Transcriptome Changes of Skeletal Muscle RNA-Seq Speculates the Mechanism of Postprandial Hyperglycemia in Diabetic Goto-Kakizaki Rats During the Early Stage of T2D
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Breeding and Tissues Collection
2.2. Measurement of Plasma Glucose and Insulin Concentration
2.3. RNA Extraction, Library Construction and Sequencing
2.4. Differentially Expressed Genes
2.5. Enrichment Analysis of Kyoto Encyclopedia of Genes and Genomes (KEGG) Pathways
2.6. Prediction of the Transcriptional Factors of Key Dysregulated Genes
2.7. Validation of RNA-Seq Results with RT-qPCR
2.8. Statistical Analysis
3. Results
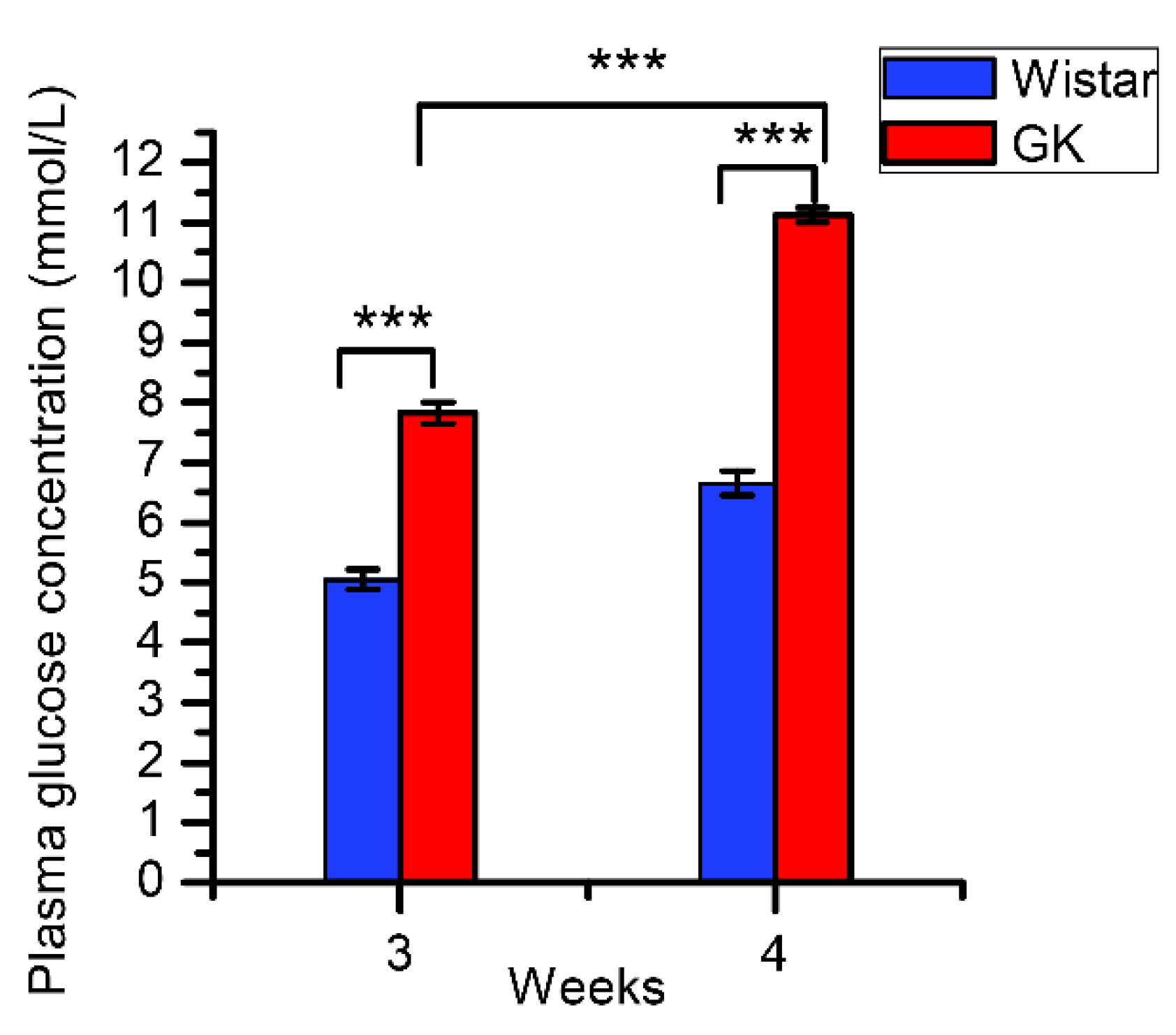
3.1. The Characterization of Rats
3.2. The Differentially Expressed Genes and Enriched Pathways
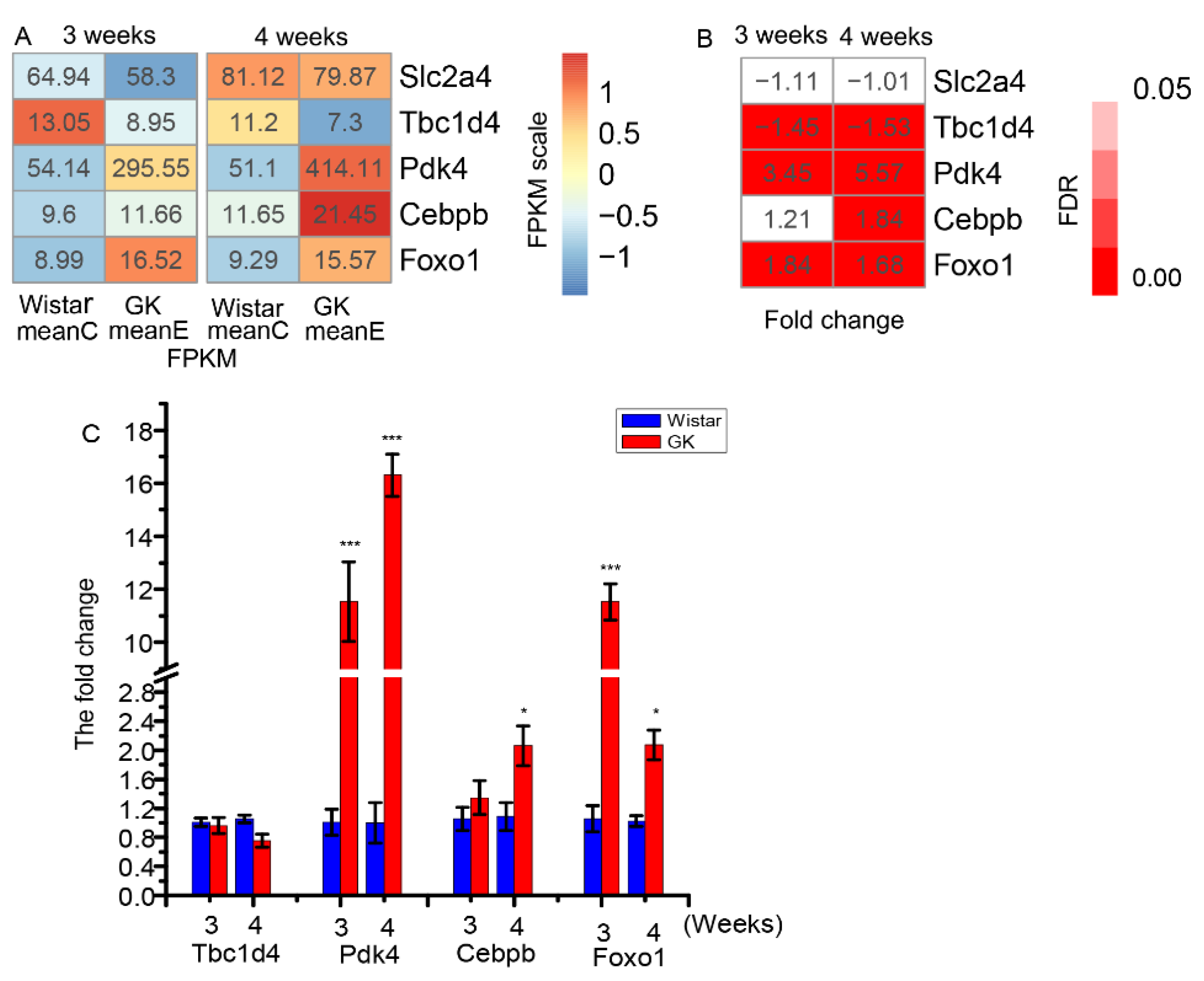
3.3. The Differentially Expressed Genes Related to Glucose Uptake and Oxidation
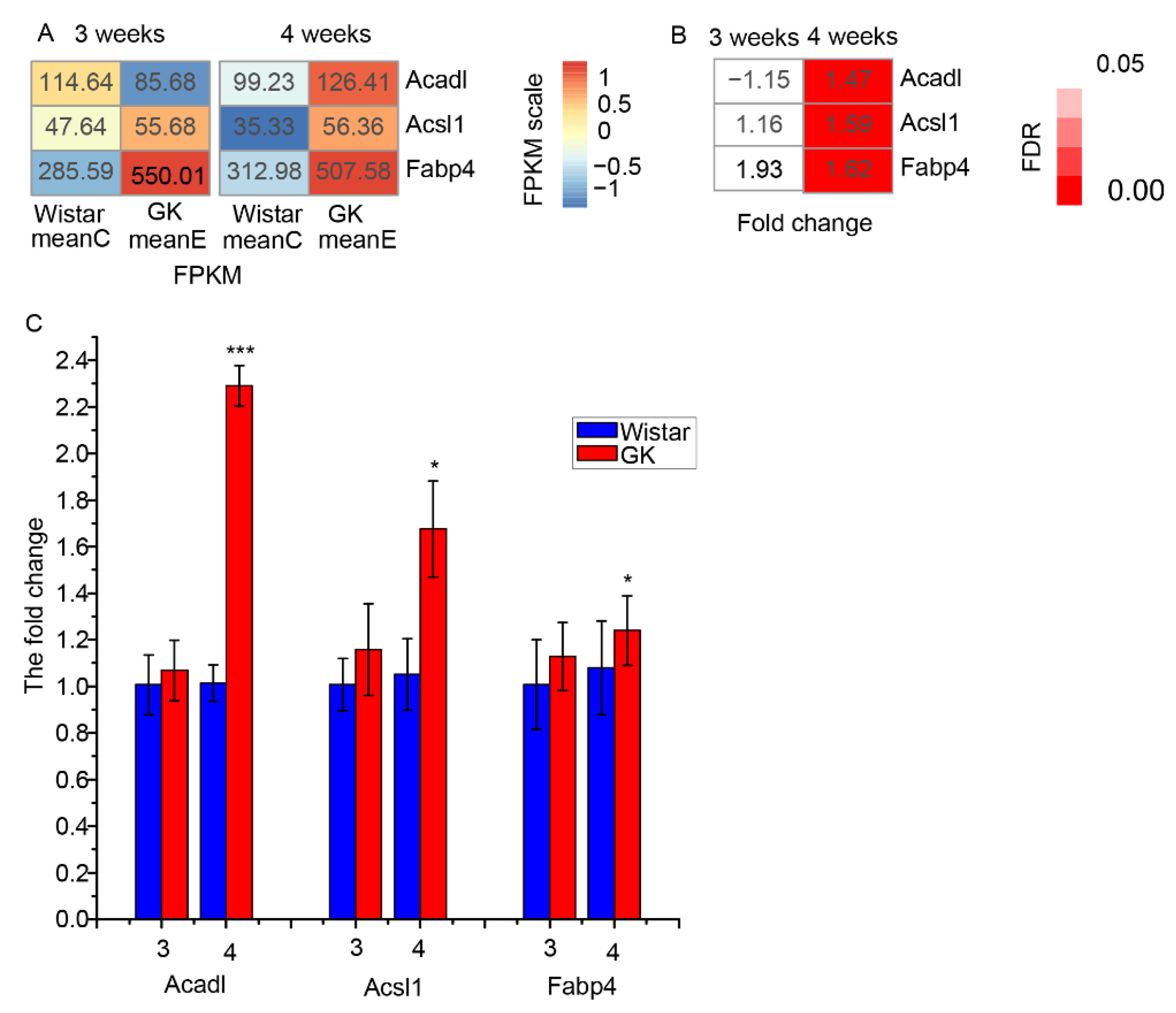
3.4. The Differentially Expressed Genes Related to Fatty Acid Oxidation
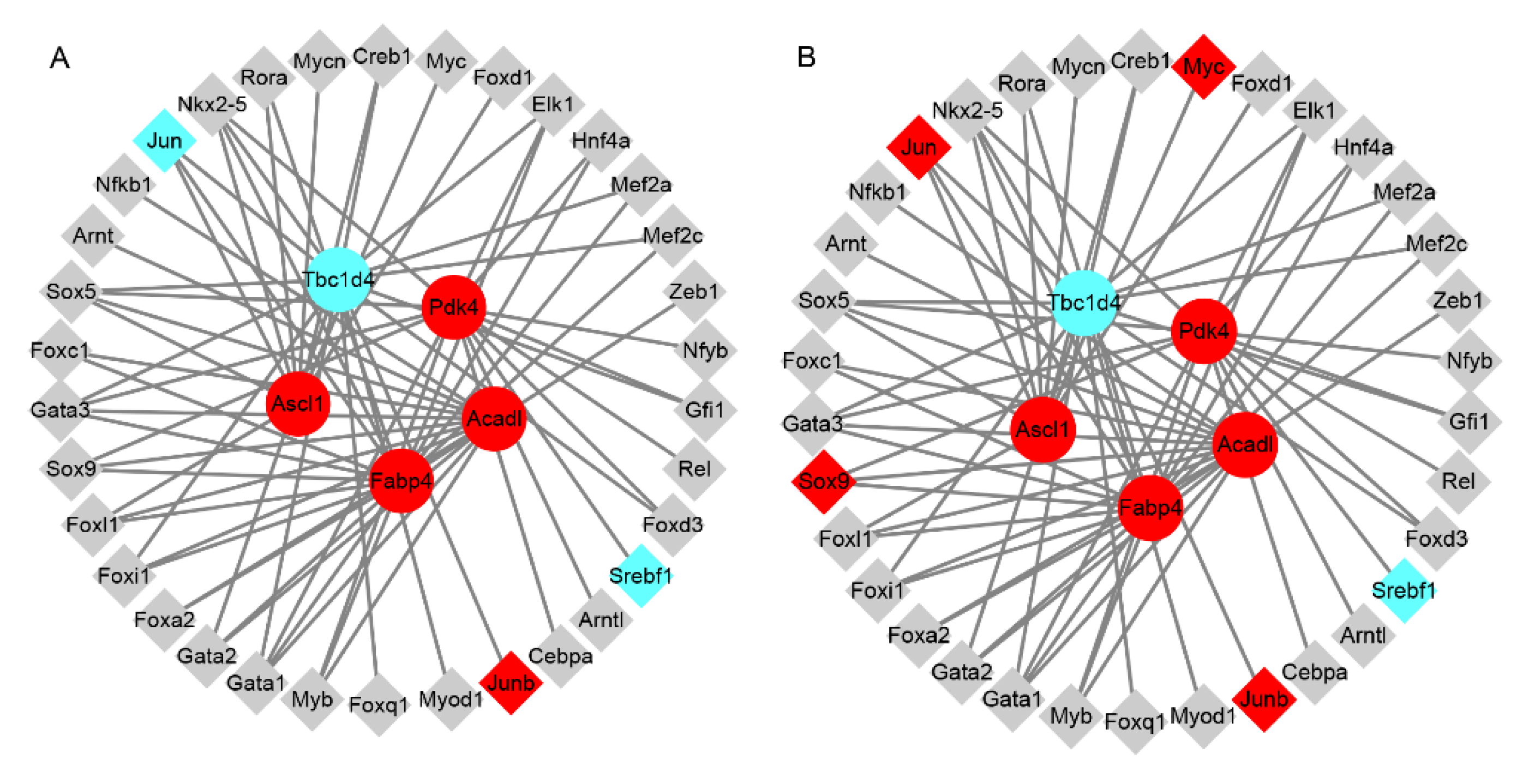
3.5. The Predicted Transcriptional Factors of Key Dysregulated Genes
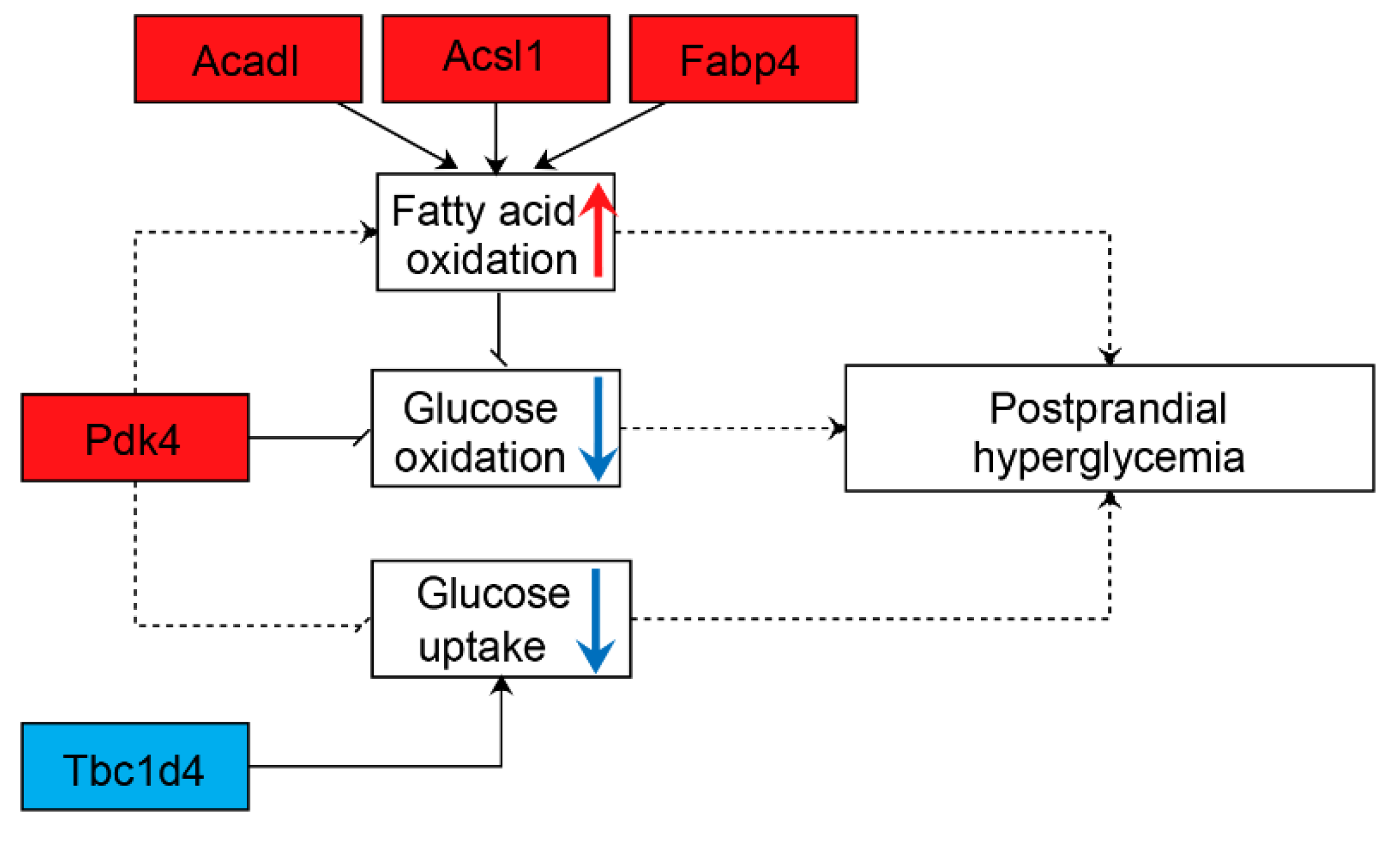
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Data Availability
References
- Cho, N.; Shaw, J.; Karuranga, S.; Huang, Y.; Fernandes, J.D.R.; Ohlrogge, A.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar]
- Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes. Care 1997, 20, 1183–1197. [CrossRef]
- Qiao, Q.; Nakagami, T.; Tuomilehto, J.; Borch-Johnsen, K.; Balkau, B.; Iwamoto, Y.; Tajima, N.; Group International Diabetes Epidemiology; Decoda Study Group. Comparison of the fasting and the 2-h glucose criteria for diabetes in different Asian cohorts. Diabetologia 2000, 43, 1470–1475. [Google Scholar] [CrossRef]
- Yang, W.; Lu, J.; Weng, J.; Jia, W.; Ji, L.; Xiao, J.; Shan, Z.; Liu, J.; Tian, H.; Ji, Q. Prevalence of diabetes amongmen and women in China. N. Engl. J. Med. 2010, 362, 1090–1101. [Google Scholar] [CrossRef]
- Qiao, Q.; Hu, G.; Tuomilehto, J.; Nakagami, T.; Balkau, B.; Borch-Johnsen, K.; Ramachandran, A.; Mohan, V.; Iyer, S.R.; Tominaga, M.; et al. Age- and sex-specific prevalence of diabetes and impaired glucose regulation in 11 Asian cohorts. Diabetes Care 2003, 26, 1770–1780. [Google Scholar]
- GoTo, Y.; Kakizaki, M.; Masaki, N. Spontaneous diabetes produced by selective breeding of normal Wistar rats. Proc. Japan Acad. 1975, 51, 80–85. [Google Scholar] [CrossRef]
- Goto, Y.; Kakizaki, M.; Masaki, N. Production of Spontaneous Diabetic Rats by Repetition of Selective Breeding. Tohoku J. Exp. Med. 1976, 119, 85–90. [Google Scholar] [CrossRef]
- Movassat, J.; Bailbe, D.; Lubrano-Berthelier, C.; Picarel-Blanchot, F.; Bertin, E.; Mourot, J.; Portha, B. Follow-up of GK rats during prediabetes highlights increased insulin action and fat deposition despite low insulin secretion. Am. J. Physiol. Metab. 2008, 294, E168–E175. [Google Scholar] [CrossRef]
- Nie, J.; Xue, B.; Sukumaran, S.; Jusko, W.J.; Dubois, D.C.; Almon, R.R. Differential muscle gene expression as a function of disease progression in Goto-Kakizaki diabetic rats. Mol. Cell. Endocrinol. 2011, 338, 10–17. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Jacot, E.; Jéquier, E.; Maeder, E.; Wahren, J.; Felber, J.P. The Effect of Insulin on the Disposal of Intravenous Glucose: Results from Indirect Calorimetry and Hepatic and Femoral Venous Catheterization. Diabetes 1981, 30, 1000–1007. [Google Scholar] [CrossRef]
- Baron, A.D.; Brechtel, G.; Wallace, P.; Edelman, S.V. Rates and tissue sites of non-insulin- and insulin-mediated glucose uptake in humans. Am. J. Physiol. Metab. 1988, 255, 769–774. [Google Scholar] [CrossRef]
- Uysal, K.T.; Scheja, L.; Wiesbrock, S.M.; Bonner-Weir, S.; Hotamisligil, G.S. Improved Glucose and Lipid Metabolism in Genetically Obese Mice Lacking aP2. Endocrinology 2000, 141, 3388–3396. [Google Scholar] [CrossRef]
- Scheja, L.; Makowski, L.; Uysal, K.T.; Wiesbrock, S.M.; Shimshek, D.R.; Meyers, D.S.; Morgan, M.; Parker, R.A.; Hotamisligil, G.S. Altered insulin secretion associated with reduced lipolytic efficiency in aP2-/- mice. Diabetes 1999, 48, 1987–1994. [Google Scholar] [CrossRef]
- Dobin, A.; Gingeras, T.R. Mapping RNA-seq Reads with STAR. Curr. Protoc. Bioinformatics 2015, 51, 11–19. [Google Scholar] [PubMed]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.-C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef]
- Frazee, A.C.; Pertea, G.; E Jaffe, A.; Langmead, B.; Salzberg, S.L.; Leek, J.T. Ballgown bridges the gap between transcriptome assembly and expression analysis. Nat. Biotechnol. 2015, 33, 243–246. [Google Scholar] [CrossRef]
- Kayala, M.A.; Baldi, P. Cyber-T web server: differential analysis of high-throughput data. Nucleic Acids Res. 2012, 40, W553–W559. [Google Scholar] [CrossRef]
- Matys, V. TRANSFAC®: transcriptional regulation, from patterns to profiles. Nucleic Acids Res. 2003, 31, 374–378. [Google Scholar] [CrossRef]
- Khan, A.; Fornes, O.; Stigliani, A.; Gheorghe, M.; Castro-Mondragon, J.A.; van der Lee, R.; Bessy, A.; Cheneby, J.; Kulkarni, S.R.; Tan, G. JASPAR 2018: Update of the open-access database of transcription factor binding profiles and its web framework. Nucleic Acids Res. 2018, 46, D260–D266. [Google Scholar] [CrossRef]
- Qi, Z.; He, J.; Zhang, Y.; Shao, Y.; Ding, S. Exercise training attenuates oxidative stress and decreases p53 protein content in skeletal muscle of type 2 diabetic Goto-Kakizaki rats. Radic. Boil. Med. 2011, 50, 794–800. [Google Scholar] [CrossRef]
- Tachibana, N.; Yamashita, Y.; Nagata, M.; Wanezaki, S.; Ashida, H.; Horio, F.; Kohno, M. Soy β-conglycinin improves glucose uptake in skeletal muscle and ameliorates hepatic insulin resistance in Goto-Kakizaki rats. Nutr. Res. 2014, 34, 160–167. [Google Scholar] [CrossRef]
- Rubin, A.; Salzberg, A.C.; Imamura, Y.; Grivitishvilli, A.; Tombran-Tink, J. Identification of novel targets of diabetic nephropathy and PEDF peptide treatment using RNA-seq. BMC Genom. 2016, 17, 1047. [Google Scholar] [CrossRef]
- Chao, L.C.; Wroblewski, K.; Ilkayeva, O.R.; Stevens, R.D.; Bain, J.; Meyer, G.A.; Schenk, S.; Martinez, L.; Vergnes, L.; Narkar, V.A.; et al. Skeletal muscle Nur77 expression enhances oxidative metabolism and substrate utilization. J. Lipid Res. 2012, 53, 2610–2619. [Google Scholar] [CrossRef]
- Fink, R.; Wallace, P.; Brechtel, G.; Olefsky, J. Evidence that glucose transport is rate-limiting for in vivo glucose uptake. Metab. Clin. Exp. 1992, 41, 897–902. [Google Scholar] [CrossRef]
- Ziel, F.H.; Venkatesan, N.; Davidson, M.B. Glucose Transport Is Rate Limiting for Skeletal Muscle Glucose Metabolism in Normal and STZ-Induced Diabetic Rats. Diabetes 1988, 37, 885–890. [Google Scholar] [CrossRef]
- Ren, J.M.; A Marshall, B.; A Gulve, E.; Gao, J.; Johnson, D.W.; O Holloszy, J.; Mueckler, M. Evidence from transgenic mice that glucose transport is rate-limiting for glycogen deposition and glycolysis in skeletal muscle. J. Boil. Chem. 1993, 268, 16113–16115. [Google Scholar]
- Randle, P.; Garland, P.; Hales, C.; Newsholme, E. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1963, 281, 785–789. [Google Scholar] [CrossRef]
- Hue, L.; Taegtmeyer, H. The Randle cycle revisited: a new head for an old hat. Am. J. Physiol. Metab. 2009, 297, E578–E591. [Google Scholar] [CrossRef]
- Monnier, L.; Lapinski, H.; Colette, C. Contributions of Fasting and Postprandial Plasma Glucose Increments to the Overall Diurnal Hyperglycemia of Type 2 Diabetic Patients: Variations with increasing levels of HbA1c. Diabetes Care 2003, 26, 881–885. [Google Scholar] [CrossRef]
- Woerle, H.J.; Pimenta, W.P.; Meyer, C.; Gosmanov, N.R.; Szoke, E.; Szombathy, T.; Mitrakou, A.; Gerich, J.E. Diagnostic and therapeutic implications of relationships between fasting, 2-hour postchallenge plasma glucose and hemoglobin a1c values. Arch. Intern. Med. 2004, 164, 1627–1632. [Google Scholar] [CrossRef]
- Monnier, L.; Colette, C.; Dunseath, G.J.; Owens, D.R. The Loss of Postprandial Glycemic Control Precedes Stepwise Deterioration of Fasting With Worsening Diabetes. Diabetes Care 2007, 30, 263–269. [Google Scholar] [CrossRef]
- Woerle, H.J.; Neumann, C.; Zschau, S.; Tenner, S.; Irsigler, A.; Schirra, J.; Gerich, J.E.; Göke, B. Impact of fasting and postprandial glycemia on overall glycemic control in type 2 diabetes Importance of postprandial glycemia to achieve target HbA1c levels. Diabetes Res. Clin. Pract. 2007, 77, 280–285. [Google Scholar] [CrossRef]
- Birnbaum, M.J. Identification of a novel gene encoding an insulin-responsive glucose transporter protein. Cell 1989, 57, 305–315. [Google Scholar] [CrossRef]
- Kaestner, K.H.; Christy, R.J.; McLenithan, J.C.; Braiterman, L.T.; Cornelius, P.; Pekala, P.H.; Lane, M.D. Sequence, tissue distribution, and differential expression of mRNA for a putative insulin-responsive glucose transporter in mouse 3T3-L1 adipocytes. Proc. Acad. Sci. 1989, 86, 3150–3154. [Google Scholar] [CrossRef]
- Abel, E.D.; Peroni, O.; Kim, J.K.; Kim, Y.-B.; Boss, O.; Hadro, E.; Minnemann, T.; Shulman, G.I.; Kahn, B.B. Adipose-selective targeting of the GLUT4 gene impairs insulin action in muscle and liver. Nat. Cell Boil. 2001, 409, 729–733. [Google Scholar] [CrossRef]
- Dugani, C.B.; Klip, A. Glucose transporter 4: cycling, compartments and controversies. EMBO Rep. 2005, 6, 1137–1142. [Google Scholar] [CrossRef]
- Graham, T.; Kahn, B. Tissue-specific Alterations of Glucose Transport and Molecular Mechanisms of Intertissue Communication in Obesity and Type 2 Diabetes. Horm. Metab. 2007, 39, 717–721. [Google Scholar] [CrossRef]
- Huang, S.; Lifshitz, L.M.; Jones, C.; Bellve, K.D.; Standley, C.; Fonseca, S.; Corvera, S.; Fogarty, K.E.; Czech, M.P. Insulin Stimulates Membrane Fusion and GLUT4 Accumulation in Clathrin Coats on Adipocyte Plasma Membranes. Mol. Cell. Boil. 2007, 27, 3456–3469. [Google Scholar] [CrossRef]
- Kim, J.K.; Zisman, A.; Fillmore, J.J.; Peroni, O.D.; Kotani, K.; Perret, P.; Zong, H.; Dong, J.; Kahn, C.R.; Kahn, B.B.; et al. Glucose toxicity and the development of diabetes in mice with muscle-specific inactivation of GLUT4. J. Clin. Investig. 2001, 108, 153–160. [Google Scholar] [CrossRef]
- Zisman, A.; Peroni, O.D.; Abel, E.D.; Michael, M.D.; Mauvais-Jarvis, F.; Lowell, B.B.; Wojtaszewski, J.F.; Hirshman, M.F.; Virkamaki, A.; Goodyear, L.J.; et al. Targeted disruption of the glucose transporter 4 selectively in muscle causes insulin resistance and glucose intolerance. Nat. Med. 2000, 6, 924–928. [Google Scholar] [CrossRef]
- Kim, Y.-B.; Peroni, O.D.; Aschenbach, W.G.; Minokoshi, Y.; Kotani, K.; Zisman, A.; Kahn, C.R.; Goodyear, L.J.; Kahn, B.B. Muscle-Specific Deletion of the Glut4 Glucose Transporter Alters Multiple Regulatory Steps in Glycogen Metabolism. Mol. Cell. Boil. 2005, 25, 9713–9723. [Google Scholar] [CrossRef] [PubMed]
- Sano, H.; Kane, S.; Mı̂inea, C.P.; Asara, J.M.; Lane, W.S.; Garner, C.W.; Sano, E.; Miinea, C.P.; Lienhard, G.E. Insulin-stimulated Phosphorylation of a Rab GTPase-activating Protein Regulates GLUT4 Translocation. J. Boil. Chem. 2003, 278, 14599–14602. [Google Scholar] [CrossRef]
- Sakamoto, K.; Holman, G.D. Emerging role for AS160/TBC1D4 and TBC1D1 in the regulation of GLUT4 traffic. Am. J. Physiol. Metab. 2008, 295, E29–37. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Ducommun, S.; Quan, C.; Xie, B.; Li, M.; Wasserman, D.H.; Sakamoto, K.; Mackintosh, C.; Chen, S. AS160 deficiency causes whole-body insulin resistance via composite effects in multiple tissues. Biochem. J. 2013, 449, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Lansey, M.N.; Walker, N.N.; Hargett, S.R.; Stevens, J.R.; Keller, S.R. Deletion of Rab GAP AS160 modifies glucose uptake and GLUT4 translocation in primary skeletal muscles and adipocytes and impairs glucose homeostasis. Am. J. Physiol. Metab. 2012, 303, E1273–E1286. [Google Scholar] [CrossRef]
- Qin, X.; Hou, Z.; Guo, Y.; Wu, J.; Gao, F.; Li, T.; Xu, J.; Liu, Z.; Zheng, H.; Zhang, X. Glucose oxidation positively regulates glucose uptake and improves cardiac function recovery after myocardial reperfusion. Am. J. Physiol. Metab. 2017, 313, E577–E585. [Google Scholar]
- Jue, T.; Stein, P.; Shulman, G.I.; Rothman, D.L.; DeFronzo, R.A.; Shulman, R.G. Quantitation of Muscle Glycogen Synthesis in Normal Subjects and Subjects with Non-Insulin-Dependent Diabetes by13C Nuclear Magnetic Resonance Spectroscopy. New Engl. J. Med. 1990, 322, 223–228. [Google Scholar]
- Macaulay, K.; Blair, A.S.; Hajduch, E.; Hundal, H.S.; Terashima, T.; Baba, O.; Sutherland, C. Constitutive Activation of GSK3 Down-regulates Glycogen Synthase Abundance and Glycogen Deposition in Rat Skeletal Muscle Cells. J. Boil. Chem. 2005, 280, 9509–9518. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Zhong, T.; Guo, J.; Li, L.; Zhang, H.; Wang, L. Transcriptional regulation of pig GYS1 gene by glycogen synthase kinase 3β (GSK3β). Mol. Cell. Biochem. 2017, 424, 203–208. [Google Scholar] [CrossRef]
- Song, X.M.; Kawano, Y.; Krook, A.; Ryder, J.W.; Efendic, S.; Roth, R.A.; Wallberg-Henriksson, H.; Zierath, J. Muscle fiber type-specific defects in insulin signal transduction to glucose transport in diabetic GK rats. Diabetes 1999, 48, 664–670. [Google Scholar] [CrossRef]
- Bitar, M.S.; Al-Saleh, E.; Al-Mulla, F. Oxidative stress—mediated alterations in glucose dynamics in a genetic animal model of type II diabetes. Life Sci. 2005, 77, 2552–2573. [Google Scholar] [CrossRef] [PubMed]
- Bitar, M.S.; Wahid, S.; Pilcher, C.W.; Al-Saleh, E.; Al-Mulla, F. α-lipoic acid mitigates insulin resistance in Goto-Kakizaki rats. Horm. Metab. Res. 2004, 36, 542–549. [Google Scholar] [CrossRef]
- Nie, J.; DuBois, D.C.; Jusko, W.J.; Almonm, R.R. Mechanistic population modeling of diabetes disease progression in Goto-Kakizaki rat muscle. Biopharm. Drug Dispos. 2011, 32, 50–63. [Google Scholar] [CrossRef]
- Li, L.O.; Grevengoed, T.J.; Paul, D.S.; Ilkayeva, O.; Koves, T.R.; Pascual, F.; Newgard, C.B.; Muoio, D.M.; Coleman, R.A. Compartmentalized acyl-CoA metabolism in skeletal muscle regulates systemic glucose homeostasis. Diabetes 2015, 64, 23–35. [Google Scholar] [CrossRef]
- Chegary, M.; te Brinke, H.; Ruiter, J.P.; Wijburg, F.A.; Stoll, M.S.; Minkler, P.E.; van Weeghel, M.; Schulz, H.; Hoppel, C.L.; Wanders, R.J. Mitochondrial long chain fatty acid β-oxidation in man and mouse. Biochim. Biophys. Acta 2009, 1791, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Perdomo, G.; Commerford, S.R.; Richard, A.M.; Adams, S.H.; Corkey, B.E.; O’Doherty, R.M.; Brown, N.F. Increased β-oxidation in muscle cells enhances insulin-stimulated glucose metabolism and protects against fatty acid-induced insulin resistance despite intramyocellular lipid accumulation. J. Biol. Chem. 2004, 279, 27177–27186. [Google Scholar] [CrossRef] [PubMed]
- Herrero, L.; Rubí, B.; Sebastián, D.; Serra, D.; Asins, G.; Maechler, P.; Prentki, M.; Hegardt, F.G. Alteration of the malonyl-CoA/carnitine palmitoyltransferase I interaction in the β-cell impairs glucose-induced insulin secretion. Diabetes 2005, 54, 462–471. [Google Scholar] [CrossRef]
- Stefanovic-Racic, M.; Perdomo, G.; Mantell, B.S.; Sipula, I.J.; Brown, N.F.; O’Doherty, R.M. A moderate increase in carnitine palmitoyltransferase 1a activity is sufficient to substantially reduce hepatic triglyceride levels. Am. J. Physiol. Metab. 2008, 294, E969–E977. [Google Scholar] [CrossRef]
- Akkaoui, M.; Cohen, I.; Esnous, C.; Lenoir, V.; Sournac, M.; Girard, J.; Prip-Buus, C. Modulation of the hepatic malonyl-CoA-carnitine palmitoyltransferase 1A partnership creates a metabolic switch allowing oxidation of de novo fatty acids. Biochem. J. 2009, 420, 429–438. [Google Scholar] [CrossRef]
- Zhao, G.; Burgess, S.C.; Rosaaen-Stowe, K.A.; Inagaki, T.; Latif, S.; Shelton, J.M.; McAnally, J.; Bassel-Duby, R.; Harris, R.A.; Kliewer, S.A.; et al. Overexpression of pyruvate dehydrogenase kinase 4 in heart perturbs metabolism and exacerbates calcineurin-induced cardiomyopathy. Am. J. Physiol. Circ. Physiol. 2008, 294, 936–943. [Google Scholar] [CrossRef]
- Macia, M.; Pecchi, É.; Vilmen, C.; Desrois, M.; Lan, C.; Portha, B.; Bernard, M.; Bendahan, D.; Giannesini, B. Insulin Resistance Is Not Associated with an Impaired Mitochondrial Function in Contracting Gastrocnemius Muscle of Goto-Kakizaki Diabetic Rats In Vivo. PLOS ONE 2015, 10, e0129579. [Google Scholar]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Meng, Y.; Fu, S.; Li, X.; Chen, Z.; Huang, L.; Du, H. Transcriptome Changes of Skeletal Muscle RNA-Seq Speculates the Mechanism of Postprandial Hyperglycemia in Diabetic Goto-Kakizaki Rats During the Early Stage of T2D. Genes 2019, 10, 406. https://doi.org/10.3390/genes10060406
Zhang W, Meng Y, Fu S, Li X, Chen Z, Huang L, Du H. Transcriptome Changes of Skeletal Muscle RNA-Seq Speculates the Mechanism of Postprandial Hyperglycemia in Diabetic Goto-Kakizaki Rats During the Early Stage of T2D. Genes. 2019; 10(6):406. https://doi.org/10.3390/genes10060406
Chicago/Turabian StyleZhang, Wenlu, Yuhuan Meng, Shuying Fu, Xingsong Li, Zixi Chen, Lizhen Huang, and Hongli Du. 2019. "Transcriptome Changes of Skeletal Muscle RNA-Seq Speculates the Mechanism of Postprandial Hyperglycemia in Diabetic Goto-Kakizaki Rats During the Early Stage of T2D" Genes 10, no. 6: 406. https://doi.org/10.3390/genes10060406
APA StyleZhang, W., Meng, Y., Fu, S., Li, X., Chen, Z., Huang, L., & Du, H. (2019). Transcriptome Changes of Skeletal Muscle RNA-Seq Speculates the Mechanism of Postprandial Hyperglycemia in Diabetic Goto-Kakizaki Rats During the Early Stage of T2D. Genes, 10(6), 406. https://doi.org/10.3390/genes10060406



