev. Plant. Phys. 1995, 46, 445–474. [Google Scholar] [CrossRef]
- Hahn, S.; Buratowski, S.; Sharp, P.A.; Guarente, L. Yeast TATA-binding protein TFIIIB binds to TATA elements with both consensus and nonconsensus DNA-sequences. Proc. Natl. Acad. Sci. USA 1989, 86, 5718–5722. [Google Scholar] [CrossRef]
- Mazarei, M.; Ying, Z.; Houtz, R.L. Functional analysis of the Rubisco large subunit N-epsilon-methyltransferase promoter from tobacco and its regulation by light in soybean hairy roots. Plant. Cell Rep. 1998, 17, 907–912. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, J.; Yu, K.; Zhang, M.; Shi, Y.; Duan, C.; Wang, J. The Effect of Light Intensity on the Expression of Leucoanthocyanidin Reductase in Grapevine Calluses and Analysis of Its Promoter Activity. Genes 2020, 11, 1156. https://doi.org/10.3390/genes11101156
Cheng J, Yu K, Zhang M, Shi Y, Duan C, Wang J. The Effect of Light Intensity on the Expression of Leucoanthocyanidin Reductase in Grapevine Calluses and Analysis of Its Promoter Activity. Genes. 2020; 11(10):1156. https://doi.org/10.3390/genes11101156
Chicago/Turabian StyleCheng, Jing, Keji Yu, Mingyue Zhang, Ying Shi, Changqing Duan, and Jun Wang. 2020. "The Effect of Light Intensity on the Expression of Leucoanthocyanidin Reductase in Grapevine Calluses and Analysis of Its Promoter Activity" Genes 11, no. 10: 1156. https://doi.org/10.3390/genes11101156
APA StyleCheng, J., Yu, K., Zhang, M., Shi, Y., Duan, C., & Wang, J. (2020). The Effect of Light Intensity on the Expression of Leucoanthocyanidin Reductase in Grapevine Calluses and Analysis of Its Promoter Activity. Genes, 11(10), 1156. https://doi.org/10.3390/genes11101156





