Physiological and Genetic Dissection of Sucrose Inputs to the Arabidopsis thaliana Circadian System
Abstract
1. Introduction
2. Methods
2.1. Plant Material
2.2. Growth Conditions and Luciferase Imaging
3. Results
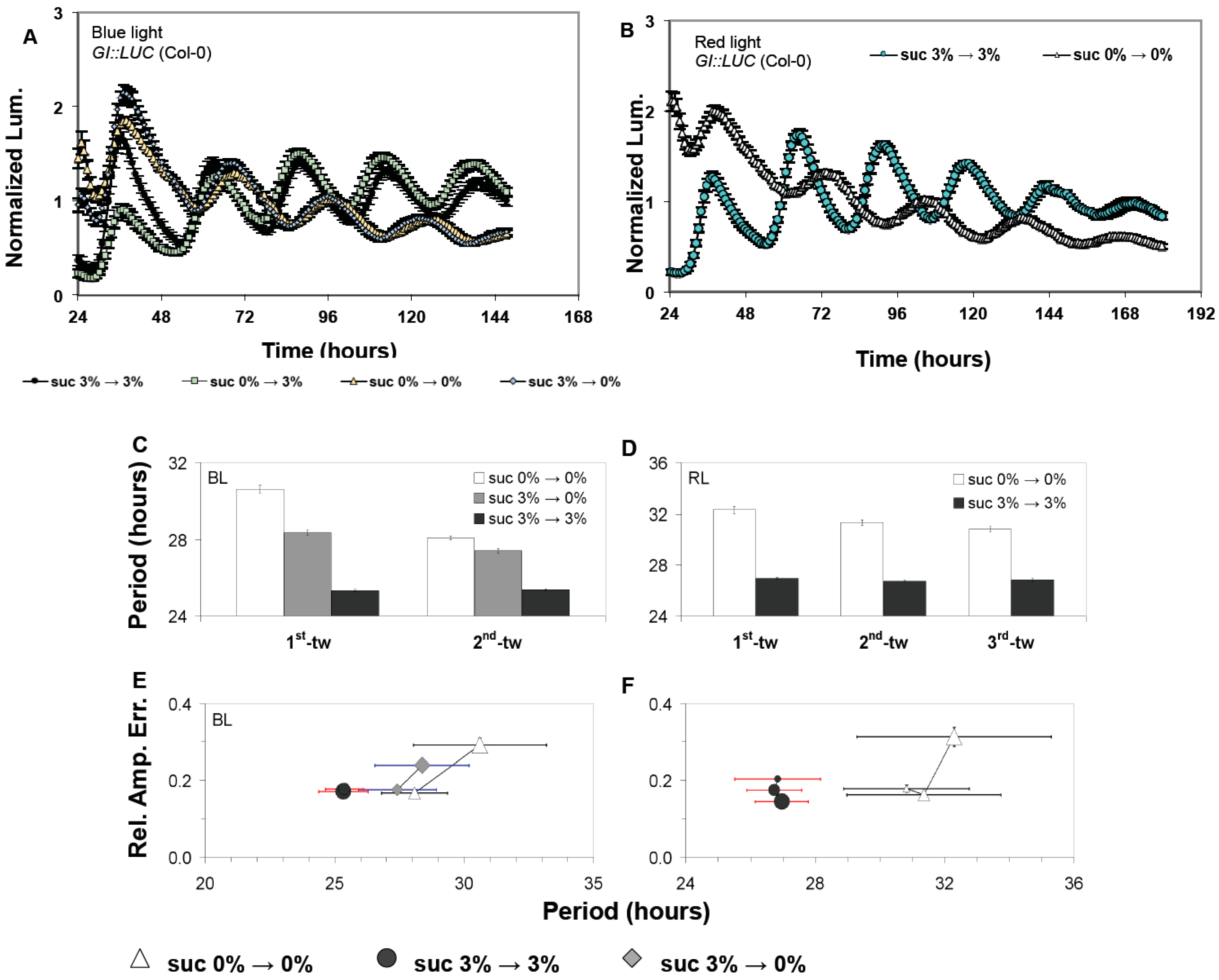
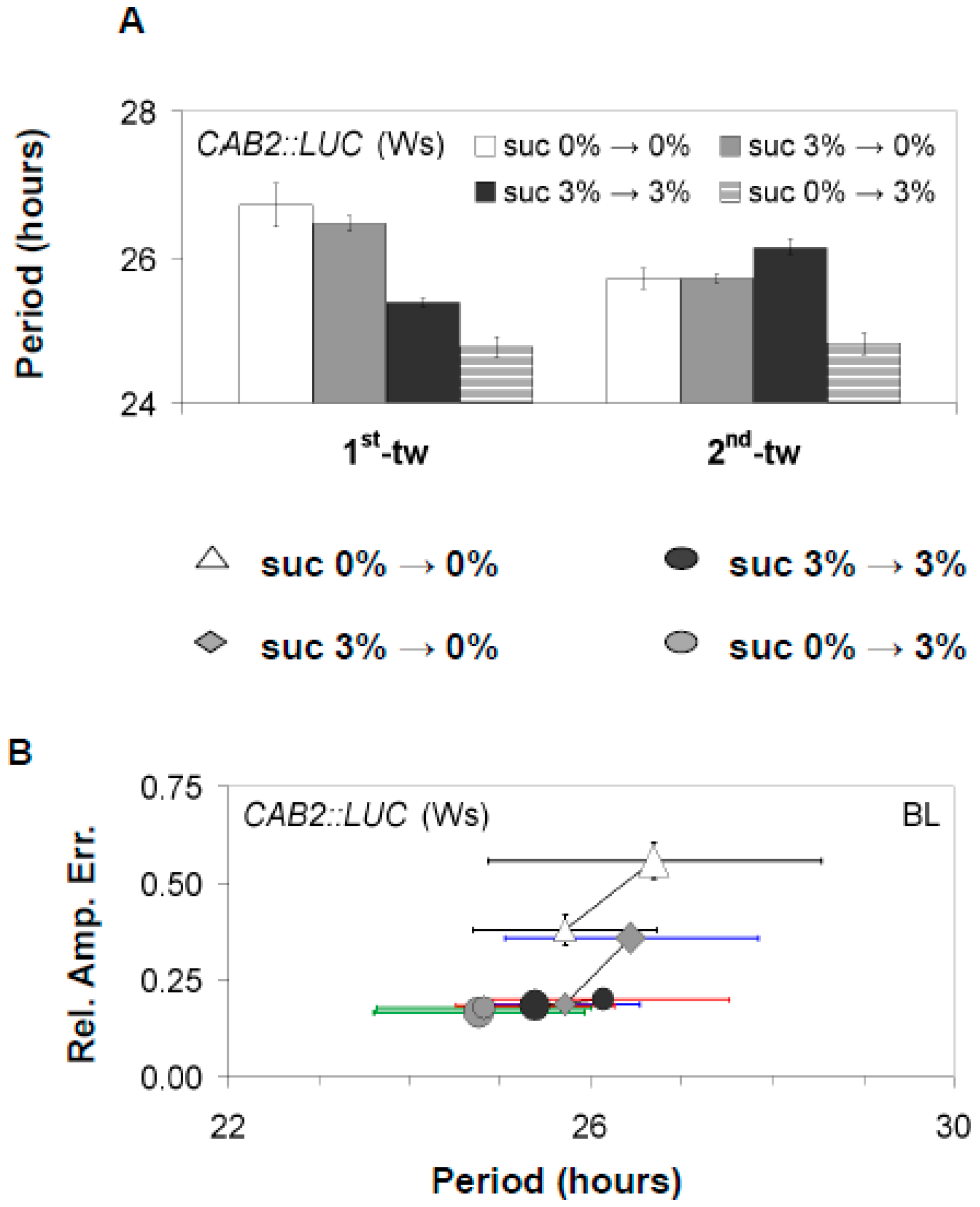
3.1. Confirmations That Sucrose Regulates Free-Running Period of the Oscillator
3.2. The Oscillator Dynamically Responds to Sucrose under Free-Running Conditions
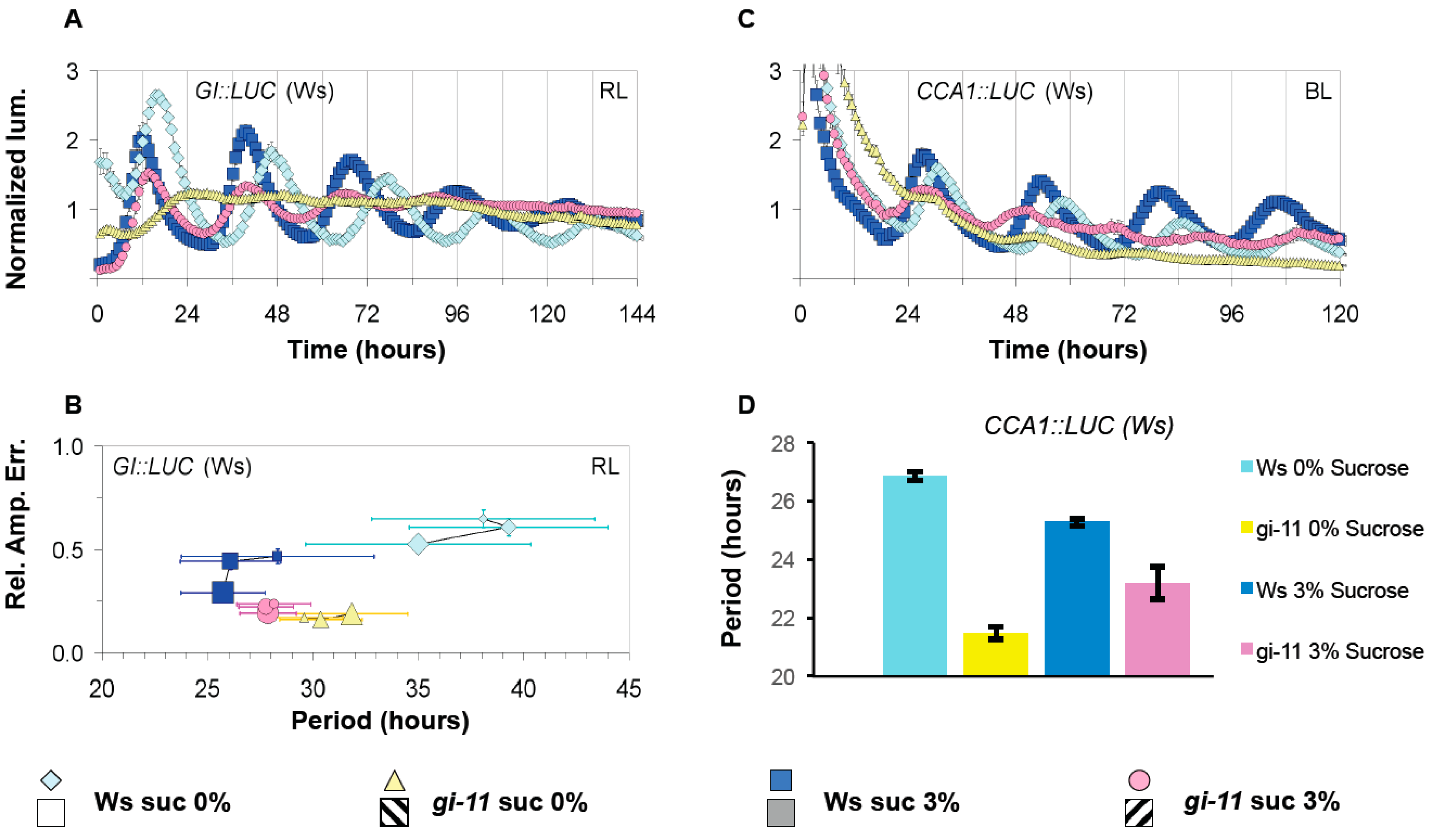
3.3. GI Has a Light Dependent Sucrose Phenotype
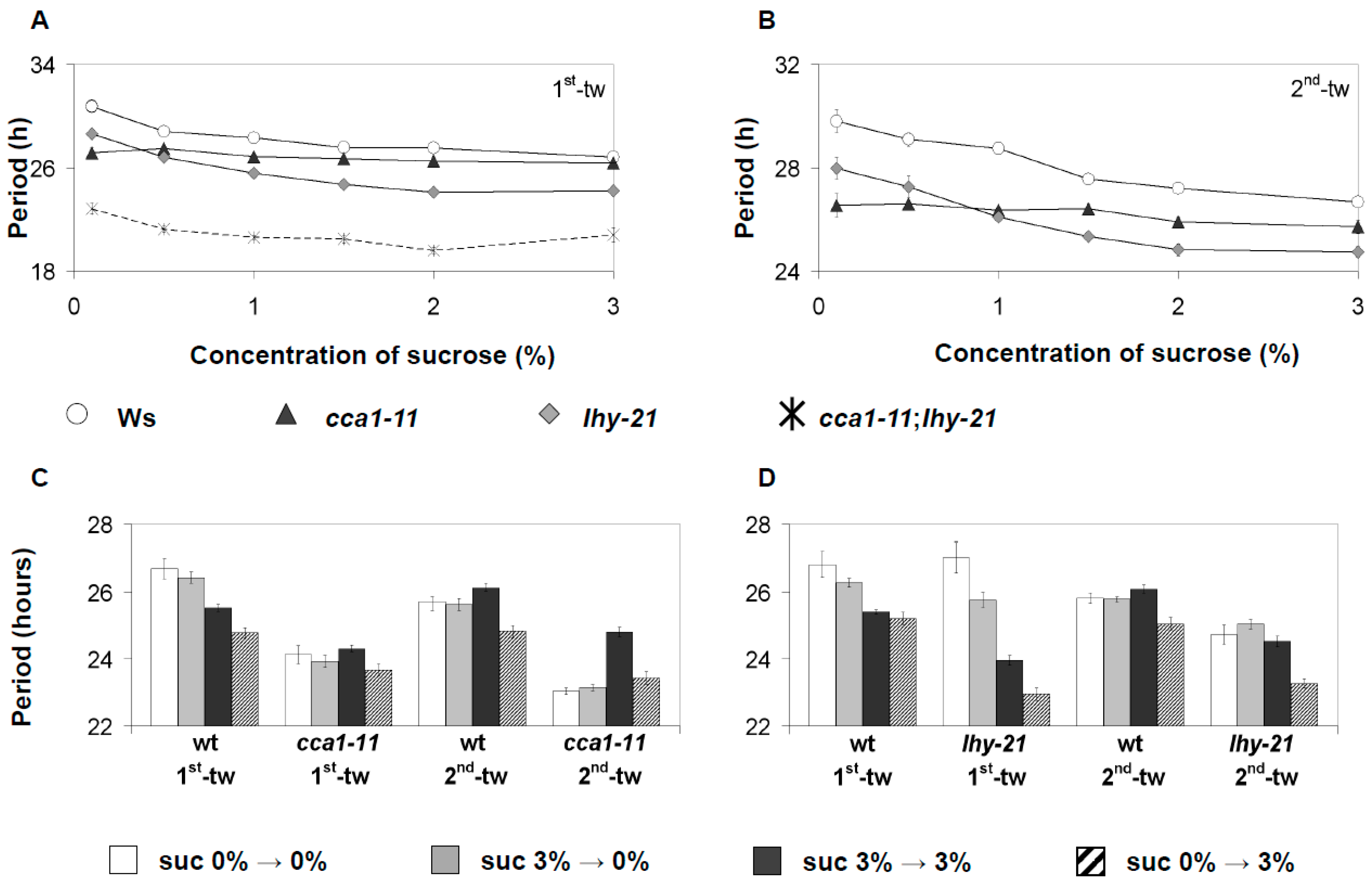
3.4. CCA1 and LHY Have Functionally Distinct Roles in Sucrose Signaling to the Oscillator
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bujdoso, N.; Davis, S.J. Mathematical modeling of an oscillating gene circuit to unravel the circadian clock network of Arabidopsis thaliana. Front. Plant Sci. 2013, 4, 3. [Google Scholar] [CrossRef]
- Hanano, S.; Domagalska, M.A.; Nagy, F.; Davis, S.J. Multiple phytohormones influence distinct parameters of the plant circadian clock. Genes Cells 2006, 11, 1381–1392. [Google Scholar] [CrossRef]
- Sanchez, A.; Shin, J.; Davis, S.J. Abiotic stress and the plant circadian clock. Plant Signal. Behav. 2011, 6, 223–231. [Google Scholar] [CrossRef]
- Shim, J.S.; Kubota, A.; Imaizumi, T. Circadian clock and photoperiodic flowering in Arabidopsis: Constans is a hub for signal integration. Plant Physiol. 2017, 173, 5–15. [Google Scholar] [CrossRef]
- Staiger, D.; Shin, J.; Johansson, M.; Davis, S.J. The circadian clock goes genomic. Genome Biol. 2013, 14, 208. [Google Scholar] [CrossRef]
- Sanchez, S.E.; Kay, S.A. The plant circadian clock: From a simple timekeeper to a complex developmental manager. Cold Spring Harb. Perspect. Biol. 2016, 8, a027748. [Google Scholar] [CrossRef]
- Habte, E.; Müller, L.M.; Shtaya, M.; Davis, S.J.; von Korff, M. Osmotic stress at the barley root affects expression of circadian clock genes in the shoot. Plant Cell Environ. 2014, 37, 1321–1337. [Google Scholar] [CrossRef]
- Müller, L.M.; von Korff, M.; Davis, S.J. Connections between circadian clocks and carbon metabolism reveal species-specific effects on growth control. J. Exp. Bot. 2014, 65, 2915–2923. [Google Scholar] [CrossRef]
- Dodd, A.N.; Salathia, N.; Hall, A.; Kevei, E.; Toth, R.; Nagy, F.; Hibberd, J.M.; Millar, A.J.; Webb, A.A. Plant circadian clocks increase photosynthesis, growth, survival, and competitive advantage. Science 2005, 309, 630–633. [Google Scholar] [CrossRef]
- Haydon, M.J.; Mielczarek, O.; Robertson, F.C.; Hubbard, K.E.; Webb, A.A.R. Photosynthetic entrainment of the Arabidopsis thaliana circadian clock. Nature 2013, 502, 689. [Google Scholar] [CrossRef]
- Tu, B.P.; Kudlicki, A.; Rowicka, M.; McKnight, S.L. Logic of the yeast metabolic cycle: Temporal compartmentalization of cellular processes. Science 2005, 310, 1152–1158. [Google Scholar] [CrossRef]
- Lloyd, D.; Murray, D.B. Redox rhythmicity: Clocks at the core of temporal coherence. Bioessays 2007, 29, 465–473. [Google Scholar] [CrossRef]
- Ding, Z.; Doyle, M.R.; Amasino, R.M.; Davis, S.J. A complex genetic interaction between Arabidopsis thaliana TOC1 and CCA1/LHY in driving the circadian clock and in output regulation. Genetics 2007, 176, 1501–1510. [Google Scholar] [CrossRef][Green Version]
- Gendron, J.M.; Pruneda-Paz, J.L.; Doherty, C.J.; Gross, A.M.; Kang, S.E.; Kay, S.A. Arabidopsis circadian clock protein, TOC1, is a DNA-binding transcription factor. Proc. Nat. Acad. Sci. USA 2012, 109, 3167–3172. [Google Scholar] [CrossRef]
- Mizoguchi, T.; Wheatley, K.; Hanzawa, Y.; Wright, L.; Mizoguchi, M.; Song, H.R.; Carre, I.A.; Coupland, G. LHY and CCA1 are partially redundant genes required to maintain circadian rhythms in Arabidopsis. Dev. Cell 2002, 2, 629–641. [Google Scholar] [CrossRef]
- Nakamichi, N.; Kiba, T.; Henriques, R.; Mizuno, T.; Chua, N.-H.; Sakakibara, H. Pseudo-response regulators 9, 7, and 5 are transcriptional repressors in the Arabidopsis circadian clock. Plant Cell 2010, 22, 594–605. [Google Scholar] [CrossRef]
- Kim, W.-Y.; Fujiwara, S.; Suh, S.-S.; Kim, J.; Kim, Y.; Han, L.; David, K.; Putterill, J.; Nam, H.G.; Somers, D.E. Zeitlupe is a circadian photoreceptor stabilized by Gigantea in blue light. Nature 2007, 449, 356. [Google Scholar] [CrossRef]
- Cha, J.Y.; Kim, J.; Kim, T.S.; Zeng, Q.; Wang, L.; Lee, S.Y.; Kim, W.Y.; Somers, D.E. GIGANTEA is a co-chaperone which facilitates maturation of zeitlupe in the Arabidopsis circadian clock. Nat. Commun. 2017, 8, 3. [Google Scholar] [CrossRef]
- Herrero, E.; Kolmos, E.; Bujdoso, N.; Yuan, Y.; Wang, M.; Berns, M.C.; Uhlworm, H.; Coupland, G.; Saini, R.; Jaskolski, M.; et al. Early flowering4 recruitment of early flowering3 in the nucleus sustains the Arabidopsis circadian clock. Plant Cell 2012, 24, 428–443. [Google Scholar] [CrossRef]
- Anwer, M.U.; Boikoglou, E.; Herrero, E.; Hallstein, M.; Davis, A.M.; James, G.V.; Nagy, F.; Davis, S.J. Natural variation reveals that intracellular distribution of ELF3 protein is associated with function in the circadian clock. eLife 2014, 3, e02206. [Google Scholar] [CrossRef]
- Boikoglou, E.; Ma, Z.; von Korff, M.; Davis, A.M.; Nagy, F.; Davis, S.J. Environmental memory from a circadian oscillator: The Arabidopsis thaliana clock differentially integrates perception of photic versus thermal entrainment. Genetics 2011, 189, 655–664. [Google Scholar] [CrossRef]
- Oakenfull, R.J.; Davis, S.J. Shining a light on the Arabidopsis circadian clock. Plant Cell Environ. 2017, 40, 2571–2585. [Google Scholar] [CrossRef]
- Webb, A.A.R.; Seki, M.; Satake, A.; Caldana, C. Continuous dynamic adjustment of the plant circadian oscillator. Nat. Commun. 2019, 10, 550. [Google Scholar] [CrossRef]
- Knight, H.; Thomson, A.J.W.; McWatters, H.G. Sensitive to freezing6 integrates cellular and environmental inputs to the plant circadian clock. Plant Physiol. 2008, 148, 293–303. [Google Scholar] [CrossRef]
- Dalchau, N.; Baek, S.J.; Briggs, H.M.; Robertson, F.C.; Dodd, A.N.; Gardner, M.J.; Stancombe, M.A.; Haydon, M.J.; Stan, G.-B.; Gonçalves, J.M.; et al. The circadian oscillator gene Gigantea mediates a long-term response of the Arabidopsis thaliana circadian clock to sucrose. Proc. Nat. Acad. Sci. USA 2011, 108, 5104–5109. [Google Scholar] [CrossRef]
- Frank, A.; Matiolli, C.C.; Viana, A.J.C.; Hearn, T.J.; Kusakina, J.; Belbin, F.E.; Wells Newman, D.; Yochikawa, A.; Cano-Ramirez, D.L.; Chembath, A.; et al. Circadian entrainment in Arabidopsis by the sugar-responsive transcription factor bZIP63. Curr. Biol. 2018, 28, 2597–2606, e2596. [Google Scholar] [CrossRef]
- Mair, A.; Pedrotti, L.; Wurzinger, B.; Anrather, D.; Simeunovic, A.; Weiste, C.; Valerio, C.; Dietrich, K.; Kirchler, T.; Nägele, T.; et al. SnRK1-triggered switch of bZIP63 dimerization mediates the low-energy response in plants. eLife 2015, 4, e05828. [Google Scholar] [CrossRef]
- Sánchez-Villarreal, A.; Davis, A.M.; Davis, S.J. AKIN10 activity as a cellular link between metabolism and circadian-clock entrainment in Arabidopsis thaliana. Plant Signal. Behav. 2018, 13, e1411448. [Google Scholar] [CrossRef]
- Sanchez-Villarreal, A.; Shin, J.; Bujdoso, N.; Obata, T.; Neumann, U.; Du, S.X.; Ding, Z.; Davis, A.M.; Shindo, T.; Schmelzer, E.; et al. TIME FOR COFFEE is an essential component in the maintenance of metabolic homeostasis in Arabidopsis thaliana. Plant J. Cell Mol. Biol. 2013, 76, 188–200. [Google Scholar] [CrossRef]
- Shin, J.; Du, S.; Bujdoso, N.; Hu, Y.; Davis, S.J. Overexpression and loss-of-function at time for coffee results in similar phenotypes in diverse growth and physiological responses. J. Plant Biol. 2013, 56, 152–159. [Google Scholar] [CrossRef]
- Shin, J.; Sánchez-Villarreal, A.; Davis, A.M.; Du, S.-X.; Berendzen, K.W.; Koncz, C.; Ding, Z.; Li, C.; Davis, S.J. The metabolic sensor AKIN10 modulates the Arabidopsis circadian clock in a light-dependent manner. Plant Cell Environ. 2017, 40, 997–1008. [Google Scholar] [CrossRef]
- Shor, E.; Paik, I.; Kangisser, S.; Green, R.; Huq, E. Phytochrome interacting factors mediate metabolic control of the circadian system in Arabidopsis. New Phytol. 2017, 215, 217–228. [Google Scholar] [CrossRef]
- Shor, E.; Potavskaya, R.; Kurtz, A.; Paik, I.; Huq, E.; Green, R. PIF-mediated sucrose regulation of the circadian oscillator is light quality and temperature dependent. Genes 2018, 9, 628. [Google Scholar] [CrossRef]
- Haydon, M.J.; Mielczarek, O.; Frank, A.; Román, Á.; Webb, A.A.R. Sucrose and ethylene signaling interact to modulate the circadian clock. Plant Physiol. 2017, 175, 947–958. [Google Scholar] [CrossRef]
- Hall, A.; Kozma-Bognár, L.; Bastow, R.M.; Nagy, F.; Millar, A.J. Distinct regulation of CAB and PHYB gene expression by similar circadian clocks. Plant J. Cell Mol. Biol. 2002, 32, 529–537. [Google Scholar] [CrossRef]
- Ding, Z.; Millar, A.J.; Davis, A.M.; Davis, S.J. Time for coffee encodes a nuclear regulator in the Arabidopsis thaliana circadian clock. Plant Cell 2007, 19, 1522–1536. [Google Scholar] [CrossRef]
- McWatters, H.G.; Kolmos, E.; Hall, A.; Doyle, M.R.; Amasino, R.M.; Gyula, P.; Nagy, F.; Millar, A.J.; Davis, S.J. ELF4 Is required for oscillatory properties of the circadian clock. Plant Physiol. 2007, 144, 391–401. [Google Scholar] [CrossRef]
- Gould, P.D.; Locke, J.C.; Larue, C.; Southern, M.M.; Davis, S.J.; Hanano, S.; Moyle, R.; Milich, R.; Putterill, J.; Millar, A.J.; et al. The molecular basis of temperature compensation in the Arabidopsis circadian clock. Plant Cell 2006, 18, 1177–1187. [Google Scholar] [CrossRef]
- Hanano, S.; Stracke, R.; Jakoby, M.; Merkle, T.; Domagalska, M.A.; Weisshaar, B.; Davis, S.J. A systematic survey in Arabidopsis thaliana of transcription factors that modulate circadian parameters. BMC Genom. 2008, 9, 182. [Google Scholar] [CrossRef]
- Gould, P.D.; Ugarte, N.; Domijan, M.; Costa, M.; Foreman, J.; MacGregor, D.; Rose, K.; Griffiths, J.; Millar, A.J.; Finkenstädt, B.; et al. Network balance via CRY signalling controls the Arabidopsis circadian clock over ambient temperatures. Mol. Syst. Biol. 2013, 9, 650. [Google Scholar] [CrossRef]
- Martin-Tryon, E.L.; Kreps, J.A.; Harmer, S.L. Gigantea acts in blue light signaling and has biochemically separable roles in circadian clock and flowering time regulation. Plant Physiol. 2007, 143, 473–486. [Google Scholar] [CrossRef]
- Hargreaves, J.K.; Knight, M.I.; Pitchford, J.W.; Oakenfull, R.J.; Davis, S.J. Clustering nonstationary circadian rhythms using locally stationary wavelet representations. Multiscale Model. Simul. 2018, 16, 184–214. [Google Scholar] [CrossRef]
- Davis, A.M.; Ronald, J.; Ma, Z.; Wilkinson, A.J.; Philippou, K.; Shindo, T.; Queitsch, C.; Davis, S.J. HSP90 contributes to entrainment of the Arabidopsis circadian clock via the morning loop. Genetics 2018, 210, 1383–1390. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Philippou, K.; Ronald, J.; Sánchez-Villarreal, A.; Davis, A.M.; Davis, S.J. Physiological and Genetic Dissection of Sucrose Inputs to the Arabidopsis thaliana Circadian System. Genes 2019, 10, 334. https://doi.org/10.3390/genes10050334
Philippou K, Ronald J, Sánchez-Villarreal A, Davis AM, Davis SJ. Physiological and Genetic Dissection of Sucrose Inputs to the Arabidopsis thaliana Circadian System. Genes. 2019; 10(5):334. https://doi.org/10.3390/genes10050334
Chicago/Turabian StylePhilippou, Koumis, James Ronald, Alfredo Sánchez-Villarreal, Amanda M. Davis, and Seth J. Davis. 2019. "Physiological and Genetic Dissection of Sucrose Inputs to the Arabidopsis thaliana Circadian System" Genes 10, no. 5: 334. https://doi.org/10.3390/genes10050334
APA StylePhilippou, K., Ronald, J., Sánchez-Villarreal, A., Davis, A. M., & Davis, S. J. (2019). Physiological and Genetic Dissection of Sucrose Inputs to the Arabidopsis thaliana Circadian System. Genes, 10(5), 334. https://doi.org/10.3390/genes10050334




