Biomphalaria glabrata Granulin Increases Resistance to Schistosoma mansoni Infection in Several Biomphalaria Species and Induces the Production of Reactive Oxygen Species by Haemocytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Ethics Statement
2.2. Snails and Parasites
2.3. Recombinant BgGRN Synthesis and Purification
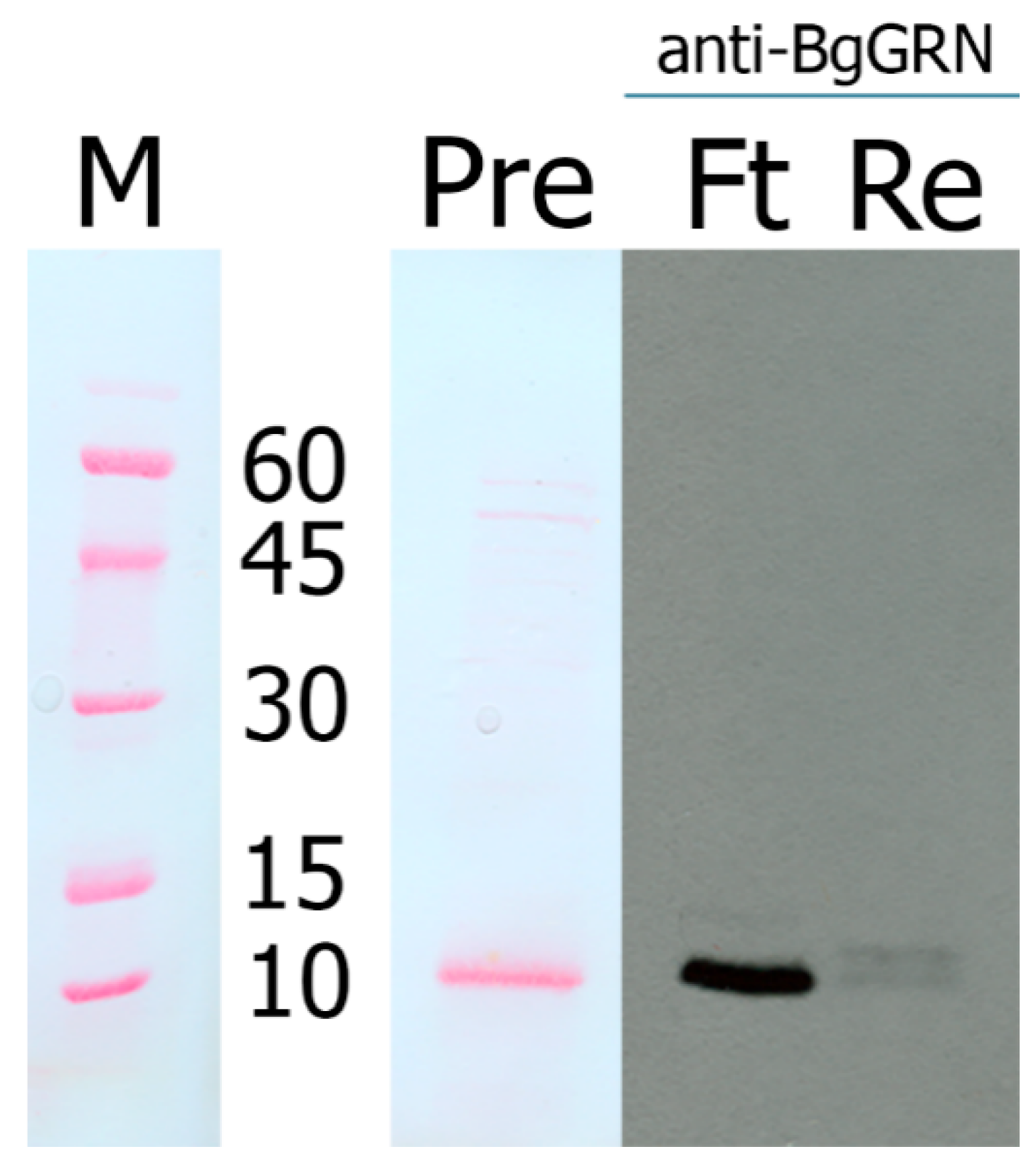
2.4. Anti-BgGRN Polyclonal Antibody Generation and Validation
2.5. Western Blot Analysis of BgGRN
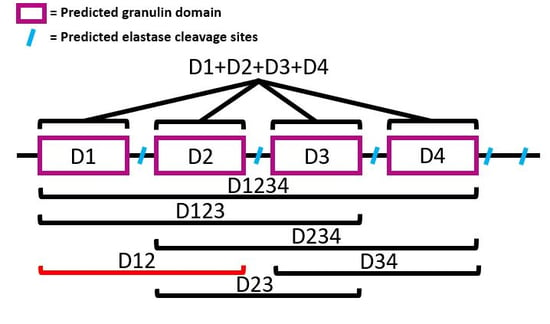
2.6. Generation of Individual BgGRN Domains
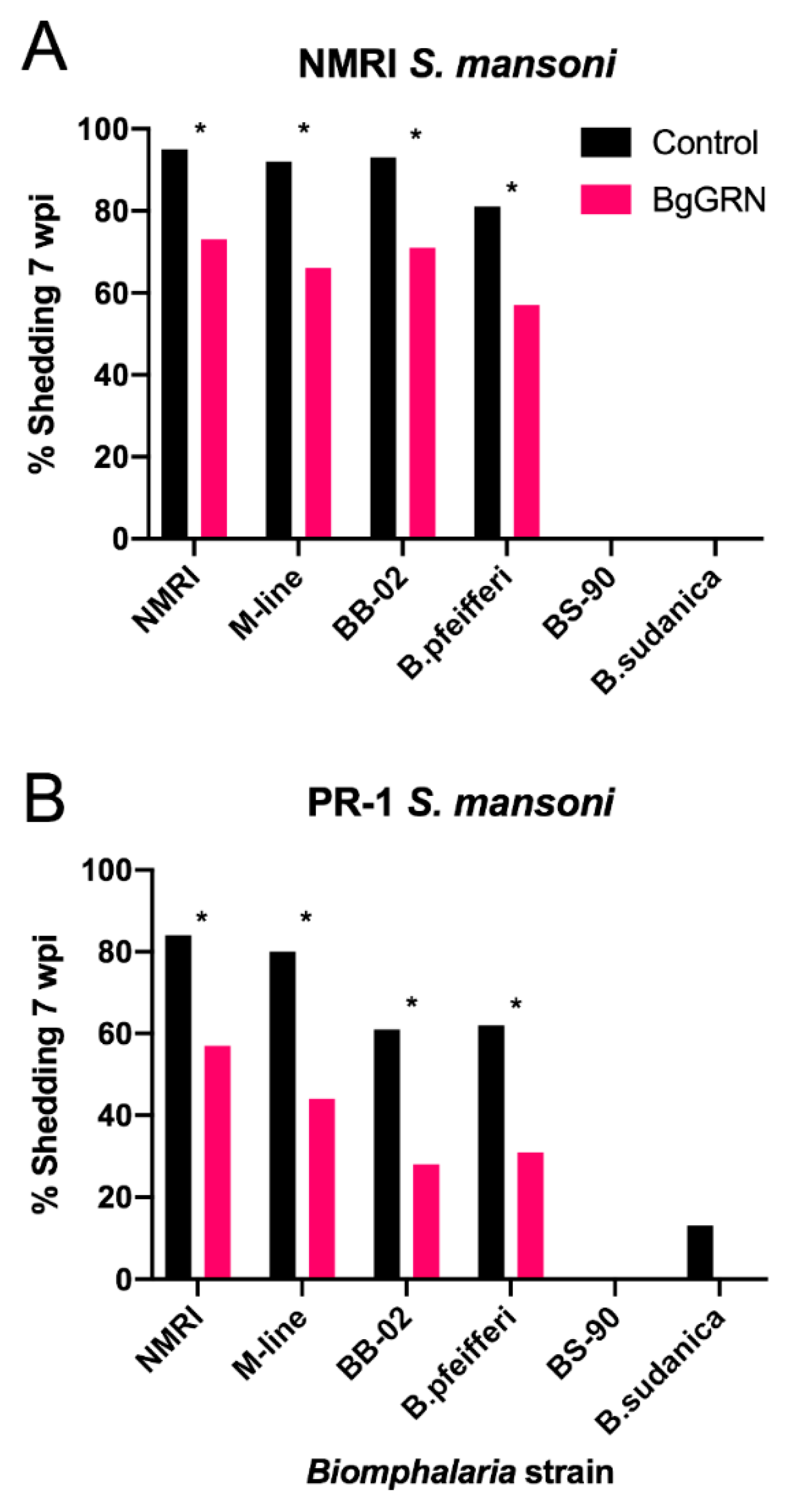
2.7. Cross Species Effect of BgGRN on S. mansoni Infection Success
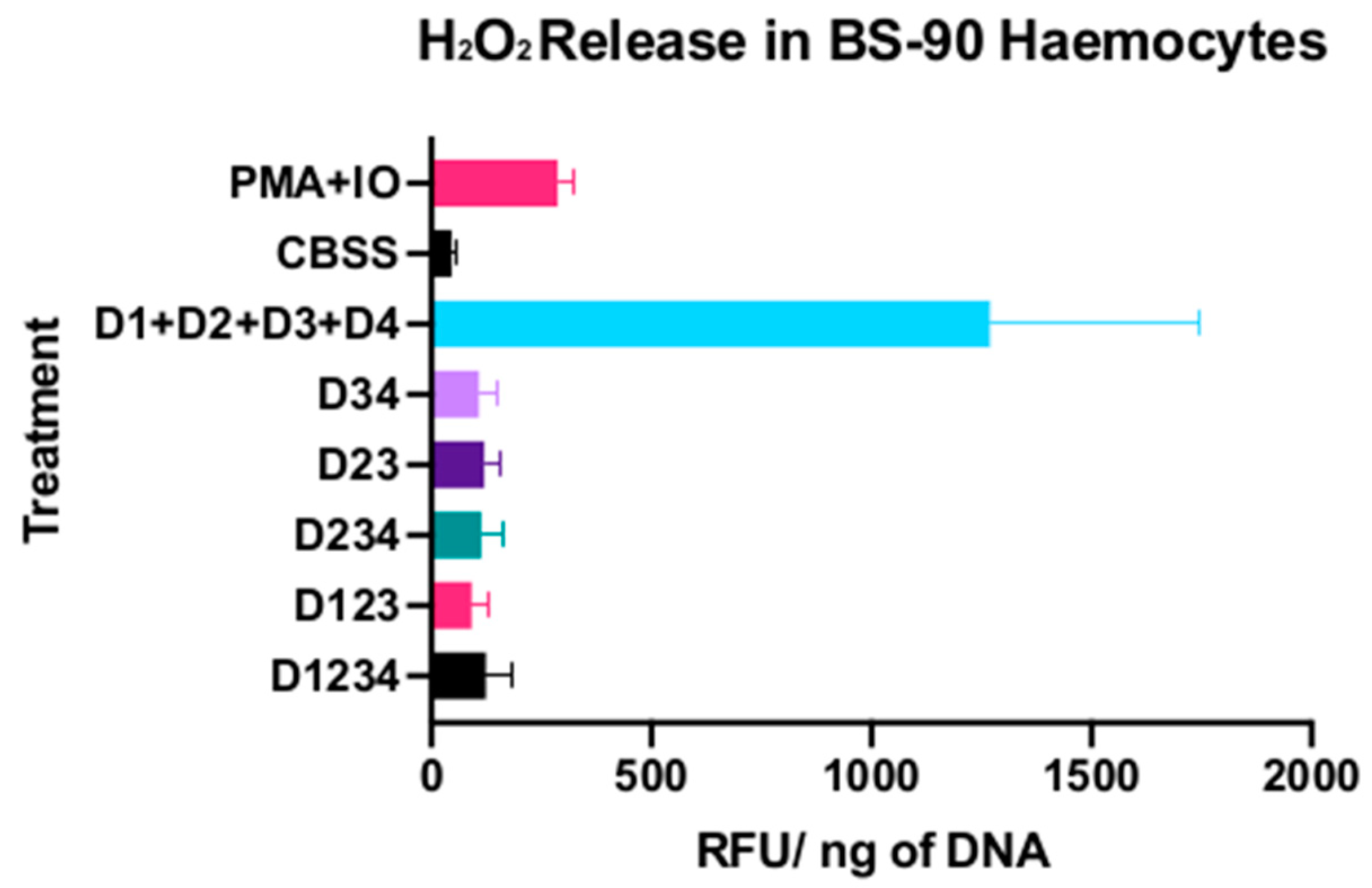
2.8. Effects of BgGRN Subunits on Reactive Oxygen Species Generation
2.9. Satistical Analysis
3. Results
3.1. Generation of Individual Subunits of BgGRN
3.2. Reactive Oxygen Species Production
3.3. Cross Species Infection Reduction
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. The Weekly Epidemiological Record (WER); WHO: Geneva, Switzerland, 2018; pp. 445–452. [Google Scholar]
- Humphries, J.E.; Yoshino, T.P. Regulation of hydrogen peroxide release in circulating hemocytes of the planorbid snail Biomphalaria glabrata. Dev. Comp. Immunol. 2008, 32, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Adema, C.M.; Hillier, L.D.W.; Jones, C.S.; Loker, E.S.; Knight, M.; Minx, P.; Oliveira, G.; Raghavan, N.; Shedlock, A.; do Amaral, L.R.; et al. Whole genome analysis of a schistosomiasis-transmitting freshwater snail. Nat. Commun. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.A.T.; Dejong, R.J.; Snyder, S.D.; Mkoji, G.M.; Loker, E.S. Schistosoma mansoni and Biomphalaria: Past history and future trends. Parasitology 2001, 123, 211–228. [Google Scholar] [CrossRef] [PubMed]
- Paranse, W.L.; Correa, L.R. Variation in Susceptibility of Populations of Australorbis glabratus to a Strain of Schistosoma mansoni. Rev. Inst. Med. Trop. Sao Paulo 1963, 5, 15–22. [Google Scholar]
- Richards, C.S.; Merritt, J.W. Genetic factors in the susceptibility of juvenile Biomphalaria glabrata to Schistosoma mansoni infection. Am. J. Trop. Med. Hyg. 1972, 21, 425–434. [Google Scholar] [CrossRef]
- Newton, W.L. The Establishment of a Strain of Australorbis glabratus Which Combines Albinism and High Susceptibility to Infection with Schistosoma mansoni. J Parasitol. 1955, 41, 526. [Google Scholar] [CrossRef]
- Cooper, L.A.; Larson, S.E.; Lewis, F.A. Male Reproductive Success of Schistosoma mansoni-Infected Biomphalaria glabrata Snails. J. Parasitol. 1996, 82, 428. [Google Scholar] [CrossRef]
- Pila, E.A.; Li, H.; Hambrook, J.R.; Wu, X.; Hanington, P.C. Schistosomiasis from a Snail’s Perspective: Advances in Snail Immunity. Trends Parasitol. 2017, 33, 845–857. [Google Scholar] [CrossRef]
- Coustau, C.; Gourbal, B.; Duval, D.; Yoshino, T.P.; Adema, C.M.; Mitta, G. Advances in gastropod immunity from the study of the interaction between the snail Biomphalaria glabrata and its parasites: A review of research progress over the last decade. Fish Shellfish Immunol. 2015, 46, 5–16. [Google Scholar] [CrossRef]
- Ong, C.H.P.; Bateman, A. Progranulin (granulin-epithelin precursor, PC-cell derived growth factor, acrogranin) in proliferation and tumorigenesis. Histol. Histopathol. 2003, 18, 1275–1288. [Google Scholar]
- Pila, E.A.; Gordy, M.A.; Phillips, V.K.; Kabore, A.L.; Rudko, S.P.; Hanington, P.C. Endogenous growth factor stimulation of hemocyte proliferation induces resistance to Schistosoma mansoni challenge in the snail host. Proc. Natl. Acad. Sci. USA 2016, 113, 5305–5310. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Bateman, A. Progranulin (granulin-epithelin precursor, PC-cell-derived growth factor, acrogranin) mediates tissue repair and tumorigenesis. J. Mol. Med. 2003, 81, 600–612. [Google Scholar] [CrossRef] [PubMed]
- Van Damme, P.; Van Hoecke, A.; Lambrechts, D.; Vanacker, P.; Bogaert, E.; Van Swieten, J.; Van Den Bosch, L.; Robberecht, W. Progranulin functions as a neurotrophic factor to regulate neurite outgrowth and enhance neuronal survival. J. Cell Biol. 2008, 81, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Zanocco-Marani, T.; Bateman, A.; Romano, G.; Valentinis, B.; He, Z.H.; Baserga, R. Biological activities and signaling pathways of the granulin/epithelin precursor. Cancer Res. 1999, 59, 5331–5340. [Google Scholar] [PubMed]
- Zhu, J.; Nathan, C.; Jin, W.; Sim, D.; Ashcroft, G.S.; Wahl, S.M.; Lacomis, L.; Erdjument-Bromage, H.; Tempst, P.; Wright, C.D.; et al. Conversion of Proepithelin to Epithelins: Roles of SLPI and Elastase in Host Defense and Wound Repair. Cell 2002, 111, 867–878. [Google Scholar] [CrossRef]
- Hrabal, R.; Chen, Z.; James, S.; Bennett, H.P.J.; Ni, F. The hairpin stack fold, a novel protein architecture for a new family of protein growth factors. Nat. Struct. Biol. 1996, 3, 747–751. [Google Scholar] [CrossRef]
- Bateman, A.; Bennett, H.P.J. Granulins: The structure and function of an emerging family of growth factors. J. Endocrinol. 1998, 158, 145–151. [Google Scholar] [CrossRef]
- Bhandari, V.; Palfree, R.G.E.; Bateman, A. Isolation and sequence of the granulin precursor cDNA from human bone marrow reveals tandem cysteine-rich granulin domains. Proc. Natl. Acad. Sci. USA 1992, 89, 1715–1719. [Google Scholar] [CrossRef]
- Plowman, G.D.; Green, J.M.; Neubauer, M.G.; Buckley, S.D.; McDonald, V.L.; Todaro, G.J.; Shoyab, M. The epithelin precursor encodes two proteins with opposing activities on epithelial cell growth. J. Biol. Chem. 1992, 267, 13073–13078. [Google Scholar]
- Pila, E.A.; Tarrabain, M.; Kabore, A.L.; Hanington, P.C. A Novel Toll-Like Receptor (TLR) Influences Compatibility between the Gastropod Biomphalaria glabrata, and the Digenean Trematode Schistosoma mansoni. PLoS Pathog. 2016, 12, 1–23. [Google Scholar] [CrossRef]
- Yang, Y.; Bazhin, A.V.; Werner, J.; Karakhanova, S. Reactive oxygen species in the immune system. Int. Rev. Immunol. 2013, 32, 249–270. [Google Scholar] [CrossRef] [PubMed]
- Hahn, U.K.; Bender, R.C.; Bayne, C.J. Production of reactive oxygen species by hemocytes of Biomphalaria glabrata: Carbohydrate-specific stimulation. Dev. Comp. Immunol. 2000, 24, 531–541. [Google Scholar] [CrossRef]
- Hahn, U.K.; Bender, R.C.; Bayne, C.J. Killing of Schistosoma mansoni Sporocysts by Hemocytes from Resistant Biomphalaria glabrata: Role of Reactive Oxygen Species. J. Parasitol. 2001, 87, 292. [Google Scholar] [CrossRef]
- Cody, J.J.; Ittiprasert, W.; Miller, A.N.; Henein, L.; Mentink-Kane, M.M.; Hsieh, M.H. The NIH-NIAID Schistosomiasis Resource Center at the Biomedical Research Institute: Molecular Redux. PLoS Negl. Trop. Dis. 2016, 10. [Google Scholar] [CrossRef]
- Pila, E.A.; Peck, S.J.; Hanington, P.C. The protein pheromone temptin is an attractant of the gastropod Biomphalaria glabrata. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2017, 203, 855–866. [Google Scholar] [CrossRef]
- Guichard, C.; Pedruzzi, E.; Dewas, C.; Fay, M.; Pouzet, C.; Bens, M.; Vandewalle, A.; Ogier-Denis, E.; Gougerot-Pocidalo, M.A.; Elbim, C. Interleukin-8-induced priming of neutrophil oxidative burst requires sequential recruitment of NADPH oxidase components into lipid rafts. J. Biol. Chem. 2005, 280, 37021–37032. [Google Scholar] [CrossRef]
- Bréchard, S.; Bueb, J.-L.; Tschirhart, E.J. Interleukin-8 primes oxidative burst in neutrophil-like HL-60 through changes in cytosolic calcium. Cell Calcium. 2005, 37, 531–540. [Google Scholar] [CrossRef]
- Moné, Y.; Ribou, A.C.; Cosseau, C.; Duval, D.; Théron, A.; Mitta, G.; Gourbal, B. An example of molecular co-evolution: Reactive oxygen species (ROS) and ROS scavenger levels in Schistosoma mansoni/Biomphalaria glabrata interactions. Int. J. Parasitol. 2011, 41, 721–730. [Google Scholar] [CrossRef]
- Bender, R.C.; Broderick, E.J.; Goodall, C.P.; Bayne, C.J. Respiratory burst of Biomphalaria glabrata hemocytes: Schistosoma mansoni-resistant snails produce more extracellular H2O2 than susceptible snails. J. Parasitol. 2005, 91, 275–279. [Google Scholar] [CrossRef]
- Dinguirard, N.; Cavalcanti, M.G.S.; Wu, X.J.; Bickham-Wright, U.; Sabat, G.; Yoshino, T.P. Proteomic analysis of Biomphalaria glabrata hemocytes during in vitroencapsulation of Schistosoma mansoni sporocysts. Front. Immunol. 2018, 29, 9. [Google Scholar]
- Zhang, S.M.; Coultas, K.A. Identification and characterization of five transcription factors that are associated with evolutionarily conserved immune signaling pathways in the schistosome-transmitting snail Biomphalaria glabrata. Mol. Immunol. 2011, 48, 1868–1881. [Google Scholar] [CrossRef] [PubMed]
- Moresco, E.M.Y.; Beutler, B. Special Delivery: Granulin Brings CpG DNA to Toll-like Receptor 9. Immunity 2011, 34, 453–455. [Google Scholar] [CrossRef] [PubMed]
- Smout, M.J.; Laha, T.; Mulvenna, J.; Sripa, B.; Suttiprapa, S.; Jones, A.; Brindley, P.J.; Loukas, A. A granulin-like growth factor secreted by the carcinogenic liver fluke, Opisthorchis viverrini, promotes proliferation of host cells. PLoS Pathog. 2009, 5. [Google Scholar] [CrossRef] [PubMed]
- Papatpremsiri, A.; Smout, M.J.; Loukas, A.; Brindley, P.J.; Sripa, B.; Laha, T. Suppression of Ov-grn-1 encoding granulin of Opisthorchis viverrini inhibits proliferation of biliary epithelial cells. Exp. Parasitol. 2015, 148, 17–23. [Google Scholar] [CrossRef]
- Abe, M.; Kuroda, R. The development of CRISPR for a mollusc establishes the formin Lsdia1 as the long-sought gene for snail dextral/sinistral coiling. Development 2019, 14, 146. [Google Scholar] [CrossRef]
- Perry, K.J.; Henry, J.Q. CRISPR/Cas9-mediated genome modification in the mollusc, Crepidula fornicata. Genesis 2015, 53, 237–244. [Google Scholar] [CrossRef]
- Alphey, L. Can CRISPR-Cas9 gene drives curb malaria? Nat. Biotechnol. 2016, 34, 149–150. [Google Scholar] [CrossRef]
- Hammond, A.; Galizi, R.; Kyrou, K.; Simoni, A.; Siniscalchi, C.; Katsanos, D.; Gribble, M.; Baker, D.; Marois, E.; Russell, S.; et al. A CRISPR-Cas9 gene drive system targeting female reproduction in the malaria mosquito vector Anopheles gambiae. Nat. Biotechnol. 2016, 34, 78–83. [Google Scholar] [CrossRef]
- Gantz, V.M.; Jasinskiene, N.; Tatarenkova, O.; Fazekas, A.; Macias, V.M.; Bier, E.; James, A.A. Highly efficient Cas9-mediated gene drive for population modification of the malaria vector mosquito Anopheles stephensi. Proc. Natl. Acad. Sci. USA 2015, 112, E6736–E6743. [Google Scholar] [CrossRef]
- N’Goran, E.K.; Utzinger, J.; N’Guessan, A.N.; Muller, I.; Zamble, K.; Lohourignon, K.L.; Traoré, M.; Sosthène, B.A.; Lengeler, C.; Tanner, M. Reinfection with Schistosoma haematobium following school-based chemotherapy with praziquantel in four highly endemic villages in Cote d’Ivoire. Trop. Med. Int. Health 2001, 6, 817–825. [Google Scholar] [CrossRef]
- Doenhoff, M.J.; Kusel, J.R.; Coles, G.C.; Ciol1, D. Resistance of Schistosoma mansoni to praziquantel: Is there a problem? Trans. R Soc. Trop. Med. Hyg. 2002, 96, 465–469. [Google Scholar] [CrossRef]

| BgGRN Domain1 + Full-Length Fwd2 | C ACCAGG AGG GAC AAC TAC ATG ACT TGC TGC AAG GCT AAT |
| BgGRN Domain1 Rev2 | GT AGC AAC GGC GGT TGA TCA |
| BgGRN Domain2 Fwd2 | C ACCAGG AGG GAT GGA TCG ATG ACG TGC TGC CAG CTG GCT |
| BgGRN Domain2 Rev2 | CT TCA CGC AGG TGC CAG CTG |
| BgGRN Domain3 Fwd2 | C ACCAGG AGG GGT GGA GCT ATG ACT TGC TGC AAG CTC CAG |
| BgGRN Domain3 Rev2 | CT TCT TGC ACT CGC CTT GAT |
| BgGRN Domain4 Fwd2 | C ACCAGG AGG GAT GGT AAC ATG ACT TGC TGC AAG TTG GCC |
| BgGRN Domain4 + Full-Length Rev2 | GC CCT TGT TGC ATG TTC CGG |
| BgGRN Domains of Interest | Expected Molecular Weight |
|---|---|
| BgGRN Full length | 44 Kda |
| BgGRN D1, D3, D4 separately | 10 Kda |
| BgGRN D2 | 9.8 Kda |
| BgGRN D1D2 | 19.7 Kda |
| BgGRN D2D3 | 18.0 Kda |
| BgGRN D3D4 | 18.8 Kda |
| BgGRN D1D2D3 | 27.9 Kda |
| BgGRN D2D3D4 | 26.7 Kda |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hambrook, J.R.; Gharamah, A.A.; Pila, E.A.; Hussein, S.; Hanington, P.C. Biomphalaria glabrata Granulin Increases Resistance to Schistosoma mansoni Infection in Several Biomphalaria Species and Induces the Production of Reactive Oxygen Species by Haemocytes. Genes 2020, 11, 38. https://doi.org/10.3390/genes11010038
Hambrook JR, Gharamah AA, Pila EA, Hussein S, Hanington PC. Biomphalaria glabrata Granulin Increases Resistance to Schistosoma mansoni Infection in Several Biomphalaria Species and Induces the Production of Reactive Oxygen Species by Haemocytes. Genes. 2020; 11(1):38. https://doi.org/10.3390/genes11010038
Chicago/Turabian StyleHambrook, Jacob R., Abdullah A. Gharamah, Emmanuel A. Pila, Solomon Hussein, and Patrick C. Hanington. 2020. "Biomphalaria glabrata Granulin Increases Resistance to Schistosoma mansoni Infection in Several Biomphalaria Species and Induces the Production of Reactive Oxygen Species by Haemocytes" Genes 11, no. 1: 38. https://doi.org/10.3390/genes11010038
APA StyleHambrook, J. R., Gharamah, A. A., Pila, E. A., Hussein, S., & Hanington, P. C. (2020). Biomphalaria glabrata Granulin Increases Resistance to Schistosoma mansoni Infection in Several Biomphalaria Species and Induces the Production of Reactive Oxygen Species by Haemocytes. Genes, 11(1), 38. https://doi.org/10.3390/genes11010038