Abstract
Field laboratories interested in using the MinION often need the internet to perform sample analysis. Thus, the lack of internet connectivity in resource-limited or remote locations renders downstream analysis problematic, resulting in a lack of sample identification in the field. Due to this dependency, field samples are generally transported back to the lab for analysis where internet availability for downstream analysis is available. These logistics problems and the time lost in sample characterization and identification, pose a significant problem for field scientists. To address this limitation, we have developed a stand-alone data analysis packet using open source tools developed by the Nanopore community that does not depend on internet availability. Like Oxford Nanopore Technologies’ (ONT) cloud-based What’s In My Pot (WIMP) software, we developed the offline MinION Detection Software (MINDS) based on the Centrifuge classification engine for rapid species identification. Several online bioinformatics applications have been developed surrounding ONT’s framework for analysis of long reads. We have developed and evaluated an offline real time classification application pipeline using open source tools developed by the Nanopore community that does not depend on internet availability. Our application has been tested on ATCC’s 20 strain even mix whole cell (ATCC MSA-2002) sample. Using the Rapid Sequencing Kit (SQK-RAD004), we were able to identify all 20 organisms at species level. The analysis was performed in 15 min using a Dell Precision 7720 laptop. Our offline downstream bioinformatics application provides a cost-effective option as well as quick turn-around time when analyzing samples in the field, thus enabling researchers to fully utilize ONT’s MinION portability, ease-of-use, and identification capability in remote locations.
1. Introduction
Field-deployable instruments are quickly demonstrating the transition in rapid point-of-care diagnostics and bio-surveillance allowing for reliable detection and accurate therapeutic countermeasures [1,2,3]. Several companies have developed deployable technologies for molecular diagnostics and biodefense that perform fast sample-to-answer analysis in the field [4,5,6]. Use of this equipment in emergency response situations, such as an outbreak exposure to endemic infectious diseases or the intentional use of bioweapons, allows for rapid turnaround time and definitive results, which are critical to the health and security of the people within the community. Unfortunately, most of these technologies are restricted to pre-set assay panels that could miss pathogens outside their target reach and do not generally identify organisms with antimicrobial resistance and enhanced virulence. While these instruments are proven and reliable, the user is confined to the targeted panels, primers, probes or antibodies that can be carried in the field with them. The data output is also limited to either a small PCR amplicon or protein target, providing a very narrow sliver of the whole genomic picture.
Metagenomics and whole genome sequencing are increasingly being used for diagnostic and clinical laboratories for the detection of pathogenic organisms [7,8,9]. These features enable the lab to conduct genomic characterization and phylogenic analysis, which is critical towards understanding evolutionary change, virulence and transmission during an outbreak. Oxford Nanopore Technologies (ONT) has recently developed sequencing technology that allows the user to sequence virtually anywhere in the world and low Earth orbit [10,11,12]. This small, portable device also enables affordable bio-surveillance on a global scale [13,14]. The MinION device has been field tested with successful sequencing of arbovirus, Ebola virus and Zika virus [6,15,16,17,18,19]. For example, in 2018, Nigeria experienced a record upsurge in cases of Lassa fever. A multinational team under the auspices of the World Health Organization (WHO) partnering with the Nigeria Center for Disease Control used metagenomic data generated by the MinION to determine the outbreak was due to independent zoonotic transmission events and not a viral strain with an increased transmission rate. The research group was able to rapidly deploy field labs and obtain epidemiological information critical to understanding the spread of the epidemic [20].
However, one of the biggest challenges still facing MinION sequencing in the field is offline software needed to analyze the raw data. Ideally, this offline software will have a simple to use graphical user interface (GUI) that allows users without a strong understanding of command line code and computer science experience to perform analysis and determine actionable results. Unfortunately, current ONT downstream bioinformatics and characterization often requires an internet connection and/or coding experience, which generates a bottleneck in real-time analysis to most individuals. Even with connectivity to an institutional laboratory, delay can mean death in critical situations.
One solution to this problem for next generation sequencing was the development of the Empowering the Development of Genomics Expertise (EDGE) including The Pan-Genomics for Infectious Agents (PanGIA) bioinformatics platform [21]. Sponsored by the Defense Threat Reduction Agency (DTRA), these platforms were designed to analyze Illumina short reads and were somewhat adapted for MinION long reads. In this paper, we demonstrate an offline downstream characterization pipeline specifically designed for MinION long reads. MINDS (MinION Detection Software) uses the open source read software Centrifuge for taxonomic classification purposes [22]. This real-time data streaming allows immediate analysis of the data, enabling rapid identification of bacteria, virus and fungi in a sample. Our MinION sequence analysis software provides offline real-time species identification and characterization on a standard laptop without the need for internet connectivity or high end computing power, thereby enabling true portability and validation of the samples in the field as well as in the lab.
2. Materials and Methods
2.1. Bacterial Sample
MSA-2002 was purchased from ATCC, Manassas, VA. The sample contains a mixture of 20 different bacterial strains distributed equally (5% ea.): Acinetobacter baumannii (ATCC 17978), Actinomyces odontolyticus (ATCC 17982), Bacillus cereus (ATCC 10987), Bacteroides vulgatus (ATCC 8482), Bifidobacterium adolescentis (ATCC 15703), Clostridium beijerinckii (ATCC 35702), Cutibacterium acnes (ATCC 11828), Deinococcus radiodurans (ATCC BAA-816), Enterococcus faecalis (ATCC 47077), Escherichia coli (ATCC 700926), Helicobacter pylori (ATCC 700392), Lactobacillus gasseri (ATCC 33323), Neisseria meningitidis (ATCC BAA-335), Porphyromonas gingivalis (ATCC 33277), Pseudomonas aeruginosa (ATCC 9027), Rhodobacter sphaeroides (ATCC 17029), Staphylococcus aureus (ATCC BAA-1556), Staphylococcus epidermidis (ATCC 12228), Streptococcus agalactiae (ATCC BAA-611), and Streptococcus mutans (ATCC 700610).
2.2. Metagenomic Sample Preparation
Working in a field-deployable laboratory requires the thorough vetting of equipment, processes and procedures prior to deployment due to the resource-limited environments normally encountered which would curtail sample preparation and analysis. The OmniLyse by Claremont BioSolutions provides several advantages when working in an austere environment. Effective lysis has been proven across a variety of cell types including gram-positive bacteria, spores, yeast, and cysts. Only 1–2 min is required to provide consistent yields of gDNA [23]. The small footprint of the OmniLyse and battery powered bead beating mechanism allows for easy use inside a glove box or biosafety cabinet when working with unknown, potentially high threat organisms. Fragmentation length can also be controlled based on the volume and lysis time of the sample. DNA cleanup was performed using Agencourt AMPure XP beads to provide high gDNA recovery and reduce the need for centrifugation. From start of extraction to final gDNA material, the total time is around 35 min. When using the Rapid Library Kit from Oxford Nanopore, the total time for library completion is one hour. Once loaded on the flow cell, the data collection can vary from a few thousand reads in an hour to over a hundred thousand reads in seven hours. Depending on the total number bases needed, the sample to answer using this method is from two to eight hours.
Genomic DNA was extracted and purified from MSA-2002 (ATCC, Manassas, VA, USA) using OmniLyse (Claremont BioSolutions, Upland, CA, USA) mechanical disrupter for 5 min in phosphate buffered saline. Agencourt AMPure XP (Beckman Coulter, Brea, CA, USA) cleanup was performed with following modifications. A 0.5 sample volume of 5M NaCl (Fisher Scientific, Hampton, NH, USA) with 0.5 sample volume of 30% PEG, 1.5M NaCl (Fisher Scientific, Hampton, NH, USA) was added to the lysed cells. Then 50 μL of resuspended AMPure XP beads were added and allowed to bind for 15 min. The beads were washed two times with fresh solution of 70% ethanol (Fisher Scientific, Hampton, NH, USA). After removal of the ethanol, 10 μL of nuclease-free water (VWR, Radnor, PA, USA) was added and incubated at 55 °C for 10 min to elute the gDNA from the beads [24]. Library preparation was performed with Rapid Sequencing Kit SQK-RAD004 (Oxford Nanopore Technologies, Oxford, UK) following manufactures protocol. 400 ng of template DNA was incubated with fragmentation mix at 30 °C for 1 min and at 80 °C for 1 min and cooled at 4 °C. The tagmented genomic DNA was mixed with the rapid adapter mix for five minutes at room temperature. The prepared DNA library was placed on ice until loaded on the flow cell. Platform QC was run on an R9.4.1 revD MinION flow cell (Oxford Nanopore Technologies, Oxford, UK) prior to each sequencing run.
2.3. Sample Sequencing and Bioinformatics Analysis
Reads acquisition (ONT’s MinKNOW core ver. 3.1.20 and base-calling (ONT’s Guppy software ver. 2.0.10) was integrated on ONT’s MinIT device (MinIT Release 19.01.10, 256 core GPU, 8 GB RAM, 512 GB SSD storage weighing 290 g) connected wirelessly to a Dell Precision 7720 laptop (Intel i7-7820HQ CPU, 4 core/8 thread, 2.9 GHz, 64 GB RAM with 3 TB SSD storage running Windows 10 Professional). The laptop was used primarily to run MINDS for downstream analysis (Figure 1). After 14 h of run time, FASTQ files were submitted to MINDS 1.0.53.
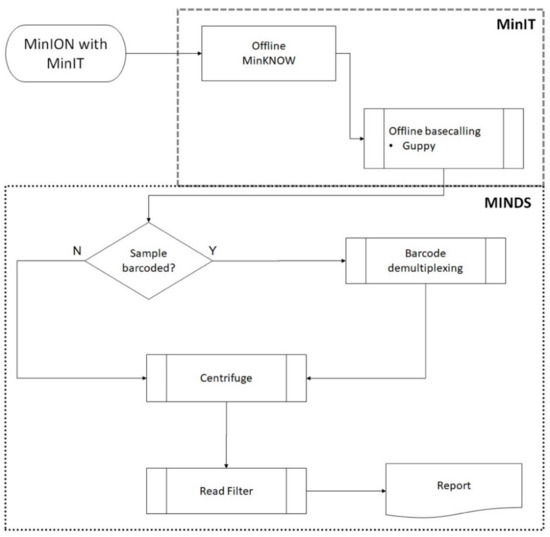
Figure 1.
Schematic demonstrating sequencing and bioinformatic workflow. Reads from the MinION were acquired with Oxford Nanopore Technologies’ (ONT’s) MinIT real-time base-calling device running MinKNOW and Guppy software. The downstream MINDS workflow processed the metagenomic sequence data read wirelessly from the MinIT to identify microorganisms present in the sample. Barcoded samples were first demultiplexed (if required) using ONT’s qcat software. Centrifuge (Johns Hopkins Center Computational Biology) mapped reads to taxonomic classifications. Background noise was filtered by removing species with only sporadic reads: having less than 0.1% of all total reads mapped and/or having less than 5 unique reads mapped.
MINDS is a user-friendly GUI written in Microsoft C# incorporating Python (version 3.6) scripts for file handling, processing, reporting and read mapping using Centrifuge 1.0.4 to perform taxonomic classification. Report graphics were generated using matplotlib and Seaborn [25,26,27]. The reads were searched against an indexed database of all RefSeq bacterial and archeal genome sequences downloaded periodically from Centrifuge developer’s website [28]. MINDS is available from the corresponding author by request.
Low level sequence data noise/background (near neighbors, and false positives and negatives) was filtered by removing sporadic mapped reads to species—those having less than 0.1% of all total reads mapped and/or less than 5 unique mapped reads.
MINDS performance was compared with ONT’s cloud-based EPI2ME What’s In My Pot (WIMP) workflow [29,30], a Centrifuge based system and also with the taxonomic sequence classifier Kraken [31]. A standard Kraken database of all complete bacterial, archaeal and viral genomes in NCBI’s Reference Sequence (RefSeq) database was built on 28 June 2019 using a 72 core, 512 Gb RAM server located at Rutgers University, New Brunswick, NJ. The MinION sequence data was analyzed by Kraken on the same server.
3. Results
Using ATCC MSA-2002 as a metagenomic mock community allowed us to test the MINDS pipeline with a variety of gram-positive and gram-negative bacteria. A 3 × 107 cells equal ratio, whole cell mix containing 20 bacterial strains was lysed for five minutes using OmniLyse. After cleanup, the nucleic acid concentration was set to 53 ng/µL and library preparation was performed using SQK-RAD004. The metagenomic mock community sample was run overnight for 14 h. In the first eight hours 170,000 reads were generated. An additional six hours of run time acquired only 3440 more reads due to the unavailable pores in the flow cell. Nanoplot [32] was used to obtain the statistical results of this run (Table 1). Over 390 million bases were called and the mean Q score of 9.6. The basecalled FASTQ files generated were then submitted to Centrifuge and Figure 2 shows the results generated after filtering. The overall analysis from sample submission to report took 15 min.

Table 1.
Nanoplot statistics for the MinIT base-calling output and post MinION Detection Software (MINDS) statistics for the Centrifuge analysis. The average multi-classified read mapped to 2.53 species (Centrifuge multi-classified reads count/Actual MinION reads that Centrifuge multi-classified).
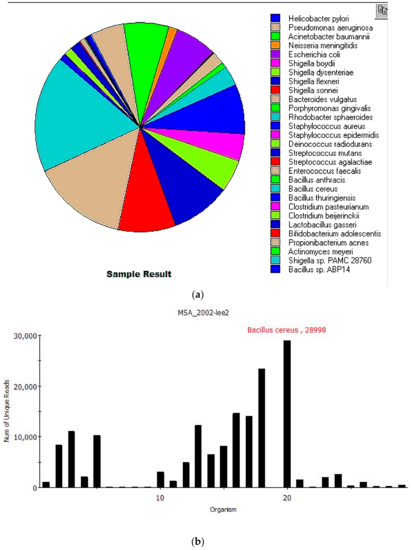
Figure 2.
MINDS report showing 19 of the 20 MSA-2002 mock species correctly identified from with an additional 10 near neighbor species identified. (a) The pie chart displays unique read abundance of each species. Actinomyces odontolyticus was not present in the Centrifuge database, so reads were assigned to closely related Actinomyces meyeri. MINDS also identified nine additional bacterial species closely related to three MSA-2002 species: five Shigella spp. from the E. coli/Shigella pan-genomic group, three Bacillus spp. from the pan-genomic Bacillus cereus group and Clostridium pasteurianum, a near-neighbor of Clostridium beijerinckii. (b) The MINDS taxonomy report displaying a read abundance histogram of the 29 species identified.
Nineteen of 20 species from the MSA-2002 mock community were correctly identified, with an additional 10 near-neighbor microorganisms also identified. Table 2 shows the Centrifuge results after filtering identifying 29 species in the sample listed by total and unique reads. Total reads are sequences classified to species level (including multi-classified reads). Unique reads are classified to a single species. Actinomyces odontolyticus was not identified because it was not present in the Centrifuge database, so reads were assigned to closely related Actinomyces meyeri. Interestingly, it was recently proposed that both species belong to Schaalia, a new genus in the Actinobacteria [33]. Comparing the genome sequences of Schaalia odontolytica (NCBI accession NZ_DS264586.1) and Schaalia meyeri (NCBI accession NZ_CP012072.1) results in a 79% sequence identity with a coverage of 74%. The low coverage is due to the genome size difference: 2.39 Mb for Schaalia odontolytica vs. 2.05 Mb for Schaalia meyeri.

Table 2.
Filtered Centrifuge results showing all 29 species identified in the MSA-2002 sample. Total reads are reads classified to species including multi-classified reads. Unique reads are those classified to a single species. The confidence score is the percentage of unique reads to total reads. The confidence grades are simply ranges of confidence scores: A = 90–100%, B = 80–90%, C = 70–80%, D = 60–70%, F = 0–60%. The last column shows the relative abundance of unique reads—the percentage of species specific unique reads to total unique reads. Except for the incorrectly identified Actinomyces meyeri, it can be seen that the other 19 MSA-2002 species had high unique reads to total reads ratios (confidence grades of B or better), while the incorrectly identified near-neighbors had low confidence grades.
MINDS also identified nine additional bacterial species closely related to three MSA-2002 species. Shigella dysenteriae, Shigella boydii, Shigella flexneri, Shigella sonnei and Shigella sp. PAMC 28760 are close relatives of MSA-2002’s E. coli and belong to a pan-genomic group [34,35]. Likewise, Bacillus thuringiensis, Bacillus anthracis and Bacillus sp. ABP14 are close relatives and belong to the Bacillus cereus group [36] Clostridium pasteurianum is a close relative of Clostridium beijerinckii and has been mistaken for it recently [37].
Offline Centrifuge was compared with other read mappers to benchmark its accuracy. Table 3 categorizes the read mapper results from highest number of MSA-2002 reads to the lowest. ONT’s “What’s In My Pot” (WIMP) cloud-based classification pipeline was used as a baseline since this module also uses Centrifuge for identification. The FASTQ files were also compared with Kraken for analysis. The three read mappers produced similar results, except the following: Kraken mapped far fewer reads to Streptococcus agalactiae, but correctly mapped 778 reads to the recently re-classified Schaalia odontolytica (formerly Actinomyces odontolyticus) [33].

Table 3.
Comparison of MINDS’ offline Centrifuge read mappers with ONT’s cloud-based Centrifuge (WIMP-What’s In My Pot) and Kraken read mappers. All three read mappers produced similar results except for Streptococcus agalactiae and Actinomyces meyeri as noted below.
Strain level identification is an important goal of taxonomic classification. For example, it would be important for a commander to know whether Bacillus anthracis Ames or Bacillus anthracis Sterne was used in an attack: the former is deadly, the latter is a vaccine strain [38]. Table 4 shows the percentage of reads mapped to strain by WIMP and Kraken. Few strains had >90% reads mapped to them. The top strain hit (highest number of strain reads mapped per species) was correct for WIMP in 13 of 20 cases and for Kraken in 9 of 20 cases.

Table 4.
Percentage of ATCC MSA-2002 reads mapped to strain by ONT’s cloud-based “What’s In My Pot” (WIMP) and offline Kraken taxonomic classifier. Strains are listed as they are referred to in NCBI’s RefSeq genome database. Reads and percentages in boldface had the highest number of strain reads mapped per species.
4. Discussion
As sequencing continues to move into the field, great effort is needed to ensure the user has the necessary equipment and software required for detection. The OmniLyse kit along with Solid Phase Reversible Immobilization (SPRI) clean up provides a small consumable footprint for DNA extraction, removing the need of centrifuge and spin columns used in traditional extraction kits. Small, portable thermocyclers also allow the library preparation with ONT’s Rapid Sequencing Kit (RAD004) performed with little space requirements. This rapid extraction and purification method does have a tradeoff as the quality of the DNA is considerably lower than the values recommended by ONT, thus affecting the throughput of DNA. However, the quantity obtained using 188 ng/μL afforded sufficient gDNA for sequencing and would allow for possible refueling of the flow cell to increase the amount of data generated.
Four repeated experiments were performed to increase the read output of the gram-positive Actinobacteria: Cutibacterium acnes, Bifidobacterium adolescentis, and Actinomyces meyeri (data not shown). However, these organisms were consistently underrepresented with respect to the total reads generated. Even with five minutes of OmniLyse cell disruption, no change in read distribution was observed. An extraction method with higher quality gDNA output for possible refueling the flow cell might be required if more genome coverage of these organisms is necessary.
The recent release of ONT’s GPU-based MinIT greatly reduces the computational burden on the portable laptop and also allows for real-time basecalling with the ability to perform 150 k bases per second verses a traditional CPU-based computer with an output of 20 k bases per second. The user also has the ease of “plug and play” feature of the MinIT and not have to worry about the laptop’s capability with the MinION. Using MinIT for real time basecalling allowed us to have the FASTQ files ready for downstream analysis as soon as the sample acquisition on MinION was stopped. Future efforts will focus on customizing the MINDS pipeline to classify reads in real-time as the FASTQ files are generated from the MinIT. This has the potential to reduce the run time and more rapidly determine results allowing for faster decision and countermeasures in the case of a biothreat detection.
Software in the field not only has to work offline, it also needs intuitive interface features that allows the end user unfamiliar with command line code the ability to quickly analyze data. The MINDS application provides easy to use graphical interface that minimizes the need for command line expertise (Figure 3). The end user simply submits the folder holding FAST5/FASTQ files along with other prevalent information such as flow cell ID, MinION serial number, etc. Once all the relevant information is submitted, the analysis can be performed by clicking the “Start” button. Based on the workflow selection, data analysis is performed, and a taxonomy report is generated.
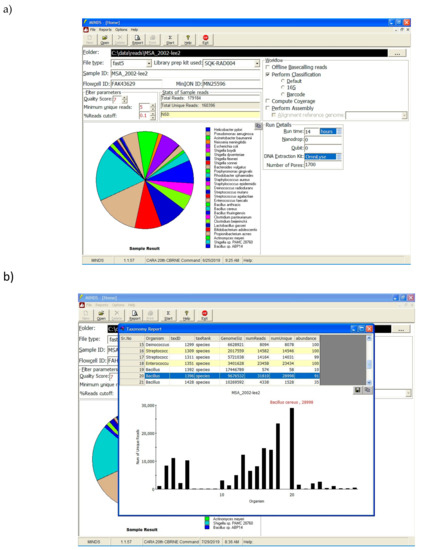
Figure 3.
(a) MINDS graphical interface allows users to straightforwardly submit the FASTQ file, input experimental metadata and conditions, generates intuitive data analysis and (b) easily interpretable reports.
The MINDS pipeline has proven its successful adaptability during ONT’s release of new software and products. Since MINDS acts like a wrapper for the latest tools developed by the ONT community, it can be easily modified to accommodate the new software and kits released by ONT. For example, Guppy replaced Albacore and MINDS seamlessly integrated the new base-caller. With changes to the Centrifuge code, MINDS was able to analyze ONT’s change from single FAST5/FASTQ to multi FAST5/FASTQ. Lastly, as demultiplexing tools evolved through the past few years, MINDS implemented various open source software changes with no change to the GUI. The rapidly changing software development for processing MinION data requires regular patching or updating the command line code that interfaces with a stable GUI, which has proven easily accomplished with MINDS over the past few years.
Centrifuge was chosen for its mapping utility on a laptop: it has a relatively small indexed database size and RAM requirement. For example, our indexed Kraken database was 227 Gb compared to the 25 Gb Centrifuge database, and RAM requirement for Centrifuge were ~4 Gb: approximately 20× less than Kraken’s. As shown, there is little discernable difference in the performance of the two read mappers. As more useful read mappers are developed, they can be easily substituted and incorporated into the MINDS interface.
5. Conclusions
MINDS software was developed for users without a scientific education or laboratory background, such as enlisted soldiers, sailors, airmen and marines. Real-world metagenomic data can be difficult to analyze and interpret, especially for a user that is unfamiliar with bioinformatic tools. In contrast, MINDS allows any user to run sample data and receive quick, actionable results with a clear interpretation. For example, MINDS generates easy to understand bar graphs and pie charts, while providing the raw read information in a very intuitive graphical form. In this report, we have demonstrated an unbiased fieldable detection capability using ONT’s MinION sequencing platform and the MINDS platform. Our system dramatically reduces the time frame needed to detect targets as well as providing a sequencing in the field capability which minimizes the burden of overseas shipping of samples back to a lab such as the Centers for Disease Control or U.S. Army Medical Research Institute for Infectious Disease. Furthermore, the intuitive GUI of MINDS allows any user to quickly perform classification on their reads generated from MinIT. Additionally, simple parameter selection allows the user to provide percent cutoff to remove background noise to minimize false-positives and false-negatives which can interfere with the identification and decision-making processes.
Several open-source software tools for classification were tested for field applications. Centrifuge performed faster than the other tools tested on the same computational hardware and did not require a large computational memory burden due to its database indexing capabilities. A small footprint database is a decisive feature for field forward computation. The offline Centrifuge classified all 20 organisms very similarly to the cloud-based Centrifuge through WIMP and also with Kraken. However, neither Centrifuge nor Kraken could convincingly classify the taxa to strain.
Future efforts will include having MINDS run data streaming from the MinION and MinIT in real time, enhancing MINDS capabilities to provide faster interpretation of the results. Also, further development in the sample preparation workflow is needed as library preparation still requires several hands-on steps with various pieces of laboratory equipment and consumables to operate in a field-forward environment. Efforts have begun to minimize this laboratory equipment burden to allow sequencing anywhere by anyone. These include future products developed by Oxford Nanopore including VolTRAX and Ubiq. Strain level identification is an important goal we hope to achieve by first assembling the reads into larger contigs before classification. Assembled contigs will provide a much smaller number, yet much longer sequences to map against the database and should provide more information rich strain determining features than the individual unassembled read sequences alone.
Author Contributions
S.V.D. designed the concept and developed the software, T.M.R. generated the samples and ran the samples on MinION. S.V.D. and T.M.R. did the validation and verification of the pipeline. R.F.S. and L.J.K. contributed to the scientific discussion, implementation of the pipeline, and edited the manuscript, L.J.K. assisted in developing the extraction method. S.V.D. and T.M.R. contributed equally in preparing this manuscript, K.B. and M.M.W. supervised the work and contributed to the manuscript.
Funding
This research was funded by US ARMY 20th CBRNE and Joint Program Executive Office for Chemical, Biological, Radiological and Nuclear Defense (JPEO-CBRND).
Acknowledgments
Authors would like to thank Rebecca Lewandowski of US Army 20th CBRNE for her feedback and using the application in the field. Andrew Murphy and Brady Redmond, JPEO-CBRND, provided steadfast program management support. Rob Muldowney graciously provided access to servers at the School of Environment and Biological Sciences/New Jersey Agricultural Experiment Station, Rutgers-The State University of New Jersey.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Primiceri, E.; Chiriacò, M.S.; Notarangelo, F.M.; Crocamo, A.; Ardissino, D.; Cereda, M.; Bramanti, A.P.; Bianchessi, M.A.; Giannelli, G.; Maruccio, G. Key enabling technologies for point-of-care diagnostics. Sensors (Basel) 2018, 18, 3607. [Google Scholar] [CrossRef] [PubMed]
- Vashist, S.K. Point-of-care diagnostics: Recent advances and trends. Biosensors (Basel) 2017, 7, 62. [Google Scholar] [CrossRef] [PubMed]
- Zarei, M. Advances in point-of-care technologies for molecular diagnostics. Biosens. Bioelectron. 2017, 98, 494–506. [Google Scholar] [CrossRef] [PubMed]
- Gay-Andrieu, F.; Magassouba, N.; Picot, V.; Phillips, C.L.; Peyrefitte, C.N.; Dacosta, B.; Doré, A.; Kourouma, F.; Ligeon-Ligeonnet, V.; Gauby, C.; et al. Clinical evaluation of the BioFire FilmArray. J. Clin. Virol. 2017, 92, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Llibre, A.; Shimakawa, Y.; Duffy, D. Potential utility of the Genedrive point-of-care test for HCV RNA detection. Gut 2018. [Google Scholar] [CrossRef]
- Russell, J.A.; Campos, B.; Stone, J.; Blosser, E.M.; Burkett-Cadena, N.; Jacobs, J.L. Unbiased strain-typing of Arbovirus directly from mosquitoes using Nanopore sequencing: A field-forward biosurveillance protocol. Sci. Rep. 2018, 8, 5417. [Google Scholar] [CrossRef] [PubMed]
- Afshinnekoo, E.; Chou, C.; Alexander, N.; Ahsanuddin, S.; Schuetz, A.N.; Mason, C.E. Precision metagenomics: Rapid metagenomic analyses for infectious disease diagnostics and public health surveillance. J. Biomol. Tech. 2017, 28, 40–45. [Google Scholar] [CrossRef]
- Gu, W.; Miller, S.; Chiu, C.Y. Clinical metagenomic next-generation sequencing for pathogen detection. Annu. Rev. Pathol. 2019, 14, 319–338. [Google Scholar] [CrossRef]
- Schlaberg, R.; Chiu, C.Y.; Miller, S.; Procop, G.W.; Weinstock, G. Validation of metagenomic next-generation sequencing tests for universal pathogen detection. Arch. Pathol. Lab. Med. 2017, 141, 776–786. [Google Scholar] [CrossRef]
- Castro-Wallace, S.L.; Chiu, C.Y.; John, K.K.; Stahl, S.E.; Rubins, K.H.; McIntyre, A.B.R.; Dworkin, J.P.; Lupisella, M.L.; Smith, D.J.; Botkin, D.J.; et al. Nanopore DNA sequencing and genome assembly on the International Space Station. Sci. Rep. 2017, 7, 18022. [Google Scholar] [CrossRef]
- Goordial, J.; Altshuler, I.; Hindson, K.; Chan-Yam, K.; Marcolefas, E.; Whyte, L.G. In situ field sequencing and life detection in remote (79°26′N) Canadian high arctic permafrost ice wedge microbial communities. Front. Microbiol. 2017, 8, 2594. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.S.; Zaikova, E.; Goerlitz, D.S.; Bai, Y.; Tighe, S.W. Real-time DNA sequencing in the Antarctic Dry Valleys using the Oxford Nanopore sequencer. J. Biomol. Tech. 2017, 28, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Brindha, J.; Kaushik, C.; Balamurali, M.M. Biosensors for pathogen surveillance. Environ. Chem. Lett. 2018, 16, 1325–1337. [Google Scholar]
- Leggett, R.M.; Clark, M.D. A world of opportunities with Nanopore sequencing. J. Exp. Bot. 2017, 68, 5419–5429. [Google Scholar] [CrossRef] [PubMed]
- Grubaugh, N.D.; Gangavarapu, K.; Quick, J.; Matteson, N.L.; De Jesus, J.G.; Main, B.J.; Tan, A.L.; Paul, L.M.; Brackney, D.E.; Grewal, S.; et al. An amplicon-based sequencing framework for accurately measuring intrahost virus diversity using PrimalSeq and iVar. Genome Biol. 2019, 20, 8. [Google Scholar] [CrossRef] [PubMed]
- Hoenen, T.; Groseth, A.; Rosenke, K.; Fischer, R.J.; Hoenen, A.; Judson, S.D.; Martellaro, C.; Falzarano, D.; Marzi, A.; Squires, R.B.; et al. Nanopore sequencing as a rapidly deployable Ebola outbreak tool. Emerg. Infect. Dis. 2016, 22, 331–334. [Google Scholar] [CrossRef] [PubMed]
- Mbala-Kingebeni, P.; Villabona-Arenas, C.J.; Vidal, N.; Likofata, J.; Nsio-Mbeta, J.; Makiala-Mandanda, S.; Mukadi, D.; Mukadi, P.; Kumakamba, C.; Djokolo, B.; et al. Rapid confirmation of the Zaire Ebola virus in the outbreak of the Equateur province in the Democratic Republic of Congo: Implications for public health interventions. Clin. Infect. Dis. 2019, 68, 330–333. [Google Scholar] [CrossRef]
- Quick, J.; Loman, N.J.; Duraffour, S.; Simpson, J.T.; Severi, E.; Cowley, L.; Bore, J.A.; Koundouno, R.; Dudas, G.; Mikhail, A.; et al. Real-time, portable genome sequencing for Ebola surveillance. Nature 2016, 530, 228–232. [Google Scholar] [CrossRef]
- Quick, J.; Grubaugh, N.D.; Pullan, S.T.; Claro, I.M.; Smith, A.D.; Gangavarapu, K.; Oliveira, G.; Robles-Sikisaka, R.; Rogers, T.F.; Beutler, N.A.; et al. Multiplex PCR method for MinION and Illumina sequencing of Zika and other virus genomes directly from clinical samples. Nat. Protoc. 2017, 12, 1261–1276. [Google Scholar] [CrossRef]
- Kafetzopoulou, L.E.; Pullan, S.T.; Lemey, P.; Suchard, M.A.; Ehichioya, D.U.; Pahlmann, M.; Thielebein, A.; Hinzmann, J.; Oestereich, L.; Wozniak, D.M.; et al. Metagenomic sequencing at the epicenter of the Nigeria 2018 Lassa fever outbreak. Science 2019, 363, 74–77. [Google Scholar] [CrossRef]
- Li, P.E.; Lo, C.C.; Anderson, J.J.; Davenport, K.W.; Bishop-Lilly, K.A.; Xu, Y.; Ahmed, S.; Feng, S.; Mokashi, V.P.; Chain, P.S. Enabling the democratization of the genomics revolution with a fully integrated web-based bioinformatics platform. Nucleic Acids Res. 2017, 45, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Song, L.; Breitwieser, F.P.; Salzberg, S.L. Centrifuge: Rapid and sensitive classification of metagenomic sequences. Genome Res. 2016, 26, 1721–1729. [Google Scholar] [CrossRef] [PubMed]
- Irwin, P.; Nguyen, L.; He, Y.; Paoli, G.; Gehring, A.; Chen, C.Y. The near-quantitative sampling of genomic DNA from various food-borne Eubacteria. BMC Microbiol. 2014, 14, 326. [Google Scholar] [CrossRef] [PubMed]
- Kerkhof, L.J.; Dillon, K.P.; Häggblom, M.M.; McGuinness, L.R. Profiling bacterial communities by MinION sequencing of ribosomal operons. Microbiome 2017, 5, 116. [Google Scholar] [CrossRef] [PubMed]
- Hunter, J.D. Matplotlib: A 2D graphics environment. Comput. Sci. Eng. 2007, 9, 90. [Google Scholar] [CrossRef]
- Matplotlib: A Python 2D Plotting Library Which Produces Publication Quality Figures in a Variety of Hardcopy Formats and Interactive Environments Across Platforms. Available online: https://matplotlib.org (accessed on 23 July 2019).
- Seaborn: A Python Data Visualization Library Based on Matplotlib. Available online: https://seaborn.pydata.org (accessed on 23 July 2019).
- Centrifuge: Classifier for Metagenomic Sequences. Available online: https://ccb.jhu.edu/software/centrifuge/manual.shtml#building-index-on-all-complete-bacterial-and-viral-genomes (accessed on 12 June 2019).
- Juul, S.; Izquierdo, F.; Hurst, A.; Dai, X.; Wright, A.; Kulesha, E.; Pettett, R.; Turner, D.J. What’s in my pot? Real-time species identification on the MinION. bioRxiv 2015, 030742. [Google Scholar] [CrossRef]
- The EPI2ME Platform a Cloud-Based Data Analysis Service for Oxford Nanopore Technologies’ Sequence Information. Available online: https://epi2me.nanoporetech.com/ (accessed on 11 June 2019).
- Wood, D.E.; Salzberg, S.L. Kraken: Ultrafast metagenomic sequence classification using exact alignments. Genome. Biol. 2014, 15, R46. [Google Scholar] [CrossRef]
- De Coster, W.; D’Hert, S.; Schultz, D.T.; Cruts, M.; Van Broeckhoven, C. NanoPack: Visualizing and processing long-read sequencing data. Bioinformatics 2018, 34, 2666–2669. [Google Scholar] [CrossRef]
- Nouioui, I.; Carro, L.; García-López, M.; Meier-Kolthoff, J.P.; Woyke, T.; Kyrpides, N.C.; Pukall, R.; Klenk, H.P.; Goodfellow, M.; Göker, M. Genome-based taxonomic classification of the phylum Actinobacteria. Front. Microbiol. 2018, 9, 2007. [Google Scholar] [CrossRef]
- Gordienko, E.N.; Kazanov, M.D.; Gelfand, M.S. Evolution of pan-genomes of Escherichia coli, Shigella spp., and Salmonella enterica. J. Bacteriol. 2013, 195, 2786–2792. [Google Scholar] [CrossRef]
- Sims, G.E.; Kim, S.H. Whole-genome phylogeny of Escherichia coli/Shigella group by feature frequency profiles (FFPs). Proc. Natl. Acad. Sci. USA 2011, 108, 8329–8334. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lai, Q.; Göker, M.; Meier-Kolthoff, J.P.; Wang, M.; Sun, Y.; Wang, L.; Shao, Z. Genomic insights into the taxonomic status of the Bacillus cereus group. Sci. Rep. 2015, 5, 14082. [Google Scholar] [CrossRef] [PubMed]
- Sedlar, K.; Kolek, J.; Provaznik, I.; Patakova, P. Reclassification of non-type strain Clostridium pasteurianum NRRL B-598 as Clostridium beijerinckii NRRL B-598. J. Biotechnol. 2017, 244, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Keim, P.; Smith, K.L.; Keys, C.; Takahashi, H.; Kurata, T.; Kaufmann, A. Molecular investigation of the Aum Shinrikyo anthrax release in Kameido, Japan. J. Clin. Microbiol. 2001, 39, 4566–4567. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).