Analysis of Hematological Traits in Polled Yak by Genome-Wide Association Studies Using Individual SNPs and Haplotypes
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Blood Sample Collection
2.2. SNP Array Genotyping and Quality Control
2.3. Statistical Analyses
2.4. Single-Marker GWAS
2.5. Haplotype Analysis
3. Results
3.1. SNP Characteristics and Phenotype Statistics
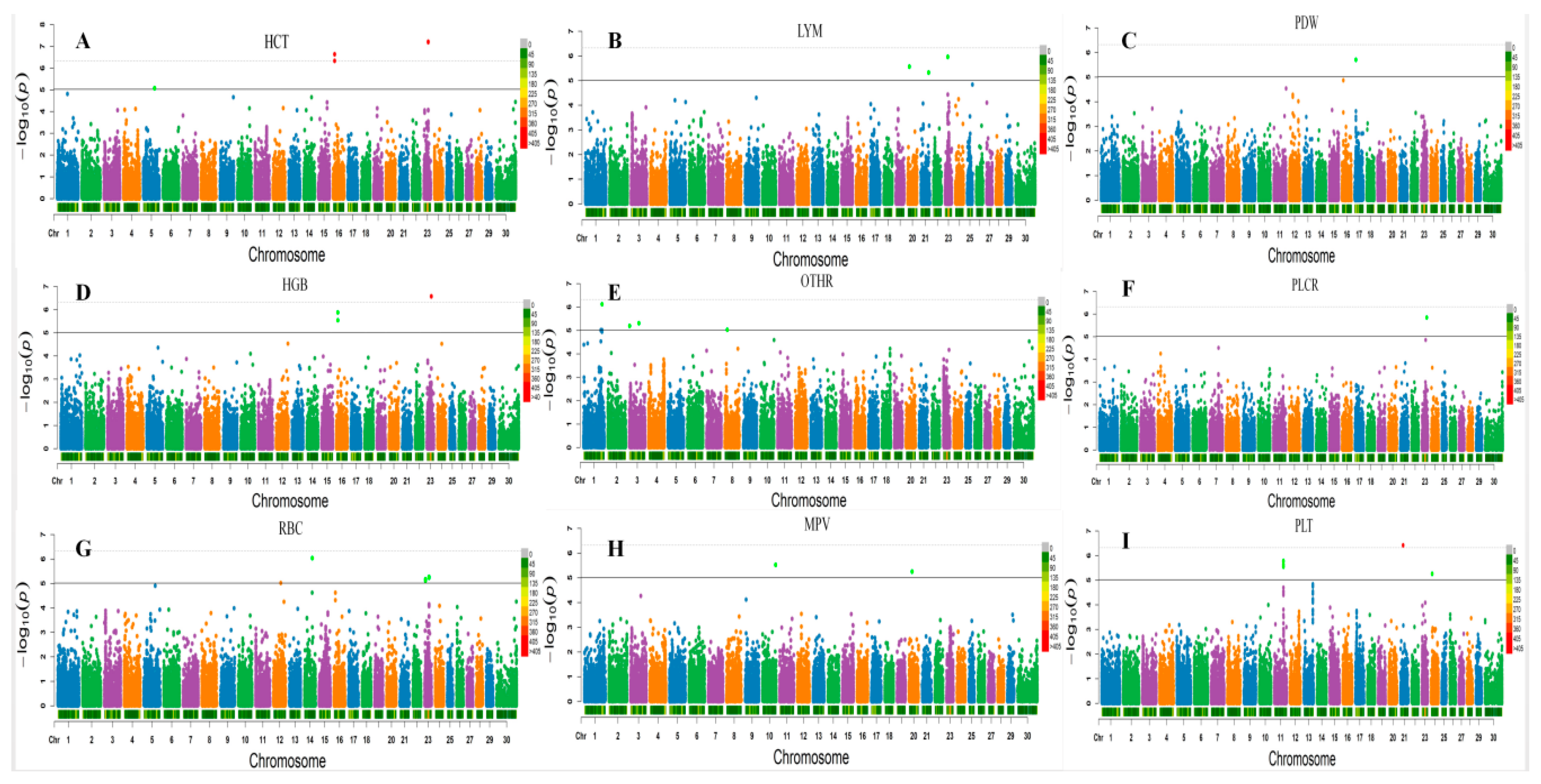
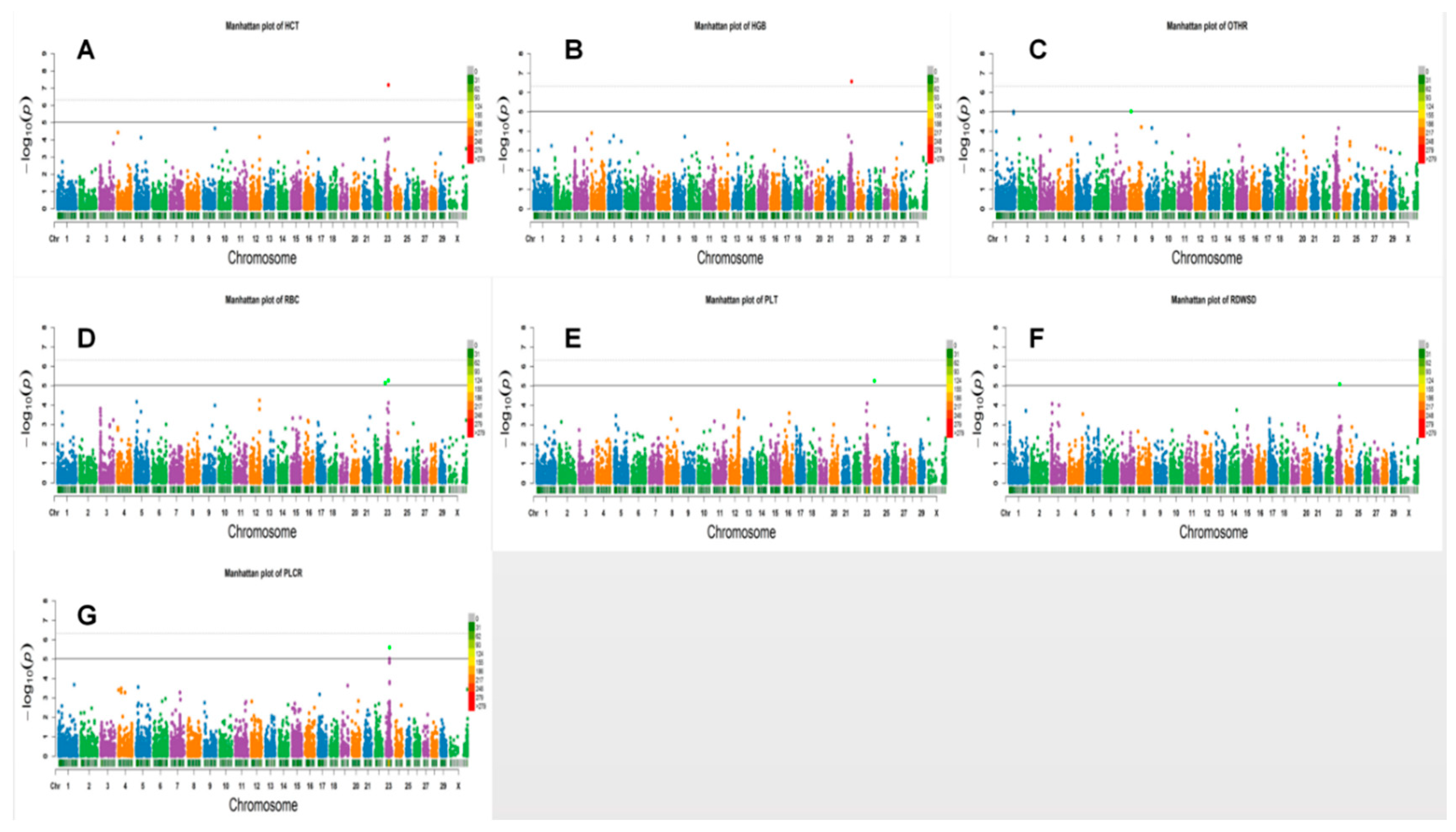
3.2. Loci for Erythrocyte Traits
3.3. White Blood Cell Counts
3.4. Platelet Traits
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Qiu, Q.; Zhang, G.; Ma, T.; Qian, W.; Wang, J.; Ye, Z.; Cao, C.; Hu, Q.; Kim, J.; Larkin, D.M. The yak genome and adaptation to life at high altitude. Nat. Genet. 2012, 44, 946. [Google Scholar] [CrossRef]
- Mizuno, S.; Ishizaki, T.; Toga, H.; Sakai, A.; Isakova, J.; Taalaibekova, E.; Baiserkeev, Z.; Kojonazarov, B.; Aldashev, A. Endogenous Asymmetric Dimethylarginine Pathway in High Altitude Adapted Yaks. Biomed. Res. Int. 2015, 2015, 196904. [Google Scholar] [CrossRef]
- Wang, Z.; Shen, X.; Liu, B.; Su, J.; Yonezawa, T.; Yu, Y.; Guo, S.; Ho, S.Y.; Vila, C.; Hasegawa, M. Phylogeographical analyses of domestic and wild yaks based on mitochondrial DNA: New data and reappraisal. J. Biogeogr. 2010, 37, 2332–2344. [Google Scholar] [CrossRef]
- Guo, S.; Savolainen, P.; Su, J.; Qian, Z.; Qi, D.; Jie, Z.; Yang, Z.; Zhao, X.; Liu, J. Origin of mitochondrial DNA diversity of domestic yaks. BMC Evol. Biol. 2006, 6, 73. [Google Scholar] [CrossRef]
- Wang, L.L.; Yu, Q.L.; Han, L.; Ma, X.L.; Song, R.D.; Zhao, S.N.; Zhang, W.H. Study on the effect of reactive oxygen species-mediated oxidative stress on the activation of mitochondrial apoptosis and the tenderness of yak meat. Food Chem. 2018, 244, 394. [Google Scholar] [CrossRef]
- Zi, X.D. Reproduction in female yaks (Bos grunniens) and opportunities for improvement. Theriogenology 2003, 59, 1303–1312. [Google Scholar] [CrossRef]
- Recavarren, S.; Arias-Stella, J. Right Ventricular Hypertrophy in People Born and Living at High Altitudes. Br. Heart J. 1964, 26, 806. [Google Scholar] [CrossRef]
- Alexander, A.F.; Jensen, R. Gross cardiac changes in cattle with high mountain (brisket) disease and in experimental cattle maintained at high altitudes. Am. J. Vet. Res. 1959, 20, 680–689. [Google Scholar]
- Greenough, P.R. Breeding for Disease Resistance in Farm Animals. Can. Vet. J. 2010, 33, 763. [Google Scholar] [CrossRef]
- Müller, M.; Brem, G. Disease resistance in farm animals. Experientia 1991, 47, 923. [Google Scholar] [CrossRef]
- Hoffman, R.; Benz Jr, E.J.; Silberstein, L.E.; Heslop, H.; Anastasi, J.; Weitz, J. Hematology: Basic Principles and Practice; Elsevier Health Sciences: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Okada, Y.; Kamatani, Y. Common genetic factors for hematological traits in Humans. J. Hum. Genet. 2012, 57, 161–169. [Google Scholar] [CrossRef]
- Evans, D.M.; Frazer, I.H.; Martin, N.G. Genetic and environmental causes of variation in basal levels of blood cells. Twin Res. 1999, 2, 250–257. [Google Scholar] [CrossRef]
- Fisch, I.R.; Freedman, S.H. Smoking, oral contraceptives, and obesity: Effects on white blood cell count. JAMA 1975, 234, 500–506. [Google Scholar] [CrossRef]
- Hall, M.A.; Ahmadi, K.R.; Norman, P.; Snieder, H.; MacGregor, A.J.; Vaughan, R.W.; Spector, T.D.; Lanchbury, J.S. Genetic influence on peripheral blood T lymphocyte levels. Genes Immun. 2000, 1, 423. [Google Scholar] [CrossRef]
- Whitfield, J.B.; Martin, N.G. Genetic and environmental influences on the size and number of cells in the blood. Genet. Epidemiol. 2010, 2. [Google Scholar] [CrossRef]
- Kamatani, Y.; Matsuda, K.; Okada, Y.; Kubo, M.; Hosono, N.; Daigo, Y.; Nakamura, Y.; Kamatani, N. Genome-wide association study of hematological and biochemical traits in a Japanese population. Nat. Genet. 2010, 42, 210–215. [Google Scholar] [CrossRef]
- Ganesh, S.K.; Zakai, N.A.; Van Rooij, F.J.; Soranzo, N.; Smith, A.V.; Nalls, M.A.; Chen, M.H.; Kottgen, A.; Glazer, N.L.; Dehghan, A.; et al. Multiple loci influence erythrocyte phenotypes in the CHARGE Consortium. Nat. Genet. 2009, 41, 1191–1198. [Google Scholar] [CrossRef]
- Jin, L.; Glessner, J.T.; Haitao, Z.; Cuiping, H.; Zhi, W.; Bradfield, J.P.; Mentch, F.D.; Yiran, G.; Cecilia, K.; Qianghua, X. GWAS of blood cell traits identifies novel associated loci and epistatic interactions in Caucasian and African-American children. Hum. Mol. Genet. 2013, 22, 1457–1464. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Liu, Z.H.; Wang, L.; Xiao, H.M.; Du, C.G.; Zhang, Y.J.; Su, R.; Li, J.Q. Expression of the RORα gene in Inner Mongolian cashmere goat hair follicles. Genet. Mol. Res. 2015, 14, 380–388. [Google Scholar] [CrossRef]
- Soranzo, N.; Rendon, A.; Gieger, C.; Jones, C.; Watkins, N.; Menzel, S.; Doring, A.; Stephens, J.; Prokisch, H.; Erber, W.; et al. A novel variant on chromosome 7q22.3 associated with mean platelet volume, counts, and function. Blood 2009, 113, 3831. [Google Scholar] [CrossRef]
- Nalls, M.A.; Wilson, J.G.; Patterson, N.J.; Tandon, A.; Zmuda, J.M.; Huntsman, S.; Garcia, M.; Hu, D.; Li, R.; Beamer, B.A.; et al. Admixture mapping of white cell count: Genetic locus responsible for lower white blood cell count in the Health ABC and Jackson Heart studies. Am. J. Hum. Genet. 2008, 82, 81–87. [Google Scholar] [CrossRef]
- Meisinger, C.; Prokisch, H.; Gieger, C.; Soranzo, N.; Mehta, D.; Rosskopf, D.; Lichtner, P.; Klopp, N.; Stephens, J.; Watkins, N.A. A Genome-wide Association Study Identifies Three Loci Associated with Mean Platelet Volume. Am. J. Hum. Genet. 2009, 84, 66–71. [Google Scholar] [CrossRef]
- Yoichiro, K.; Sukanya, W.; Hidenori, O.; Takahisa, K.; Atsushi, T.; Naoya, H.; Michiaki, K.; Tatsuhiko, T.; Naoyuki, K.; Hiromitsu, K. A genome-wide association study identifies variants in the HLA-DP locus associated with chronic hepatitis B in Asians. Nat. Genet. 2009, 41, 591–595. [Google Scholar] [CrossRef]
- Soranzo, N.; Spector, T.D.; Mangino, M.; Kühnel, B.; Rendon, A.; Teumer, A.; Willenborg, C.; Wright, B.; Chen, L.; Li, M.; et al. A genome-wide meta-analysis identifies 22 loci associated with eight hematological parameters in the HaemGen consortium. Nat. Genet. 2009, 41, 1182–1190. [Google Scholar] [CrossRef]
- Hu, Z.; Fritz-Waters, E.; Reecy, J. AnimalQTLdb: A livestock QTL database tool set for positional QTL information mining and beyond. Nucleic Acids Res. 2007, 35, 604–609. [Google Scholar] [CrossRef]
- Yang, S.; Ren, J.; Yan, X.; Huang, X.; Zou, Z.; Zhang, Z.; Yang, B.; Huang, L. Quantitative trait loci for porcine white blood cells and platelet-related traits in a White Duroc×Erhualian F2 resource population. Anim. Genet. 2009, 40, 273–278. [Google Scholar] [CrossRef]
- Zou, Z.; Ren, J.; Yan, X.; Huang, X.; Yang, S.; Zhang, Z.; Yang, B.; Li, W.; Huang, L. Quantitative trait loci for porcine baseline erythroid traits at three growth ages in a White Duroc × Erhualian F 2 resource population. Mamm. Genome 2008, 19, 640–646. [Google Scholar] [CrossRef]
- Wang, J.Y.; Luo, Y.R.; Fu, W.X.; Lu, X.; Zhou, J.P.; Ding, X.D.; Liu, J.F.; Zhang, Q. Genome-wide association studies for hematological traits in swine. Anim. Genet. 2013, 44, 34–43. [Google Scholar] [CrossRef]
- Orrù, L.; Abeni, F.; Catillo, G.; Grandoni, F.; Crisà, A.; De Matteis, G.; Scatà, M.; Napolitano, F.; Moioli, B. Leptin gene haplotypes are associated with change in immunological and hematological variables in dairy cow during the peripartum period. J. Anim. Sci. 2012, 90, 16–26. [Google Scholar] [CrossRef]
- Moore, D.D.; Dowhan, D. Preparation and analysis of DNA. In Current Protocols in Molecular Biology; Ausubel, F.M., Brent, R., Kingston, R.E., Moore, D.D., Seidman, J.G., Smith, J.A., Struhl, R., Eds.; John Wiley & Sons: New York, NY, USA, 1997; Volume 1, pp. 241–245. ISBN 0-471-50338-X. [Google Scholar]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.R.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.W.; Daly, M.J.; et al. PLINK: A Tool Set for Whole-Genome Association and Population-Based Linkage Analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef]
- Yang, Q.; Cui, J.; Chazaro, I.; Cupples, L.A.; Demissie, S. Power and type I error rate of false discovery rate approaches in genome-wide association studies. BMC Genet. 2005, 6, 1–4. [Google Scholar] [CrossRef]
- Breslow, N.E.; Clayton, D.G. Approximate Inference in Generalized Linear Mixed Models. J. Am. Stat. Assoc. 1993, 88, 9–25. [Google Scholar] [CrossRef]
- Bradbury, P.; Zhang, Z.; Kroon, D.; Casstevens, T.Y.; Buckler, E. TASSEL: Software for association mapping of complex traits in diverse samples. Bioinformatics 2007, 23, 2633–2635. [Google Scholar] [CrossRef]
- Alexander, D.H.; Novembre, J.K. Fast model-based estimation of ancestry in unrelated individuals. Genome Res. 2009, 19, 1655–1664. [Google Scholar] [CrossRef]
- Long, J.; Williams, R.; Urbanek, M. An E-M algorithm and testing strategy for multiple-locus haplotypes. Am. J. Hum. Genet. 1995, 56, 799–810. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, Z.; Yan, X.; Chen, H.; Zhang, W.; Yuan, H.; Huang, L. Genome-wide association studies for hematological traits in Chinese Sutai pigs. BMC Genet. 2014, 15, 41. [Google Scholar] [CrossRef]
- Zhang, Z.; Hong, Y.; Gao, J.; Xiao, S.; Ma, J.; Zhang, W.; Ren, J.; Huang, L. Genome-wide association study reveals constant and specific loci for hematological traits at three time stages in a White Duroc × Erhualian F2 resource population. PLoS ONE 2013, 8, e63665. [Google Scholar] [CrossRef]
- Hoeltke, C.; Kopka, W.K.; Heindel, W.; Schober, O.; Schaefers, M.; Bremer, C. Biodistribution of A Nonpeptidic Fluorescent Endothelin A Receptor Imaging Probe. Mol. Imaging 2009, 8, 27–34. [Google Scholar] [CrossRef]
- Yanagisawa, M.; Kurihara, H.; Kimura, S.; Tomobe, Y.; Kobayashi, M.; Mitsui, Y.; Yazaki, Y.; Goto, K.; Masaki, T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 1988, 6163. [Google Scholar] [CrossRef]
- Maclean, M.R.; Mcculloch, K.M.; Baird, M. Endothelin ETA- and ETB-receptor-mediated vasoconstriction in rat pulmonary arteries and arterioles. J. Cardiovasc. Pharm. 1994, 23, 838–845. [Google Scholar] [CrossRef]
- Watts, S.W. Endothelin receptors: what’s new and what do we need to know? Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, 254–260. [Google Scholar] [CrossRef]
- Nagalakshmi, S.; Derk, C.T. Endothelin receptor antagonists as disease modifiers in systemic sclerosis. Inflamm. Allergy Drug Targets 2011, 10. [Google Scholar] [CrossRef]
- Cleland, J.G.; Daubert, J.C.; Erdmann, E.; Freemantle, N.; Gras, D.; Kappenberger, L.; Tavazzi, L. The effect of cardiac resynchronization on morbidity and mortality in heart failure. Dig. World Core Med. J. 2005, 114, 216–218. [Google Scholar] [CrossRef]
- Liu, J.Q. Variations of levels of lipoprotein, platelet and zinc in 95 acute myocardial infarction patients in high plateau. China Trop. Med. 2010, 1001, 99–109. [Google Scholar] [CrossRef]
- Bodin, S.; Tronchère, H.; Payrastre, B. Lipid rafts are critical membrane domains in blood platelet activation processes. Biochim. Et Biophys. Acta 2003, 1610, 247–257. [Google Scholar] [CrossRef]
- Menzi, F.; Besuchet-Schmutz, N.; Fragnière, M.; Hofstetter, S.; Jagannathan, V.; Mock, T.; Raemy, A.; Studer, E.; Mehinagic, K.; Regenscheit, N.; et al. A transposable element insertion in APOB causes cholesterol deficiency in Holstein cattle. Anim. Genet. 2016, 47. [Google Scholar] [CrossRef]
- Gross, J.; Schwinn, A.C.; Schmitz-Hsu, F.; Menzi, F.; Drögemüller, C.; Albrecht, C.; Bruckmaier, R. Rapid Communication: Cholesterol deficiency–associated APOB mutation impacts lipid metabolism in Holstein calves and breeding bulls. J. Anim. Sci. 2016, 94. [Google Scholar] [CrossRef]
- Gondi, C.S.; Lakka, S.N.; Siddique, K.; Dinh, D.H.; Olivero, W.C.; Gujrati, M.; Rao, J.S. Expression of antisense uPAR and antisense uPA from a bicistronic adenoviral construct inhibits glioma cell invasion, tumor growth, and angiogenesis. Oncogene 2003, 22, 5967–5975. [Google Scholar] [CrossRef]
- Tang, J.H.; He, W.J.; Huang, H.; Tan, C.C.; Duan, Q.; Wang, K.J.; Yuan, X.Y.; Zhu, X.J. Important roles of glycosylphosphatidylinositol (GPI)-specific phospholipase D and some GPI-anchored proteins in the pathogenesis of hepatocellular carcinoma. Clin. Biochem. 2009, 42, 400–407. [Google Scholar] [CrossRef]
- Kordbacheh, F.; O’Meara, C.H.; Coupland, L.A.; Lelliott, P.M.; Parish, C.R. Extracellular histones induce erythrocyte fragility and anemia. Blood 2017, 130. [Google Scholar] [CrossRef]
| Traits | Abbreviation | Value 1 (No. 2) | CV 3 |
|---|---|---|---|
| Mean corpuscular hemoglobin concentration (g/L) | MCHC | 318.061 ± 9.281 (313) | 2.92 |
| Red blood cell volume distribution width-SD (fL) | RDWSD | 31.427 ± 2.362 (315) | 7.52 |
| Mean corpuscular volume (fl) | MCV | 42.725 ± 3.25 (315) | 7.61 |
| Red cell distribution coefficient of variation | RDWCV | 0.187 ± 0.015 (315) | 7.75 |
| Mean platelet volume (fl) | MPV | 7.146 ± 0.627 (171) | 8.77 |
| Mean corpuscular hemoglobin (pg) | MCH | 13.611 ± 1.312 (315) | 9.64 |
| Red blood cell count (1012/L) | RBC | 10.031 ± 1.266 (314) | 12.62 |
| Hematocrit (%) | HCT | 0.429 ± 0.056 (309) | 12.83 |
| Hemoglobin (g/L) | HGB | 136.217 ± 18.577 (314) | 13.64 |
| Platelet distribution width (%) | PDW | 8.777 ± 1.314 (169) | 14.97 |
| White blood cell count (109/L) | WBC | 9.04 ± 2.197 (278) | 24.31 |
| Medium white blood cell count (109/L) | OTHR | 4.603 ± 1.505 (270) | 32.69 |
| Lymphocyte count (109/L) | LYM | 4.538 ± 1.588 (276) | 34.99 |
| Platelet count (109/L) | PLT | 326.193 ± 170.149 (311) | 52.17 |
| Platelet-large cell ratio (%) | PLCR | 0.068 ± 0.039 (111) | 57.71 |
| Traits 1 | SNP 2 | CHR 3 | Position 4 | p Value | VAR (%) 5 | Nearest Gene 6 | |
|---|---|---|---|---|---|---|---|
| Name | Distance (bp) | ||||||
| HCT * | BOVINEHD0500023978 | 5 | 84784989 | 8.51× 10−6 | 7.44 | IFLTD1 | 69,046 |
| HCT ** | BOVINEHD4100016160 | 23 | 32994655 | 6.21× 10−8 | 10.48 | GPLD1 | WITHIN |
| HCT ** | BOVINEHD2300009636 | 23 | 32996521 | 6.55 × 10−8 | 10.38 | GPLD1 | WITHIN |
| HGB ** | BOVINEHD4100016160 | 23 | 32994655 | 2.63× 10−7 | 9.48 | GPLD1 | WITHIN |
| HGB ** | BOVINEHD2300009636 | 23 | 32996521 | 2.76 × 10−7 | 9.38 | GPLD1 | WITHIN |
| RBC * | BOVINEHD1400016753 | 14 | 60395118 | 9.25 × 10−7 | 7.52 | LOC782927 | WITHIN |
| RBC * | BOVINEHD2300009636 | 23 | 32996521 | 5.40 × 10−6 | 7.59 | GPLD1 | WITHIN |
| RBC * | BOVINEHD4100016160 | 23 | 32994655 | 5.84 × 10−6 | 7.59 | GPLD1 | WITHIN |
| RBC * | BOVINEHD2300001545 | 23 | 6375280 | 6.80 × 10−6 | 7.45 | C23H6ORF142 | 42 |
| RBC * | BOVINEHD2300001550 | 23 | 6383172 | 7.65 × 10−6 | 7.38 | C23H6ORF142 | 42 |
| MPV * | BOVINEHD1000028551 | 10 | 98690434 | 3.00 × 10−6 | 14.31 | LOC788481 | 13,887 |
| MPV * | BOVINEHD2000007009 | 20 | 23305743 | 5.68 × 10−6 | 11.73 | IL31RA | 63 |
| PDW * | BOVINEHD1700003099 | 17 | 10778156 | 1.98 × 10−6 | 13.35 | EDNRA | WITHIN |
| PLT * | BOVINEHD1100022362 | 11 | 77897626 | 1.68 × 10−6 | 7.29 | APOB | WITHIN |
| PLT * | BOVINEHD2100007261 | 21 | 25044956 | 3.87 × 10−7 | 9.3 | SH3GL3 | 11,156 |
| PLT * | BOVINEHD2400002733 | 24 | 9663513 | 5.51 × 10−6 | 7.69 | LOC538958 | 77,309 |
| LYM * | BOVINEHD2000004120 | 20 | 12840434 | 2.75 × 10−6 | 9.13 | LOC529061 | WITHIN |
| LYM * | BOVINEHD2100017671 | 21 | 61024785 | 4.74 × 10−6 | 8.76 | LOC781495 | WITHIN |
| LYM * | BOVINEHD2300005782 | 23 | 21870423 | 1.11 × 10−6 | 8.48 | MUT | 68,056 |
| OTHR * | BOVINEHD0100040184 | 1 | 140543231 | 7.71 × 10−7 | 10.17 | ATP2C1 | 20,601 |
| OTHR * | BOVINEHD0300001040 | 3 | 3461567 | 6.51 × 10−6 | 8.7 | LOC100295170 | 2804 |
| OTHR * | BOVINEHD0300021395 | 3 | 73543262 | 4.99 × 10−6 | 8.89 | NEGR1 | WITHIN |
| OTHR * | BOVINEHD0800005780 | 8 | 18417107 | 9.50 × 10−6 | 8.44 | TUSC1 | 151,827 |
| Traits 1 | NSNP 2 | CHR3 | START 4 | END 5 | SNP1 6 | SNP2 7 | VAR (%) 8 | p Value | Nearest Gene 9 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Name | Distance (bp) | |||||||||
| RBC * | 2 | 23 | 6045141 | 6051410 | BovineHD2300001442 | BovineHD2300001444 | 7.41 | 7.16 × 10−6 | TINAG | WITHIN |
| RBC * | 2 | 23 | 6045141 | 6051410 | BovineHD2300001442 | BovineHD2300001444 | 7.39 | 7.45 × 10−6 | TINAG | WITHIN |
| RBC * | 2 | 23 | 32982199 | 32983832 | BovineHD2300009627 | BovineHD2300009628 | 7.58 | 5.40 × 10−6 | GPLD1 | WITHIN |
| HGB ** | 2 | 23 | 32982199 | 32983832 | BovineHD2300009627 | BovineHD2300009628 | 9.38 | 2.76 × 10−7 | GPLD1 | WITHIN |
| HCT ** | 2 | 23 | 32982199 | 32983832 | BovineHD2300009627 | BovineHD2300009628 | 10.37 | 6.55 × 10−8 | GPLD1 | WITHIN |
| PLT * | 2 | 24 | 7751939 | 7753085 | BovineHD2400002173 | ARS-BFGL-NGS-112539 | 7.69 | 5.51 × 10−6 | LOC100336967 | WITHIN |
| OTHR * | 5 | 8 | 17716845 | 17825120 | BovineHD4100006566 | ARS-BFGL-NGS-10089 | 8.43 | 9.50 × 10−6 | LOC785941 | 8868 |
| RDWSD * | 7 | 23 | 31504452 | 31558355 | BovineHD2300009102 | BovineHD2300009103 | 6.43 | 8.17 × 10−6 | HIST1H1E,HIST1H2BI | WITHIN |
| PLCR * | 2 | 23 | 32935769 | 32941851 | BovineHD2300009596 | BovineHD2300009599 | 19.08 | 2.53 × 10−6 | LOC520603 | WITHIN |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Jia, C.; Fu, D.; Chu, M.; Ding, X.; Wu, X.; Guo, X.; Pei, J.; Bao, P.; Liang, C.; et al. Analysis of Hematological Traits in Polled Yak by Genome-Wide Association Studies Using Individual SNPs and Haplotypes. Genes 2019, 10, 463. https://doi.org/10.3390/genes10060463
Ma X, Jia C, Fu D, Chu M, Ding X, Wu X, Guo X, Pei J, Bao P, Liang C, et al. Analysis of Hematological Traits in Polled Yak by Genome-Wide Association Studies Using Individual SNPs and Haplotypes. Genes. 2019; 10(6):463. https://doi.org/10.3390/genes10060463
Chicago/Turabian StyleMa, Xiaoming, Congjun Jia, Donghai Fu, Min Chu, Xuezhi Ding, Xiaoyun Wu, Xian Guo, Jie Pei, Pengjia Bao, Chunnian Liang, and et al. 2019. "Analysis of Hematological Traits in Polled Yak by Genome-Wide Association Studies Using Individual SNPs and Haplotypes" Genes 10, no. 6: 463. https://doi.org/10.3390/genes10060463
APA StyleMa, X., Jia, C., Fu, D., Chu, M., Ding, X., Wu, X., Guo, X., Pei, J., Bao, P., Liang, C., & Yan, P. (2019). Analysis of Hematological Traits in Polled Yak by Genome-Wide Association Studies Using Individual SNPs and Haplotypes. Genes, 10(6), 463. https://doi.org/10.3390/genes10060463





