An Entamoeba-Specific Mitosomal Membrane Protein with Potential Association to the Golgi Apparatus
Abstract
1. Introduction
2. Materials and Methods
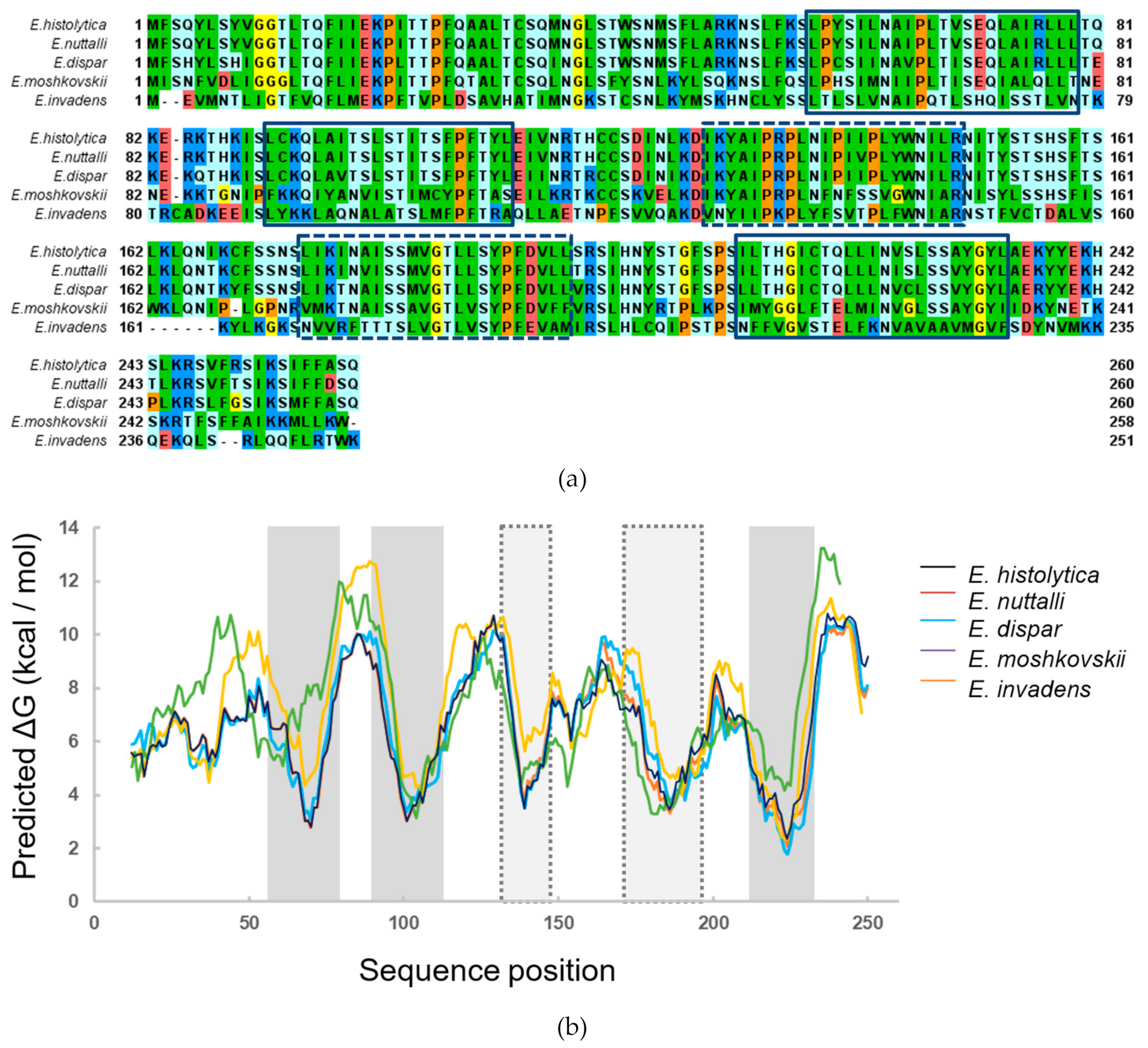
2.1. In Silico Predictions and Analyses
2.2. Amoeba Cultivation, Plasmid Construction, and Amoeba Transfection
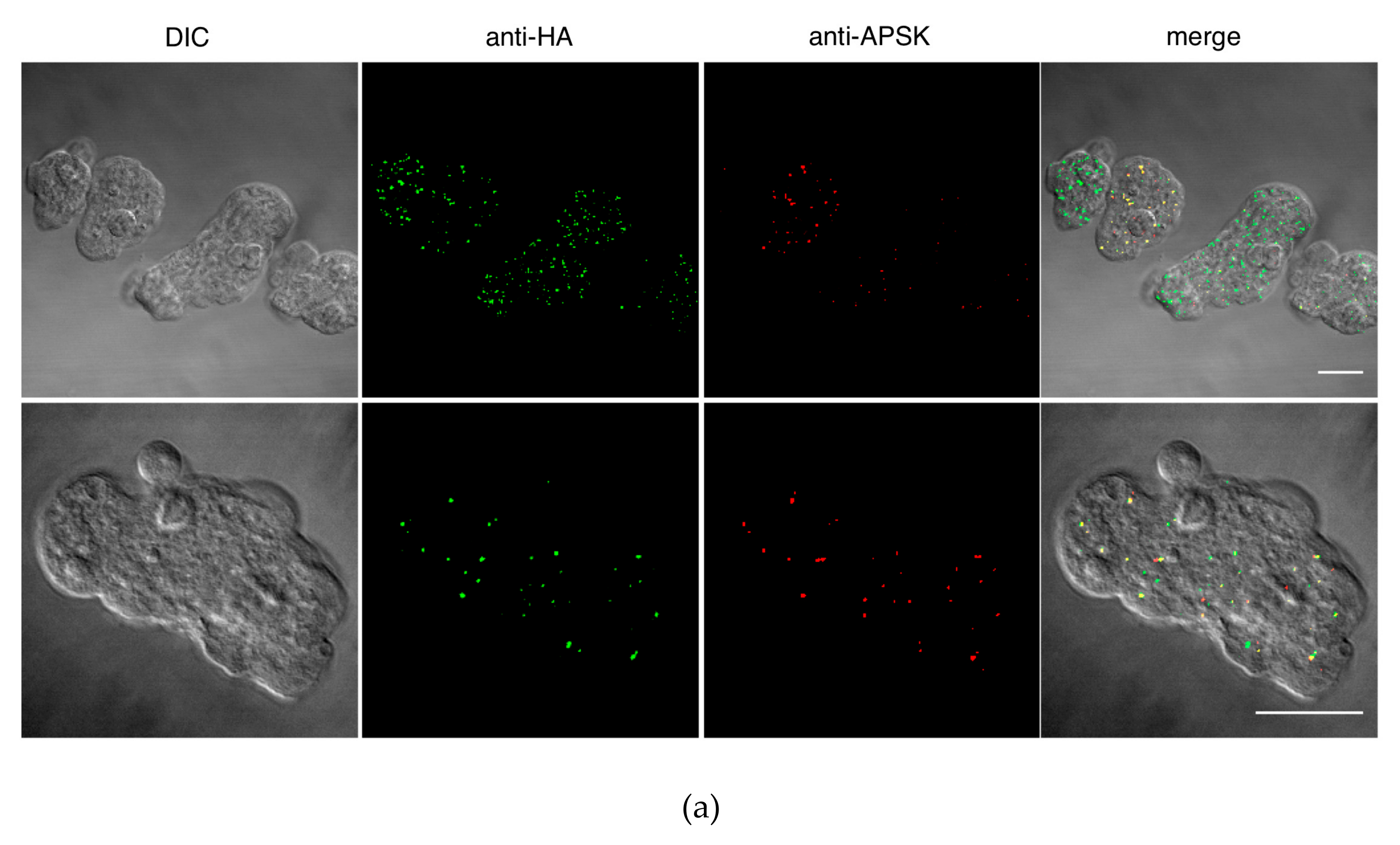
2.3. Immunoflourescence Assay
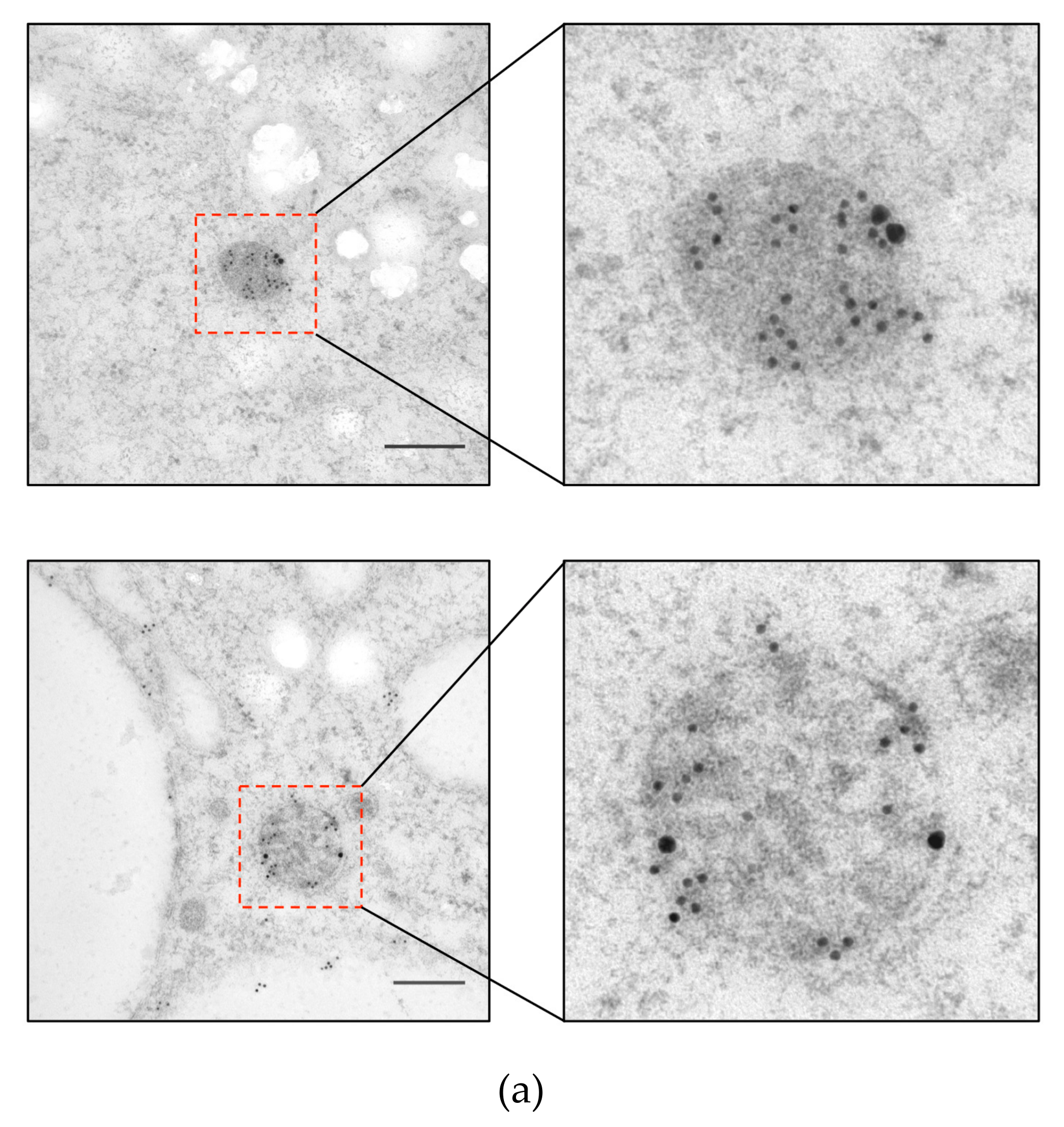
2.4. Immunoelectron Microscopy
2.5. Percoll-Gradient Fractionation
2.6. Sodium Carbonate (Na2CO3) Treatment of Organelle-Enriched Fractions
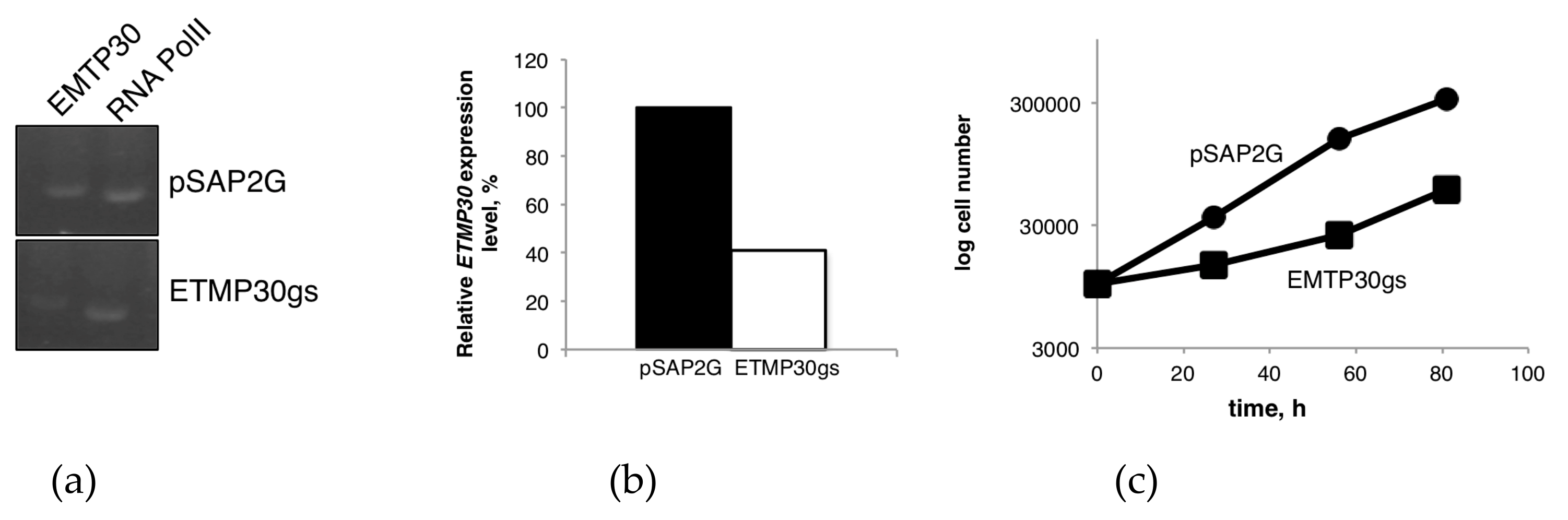
2.7. Quantitative Real-Time PCR (qRT-PCR) Analysis
2.8. Immunoprecipitation (IP) of ETMP30-HA by Anti-HA Antibody
3. Results
3.1. ETMP30 is Predicted to Be an Entamoeba-Specific Multi-Pass Membrane Protein
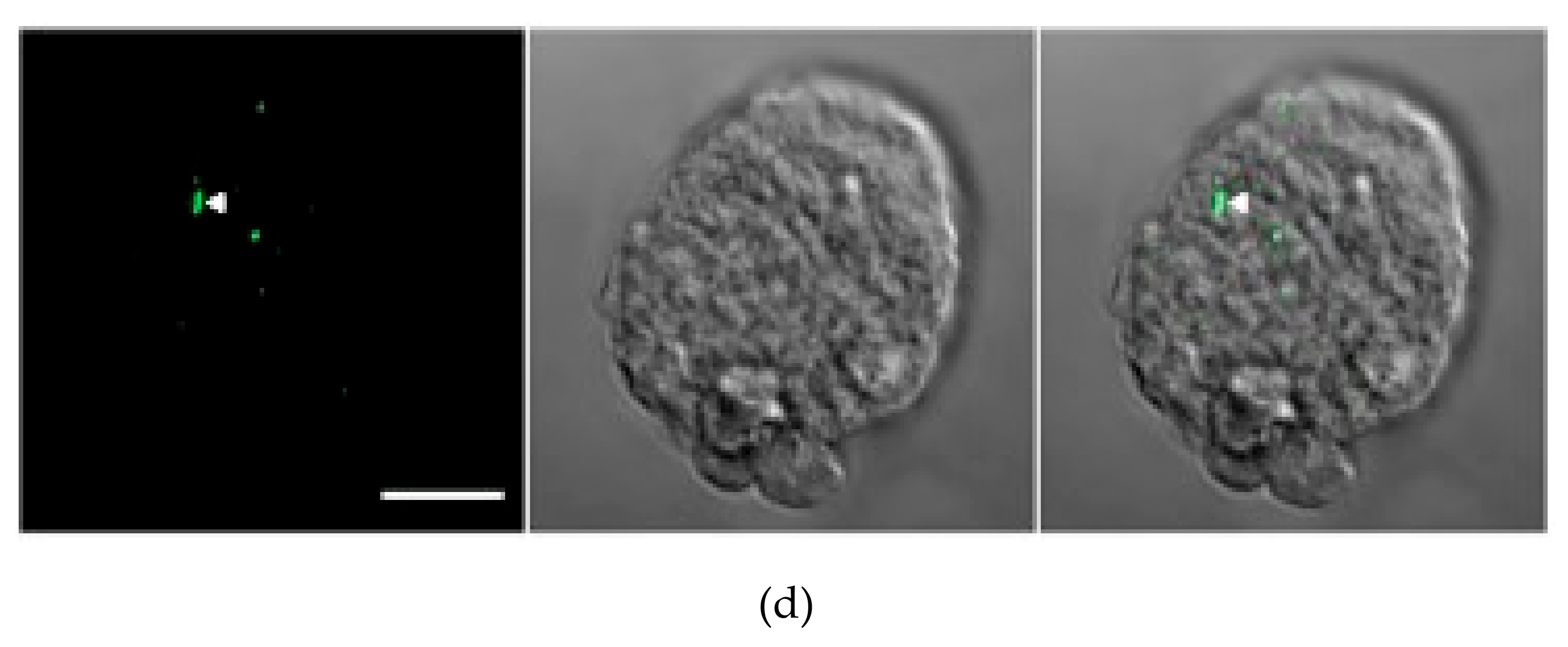
3.2. ETMP30 Is Localized to the Mitosomal Membranes
3.3. Transcriptional Gene Silencing of ETMP30 Leads to Growth Retardation and Slight Elongation of Mitosomes
3.4. ETMP30 Interacts with a Cation-Transporting P-Type ATPase
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Susin, S.A.; Lorenzo, H.K.; Zamzami, N.; Marzo, I.; Snow, B.E.; Brothers, G.M.; Mangion, J.; Jacotot, E.; Costantini, P.; Loeffler, M.; et al. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature 1999, 397, 441–446. [Google Scholar] [CrossRef]
- Lill, R.; Kispal, G. Maturation of cellular Fe-S proteins: An essential function of mitochondria. Trends Biochem. Sci. 2000, 25, 352–356. [Google Scholar] [CrossRef]
- Santos, H.J.; Makiuchi, T.; Nozaki, T. Reinventing an organelle: The reduced mitochondrion in parasitic protists. Trends Parasitol. 2018, 34, 1038–1055. [Google Scholar] [CrossRef]
- Stanley, S.L. Amoebiasis. Lancet 2003, 361, 1025–1034. [Google Scholar] [CrossRef]
- Makiuchi, T.; Mi-ichi, F.; Nakada-Tsukui, K.; Nozaki, T. Novel TPR-containing subunit of TOM complex functions as cytosolic receptor for Entamoeba mitosomal transport. Sci. Rep. 2013, 3, 1129. [Google Scholar] [CrossRef] [PubMed]
- Santos, H.J.; Imai, K.; Makiuchi, T.; Tomii, K.; Horton, P.; Nozawa, A.; Ibrahim, M.; Tozawa, Y.; Nozaki, T. A novel mitosomal β-barrel outer membrane protein in Entamoeba. Sci. Rep. 2015, 5, 1–10. [Google Scholar] [CrossRef][Green Version]
- Mi-ichi, F.; Yousuf, M.A.; Nakada-Tsukui, K.; Nozaki, T. Mitosomes in Entamoeba histolytica contain a sulfate activation pathway. Proc. Natl. Acad. Sci. USA 2009, 106, 21731–21736. [Google Scholar] [CrossRef] [PubMed]
- Mi-ichi, F.; Miyamoto, T.; Takao, S.; Jeelani, G.; Hashimoto, T.; Hara, H.; Nozaki, T.; Yoshida, H. Entamoeba mitosomes play an important role in encystation by association with cholesteryl sulfate synthesis. Proc. Natl. Acad. Sci. USA 2015, 112, E2884–E2890. [Google Scholar] [CrossRef]
- Lithgow, T.; Schneider, A. Evolution of macromolecular import pathways in mitochondria, hydrogenosomes and mitosomes. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010, 365, 799–817. [Google Scholar] [CrossRef] [PubMed]
- Mi-ichi, F.; Makiuchi, T.; Furukawa, A.; Sato, D.; Nozaki, T. Sulfate activation in mitosomes plays an important role in the proliferation of Entamoeba histolytica. PLoS Negl. Trop. Dis. 2011, 5, e1263. [Google Scholar] [CrossRef]
- Dolezal, P.; Dagley, M.J.; Kono, M.; Wolynec, P.; Likic, V.A.; Foo, J.H.; Sedinova, M.; Tachezy, J.; Bachmann, A.; Bruchhaus, I.; et al. The essentials of protein import in the degenerate mitochondrion of Entamoeba histolytica. PLoS Pathog. 2010, 6, e1000812. [Google Scholar] [CrossRef] [PubMed]
- Höhr, A.I.C.; Straub, S.P.; Warscheid, B.; Becker, T.; Wiedemann, N. Assembly of β-barrel proteins in the mitochondrial outer membrane. Biochim. Biophys. Acta Mol. Cell Res. 2015, 1853, 74–88. [Google Scholar] [CrossRef]
- Mi-ichi, F.; Nozawa, A.; Yoshida, H.; Tozawa, Y.; Nozaki, T. Evidence that Entamoeba histolytica mitochondrial carrier family links mitosomal and cytosolic pathways through exchange of PAPS and ATP. Eukaryot. Cell 2015, 14, 1144–1150. [Google Scholar] [CrossRef] [PubMed]
- Santos, H.J.; Imai, K.; Hanadate, Y.; Fukasawa, Y.; Oda, T.; Mi-ichi, F.; Nozaki, T. Screening and discovery of lineage-specific mitosomal membrane proteins in Entamoeba histolytica. Mol. Biochem. Parasitol. 2016, 209, 10–17. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, C.; Benning, C. TGD4 involved in endoplasmic reticulum-to-chloroplast lipid trafficking is a phosphatidic acid binding protein. Plant J. 2012, 70, 614–623. [Google Scholar] [CrossRef]
- Rodríguez, M.A.; Martínez-Higuera, A.; Valle-Solis, M.I.; Hernandes-Alejandro, M.; Chávez-Munguía, B.; Figueroa-Gutiérrez, A.H.; Salas-Casas, A. A putative calcium-ATPase of the secretory pathway family may regulate calcium/manganese levels in the Golgi apparatus of Entamoeba histolytica. Parasitol. Res. 2018, 117, 3381–3389. [Google Scholar] [CrossRef]
- Finn, R.D.; Clements, J.; Eddy, S.R. HMMER web server: interactive sequence similarity searching. Nucleic Acids Res. 2011, 39, W29–W37. [Google Scholar] [CrossRef]
- Remmert, M.; Biegert, A.; Hauser, A.; Söding, J. HHblits: Lightning-fast iterative protein sequence searching by HMM-HMM alignment. Nat. Methods 2012, 9, 173–175. [Google Scholar] [CrossRef]
- Aurrecoechea, C.; Barreto, A.; Brestelli, J.; Brunk, B.P.; Caler, E.V.; Fischer, S.; Gajria, B.; Gao, X.; Gingle, A.; Grant, G.; et al. AmoebaDB and MicrosporidiaDB: Functional genomic resources for Amoebozoa and Microsporidia species. Nucl. Acids Res. 2011, 39, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinf. 2009, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hessa, T.; Meindl-Beinker, N.M.; Bernsel, A.; Kim, H.; Sato, Y.; Lerch-Bader, M.; Nilsson, I.; White, S.; Hejine, G.V. Molecular code for transmembrane-helix recognition by the Sec61 translocon. Nature 2007, 450, 1026–1030. [Google Scholar] [CrossRef] [PubMed]
- Söding, J.; Biegert, A.; Lupas, A.N. The HHpred interactive server for protein homology detection and structure prediction. Nucl. Acids Res. 2005, 33, 244–248. [Google Scholar] [CrossRef]
- Biegert, A.; Söding, J. De novo identification of highly diverged protein repeats by probabilistic consistency. Bioinformatics 2008, 24, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Diamond, L.S.; Harlow, D.R.; Cunnick, C.C. A new medium for the axenic cultivation of Entamoeba histolytica and other Entamoeba. Trans. R. Soc. Trop. Med. Hyg. 1978, 72, 431–432. [Google Scholar] [CrossRef]
- Bracha, R.; Nuchamowitz, Y.; Anbar, M.; Mirelman, D. Transcriptional silencing of multiple genes in trophozoites of Entamoeba histolytica. PLoS Pathog. 2006, 2, e48. [Google Scholar] [CrossRef]
- Nakada-Tsukui, K.; Okada, H.; Mitra, B.N.; Nozaki, T. Phosphatidylinositol-phosphates mediate cytoskeletal reorganization during phagocytosis via a unique modular protein consisting of RhoGEF/DH and FYVE domains in the parasitic protozoon Entamoeba histolytica. Cell. Microbiol. 2009, 11, 1471–1491. [Google Scholar] [CrossRef]
- Nozaki, T.; Asai, T.; Kobayashi, S.; Ikegami, F.; Noji, M.; Saito, K.; Takeuchi, T. Molecular cloning and characterization of the genes encoding two isoforms of cysteine synthase in the enteric protozoan parasite Entamoeba histolytica. Mol. Biochem. Parasitol. 1998, 97, 33–44. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real- time quantitative PCR and the 2−ΔΔC T method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.M.; Procter, J.B.; Martin, D.M.A.; Clamp, M.; Barton, G.J. Jalview Version 2-a multiple sequence alignment editor and analysis workbench. Bioinformatics 2009, 25, 1189–1191. [Google Scholar] [CrossRef] [PubMed]
- Makiuchi, T.; Santos, H.J.; Tachibana, H.; Nozaki, T. Hetero-oligomer of dynamin-related proteins participates in the fission of highly divergent mitochondria from Entamoeba histolytica. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Higuera, A.; Salas-Casas, A.; Calixto-Gálvez, M.; Chávez-Munguía, B.; Pérez-Ishiwara, D.G.; Ximénez, C.; Rodríguez, M.A. Identification of calcium-transporting ATPases of Entamoeba histolytica and cellular localization of the putative SERCA. Exp. Parasitol. 2013, 135, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Li, Y.; Hattori, N. Lysosomal defects in ATP13A2 and GBA associated familial Parkinson’s disease. J. Neural Transm. 2017, 124, 1395–1400. [Google Scholar] [CrossRef]
- Fukasawa, Y.; Oda, T.; Tomii, K.; Imai, K. Origin and evolutionary alteration of the mitochondrial import system in eukaryotic lineages. Mol. Biol. Evol. 2017, 34, 1574–1586. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, F.; Pierri, C.L. Structure and function of mitochondrial carriers - Role of the transmembrane helix P and G residues in the gating and transport mechanism. FEBS Lett. 2010, 584, 1931–1939. [Google Scholar] [CrossRef]
- Perdomo, D.; Aït-Ammar, N.; Syan, S.; Sachse, M.; Jhingan, G.; Guillén, N. Cellular and proteomics analysis of the endomembrane system from the unicellular Entamoeba histolytica. J. Proteomics 2015, 112, 125–140. [Google Scholar] [CrossRef]
- Bredeston, L.M.; Caffaro, C.E.; Samuelson, J.; Hirschberg, C.B. Golgi and endoplasmic reticulum functions take place in different subcellular compartments of Entamoeba histolytica. J. Biol. Chem. 2005, 280, 32168–32176. [Google Scholar] [CrossRef]
- Teixeira, J.E.; Huston, C.D. Evidence of a continuous endoplasmic reticulum in the protozoan parasite Entamoeba histolytica. Am. Soc. Microbiol. 2008, 7, 1222–1226. [Google Scholar] [CrossRef]
- González, E.; García De Leon, M.D.C.; Meza, I.; Ocadiz-Delgado, R.; Gariglio, P.; Silva-Olivares, A.; Galindo-Gómez, S.; Shibayama, M.; Morán, P.; Valadez, A.; et al. Entamoeba histolytica calreticulin: an endoplasmic reticulum protein expressed by trophozoites into experimentally induced amoebic liver abscesses. Parasitol. Res. 2011, 108, 439–449. [Google Scholar] [CrossRef]
- Libros-Ziv, P.; Villalobo, E.; Mirelman, D. Entamoeba histolytica: identification and characterization of an N-ethylmaleimide sensitive fusion protein homologue. Exp. Parasitol. 2005, 110, 276–279. [Google Scholar] [CrossRef] [PubMed]
- Perdomo, D.; Aït-Ammar, N.; Syan, S.; Sachse, M.; Jhingan, G.D.; Guillén, N. Data set for the proteomics analysis of the endomembrane system from the unicellular Entamoeba histolytica. Data Br. 2015, 1, 29–36. [Google Scholar] [CrossRef]
- Katritch, V.; Cherezov, V.; Stevens, R.C. Structure-function of the G protein–coupled receptor superfamily. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 531–556. [Google Scholar] [CrossRef]
- Rout, S.; Zumthor, J.P.; Schraner, E.M.; Faso, C.; Hehl, A.B. An interactome-centered protein discovery approach reveals novel components involved in mitosome function and homeostasis in Giardia lamblia. PLoS Pathog. 2016, 12, e1006036. [Google Scholar] [CrossRef] [PubMed]
- Dolman, N.J.; Gerasimenko, J.V.; Gerasimenko, O.V.; Voronina, S.G.; Petersen, O.H.; Tepikin, A.V. Stable Golgi-mitochondria complexes and formation of Golgi Ca2+ gradients in pancreatic acinar cells. J. Biol. Chem. 2005, 280, 15794–15799. [Google Scholar] [CrossRef] [PubMed]


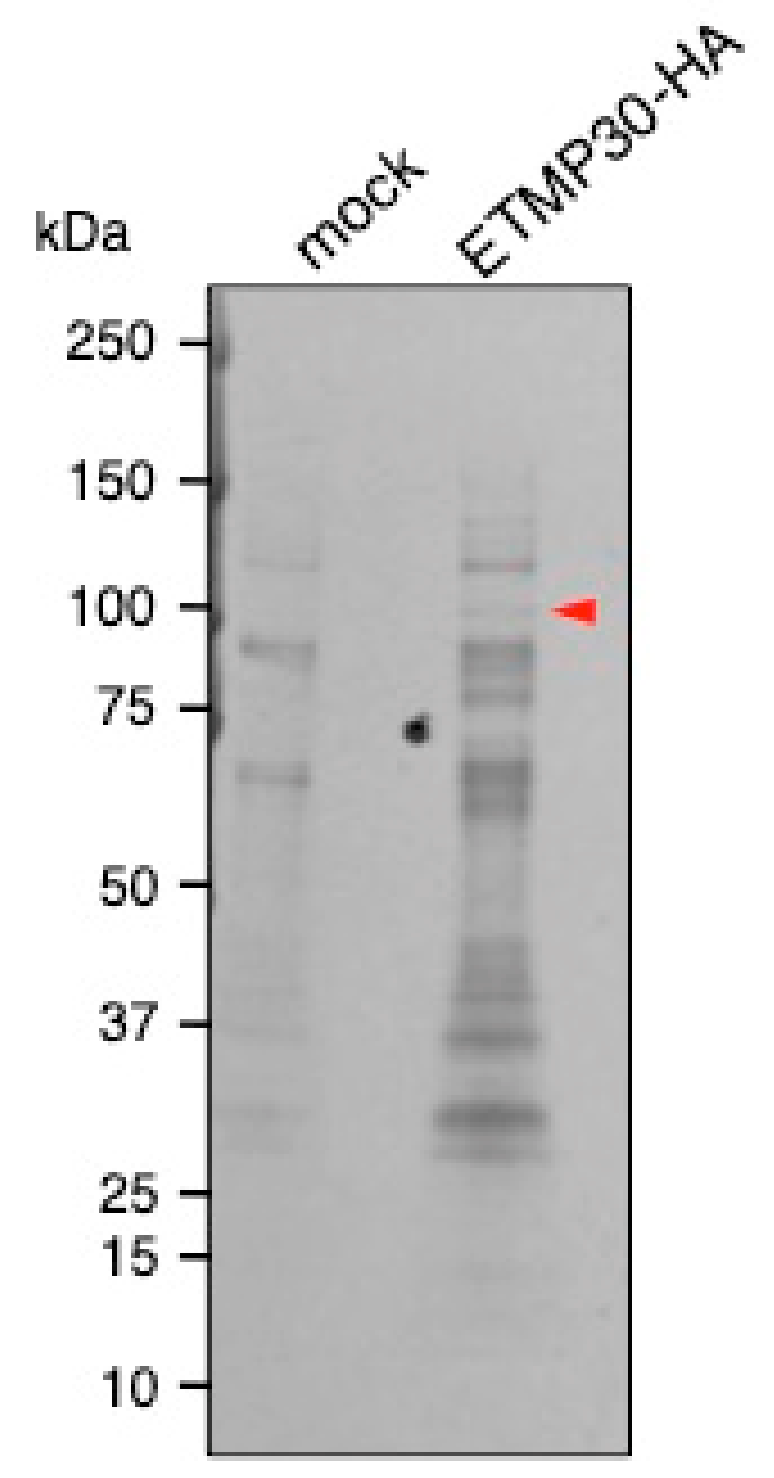
| Annotation | Gene ID | Molecular Weight (kDa) | Coverage (%) | Peptide Number (ETMP30-HA/mock) |
|---|---|---|---|---|
| Cation-transporting P-type ATPase | EHI_065670 | 126 | 9 | 8/0 |
| Pyruvate:ferredoxin oxidoreductase | EHI_051060 | 128 | 3 | 3/0 |
| Plasma membrane calcium-transporting ATPase | EHI_030830 | 114 | 2 | 2/0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, H.J.; Hanadate, Y.; Imai, K.; Nozaki, T. An Entamoeba-Specific Mitosomal Membrane Protein with Potential Association to the Golgi Apparatus. Genes 2019, 10, 367. https://doi.org/10.3390/genes10050367
Santos HJ, Hanadate Y, Imai K, Nozaki T. An Entamoeba-Specific Mitosomal Membrane Protein with Potential Association to the Golgi Apparatus. Genes. 2019; 10(5):367. https://doi.org/10.3390/genes10050367
Chicago/Turabian StyleSantos, Herbert J., Yuki Hanadate, Kenichiro Imai, and Tomoyoshi Nozaki. 2019. "An Entamoeba-Specific Mitosomal Membrane Protein with Potential Association to the Golgi Apparatus" Genes 10, no. 5: 367. https://doi.org/10.3390/genes10050367
APA StyleSantos, H. J., Hanadate, Y., Imai, K., & Nozaki, T. (2019). An Entamoeba-Specific Mitosomal Membrane Protein with Potential Association to the Golgi Apparatus. Genes, 10(5), 367. https://doi.org/10.3390/genes10050367





