Biodosimetry of Low Dose Ionizing Radiation Using DNA Repair Foci in Human Lymphocytes
Abstract
1. Introduction
2. Materials and Methods
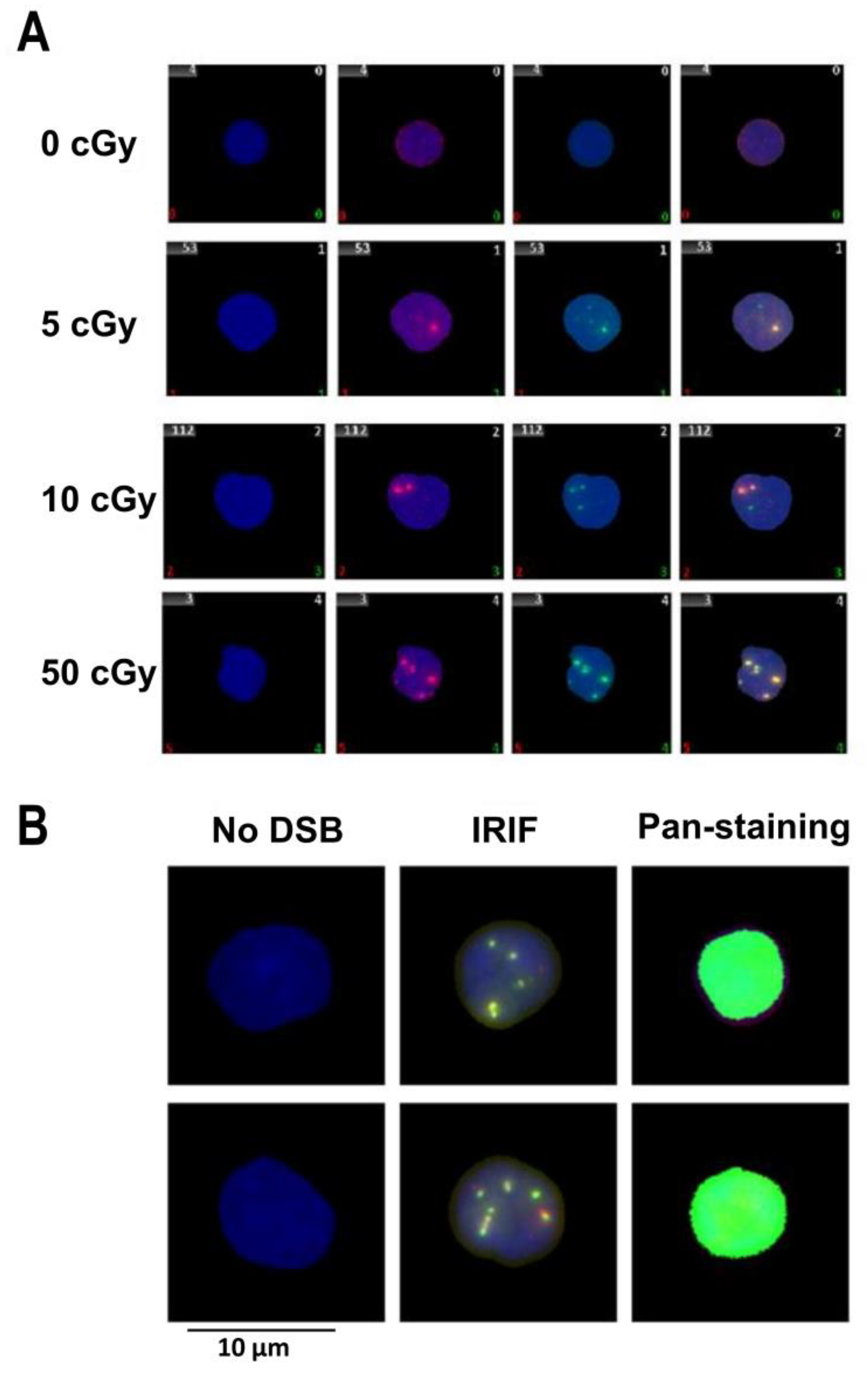
3. Results
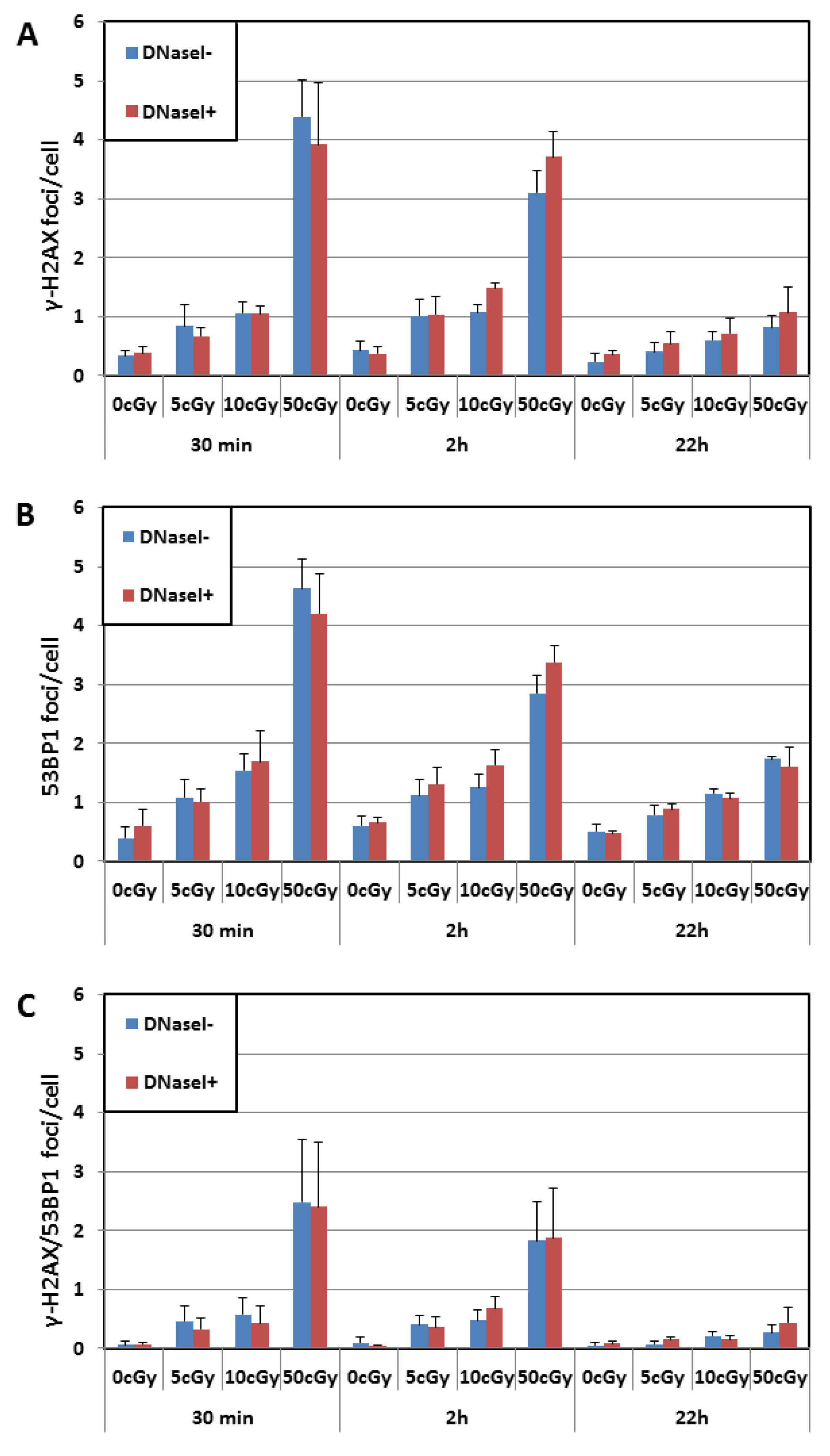
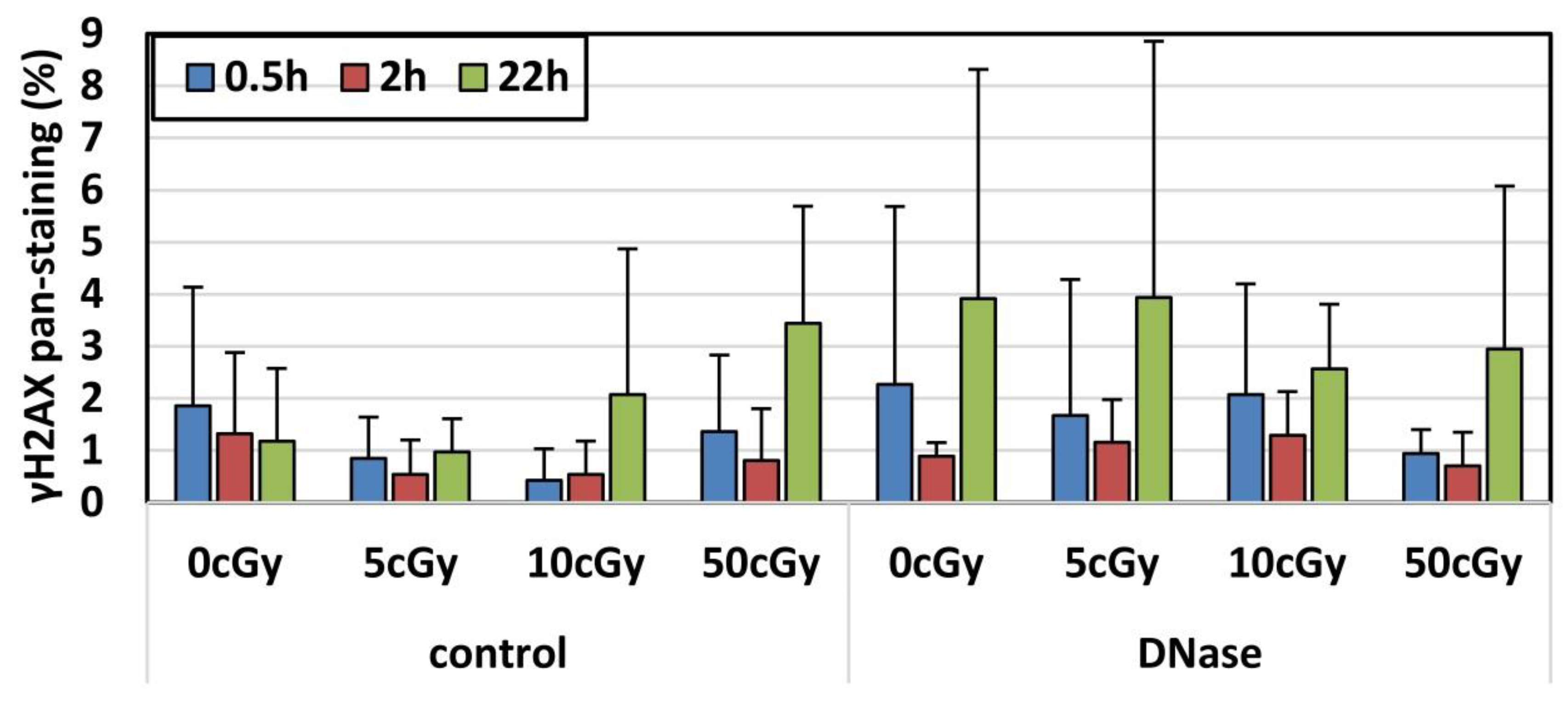
3.1. Effects of Factors: Dose, Post-Irradiation Time, DNase
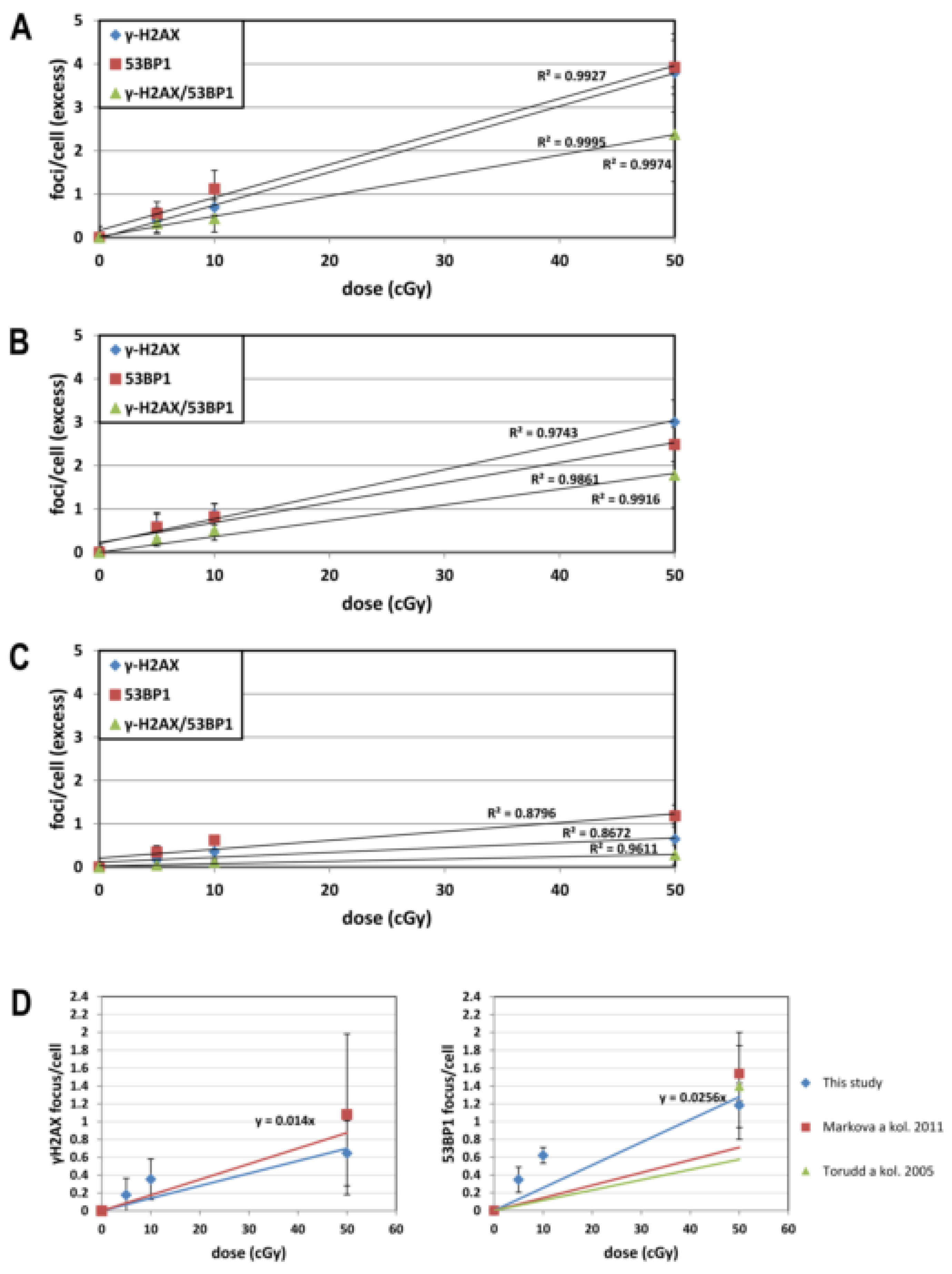
3.2. Dose Response for IRIF
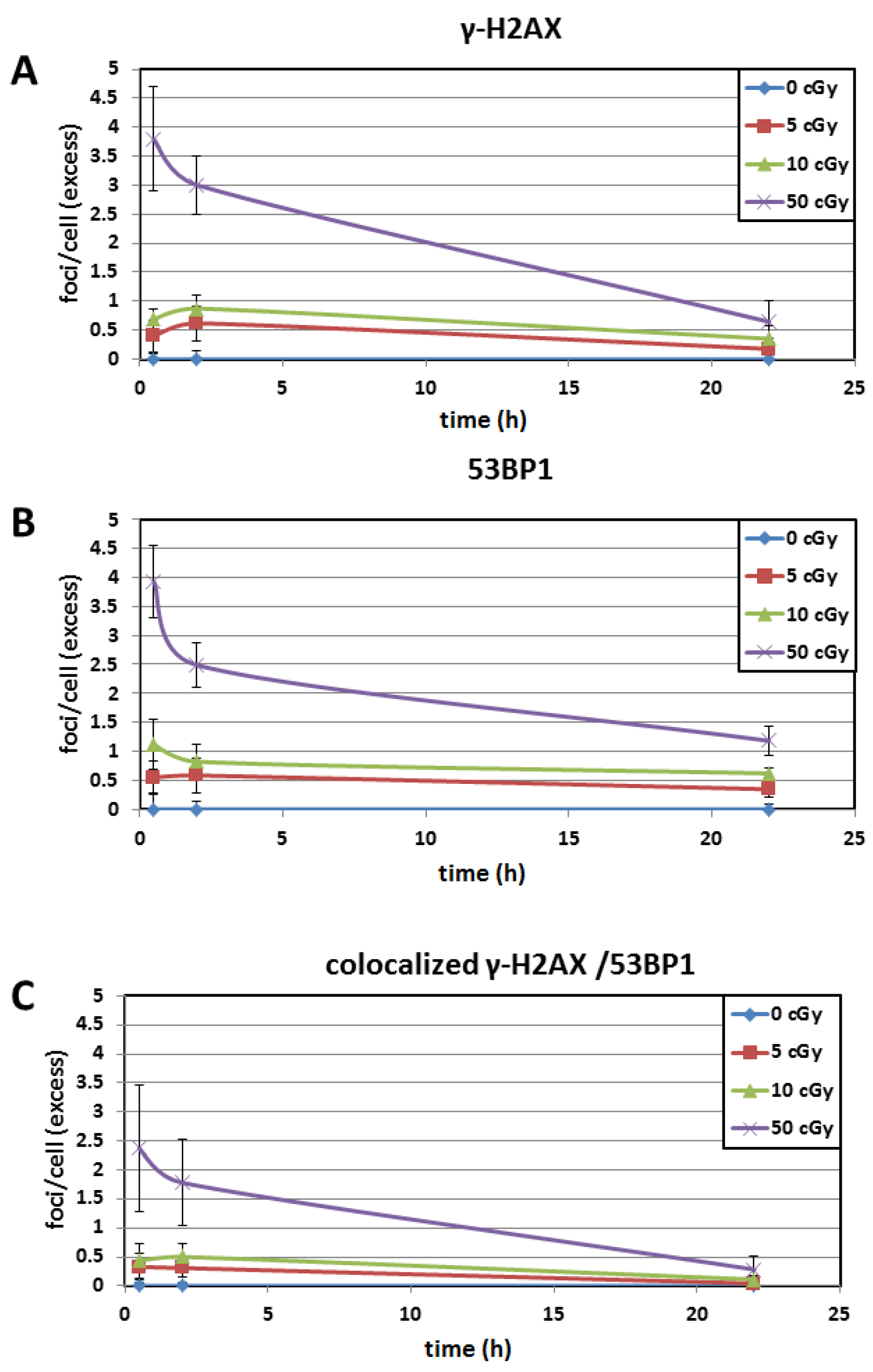
3.3. Time Kinetics
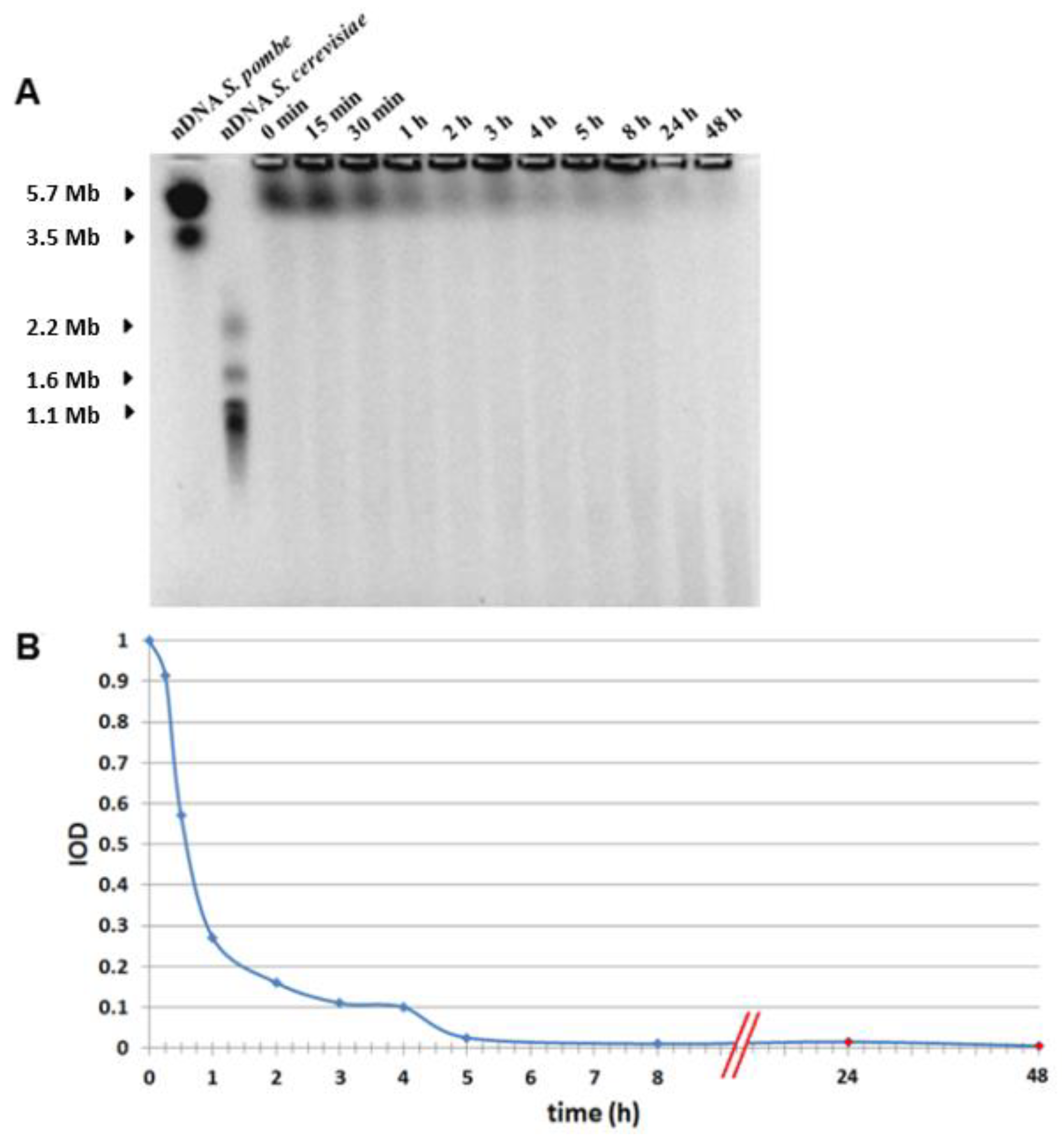
3.4. PFGE
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bekker-Jensen, S.; Lukas, C.; Kitagawa, R.; Melander, F.; Kastan, M.B.; Bartek, J.; Lukas, J. Spatial organization of the mammalian genome surveillance machinery in response to DNA strand breaks. J. Cell Biol. 2006, 173, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Rogakou, E.P.; Boon, C.; Redon, C.; Bonner, W.M. Megabase chromatin domains involved in DNA double-strand breaks in vivo. J. Cell Biol. 1999, 146, 905–916. [Google Scholar] [CrossRef] [PubMed]
- Rothkamm, K.; Lobrich, M. Evidence for a lack of DNA double-strand break repair in human cells exposed to very low x-ray doses. Proc. Natl. Acad. Sci. USA 2003, 100, 5057–5062. [Google Scholar] [CrossRef] [PubMed]
- Rothkamm, K.; Balroop, S.; Shekhdar, J.; Fernie, P.; Goh, V. Leukocyte DNA damage after multi-detector row CT: A quantitative biomarker of low-level radiation exposure. Radiology 2007, 242, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Depuydt, J.; Baert, A.; Vandersickel, V.; Thierens, H.; Vral, A. Relative biological effectiveness of mammography X-rays at the level of DNA and chromosomes in lymphocytes. Int. J. Radiat. Biol. 2013, 89, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Vandevoorde, C.; Gomolka, M.; Roessler, U.; Samaga, D.; Lindholm, C.; Fernet, M.; Hall, J.; Pernot, E.; El-Saghire, H.; Baatout, S.; et al. EPI-CT: In vitro assessment of the applicability of the γ-H2AX-foci assay as cellular biomarker for exposure in a multicentre study of children in diagnostic radiology. Int. J. Radiat. Biol. 2015, 91, 653–663. [Google Scholar] [CrossRef]
- Markova, E.; Somsedikova, A.; Vasilyev, S.; Pobijakova, M.; Lackova, A.; Lukacko, P.; Belyaev, I. DNA repair foci and late apoptosis/necrosis in peripheral blood lymphocytes of breast cancer patients undergoing radiotherapy. Int. J. Radiat. Biol. 2015, 91, 934–945. [Google Scholar] [CrossRef] [PubMed]
- Ivashkevich, A.N.; Martin, O.A.; Smith, A.J.; Redon, C.E.; Bonner, W.M.; Martin, R.F.; Lobachevsky, P.N. ΓH2AX foci as a measure of DNA damage: A computational approach to automatic analysis. Mutat. Res. 2011, 711, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Barnard, S.; Ainsbury, E.; Al-Hafidh, J.; Hadjidekova, V.; Hristova, R.; Lindholm, C.; Gil, O.M.; Moquet, J.; Moreno, M.; Rößler, U. The first γ-H2AX biodosimetry intercomparison exercise of the developing European biodosimetry network RENEB. Radiat. Prot. Dosim. 2014, 164, 265–270. [Google Scholar] [CrossRef]
- Viau, M.; Testard, I.; Shim, G.; Morat, L.; Normil, M.D.; Hempel, W.M.; Sabatier, L. Global quantification of γH2AX as a triage tool for the rapid estimation of received dose in the event of accidental radiation exposure. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2015, 793, 123–131. [Google Scholar] [CrossRef]
- Lamkowski, A.; Forcheron, F.; Agay, D.; Ahmed, E.A.; Drouet, M.; Meineke, V.; Scherthan, H. DNA damage focus analysis in blood samples of minipigs reveals acute partial body irradiation. PLoS ONE 2014, 9, e87458. [Google Scholar] [CrossRef] [PubMed]
- Durdik, M.; Kosik, P.; Gursky, J.; Vokalova, L.; Markova, E.; Belyaev, I. Imaging flow cytometry as a sensitive tool to detect low-dose-induced DNA damage by analyzing 53BP1 and γH2AX foci in human lymphocytes. Cytom. Part A J. Int. Soc. Anal. Cytol. 2015, 87, 1070–1078. [Google Scholar] [CrossRef] [PubMed]
- Horn, S.; Barnard, S.; Rothkamm, K. Γ-H2AX-based dose estimation for whole and partial body radiation exposure. PLoS ONE 2011, 6, e25113. [Google Scholar] [CrossRef] [PubMed]
- Wojewodzka, M.; Sommer, S.; Kruszewski, M.; Sikorska, K.; Lewicki, M.; Lisowska, H.; Wegierek-Ciuk, A.; Kowalska, M.; Lankoff, A. Defining Blood Processing Parameters for Optimal Detection of γ-H2AX Foci: A Small Blood Volume Method. Radiat. Res. 2015, 184, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Bouwman, P.; Aly, A.; Escandell, J.M.; Pieterse, M.; Bartkova, J.; van der Gulden, H.; Hiddingh, S.; Thanasoula, M.; Kulkarni, A.; Yang, Q.; et al. 53BP1 loss rescues BRCA1 deficiency and is associated with triple-negative and BRCA-mutated breast cancers. Nat. Struct. Mol. Biol. 2010, 17, 688–695. [Google Scholar] [CrossRef]
- Bunting, S.F.; Callen, E.; Wong, N.; Chen, H.T.; Polato, F.; Gunn, A.; Bothmer, A.; Feldhahn, N.; Fernandez-Capetillo, O.; Cao, L.; et al. 53BP1 inhibits homologous recombination in Brca1-deficient cells by blocking resection of DNA breaks. Cell 2010, 141, 243–254. [Google Scholar] [CrossRef]
- Chapman, J.R.; Sossick, A.J.; Boulton, S.J.; Jackson, S.P. BRCA1-associated exclusion of 53BP1 from DNA damage sites underlies temporal control of DNA repair. J. Cell Sci. 2012, 125, 3529–3534. [Google Scholar] [CrossRef]
- Belyaev, I.Y. Radiation-induced DNA repair foci: Spatio-temporal aspects of formation, application for assessment of radiosensitivity and biological dosimetry. Mutat. Res. 2010, 704, 132–141. [Google Scholar] [CrossRef]
- Lorat, Y.; Fleckenstein, J.; Görlinger, P.; Rübe, C.; Rübe, C. Concomitant chemotherapy increases radiotherapy-mediated DNA-damage in peripheral blood lymphocytes. BioRxiv 2019, 540575. [Google Scholar] [CrossRef]
- Kuefner, M.A.; Brand, M.; Engert, C.; Kappey, H.; Uder, M.; Distel, L.V. The effect of calyculin A on the dephosphorylation of the histone γ-H2AX after formation of X-ray-induced DNA double-strand breaks in human blood lymphocytes. Int. J. Radiat. Biol. 2013, 89, 424–432. [Google Scholar] [CrossRef]
- Brand, M.; Sommer, M.; Achenbach, S.; Anders, K.; Lell, M.; Lobrich, M.; Uder, M.; Kuefner, M.A. X-ray induced DNA double-strand breaks in coronary CT angiography: Comparison of sequential, low-pitch helical and high-pitch helical data acquisition. Eur. J. Radiol. 2012, 81, e357–e362. [Google Scholar] [CrossRef] [PubMed]
- Lobrich, M.; Rief, N.; Kuhne, M.; Heckmann, M.; Fleckenstein, J.; Rube, C.; Uder, M. In vivo formation and repair of DNA double-strand breaks after computed tomography examinations. Proc. Natl. Acad. Sci. USA 2005, 102, 8984–8989. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, V.; Wilkins, R.C. A comprehensive review of the literature on the biological effects from dental X-ray exposures. Int. J. Radiat. Biol. 2019, 95, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Rehani, M.M.; Yang, K.; Melick, E.R.; Heil, J.; Salat, D.; Sensakovic, W.F.; Liu, B. Patients undergoing recurrent CT scans: Assessing the magnitude. Eur. Radiol. 2019, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Janosikova, L.; Juricekova, M.; Horvathova, M.; Nikodemova, D.; Klepanec, A.; Salat, D. Risk Evaluation in the Low-Dose Range Ct for Radiation-Exposed Children, Based on DNA Damage. Radiat. Prot. Dosim. 2019, 29, 1–5. [Google Scholar]
- Grudzenski, S.; Raths, A.; Conrad, S.; Rube, C.E.; Lobrich, M. Inducible response required for repair of low-dose radiation damage in human fibroblasts. Proc. Natl. Acad. Sci. USA 2010, 107, 14205–14210. [Google Scholar] [CrossRef] [PubMed]
- Ganassi, E.E.; Zaichkina, S.I.; Rozanova, O.M. Modeling, using nucleases, of the effect of radiation on Chinese hamster fibroblasts. On the role of single-stranded DNA breaks, induced by DNAase I, in the initiation of chromosome aberrations. Radiobiologiia 1989, 29, 742–745. [Google Scholar]
- Zhao, X.; Yang, G.; Toyooka, T.; Ibuki, Y. New mechanism of γ-H2AX generation: Surfactant-induced actin disruption causes deoxyribonuclease I translocation to the nucleus and forms DNA double-strand breaks. Mutat. Res. Genetic Toxicol. Environ. Mutagen. 2015, 794, 1–7. [Google Scholar] [CrossRef]
- Vasilyev, S.A.; Kubes, M.; Markova, E.; Belyaev, I. DNA damage response in CD133 + stem/progenitor cells from umbilical cord blood: Low level of endogenous foci and high recruitment of 53BP1. Int. J. Radiat. Biol. 2013, 89, 301–309. [Google Scholar] [CrossRef]
- Markova, E.; Schultz, N.; Belyaev, I.Y. Kinetics and dose-response of residual 53BP1/γ-H2AX foci: Co-localization, relationship with DSB repair and clonogenic survival. Int. J. Radiat. Biol. 2007, 83, 319–329. [Google Scholar] [CrossRef]
- Markova, E.; Torudd, J.; Belyaev, I. Long time persistence of residual 53BP1/γ-H2AX foci in human lymphocytes in relationship to apoptosis, chromatin condensation and biological dosimetry. Int. J. Radiat. Biol. 2011, 87, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Torudd, J.; Protopopova, M.; Sarimov, R.; Nygren, J.; Eriksson, S.; Markova, E.; Chovanec, M.; Selivanova, G.; Belyaev, I.Y. Dose-response for radiation-induced apoptosis, residual 53BP1 foci and DNA-loop relaxation in human lymphocytes. Int. J. Radiat. Biol. 2005, 81, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Kinner, A.; Wu, W.; Staudt, C.; Iliakis, G. Γ-H2AX in recognition and signaling of DNA double-strand breaks in the context of chromatin. Nucleic Acids Res. 2008, 36, 5678–5694. [Google Scholar] [CrossRef] [PubMed]
- Hoang, J.K.; Reiman, R.E.; Nguyen, G.B.; Januzis, N.; Chin, B.B.; Lowry, C.; Yoshizumi, T.T. Lifetime Attributable Risk of Cancer from Radiation Exposure During Parathyroid Imaging: Comparison of 4D CT and Parathyroid Scintigraphy. AJR Am. J. Roentgenol. 2015, 204, W579–W585. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Yoshizumi, T.T.; Frush, D.P.; Toncheva, G.; Yin, F.F. Radiation dose from cone beam CT in a pediatric phantom: Risk estimation of cancer incidence. AJR Am. J. Roentgenol. 2010, 194, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Bagherzadeh, S.; Jabbari, N.; Khalkhali, H.R. Estimation of lifetime attributable risks (LARs) of cancer associated with abdominopelvic radiotherapy treatment planning computed tomography (CT) simulations. Int. J. Radiat. Biol. 2018, 94, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Smith-Bindman, R.; Lipson, J.; Marcus, R.; Kim, K.P.; Mahesh, M.; Gould, R.; Berrington de Gonzalez, A.; Miglioretti, D.L. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch. Intern. Med. 2009, 169, 2078–2086. [Google Scholar] [CrossRef]
- Vandevoorde, C.; Vral, A.; Vandekerckhove, B.; Philippe, J.; Thierens, H. Radiation Sensitivity of Human CD34(+) Cells Versus Peripheral Blood T Lymphocytes of Newborns and Adults: DNA Repair and Mutagenic Effects. Radiat. Res. 2016, 185, 580–590. [Google Scholar] [CrossRef]
- Jakl, L.; Lobachevsky, P.; Vokalova, L.; Durdik, M.; Markova, E.; Belyaev, I. Validation of JCountPro software for efficient assessment of ionizing radiation-induced foci in human lymphocytes. Int. J. Radiat. Biol. 2016, 92, 766–773. [Google Scholar] [CrossRef]
- Rube, C.E.; Fricke, A.; Widmann, T.A.; Furst, T.; Madry, H.; Pfreundschuh, M.; Rube, C. Accumulation of DNA damage in hematopoietic stem and progenitor cells during human aging. PLoS ONE 2011, 6, e17487. [Google Scholar] [CrossRef]
- Sorokina, S.; Markova, E.; Gursky, J.; Dobrovodsky, J.; Belyaev, I. Relative biological efficiency of protons at low and therapeutic doses in induction of 53BP1/γ-H2AX foci in lymphocytes from umbilical cord blood. Int. J. Radiat. Biol. 2013, 89, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yin, L.; Zhang, J.; Zhang, Y.; Zhang, X.; Ding, D.; Gao, Y.; Li, Q.; Chen, H. The profiles of γ-H2AX along with ATM/DNA-PKcs activation in the lymphocytes and granulocytes of rat and human blood exposed to γ rays. Radiat. Environ. Biophys. 2016, 55, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Durdik, M.; Kosik, P.; Kruzliakova, J.; Jakl, L.; Markova, E.; Belyaev, I. Hematopoietic stem/progenitor cells are less prone to undergo apoptosis than lymphocytes despite similar DNA damage response. Oncotarget 2017, 8, 48846–48853. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Roch-Lefevre, S.; Mandina, T.; Voisin, P.; Gaetan, G.; Mesa, J.E.; Valente, M.; Bonnesoeur, P.; Garcia, O.; Voisin, P.; Roy, L. Quantification of γ-H2AX foci in human lymphocytes: A method for biological dosimetry after ionizing radiation exposure. Radiat. Res. 2010, 174, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Joiner, M.C.; Marples, B.; Lambin, P.; Short, S.C.; Turesson, I. Low-dose hypersensitivity: Current status and possible mechanisms. Int. J. Radiat. Oncol. Biol. Phys. 2001, 49, 379–389. [Google Scholar] [CrossRef]
- Lobrich, M.; Jeggo, P.A. The impact of a negligent G2/M checkpoint on genomic instability and cancer induction. Nat. Rev. Cancer 2007, 7, 861–869. [Google Scholar] [CrossRef]
- Marples, B.; Wouters, B.G.; Collis, S.J.; Chalmers, A.J.; Joiner, M.C. Low-dose hyper-radiosensitivity: A consequence of ineffective cell cycle arrest of radiation-damaged G2-phase cells. Radiat. Res. 2004, 161, 247–255. [Google Scholar] [CrossRef]
- Short, S.C.; Woodcock, M.; Marples, B.; Joiner, M.C. Effects of cell cycle phase on low-dose hyper-radiosensitivity. Int. J. Radiat. Biol. 2003, 79, 99–105. [Google Scholar] [CrossRef]
| Variables | Multifactorial ANOVA | Categorical Factors | p-Values Adjusted by FDR | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| γH2AX * | 53BP1 * | γH2AX/53BP1 * | Pan-st. (%) | Multivariate | Univariate | Time | Dose | Treatment | Time | Dose | Treatment |
| + | + | + | - | + | - | all | + | + | 0.00000 | 0.00000 | 0.74331 |
| + | - | - | - | - | + | all | + | + | 0.00000 | 0.00000 | 0.65262 |
| - | + | - | - | - | + | all | + | + | 0.00000 | 0.00000 | 0.92818 |
| - | - | + | - | - | + | all | + | + | 0.00006 | 0.00000 | 0.96429 |
| - | - | - | + | - | + | all | + | + | 0.10375 | 0.93183 | 0.70285 |
| + | + | + | - | + | - | 30 min | + | + | - | 0.00000 | 0.90202 |
| + | - | - | - | - | + | 30 min | + | + | - | 0.00000 | 1.00000 |
| - | + | - | - | - | + | 30 min | + | + | - | 0.00000 | 1.00000 |
| - | - | + | - | - | + | 30 min | + | + | - | 0.00011 | 1.00000 |
| - | - | - | + | - | + | 30 min | + | + | - | 0.84416 | 1.00000 |
| + | + | + | - | + | - | 2 h | + | + | - | 0.00000 | 0.15215 |
| + | - | - | - | - | + | 2 h | + | + | - | 0.00000 | 0.19180 |
| - | + | - | - | - | + | 2 h | + | + | - | 0.00000 | 0.14320 |
| - | - | + | - | - | + | 2 h | + | + | - | 0.00006 | 0.83501 |
| - | - | - | + | - | + | 2 h | + | + | - | 0.91106 | 0.70876 |
| + | + | + | - | + | - | 22 h | + | + | - | 0.00000 | 1.00000 |
| + | - | - | - | - | + | 22 h | + | + | - | 0.00000 | 1.00000 |
| - | + | - | - | - | + | 22 h | + | + | - | 0.00000 | 1.00000 |
| - | - | + | - | - | + | 22 h | + | + | - | 0.00000 | 0.97244 |
| - | - | - | + | - | + | 22 h | + | + | - | 0.92655 | 0.64110 |
| FDR | p-Values Obtained by t-Test Adjusted by FDR | ||||
|---|---|---|---|---|---|
| Time | Dose | γH2AX | 53BP1 | γH2AX/53BP1 | Pan-Staining |
| 0.5 h | 5 cGy | 0.02 | 0.02 | 0.02 | 0.634 |
| 10 cGy | 0.0001 | 0.002 | 0.02 | 0.641 | |
| 50 cGy | 0.00002 | 0.000002 | 0.002 | 0.572 | |
| 2 h | 5c Gy | 0.004 | 0.006 | 0.006 | 0.705 |
| 10 cGy | 0.0002 | 0.001 | 0.002 | 0.767 | |
| 50 cGy | 0.000002 | 0.000004 | 0.001 | 0.635 | |
| 22 h | 5 cGy | 0.147 | 0.003 | 0.364 | 0.962 |
| 10 cGy | 0.02 | 0.00001 | 0.02 | 0.912 | |
| 50 cGy | 0.008 | 0.00001 | 0.03 | 0.772 | |
| Dose Response | Time | γH2AX | 53BP1 | γH2AX/53BP1 |
|---|---|---|---|---|
| up to 10 cGy | 30 min | y = 0.0712x | y = 0.1114x | y = 0.0476x |
| 2 h | y = 0.0892x | y = 0.0948x | y = 0.0524x | |
| 22 h | y = 0.0354x | y = 0.0635x | y = 0.0106x | |
| up to 50 cGy | 30 min | y = 0.0756x | y = 0.08x | y = 0.0475x |
| 2 h | y = 0.0618x | y = 0.0516x | y = 0.0364x | |
| 22 h | y = 0.014x | y = 0.0256x | y = 0.0059x |
| p-Value Obtained by Fisher’s LSD Test | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| γH2AX | 53BP1 | Co-Localization | ||||||||
| 30 min | 2 h | 22 h | 30 min | 2 h | 22 h | 30 min | 2 h | 22 h | ||
| 0 cGy | 30 min | - | 0.558 | 0.484 | - | 0.366 | 0.739 | - | 0.896 | 0.459 |
| 2 h | 0.558 | - | 0.749 | 0.366 | - | 0.113 | 0.896 | - | 0.540 | |
| 22 h | 0.484 | 0.749 | - | 0.739 | 0.113 | - | 0.459 | 0.540 | - | |
| 5 cGy | 30 min | - | 0.330 | 0.826 | - | 0.561 | 0.471 | - | 0.950 | 0.255 |
| 2 h | 0.330 | - | 0.355 | 0.561 | - | 0.178 | 0.950 | - | 0.146 | |
| 22 h | 0.826 | 0.355 | - | 0.471 | 0.178 | - | 0.255 | 0.146 | - | |
| 10 cGy | 30 min | - | 0.138 | 0.537 | - | 0.591 | 0.316 | - | 0.775 | 0.251 |
| 2 h | 0.138 | - | 0.599 | 0.591 | - | 0.078 | 0.775 | - | 0.062 | |
| 22 h | 0.537 | 0.599 | - | 0.316 | 0.078 | - | 0.251 | 0.062 | - | |
| 50 cGy | 30 min | - | 0.236 | 0.008 | - | 0.039 | 0.003 | - | 0.558 | 0.063 |
| 2 h | 0.236 | - | 0.005 | 0.039 | - | 0.002 | 0.558 | - | 0.061 | |
| 22 h | 0.008 | 0.005 | - | 0.003 | 0.002 | - | 0.063 | 0.061 | - | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakl, L.; Marková, E.; Koláriková, L.; Belyaev, I. Biodosimetry of Low Dose Ionizing Radiation Using DNA Repair Foci in Human Lymphocytes. Genes 2020, 11, 58. https://doi.org/10.3390/genes11010058
Jakl L, Marková E, Koláriková L, Belyaev I. Biodosimetry of Low Dose Ionizing Radiation Using DNA Repair Foci in Human Lymphocytes. Genes. 2020; 11(1):58. https://doi.org/10.3390/genes11010058
Chicago/Turabian StyleJakl, Lukáš, Eva Marková, Lucia Koláriková, and Igor Belyaev. 2020. "Biodosimetry of Low Dose Ionizing Radiation Using DNA Repair Foci in Human Lymphocytes" Genes 11, no. 1: 58. https://doi.org/10.3390/genes11010058
APA StyleJakl, L., Marková, E., Koláriková, L., & Belyaev, I. (2020). Biodosimetry of Low Dose Ionizing Radiation Using DNA Repair Foci in Human Lymphocytes. Genes, 11(1), 58. https://doi.org/10.3390/genes11010058





