The Duplicated Y-specific amhy Gene Is Conserved and Linked to Maleness in Silversides of the Genus Odontesthes
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Species Identification
2.2. Phenotypic Sex Determination
2.3. DNA Extraction and Sex Genotyping by amhy Amplification
3. Results
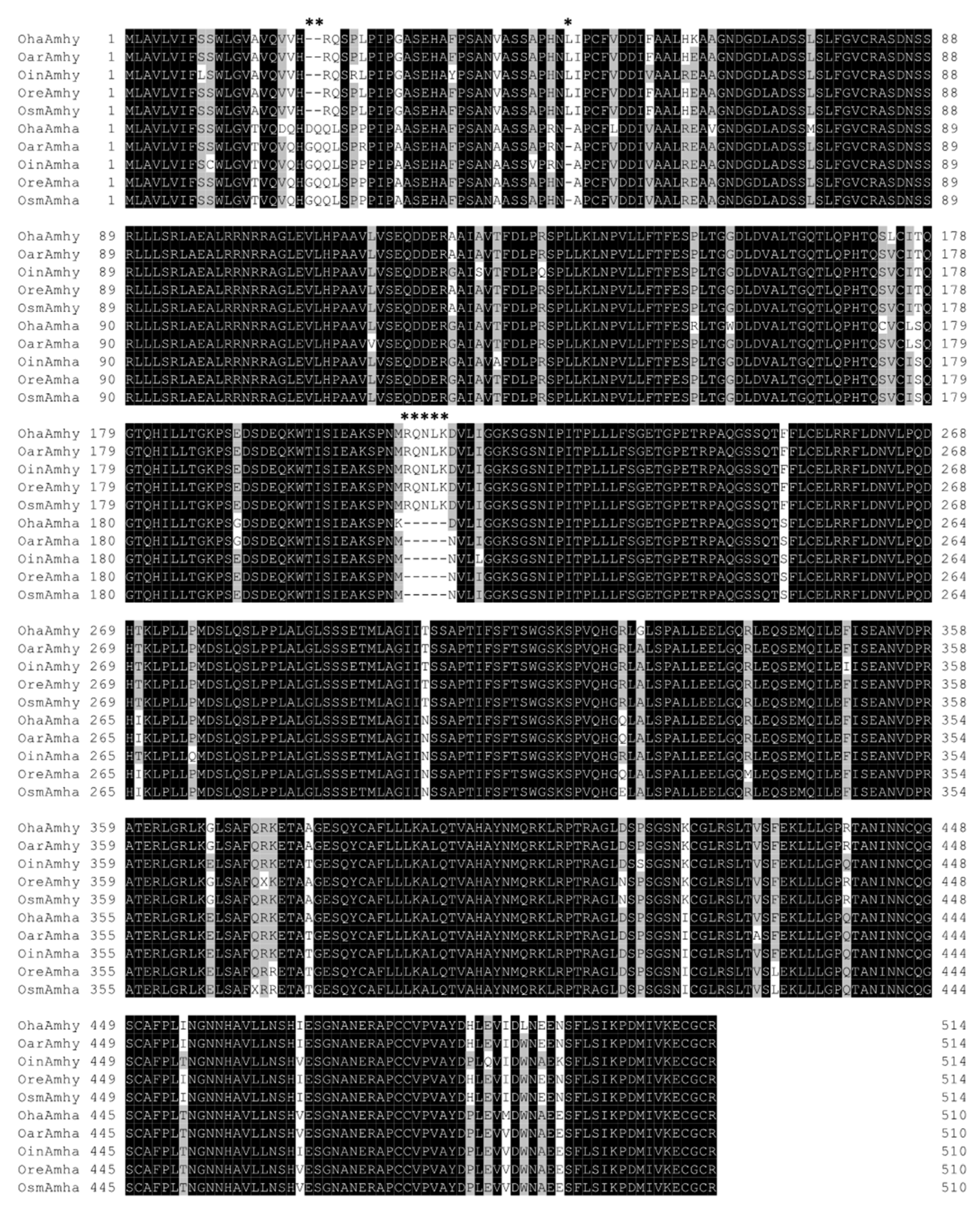
3.1. Amplification and Sequencing of amhy and amha Genes
3.2. Correlation Between the Presence of amhy and Phenotypic Sex
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hattori, R.S.; Strüssmann, C.A.; Fernandino, J.I.; Somoza, G.M. Genotypic sex determination in teleosts: Insights from the testis-determining amhy gene. Gen. Comp. Endocrinol. 2013, 192, 55–59. [Google Scholar] [CrossRef]
- Kikuchi, K.; Hamaguchi, S. Novel sex-determining genes in fish and sex chromosome evolution. Dev. Dyn. 2013, 242, 339–353. [Google Scholar] [CrossRef]
- Devlin, R.H.; Nagahama, Y. Sex determination and sex differentiation in fish. Aquaculture 2002, 208, 191–364. [Google Scholar] [CrossRef]
- Takehana, Y.; Matsuda, M.; Myosho, T.; Suster, M.L.; Kawakami, K.; Shin-I, T.; Kohara, Y.; Kuroki, Y.; Toyoda, A.; Fujiyama, A.; et al. Co-option of Sox3 as the male-determining factor on the y chromosome in the fish Oryzias dancena. Nat. Commun. 2014, 5, 4157. [Google Scholar] [CrossRef]
- Matsuda, M.; Nagahama, Y.; Shinomiya, A.; Sato, T. DMY is a Y-specific DM-domain gene required for male development in the medaka fish. Nature 2002, 399, 559–563. [Google Scholar] [CrossRef]
- Myosho, T.; Otake, H.; Masuyama, H.; Matsuda, M.; Kuroki, Y.; Fujiyama, A.; Naruse, K.; Hamaguchi, S.; Sakaizumi, M. Tracing the emergence of a novel sex-determining gene in medaka, Oryzias luzonensis. Genetics 2012, 191, 163–170. [Google Scholar] [CrossRef]
- Yano, A.; Guyomard, R.; Nicol, B.; Jouanno, E.; Quillet, E.; Klopp, C.; Cabau, C.; Bouchez, O.; Fostier, A.; Guiguen, Y. An immune-related gene evolved into the master sex-determining gene in rainbow trout, Oncorhynchus mykiss. Curr. Biol. 2012, 22, 1423–1428. [Google Scholar] [CrossRef]
- Yano, A.; Nicol, B.; Jouanno, E.; Quillet, E.; Fostier, A.; Guyomard, R.; Guiguen, Y. The sexually dimorphic on the Y-chromosome gene (sdY) is a conserved male-specific Y-chromosome sequence in many salmonids. Evol. Appl. 2013, 6, 486–496. [Google Scholar] [CrossRef]
- Pfennig, F.; Standke, A.; Gutzeit, H.O. The role of Amh signaling in teleost fish—Multiple functions not restricted to the gonads. Gen. Comp. Endocrinol. 2015, 223, 87–107. [Google Scholar] [CrossRef]
- Hattori, R.S.; Murai, Y.; Oura, M.; Masuda, S.; Majhi, S.K.; Sakamoto, T.; Fernandino, J.I.; Somoza, G.M.; Yokota, M.; Strussmann, C.A. A Y-linked anti-Mullerian hormone duplication takes over a critical role in sex determination. Proc. Natl. Acad. Sci. USA 2012, 109, 2955–2959. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Zhang, Y.; Sarida, M.; Hattori, R.S.; Strüssmann, C.A. Coexistence of genotypic and temperature-dependent sex determination in pejerrey Odontesthes bonariensis. PLoS ONE 2014, 9, e102574. [Google Scholar] [CrossRef]
- Bej, D.K.; Miyoshi, K.; Hattori, R.S.; Strüssmann, C.A.; Yamamoto, Y. A Duplicated, Truncated amh Gene Is Involved in Male Sex Determination in an Old World Silverside. G3 Genes Genomes Genet. 2017, 7, 2489–2495. [Google Scholar] [CrossRef]
- Li, M.; Sun, Y.; Zhao, J.; Shi, H.; Zeng, S.; Ye, K.; Jiang, D.; Zhou, L.; Sun, L.; Tao, W.; et al. A Tandem Duplicate of Anti-Müllerian Hormone with a Missense SNP on the Y Chromosome Is Essential for Male Sex Determination in Nile Tilapia, Oreochromis niloticus. PLoS Genet. 2015, 11, e1005678. [Google Scholar] [CrossRef]
- Pan, Q.; Feron, R.; Yano, A.; Guyomard, R.; Jouanno, E.; Vigouroux, E.; Wen, M.; Busnel, J.-M.; Bobe, J.; Concordet, J.-P.; et al. Identification of the master sex determining gene in Northern pike (Esox lucius) reveals restricted sex chromosome differentiation. PLoS Genet. 2019, 15, e1008013. [Google Scholar] [CrossRef]
- Kamiya, T.; Kai, W.; Tasumi, S.; Oka, A.; Matsunaga, T.; Mizuno, N.; Fujita, M.; Suetake, H.; Suzuki, S.; Hosoya, S.; et al. A trans-species missense SNP in Amhr2 is associated with sex determination in the tiger Pufferfish, Takifugu rubripes (Fugu). PLoS Genet. 2012, 8, e1002798. [Google Scholar] [CrossRef]
- Ieda, R.; Hosoya, S.; Tajima, S.; Atsumi, K.; Kamiya, T.; Nozawa, A.; Aoki, Y.; Tasumi, S.; Koyama, T.; Nakamura, O.; et al. Identification of the sex-determining locus in grass puffer (Takifugu niphobles) provides evidence for sex-chromosome turnover in a subset of Takifugu species. PLoS ONE 2018, 13, e0190635. [Google Scholar] [CrossRef]
- Hattori, R.S.; Tashiro, S.; Zhang, Y.; Kakuta, N.; Yokota, M.; Strüssmann, C.A.; Yamamoto, Y. Demonstration of viability and fertility and development of a molecular tool to identify YY supermales in a fish with both genotypic and environmental sex determination. Ecol. Evol. 2018, 8, 7522–7528. [Google Scholar] [CrossRef]
- Dyer, B.S. Systematic revision of the South American silversides (Teleostei, Atheriniformes). Biocell 2006, 30, 69–88. [Google Scholar]
- Muñoz, C.; Nirchio, M.; Pérez, J.E.; Ron, E.; Oliveira, C.; Ferreira, I.A. Cytogenetic characterization of the silverside fish Odontesthes regia (Humboldt, 1833) (Teleostei: Atheriniformes: Atherinopsidae) from Iquique, Chile. Rev. Biol. Mar. Oceanogr. 2006, 41, 57–62. [Google Scholar] [CrossRef]
- Sola, L.; Natili, G.L.; Cataudella, S. Cytogenetical characterization of Odontesthes bonariensis (Pisces, Atherinidae), an Argentine species introduced in Italy. Genetica 1988, 77, 217–224. [Google Scholar] [CrossRef]
- Campanella, D.; Hughes, L.C.; Unmack, P.J.; Bloom, D.D.; Piller, K.R.; Ortí, G. Multi-locus fossil-calibrated phylogeny of Atheriniformes (Teleostei, Ovalentaria). Mol. Phylogenet. Evol. 2015, 86, 8–23. [Google Scholar] [CrossRef]
- Fernandino, J.I.; Hattori, R.S.; Strüssmann, C.A.; Yamamoto, Y.; Somoza, G.M. Sex determination in fish: Odontesthes spp. (Atherinopsidae) as experimental models. Anim. Reprod. 2015, 12, 24–27. [Google Scholar]
- Yamamoto, Y.; Hattori, R.S.; Patiño, R.; Strüssmann, C.A. Environmental regulation of sex determination in fishes: Insights from Atheriniformes. Curr. Top. Dev. Biol. 2019, 134, 49–69. [Google Scholar]
- Mawaribuchi, S.; Yoshimoto, S.; Ohashi, S.; Takamatsu, N.; Ito, M. Molecular evolution of vertebrate sex-determining genes. Chromosome Res. 2012, 20, 139–151. [Google Scholar] [CrossRef]
- Conover, D.O.; Voorhees, D.A.V.; Ehtisham, A. Sex Ratio Selection and the Evolution of Environmental Sex Determination in Laboratory Populations of Menidia menidia. Evolution (N. Y.) 2006, 46, 1722–1730. [Google Scholar]
- Corona-Herrera, G.A.; Tello-Ballinas, J.A.; Hattori, R.S.; Martínez-Palacios, C.A.; Strüssmann, C.A.; Cárdenas-Reygadas, R.R.; Martínez-Chávez, C.C. Gonadal differentiation and temperature effects on sex determination in the freshwater pike silverside Chirostoma estor Jordan 1880. Environ. Biol. Fishes 2016, 99, 463–471. [Google Scholar] [CrossRef]
- Brown, E.E.; Baumann, H.; Conover, D.O. Temperature and photoperiod effects on sex determination in a fish. J. Exp. Mar. Bio. Ecol. 2014, 461, 39–43. [Google Scholar] [CrossRef]
- Strüssmann, C.A.; Patiño, R. Sex determination, Environmental. In Encyclopedia of Reproduction; Knobil, E., Neil, J.D., Eds.; Academic Press: San Diego, CA, USA, 1999; pp. 402–409. [Google Scholar]
- Peterson, T.C.; Stott, P.A.; Herring, S. Explaining Extreme Events of 2011 from a Climate Perspective. Bull. Am. Meteorol. Soc. 2012, 93, 1041–1067. [Google Scholar] [CrossRef]
- Gelca, R.; Hayhoe, K.; Scott-Fleming, I.; Crow, C.; Dawson, D.; Patiño, R. Climate-water quality relationships in Texas reservoirs. Hydrol. Process. 2016, 30, 12–29. [Google Scholar] [CrossRef]
- Strüssmann, C.A.; Conover, D.O.; Somoza, G.M.; Miranda, L.A. Implications of climate change for the reproductive capacity and survival of New World silversides (family Atherinopsidae). J. Fish Biol. 2010, 77, 1818–1834. [Google Scholar] [CrossRef]
- Duffy, T.A.; Mcelroy, A.E.; Conover, D.O. Variable susceptibility and response to estrogenic chemicals in Menidia Menidia. Mar. Ecol. Prog. Ser. 2009, 380, 245–254. [Google Scholar] [CrossRef]
- Fernandino, J.I.; Hattori, R.S. Sex determination in Neotropical fish: Implications ranging from aquaculture technology to ecological assessment. Gen. Comp. Endocrinol. 2019, 273, 172–183. [Google Scholar] [CrossRef]
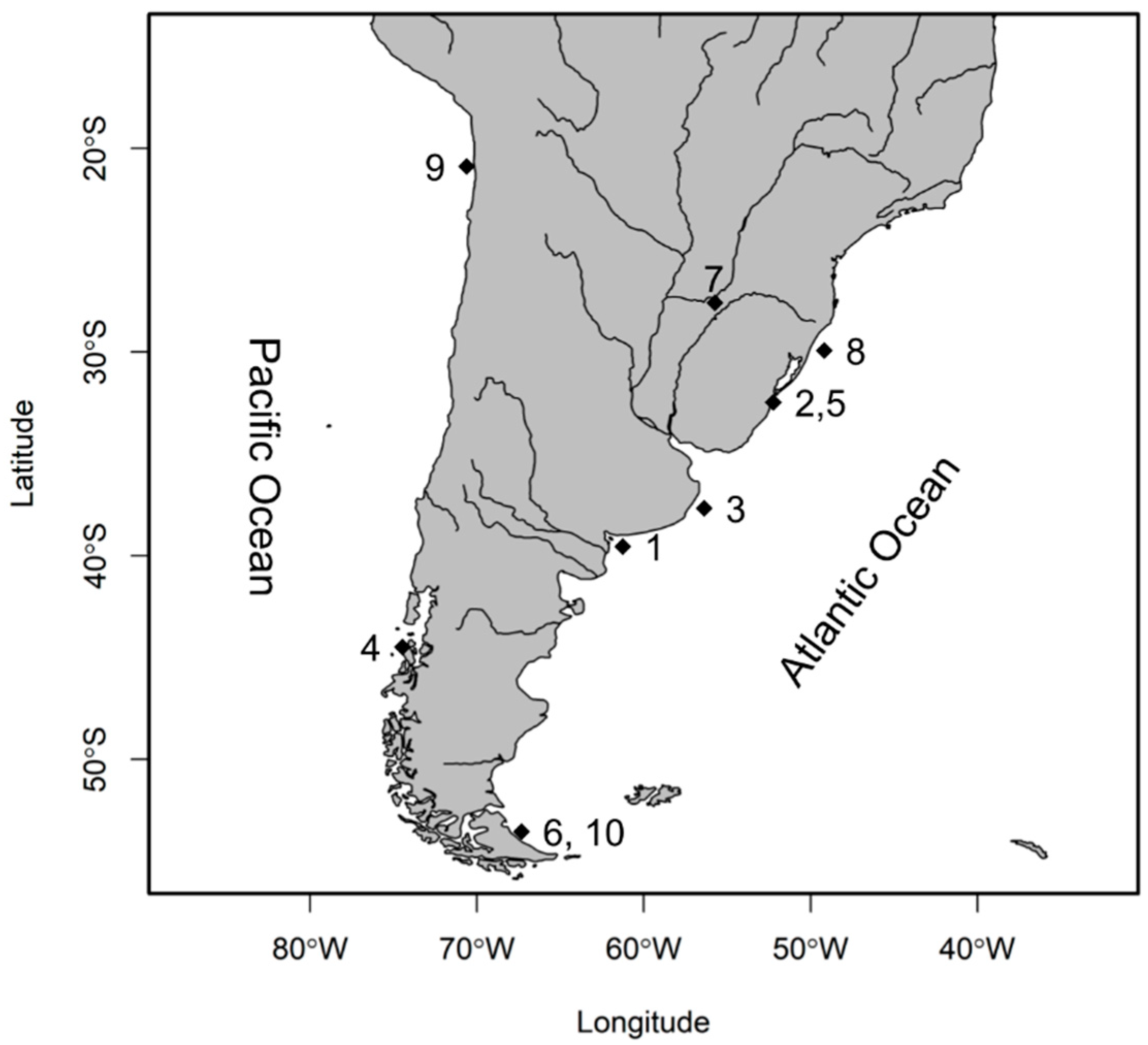

| Species | Sampling Locality/Origin | Environment | Number of Samples (F + M + ND) |
|---|---|---|---|
| O. argentinensis | Los Pocitos, San Blás (ARG) | Marine | 10 (6 + 4 + 0) |
| O. humensis | Mirin Lagoon, Taim (BRA) | Inland water | 20 (4 + 14 + 2) |
| O. incisa | Mar del Plata (ARG) | Marine | 35 (26 + 9+ 0) |
| O. mauleanum | Valdivia (CHI) | Estuarine | 24 (8 + 14 + 2) |
| O. mirinensis | Mirin Lagoon, Taim (BRA) | Inland water | 21 (7 + 14 + 0) |
| O. nigricans | Punta María (ARG) | Marine | 12 (7 + 5 + 0) |
| O. perugiae | Cambe Cué Lagoon, Corrientes (ARG) | Inland water | 16 (0 + 0 + 16) |
| Paraná River, Encarnación (PAR) | Inland water | 10 (3 + 7 + 0) | |
| O. piquava | Tramandaí Lagoon, Tramandaí (BRA) | Estuarine | 21 (13 + 7 + 1) |
| O. regia | Playa Blanca, Iquique (CHI) | Marine | 36 (31 + 5 + 0) |
| O. smitti | Mar del Plata (ARG) | Marine | 4 (2 + 2 + 0) |
| Punta María (ARG) | Marine | 12 (6 + 6 + 0) | |
| Total | 246 (114 + 110 + 22) |
| Species (Population) | amhy+ (XY/YY) | Sex Reversal (%) | amhy– (XX) | Sex Reversal (%) | ||
|---|---|---|---|---|---|---|
| F | M | F | M | |||
| O. argentinensis | 0 | 4 | 0 | 6 | 0 | 0 |
| O. humensis | 1 | 12 | 1 (7.7) | 2 | 1 | 1 (33.3) |
| O. incisa * | 0 | 6 | 0 | 21 | 0 | 0 |
| O. mauleanum | 0 | 3 | 0 | 5 | 0 | 0 |
| O. mirinensis | 3 | 9 | 3 (33.3) | 4 | 5 | 5 (55.6) |
| O. nigricans | 0 | 2 | 0 | 6 | 4 | 4 (40) |
| O. perugiae (Paraná River pop.) | 0 | 7 | 0 | 3 | 0 | 0 |
| O. piquava * | 0 | 2 | 0 | 13 | 0 | 0 |
| O. regia * | 0 | 5 | 0 | 31 | 0 | 0 |
| O. smitti (Mar del Plata pop.) | 0 | 2 | 0 | 2 | 0 | 0 |
| (Punta Maria pop.) | 0 | 6 | 0 | 6 | 0 | 0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hattori, R.S.; Somoza, G.M.; Fernandino, J.I.; Colautti, D.C.; Miyoshi, K.; Gong, Z.; Yamamoto, Y.; Strüssmann, C.A. The Duplicated Y-specific amhy Gene Is Conserved and Linked to Maleness in Silversides of the Genus Odontesthes. Genes 2019, 10, 679. https://doi.org/10.3390/genes10090679
Hattori RS, Somoza GM, Fernandino JI, Colautti DC, Miyoshi K, Gong Z, Yamamoto Y, Strüssmann CA. The Duplicated Y-specific amhy Gene Is Conserved and Linked to Maleness in Silversides of the Genus Odontesthes. Genes. 2019; 10(9):679. https://doi.org/10.3390/genes10090679
Chicago/Turabian StyleHattori, Ricardo S., Gustavo M. Somoza, Juan I. Fernandino, Dario C. Colautti, Kaho Miyoshi, Zhuang Gong, Yoji Yamamoto, and Carlos A. Strüssmann. 2019. "The Duplicated Y-specific amhy Gene Is Conserved and Linked to Maleness in Silversides of the Genus Odontesthes" Genes 10, no. 9: 679. https://doi.org/10.3390/genes10090679
APA StyleHattori, R. S., Somoza, G. M., Fernandino, J. I., Colautti, D. C., Miyoshi, K., Gong, Z., Yamamoto, Y., & Strüssmann, C. A. (2019). The Duplicated Y-specific amhy Gene Is Conserved and Linked to Maleness in Silversides of the Genus Odontesthes. Genes, 10(9), 679. https://doi.org/10.3390/genes10090679





