Arterial Elasticity in Ehlers-Danlos Syndromes
Abstract
1. Introduction
2. Materials and Methods
2.1. PWV Measurements
2.2. Orthostatic Vital Sign Measurements
2.3. Data Analysis
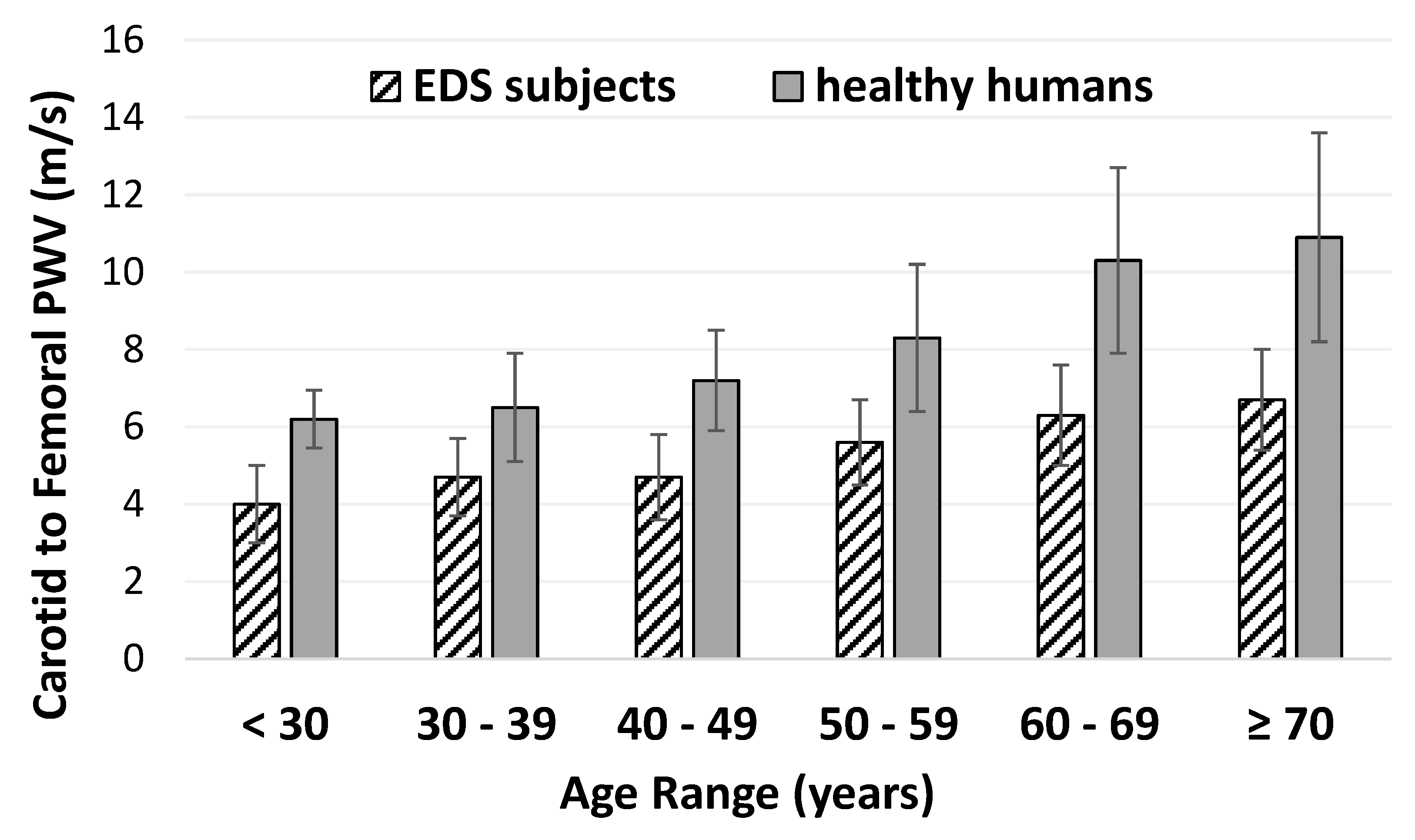
3. Results
3.1. Pulse Wave Velocity in EDS
3.2. Orthostatic Blood Pressure in EDS
3.3. Correlations between Pulse Wave Velocity and Blood Pressure
4. Discussion
4.1. Overall Findings
4.2. Significance of Decreased Pulse Wave Velocity in Ehlers-Danlos Syndrome
4.3. Association between Pulse Wave Velocity and Blood Pressure
4.4. Comparisons between EDS Types
4.5. Strengths
4.6. Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BP | Blood pressure |
| EDS | Ehlers–Danlos syndromes |
| OI | Orthostatic intolerance |
| POTS | Postural orthostatic tachycardia syndrome |
| PWV | Pulse wave velocity |
References
- Malfait, F.; Francomano, C.; Byers, P.; Belmont, J.; Berglund, B.; Black, J.; Bloom, L.; Bowen, J.M.; Brady, A.F.; Burrows, N.P.; et al. The 2017 international classification of the Ehlers-Danlos syndromes. Am. J. Med. Genet. C Semin. Med. Genet. 2017, 175, 8–26. [Google Scholar] [CrossRef] [PubMed]
- Murray, B.; Yashar, B.M.; Uhlmann, W.R.; Clauw, D.J.; Petty, E.M. Ehlers-Danlos syndrome, hypermobility type: A characterization of the patients’ lived experience. Am. J. Med. Genet. A 2013, 161, 2981–2988. [Google Scholar] [CrossRef] [PubMed]
- Roma, M.; Marden, C.L.; De Wandele, I.; Francomano, C.A.; Rowe, P.C. Postural tachycardia syndrome and other forms of orthostatic intolerance in Ehlers-Danlos syndrome. Auton. Neurosci. 2018, 215, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Wallman, D.; Weinberg, J.; Hohler, A.D. Ehlers-Danlos Syndrome and Postural Tachycardia Syndrome: A relationship study. J. Neurol. Sci. 2014, 340, 99–102. [Google Scholar] [CrossRef]
- Gazit, Y.; Nahir, A.M.; Grahame, R.; Jacob, G. Dysautonomia in the joint hypermobility syndrome. Am. J. Med. 2003, 115, 33–40. [Google Scholar] [CrossRef]
- De Wandele, I.; Rombaut, L.; De Backer, T.; Peersman, W.; Da Silva, H.; De Mits, S.; De Paepe, A.; Calders, P.; Malfait, F. Orthostatic intolerance and fatigue in the hypermobility type of Ehlers-Danlos Syndrome. Rheumatology 2016, 55, 1412–1420. [Google Scholar] [CrossRef]
- Celletti, C.; Camerota, F.; Castori, M.; Censi, F.; Gioffre, L.; Calcagnini, G.; Strano, S. Orthostatic Intolerance and Postural Orthostatic Tachycardia Syndrome in Joint Hypermobility Syndrome/Ehlers-Danlos Syndrome, Hypermobility Type: Neurovegetative Dysregulation or Autonomic Failure? BioMed Res. Int. 2017, 2017, 9161865. [Google Scholar] [CrossRef]
- Rowe, P.C.; Barron, D.F.; Calkins, H.; Maumenee, I.H.; Tong, P.Y.; Geraghty, M.T. Orthostatic intolerance and chronic fatigue syndrome associated with Ehlers-Danlos syndrome. J. Pediatr. 1999, 135, 494–499. [Google Scholar] [CrossRef]
- Laurent, S.; Cockcroft, J.; Van Bortel, L.; Boutouyrie, P.; Giannattasio, C.; Hayoz, D.; Pannier, B.; Vlachopoulos, C.; Wilkinson, I.; Struijker-Boudier, H.; et al. Expert consensus document on arterial stiffness: Methodological issues and clinical applications. Eur. Heart J. 2006, 27, 2588–2605. [Google Scholar] [CrossRef]
- Hirata, K.; Kawakami, M.; O’Rourke, M.F. Pulse wave analysis and pulse wave velocity: A review of blood pressure interpretation 100 years after Korotkov. Circ. J. 2006, 70, 1231–1239. [Google Scholar] [CrossRef]
- Cheng, J.L.; Au, J.S.; Guzman, J.C.; Morillo, C.A.; MacDonald, M.J. Cardiovascular profile in postural orthostatic tachycardia syndrome and Ehlers-Danlos syndrome type III. Clin. Auton. Res. 2017, 27, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Francois, B.; De Paepe, A.; Matton, M.T.; Clement, D. Pulse wave velocity recordings in a family with ecchymotic Ehlers-Danlos syndrome. Int. Angiol. 1986, 5, 1–5. [Google Scholar] [PubMed]
- Mirault, T.; Pernot, M.; Frank, M.; Couade, M.; Niarra, R.; Azizi, M.; Emmerich, J.; Jeunemaitre, X.; Fink, M.; Tanter, M.; et al. Carotid stiffness change over the cardiac cycle by ultrafast ultrasound imaging in healthy volunteers and vascular Ehlers-Danlos syndrome. J. Hypertens. 2015, 33, 1890–1896. [Google Scholar] [CrossRef] [PubMed]
- Bascom, R.; Schubart, J.R.; Mills, S.; Smith, T.; Zukley, L.M.; Francomano, C.A.; McDonnell, N. Heritable disorders of connective tissue: Description of a data repository and initial cohort characterization. Am. J. Med. Genet. A 2019, 179, 552–560. [Google Scholar] [CrossRef]
- Beighton, P.; De Paepe, A.; Steinmann, B.; Tsipouras, P.; Wenstrup, R.J. Ehlers-Danlos syndromes: Revised nosology, Villefranche, 1997. Ehlers-Danlos National Foundation (USA) and Ehlers-Danlos Support Group (UK). Am. J. Med. Genet. 1998, 77, 31–37. [Google Scholar] [CrossRef]
- David, M.; Malti, O.; AlGhatrif, M.; Wright, J.; Canepa, M.; Strait, J.B. Pulse wave velocity testing in the Baltimore longitudinal study of aging. J. Vis. Exp. 2014, e50817. [Google Scholar] [CrossRef]
- Reference Values for Arterial Stiffness Collaboration. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘establishing normal and reference values’. Eur. Heart J. 2010, 31, 2338–2350. [Google Scholar] [CrossRef]
- Mitchell, G.F.; Hwang, S.J.; Vasan, R.S.; Larson, M.G.; Pencina, M.J.; Hamburg, N.M.; Vita, J.A.; Levy, D.; Benjamin, E.J. Arterial stiffness and cardiovascular events: The Framingham Heart Study. Circulation 2010, 121, 505–511. [Google Scholar] [CrossRef]
- Willum-Hansen, T.; Staessen, J.A.; Torp-Pedersen, C.; Rasmussen, S.; Thijs, L.; Ibsen, H.; Jeppesen, J. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation 2006, 113, 664–670. [Google Scholar] [CrossRef]
- Laurent, S.; Boutouyrie, P.; Asmar, R.; Gautier, I.; Laloux, B.; Guize, L.; Ducimetiere, P.; Benetos, A. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension 2001, 37, 1236–1241. [Google Scholar] [CrossRef]
- Sheldon, R.S.; Grubb, B.P.; Olshansky, B.; Shen, W.K.; Calkins, H.; Brignole, M.; Raj, S.R.; Krahn, A.D.; Morillo, C.A.; Stewart, J.M.; et al. 2015 heart rhythm society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm 2015, 12, e41–e63. [Google Scholar] [CrossRef] [PubMed]
| Classical (n = 10) | Hypermobile (n = 13) | Vascular (n = 8) | Other/Unspecified (n = 29) | P Value | All Patients (n = 60) | |
|---|---|---|---|---|---|---|
| Age (years) | 42 (15–63) | 34 (13–51) | 38 (13–70) | 34 (13–66) | 0.557 | 40 (13–70) |
| Sex (M/F) | 0/9 | 2/11 | 2/7 | 7/22 | - | 11/49 |
| Height (m) | 1.63 ± 0.04 | 1.67 ± 0.05 | 1.62 ± 0.12 | 1.66 ± 0.08 | 0.336 | 1.65 ± 0.08 |
| Weight (kg) | 74.6 ± 23.5 | 68.8 ± 12.6 | 65.3 ± 16.4 | 68.0 ± 21.3 | 0.804 | 70.1 ± 21.8 |
| BMI (kg/m2) | 27.9 ± 8.0 | 24.5 ± 4.0 | 24.5 ± 4.1 | 24.7 ± 7.7 | 0.593 | 25.1 ± 6.6 |
| Supine Hemodynamics | ||||||
| Systolic BP (mmHg) | 116 ± 11 | 121 ± 7 | 117 ± 11 | 120 ± 13 | 0.655 | 119 ± 11 |
| Diastolic BP (mmHg) | 68 ± 8 | 68 ± 10 | 62 ± 11 | 67 ± 7 | 0.356 | 66 ± 8 |
| HR (beats/min) | 77 ± 10 | 77 ± 10 | 66 ± 8 | 74 ± 14 | 0.200 | 74 ± 13 |
| Central PWV (m/s) | 4.91 ± 0.56 | 4.82 ± 0.38 | 4.89 ± 0.47 | 4.59 ± 0.20 | 0.873 | 4.73 ± 0.16 |
| Peripheral PWV (m/s) | 7.45 ± 0.39 | 7.24 ± 0.33 | 7.39± 0.29 | 7.12 ± 0.17 | 0.810 | 7.23 ± 1.00 |
| Standing Hemodynamics | ||||||
| Systolic BP (mmHg) | 110 ± 19 | 118 ± 21 | 93 ± 24 | 121 ± 14 | 0.003 | 115 ± 20 |
| Diastolic BP (mmHg) | 75 ± 11 | 81 ± 12 | 69 ± 14 | 73 ± 10 | 0.087 | 75 ± 12 |
| HR (beats/min) | 91 ± 14 | 92 ± 12 | 85 ± 17 | 89 ± 16 | 0.719 | 90 ± 15 |
| Carotid to Femoral PWV | Carotid to Radial PWV | |
|---|---|---|
| SBP (supine) | 0.387 * | 0.076 |
| SBP (seated) | 0.399 * | 0.098 |
| SBP (standing) | 0.199 | 0.008 |
| Δ SBP (standing-seated) | −0.077 | −0.066 |
| DBP (supine) | 0.400 * | 0.322 * |
| DBP (seated) | 0.204 | 0.383 * |
| DBP (standing) | 0.078 | 0.323 * |
| Δ DBP (standing-seated) | −0.158 | −0.062 |
| HR (supine) | 0.015 | 0.185 |
| HR (seated) | 0.044 | 0.234 |
| HR (standing) | −0.039 | 0.165 |
| Δ HR (standing-seated) | −0.111 | −0.048 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miller, A.J.; Schubart, J.R.; Sheehan, T.; Bascom, R.; Francomano, C.A. Arterial Elasticity in Ehlers-Danlos Syndromes. Genes 2020, 11, 55. https://doi.org/10.3390/genes11010055
Miller AJ, Schubart JR, Sheehan T, Bascom R, Francomano CA. Arterial Elasticity in Ehlers-Danlos Syndromes. Genes. 2020; 11(1):55. https://doi.org/10.3390/genes11010055
Chicago/Turabian StyleMiller, Amanda J., Jane R. Schubart, Timothy Sheehan, Rebecca Bascom, and Clair A. Francomano. 2020. "Arterial Elasticity in Ehlers-Danlos Syndromes" Genes 11, no. 1: 55. https://doi.org/10.3390/genes11010055
APA StyleMiller, A. J., Schubart, J. R., Sheehan, T., Bascom, R., & Francomano, C. A. (2020). Arterial Elasticity in Ehlers-Danlos Syndromes. Genes, 11(1), 55. https://doi.org/10.3390/genes11010055





