Natural Variation and Domestication Selection of ZmPGP1 Affects Plant Architecture and Yield-Related Traits in Maize
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and the Phenotypic Evaluation
2.2. DNA Isolation and ZmPGP1 Resequencing
2.3. Analysis of Sequence Data
2.4. Marker–Trait Association Analysis in Inbred Lines
3. Results
3.1. Sequence Polymorphisms of ZmPGP1
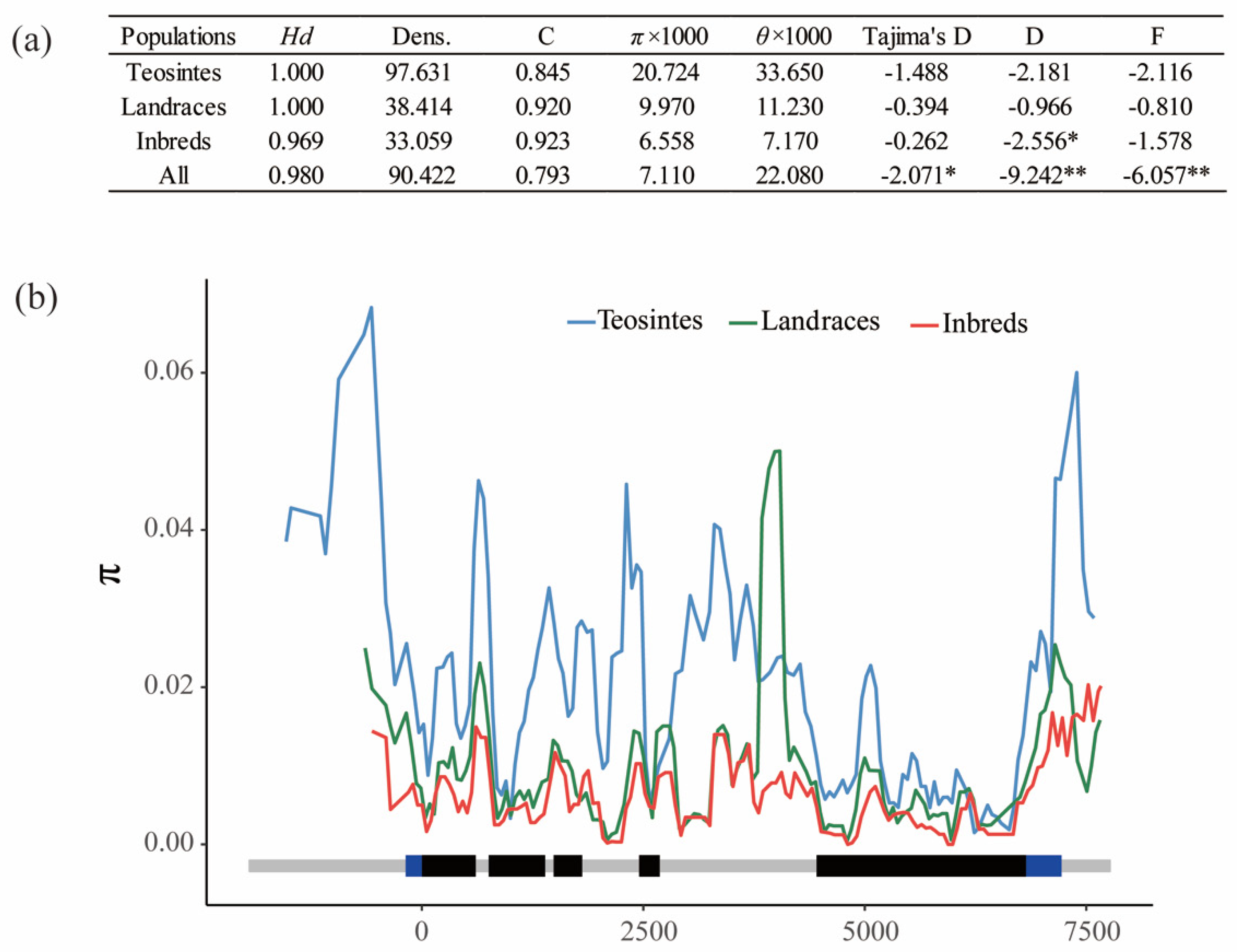
3.2. Nucleotide Diversity and Selection of ZmPGP1 in Inbred Lines, Landraces and Teosinte
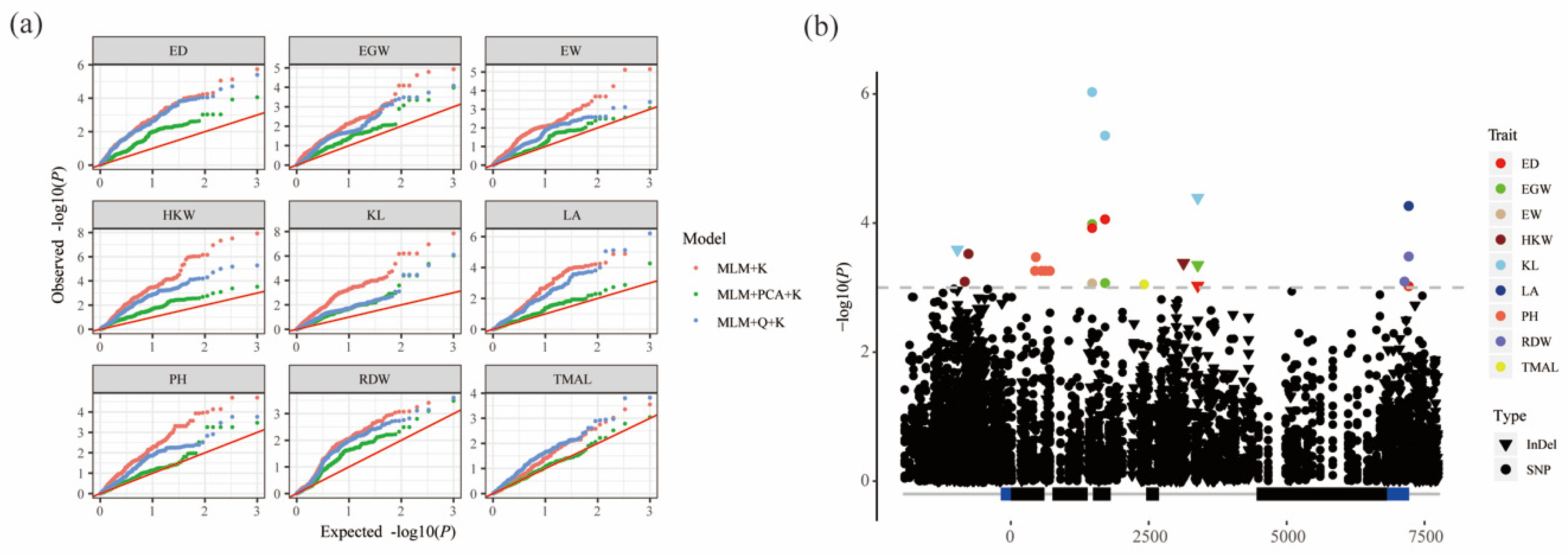
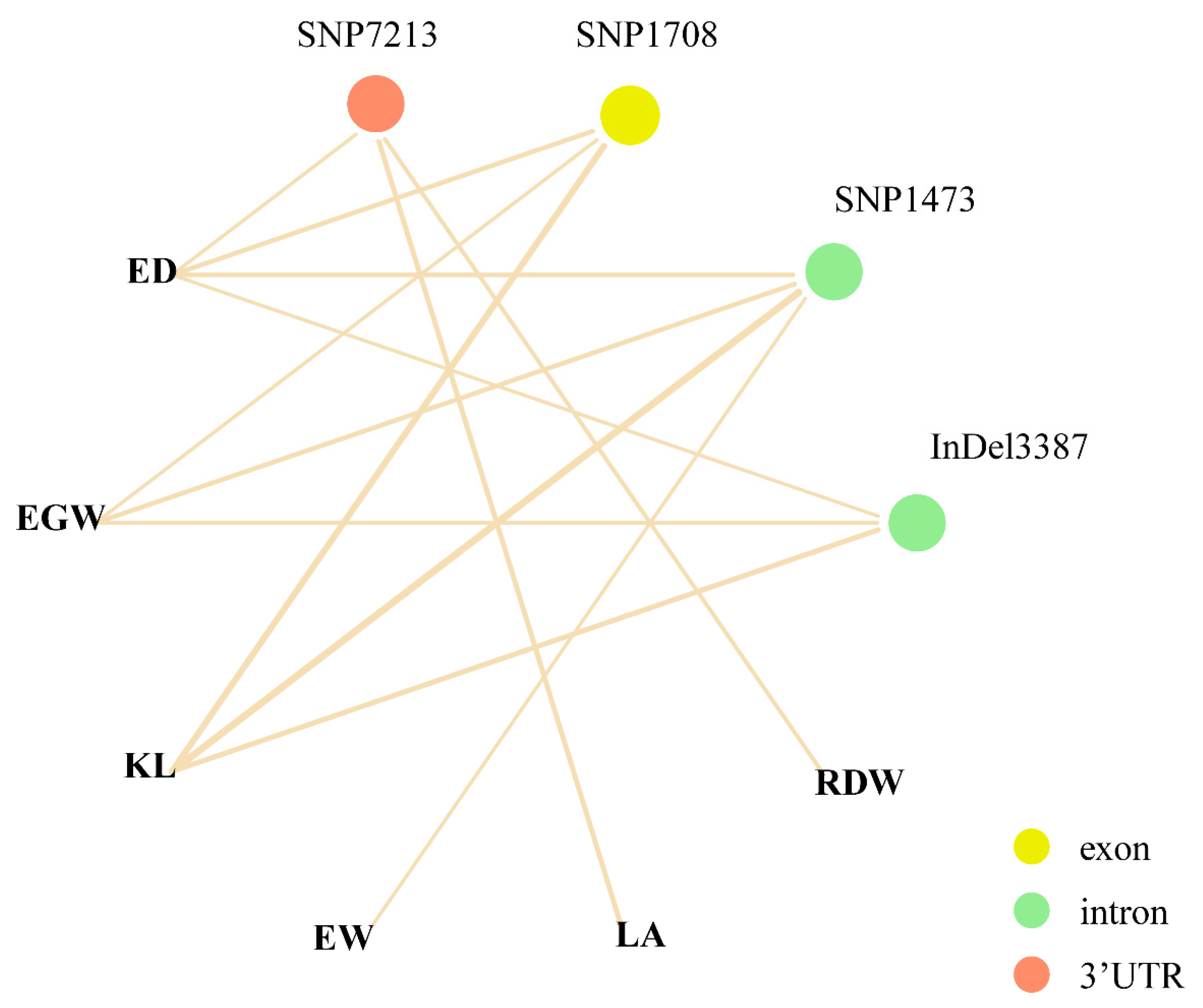
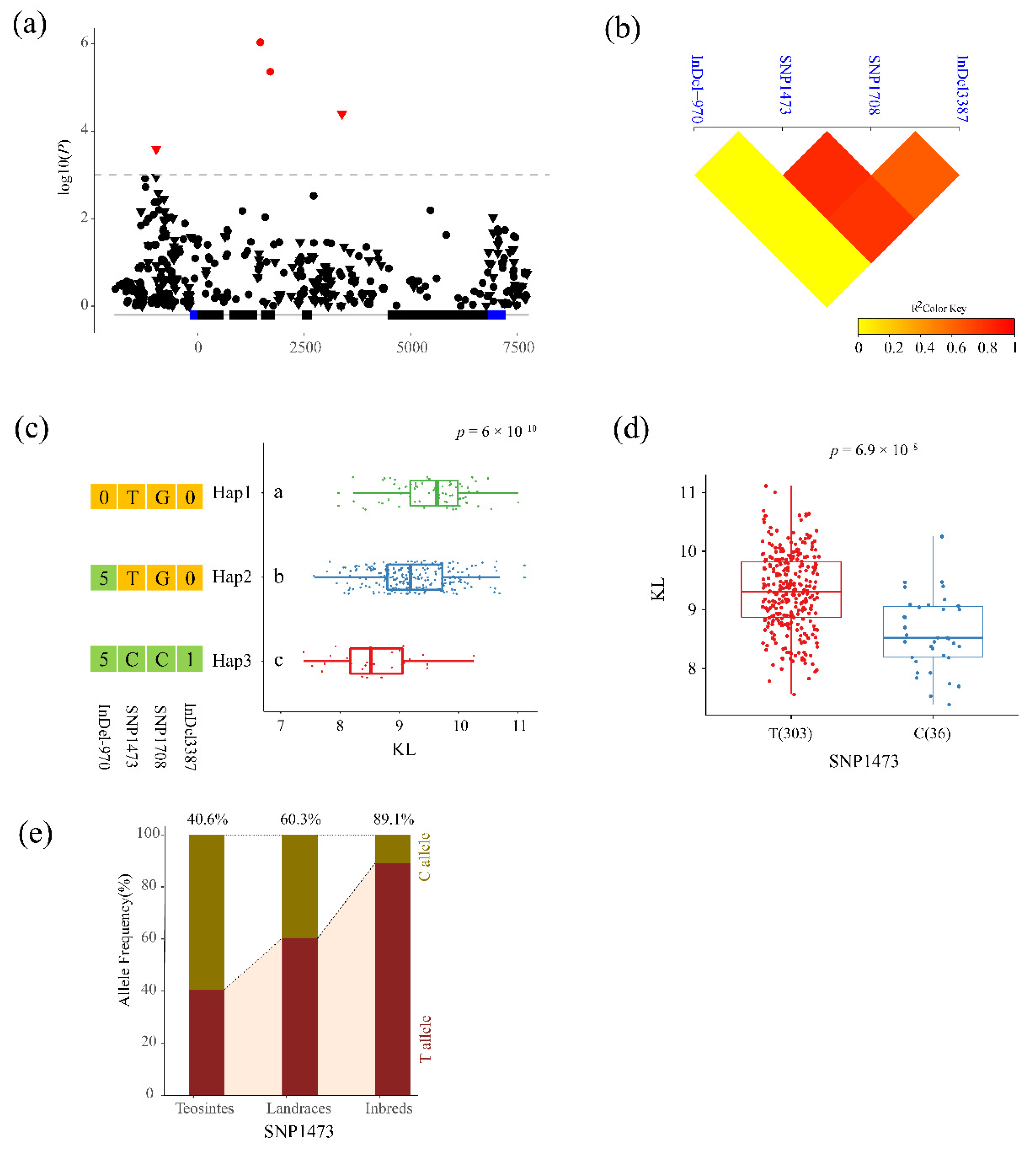
3.3. Association Analysis of Phenotypic Traits with ZmPGP1
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Matsuoka, Y.; Vigouroux, Y.; Goodman, M.M.; Sanchez, G.J.; Buckler, E.; Doebley, J. A single domestication for maize shown by multilocus microsatellite genotyping. Proc. Natl. Acad. Sci. USA 2002, 99, 6080–6084. [Google Scholar] [CrossRef]
- Fan, L.J.; Bao, J.D.; Wang, Y.; Yao, J.Q.; Gui, Y.J.; Hu, W.M.; Zhu, J.Q.; Zeng, M.Q.; Li, Y.; Xu, Y.B. Post-Domestication Selection in the Maize Starch Pathway. PLoS ONE 2009, 4, e7612. [Google Scholar] [CrossRef] [PubMed]
- Hufford, M.B.; Xu, X.; van Heerwaarden, J.; Pyhajarvi, T.; Chia, J.M.; Cartwright, R.A.; Elshire, R.J.; Glaubitz, J.C.; Guill, K.E.; Kaeppler, S.M.; et al. Comparative population genomics of maize domestication and improvement. Nat. Genet. 2012, 44, 808. [Google Scholar] [CrossRef]
- Haarhoff, S.J.; Swanepoel, P.A. Plant Population and Maize Grain Yield: A Global Systematic Review of Rainfed Trials. Crop. Sci. 2018, 58, 1819–1829. [Google Scholar] [CrossRef]
- Duvick, D.N. Genetic Progress in Yield of United States Maize (Zea mays L.). Maydica 2005, 50, 193–202. [Google Scholar]
- Messmer, R.; Fracheboud, Y.; Banziger, M.; Vargas, M.; Stamp, P.; Ribaut, J.M. Drought stress and tropical maize: QTL-by-environment interactions and stability of QTLs across environments for yield components and secondary traits. Theor. Appl. Genet. 2009, 119, 913–930. [Google Scholar] [CrossRef]
- Zhang, C.S.; Zhou, Z.Q.; Yong, H.J.; Zhang, X.C.; Hao, Z.F.; Zhang, F.J.; Li, M.S.; Zhang, D.G.; Li, X.H.; Wang, Z.H.; et al. Analysis of the genetic architecture of maize ear and grain morphological traits by combined linkage and association mapping. Theor. Appl. Genet. 2017, 130, 1011–1029. [Google Scholar] [PubMed]
- Duvick, D.N. The contribution of breeding to yield advances in maize (Zea mays L.). Adv. Agron. 2005, 86, 83–145. [Google Scholar]
- Xiao, Y.J.; Tong, H.; Yang, X.H.; Xu, S.Z.; Pan, Q.C.; Qiao, F.; Raihan, M.S.; Luo, Y.; Liu, H.J.; Zhang, X.H.; et al. Genome-wide dissection of the maize ear genetic architecture using multiple populations. New Phytol. 2016, 210, 1095–1106. [Google Scholar] [CrossRef]
- Pan, Q.C.; Xu, Y.C.; Li, K.; Peng, Y.; Zhan, W.; Li, W.Q.; Li, L.; Yan, J.B. The Genetic Basis of Plant Architecture in 10 Maize Recombinant Inbred Line Populations. Plant Physiol. 2017, 175, 858–873. [Google Scholar] [CrossRef]
- Martinez, A.K.; Soriano, J.M.; Tuberosa, R.; Koumproglou, R.; Jahrmann, T.; Salvi, S. Yield QTLome distribution correlates with gene density in maize. Plant Sci. 2016, 242, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Bommert, P.; Je, B.I.; Goldshmidt, A.; Jackson, D. The maize G α gene COMPACT PLANT2 functions in CLAVATA signalling to control shoot meristem size. Nature 2013, 502, 555. [Google Scholar] [CrossRef] [PubMed]
- Je, B.I.; Gruel, J.; Lee, Y.K.; Bommert, P.; Arevalo, E.D.; Eveland, A.L.; Wu, Q.Y.; Goldshmidt, A.; Meeley, R.; Bartlett, M.; et al. Signaling from maize organ primordia via FASCIATED EAR3 regulates stem cell proliferation and yield traits. Nat. Genet. 2016, 48, 785. [Google Scholar] [CrossRef]
- McSteen, P. Branching out: The ramosa pathway and the evolution of grass inflorescence morphology. Plant Cell 2006, 18, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Du, Y.F.; Shen, X.M.; Li, M.F.; Sun, W.; Huang, J.; Liu, Z.J.; Tao, Y.S.; Zheng, Y.L.; Yan, J.B.; et al. KRN4 Controls Quantitative Variation in Maize Kernel Row Number. PLoS Genet. 2015, 11, e1005670. [Google Scholar] [CrossRef]
- Multani, D.S.; Briggs, S.P.; Chamberlin, M.A.; Blakeslee, J.J.; Murphy, A.S.; Johal, G.S. Loss of an MDR transporter in compact stalks of maize br2 and sorghum dw3 mutants. Science 2003, 302, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Lawit, S.J.; Wych, H.M.; Xu, D.P.; Kundu, S.; Tomes, D.T. Maize DELLA Proteins dwarf plant8 and dwarf plant9 as Modulators of Plant Development. Plant Cell Physiol. 2010, 51, 1854–1868. [Google Scholar] [CrossRef]
- Bommert, P.; Lunde, C.; Nardmann, J.; Vollbrecht, E.; Running, M.; Jackson, D.; Hake, S.; Werr, W. thick tassel dwarf1 encodes a putative maize ortholog of the Arabidopsis CLAVATA1 leucine-rich repeat receptor-like kinase. Development 2005, 132, 1235–1245. [Google Scholar] [CrossRef] [PubMed]
- McSteen, P.; Hake, S. Barren inflorescence2 regulates axillary meristem development in the maize inflorescence. Development 2001, 128, 2881–2891. [Google Scholar]
- Gallavotti, A.; Zhao, Q.; Kyozuka, J.; Meeley, R.B.; Ritter, M.; Doebley, J.F.; Pe, M.E.; Schmidt, R.J. The role of barren stalk1 in the architecture of maize. Nature 2004, 432, 630–635. [Google Scholar] [CrossRef]
- Chuck, G.; Whipple, C.; Jackson, D.; Hake, S. The maize SBP-box transcription factor encoded by tasselsheath4 regulates bract development and the establishment of meristem boundaries. Development 2010, 137, 1243–1250. [Google Scholar] [CrossRef]
- Doebley, J.; Stec, A.; Hubbard, L. The evolution of apical dominance in maize. Nature 1997, 386, 485. [Google Scholar] [CrossRef]
- Wang, H.; Nussbaum-Wagler, T.; Li, B.L.; Zhao, Q.; Vigouroux, Y.; Faller, M.; Bomblies, K.; Lukens, L.; Doebley, J.F. The origin of the naked grains of maize. Nature 2005, 436, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Wills, D.M.; Whipple, C.J.; Takuno, S.; Kursel, L.E.; Shannon, L.M.; Ross-Ibarra, J.; Doebley, J.F. From Many, One: Genetic Control of Prolificacy during Maize Domestication. PLoS Genet. 2013, 9, e1003604. [Google Scholar] [CrossRef] [PubMed]
- Chang, G.; Roth, C.B. Structure of MsbA from E. coli: A homolog of the multidrug resistance ATP binding cassette (ABC) transporters. Science 2001, 293, 1793–1800. [Google Scholar] [CrossRef] [PubMed]
- Aller, S.G.; Yu, J.; Ward, A.; Weng, Y.; Chittaboina, S.; Zhuo, R.P.; Harrell, P.M.; Trinh, Y.T.; Zhang, Q.H.; Urbatsch, I.L.; et al. Structure of P-glycoprotein reveals a molecular basis for poly-specific drug binding. Science 2009, 323, 1718–1722. [Google Scholar] [CrossRef]
- Knoller, A.S.; Blakeslee, J.J.; Richards, E.L.; Peer, W.A.; Murphy, A.S. Brachytic2/ZmABCB1 functions in IAA export from intercalary meristems. J. Exp. Bot. 2010, 61, 3689–3696. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Zhang, X.; Zhang, Z.H.; Liu, H.H.; Lin, Z.W. A new allele of the Brachytic2 gene in maize can efficiently modify plant architecture. Heredity 2018, 121, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Balzan, S.; Carraro, N.; Salleres, B.; Dal Cortivo, C.; Tuinstra, M.R.; Johal, G.; Varotto, S. Genetic and phenotypic characterization of a novel brachytic2 allele of maize. Plant Growth Regul. 2018, 86, 81–92. [Google Scholar] [CrossRef]
- Pilu, R.; Cassani, E.; Villa, D.; Curiale, S.; Panzeri, D.; Cerino Badone, F.; Landoni, M. Isolation and characterization of a new mutant allele of brachytic 2 maize gene. Mol. Breeding 2007, 20, 83–91. [Google Scholar] [CrossRef]
- Zhang, M.L.; Lu, X.D.; Li, C.L.; Zhang, B.; Zhang, C.Y.; Zhang, X.S.; Ding, Z.J. Auxin efflux carrier ZmPGP1 mediates root growth inhibition under aluminum stress. Plant Physiol. 2018, 177, 819–832. [Google Scholar] [CrossRef] [PubMed]
- Li, P.C.; Pan, T.; Wang, H.M.; Wei, J.; Chen, M.J.; Hu, X.H.; Zhao, Y.; Yang, X.Y.; Yin, S.Y.; Xu, Y.; et al. Natural variation of ZmHKT1 affects root morphology in maize at the seedling stage. Planta 2019, 249, 879–889. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.; Scholl, U.I.; Ji, W.Z.; Liu, T.W.; Tikhonova, I.R.; Zumbo, P.; Nayir, A.; Bakkaloglu, A.; Ozen, S.; Sanjad, S.; et al. Genetic diagnosis by whole exome capture and massively parallel DNA sequencing. Proc. Natl. Acad. Sci. USA 2009, 106, 19096–19101. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Librado, P.; Rozas, J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 2009, 25, 1451–1452. [Google Scholar] [CrossRef] [PubMed]
- Tajima, F. Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 1989, 123, 585–595. [Google Scholar] [PubMed]
- Fu, Y.X.; Li, W.H. Statistical tests of neutrality of mutations. Genetics 1993, 133, 693–709. [Google Scholar]
- Yang, C.J.; Samayoa, L.F.; Bradbury, P.J.; Olukolu, B.A.; Xue, W.; York, A.M.; Tuholski, M.R.; Wang, W.D.; Daskalska, L.L.; Neumeyer, M.A.; et al. The genetic architecture of teosinte catalyzed and constrained maize domestication. Proc. Natl. Acad. Sci. USA 2019, 116, 5643–5652. [Google Scholar] [CrossRef]
- Swanson-Wagner, R.; Briskine, R.; Schaefer, R.; Hufford, M.B.; Ross-Ibarra, J.; Myers, C.L.; Tiffin, P.; Springer, N.M. Reshaping of the maize transcriptome by domestication. Proc. Natl. Acad. Sci. USA 2012, 109, 11878–11883. [Google Scholar] [CrossRef]
- Yamasaki, M.; Wright, S.I.; Mcmullen, M.D. Genomic screening for artificial selection during domestication and improvement in maize. Ann. Bot. 2007, 100, 967–973. [Google Scholar] [CrossRef]
- Wolters, H.; Jurgens, G. Survival of the flexible: Hormonal growth control and adaptation in plant development. Nat. Rev. Genet. 2009, 10, 305–317. [Google Scholar] [PubMed]
- Qi, J.J.; Liu, X.; Shen, D.; Miao, H.; Xie, H.Y.; Li, X.X.; Zeng, P.; Wang, S.H.; Shang, Y.; Gu, X.F.; et al. A genomic variation map provides insights into the genetic basis of cucumber domestication and diversity. Nat. Genet. 2013, 45, 1510. [Google Scholar]
- Zhou, Z.K.; Jiang, Y.; Wang, Z.; Gou, Z.H.; Lyu, J.; Li, W.Y.; Yu, Y.J.; Shu, L.P.; Zhao, Y.J.; Ma, Y.M.; et al. Resequencing 302 wild and cultivated accessions identifies genes related to domestication and improvement in soybean. Nat. Biotechnol. 2015, 33, 408. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Weber, A.L.; McMullen, M.D.; Guill, K.; Doebley, J. MADS-box genes of maize: Frequent targets of selection during domestication. Genet. Res. 2011, 93, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, M.; Tenaillon, M.I.; Bi, I.V.; Schroeder, S.G.; Villeda, H.; Doebley, J.; McMullen, M.D. A large-scale screen for artificial selection in maize identifies candidate agronomic loci for domestication and crop improvement. Plant Cell 2005, 17, 2859–2872. [Google Scholar] [CrossRef]
- Wang, H.W.; Li, K.; Hu, X.J.; Liu, Z.F.; Wu, Y.J.; Huang, C.L. Genome-wide association analysis of forage quality in maize mature stalk. BMC Plant Biol. 2016, 16, 227. [Google Scholar]
- Xing, A.Q.; Gao, Y.F.; Ye, L.F.; Zhang, W.P.; Cai, L.C.; Ching, A.; Llaca, V.; Johnson, B.; Liu, L.; Yang, X.H.; et al. A rare SNP mutation in Brachytic2 moderately reduces plant height and increases yield potential in maize. J. Exp. Bot. 2015, 66, 3791–3802. [Google Scholar] [CrossRef]
- Yan, J.B.; Warburton, M.; Crouch, J. Association Mapping for Enhancing Maize (Zea mays L.) Genetic Improvement. Crop Sci. 2011, 51, 433–449. [Google Scholar] [CrossRef]
- Yan, J.B.; Kandianis, C.B.; Harjes, C.E.; Bai, L.; Kim, E.H.; Yang, X.H.; Skinner, D.J.; Fu, Z.Y.; Mitchell, S.; Li, Q.; et al. Rare genetic variation at Zea mays crtRB1 increases β-carotene in maize grain. Nat. Genet. 2010, 42, 322. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Upstream | 5′UTR | Coding Region | 3′UTR | Downstream | Entire Region |
|---|---|---|---|---|---|---|
| Total length of amplicons (bp) | 1762 | 182 | 6821 | 400 | 545 | 9710 |
| Number of all of the sequence variants | 69 | 32 | 779 | 86 | 104 | 1070 |
| Frequency of all of the sequence variants | 0.039 | 0.176 | 0.114 | 0.215 | 0.191 | 0.110 |
| Number of nucleotide substitutions (bp) | 43 | 19 | 663 | 67 | 86 | 878 |
| Frequency of polymorphic sites per bp | 0.024 | 0.104 | 0.097 | 0.168 | 0.158 | 0.090 |
| Number of indels | 26 | 13 | 116 | 19 | 18 | 192 |
| Number of indels sites | 34 | 42 | 445 | 36 | 73 | 640 |
| Average indel length | 1.308 | 3.231 | 3.836 | 1.895 | 4.056 | 3.333 |
| Frequency of indels per bp | 0.015 | 0.071 | 0.017 | 0.048 | 0.033 | 0.020 |
| π × 1000 | 12.890 | 7.990 | 6.020 | 15.270 | 15.660 | 7.110 |
| θ × 1000 | 40.870 | 22.720 | 19.110 | 36.120 | 53.510 | 22.080 |
| Tajima’s D | −1.905 * | −1.620 | −2.089 * | −1.659 | −2.065 * | −2.071 * |
| Fu and Li’s D | −5.458 ** | −2.149 | −8.874 ** | −8.232 ** | −7.498 ** | −9.242 ** |
| Fu and Li’s F | −4.605 ** | −2.342 * | −5.917 ** | −6.037 ** | −5.699 ** | −6.057 ** |
| Trait | Marker | Allele | p Value | −lg (P) | R2 | Region | Position a |
|---|---|---|---|---|---|---|---|
| ED | SNP1473 | T/C | 1.20 × 10−4 | 3.92 | 4.74% | intron2 | 1473 |
| ED | SNP1708 | G/C | 8.75 × 10−5 | 4.06 | 4.93% | exon3 | 1708 |
| ED | InDel3387 | -/G | 9.34 × 10−4 | 3.03 | 3.49% | intron4 | 3387 |
| ED | SNP7213 | T/A | 9.52 × 10−4 | 3.02 | 3.47% | 3′UTR | 7213 |
| EGW | SNP1473 | T/C | 1.04 × 10−4 | 3.98 | 4.06% | intron2 | 1473 |
| EGW | SNP1708 | G/C | 8.53 × 10−4 | 3.07 | 2.98% | exon3 | 1708 |
| EGW | InDel3387 | -/G | 4.47 × 10−4 | 3.35 | 3.31% | intron4 | 3387 |
| EW | SNP1473 | T/C | 8.64 × 10−4 | 3.06 | 3.28% | intron2 | 1473 |
| HKW | SNP-769 | C/T | 3.01 × 10−4 | 3.52 | 4.12% | upstream | −769 |
| HKW | SNP-836 | C/A | 8.11 × 10−4 | 3.09 | 3.19% | upstream | −836 |
| HKW | InDel3129 | T/- | 4.17 × 10−4 | 3.38 | 3.42% | intron4 | 3129 |
| KL | InDel-970 | GACAG/----- | 2.58 × 10−4 | 3.59 | 3.78% | upstream | −970 |
| KL | SNP1473 | T/C | 9.34 × 10−7 | 6.03 | 6.91% | intron2 | 1473 |
| KL | SNP1708 | G/C | 4.42 × 10−6 | 5.36 | 6.03% | exon3 | 1708 |
| KL | InDel3387 | -/G | 4.06 × 10−5 | 4.39 | 4.79% | intron4 | 3387 |
| LA | SNP7213 | T/A | 5.44 × 10−5 | 4.26 | 3.94% | 3′UTR | 7213 |
| PH | SNP453 | C/G | 3.38 × 10−4 | 3.47 | 3.21% | exon1 | 453 |
| RDW | SNP7137 | C/G | 8.07 × 10−4 | 3.09 | 4.21% | 3′UTR | 7137 |
| RDW | SNP7213 | T/A | 3.30 × 10−4 | 3.48 | 4.85% | 3′UTR | 7213 |
| TMAL | SNP2414 | G/A | 8.94 × 10−4 | 3.05 | 4.36% | intron3 | 2414 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; Wei, J.; Wang, H.; Fang, Y.; Yin, S.; Xu, Y.; Liu, J.; Yang, Z.; Xu, C. Natural Variation and Domestication Selection of ZmPGP1 Affects Plant Architecture and Yield-Related Traits in Maize. Genes 2019, 10, 664. https://doi.org/10.3390/genes10090664
Li P, Wei J, Wang H, Fang Y, Yin S, Xu Y, Liu J, Yang Z, Xu C. Natural Variation and Domestication Selection of ZmPGP1 Affects Plant Architecture and Yield-Related Traits in Maize. Genes. 2019; 10(9):664. https://doi.org/10.3390/genes10090664
Chicago/Turabian StyleLi, Pengcheng, Jie Wei, Houmiao Wang, Yuan Fang, Shuangyi Yin, Yang Xu, Jun Liu, Zefeng Yang, and Chenwu Xu. 2019. "Natural Variation and Domestication Selection of ZmPGP1 Affects Plant Architecture and Yield-Related Traits in Maize" Genes 10, no. 9: 664. https://doi.org/10.3390/genes10090664
APA StyleLi, P., Wei, J., Wang, H., Fang, Y., Yin, S., Xu, Y., Liu, J., Yang, Z., & Xu, C. (2019). Natural Variation and Domestication Selection of ZmPGP1 Affects Plant Architecture and Yield-Related Traits in Maize. Genes, 10(9), 664. https://doi.org/10.3390/genes10090664




