A Novel Aquaporin 12-like Protein from Chilo suppressalis: Characterization and Functional Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. RNA Isolation and Molecular Cloning of Full-Length CsAqp12L
2.3. Sample Preparation and Exposure to Temperature and RH
2.4. Real-Time Quantitative PCR (qPCR)
2.5. Expression in Xenopus Oocytes
2.6. Bioinformatic and Phylogenetic Analyses
2.7. Data Analysis
3. Results
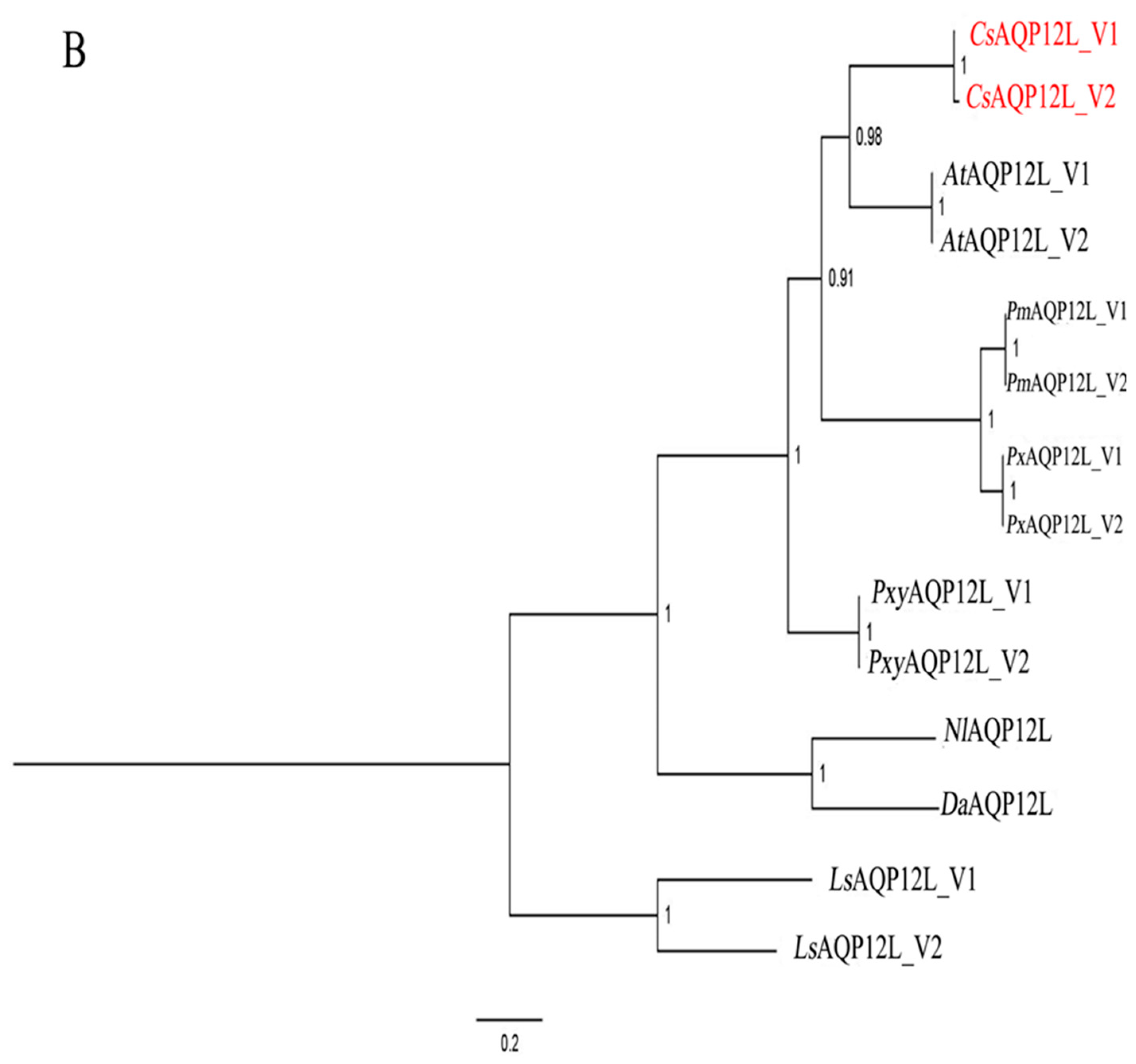
3.1. Sequence and Phylogenetic Analysis of CsAQP12L
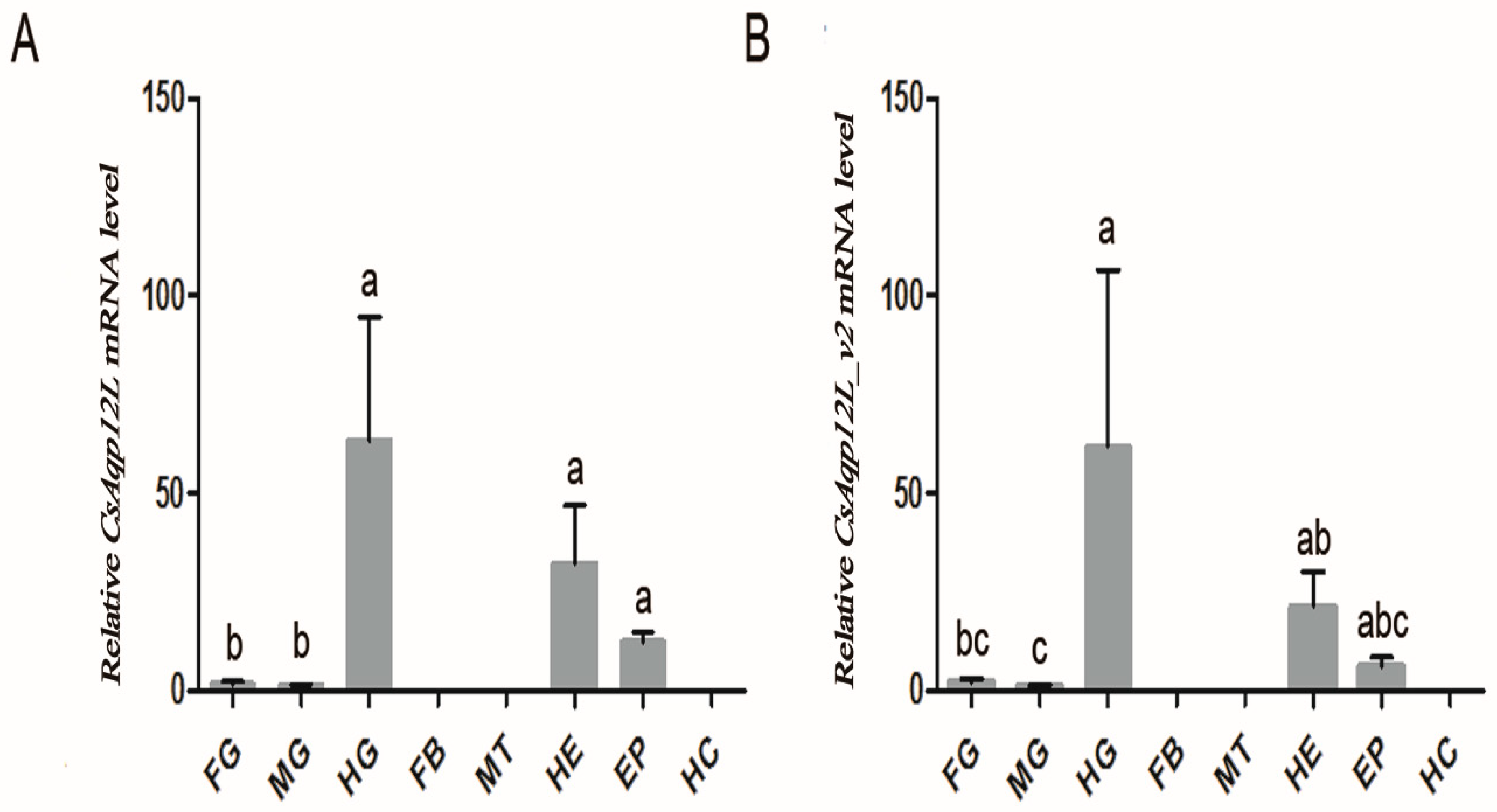
3.2. CsAqp12L Expression in Tissues and Organs of C. suppressalis
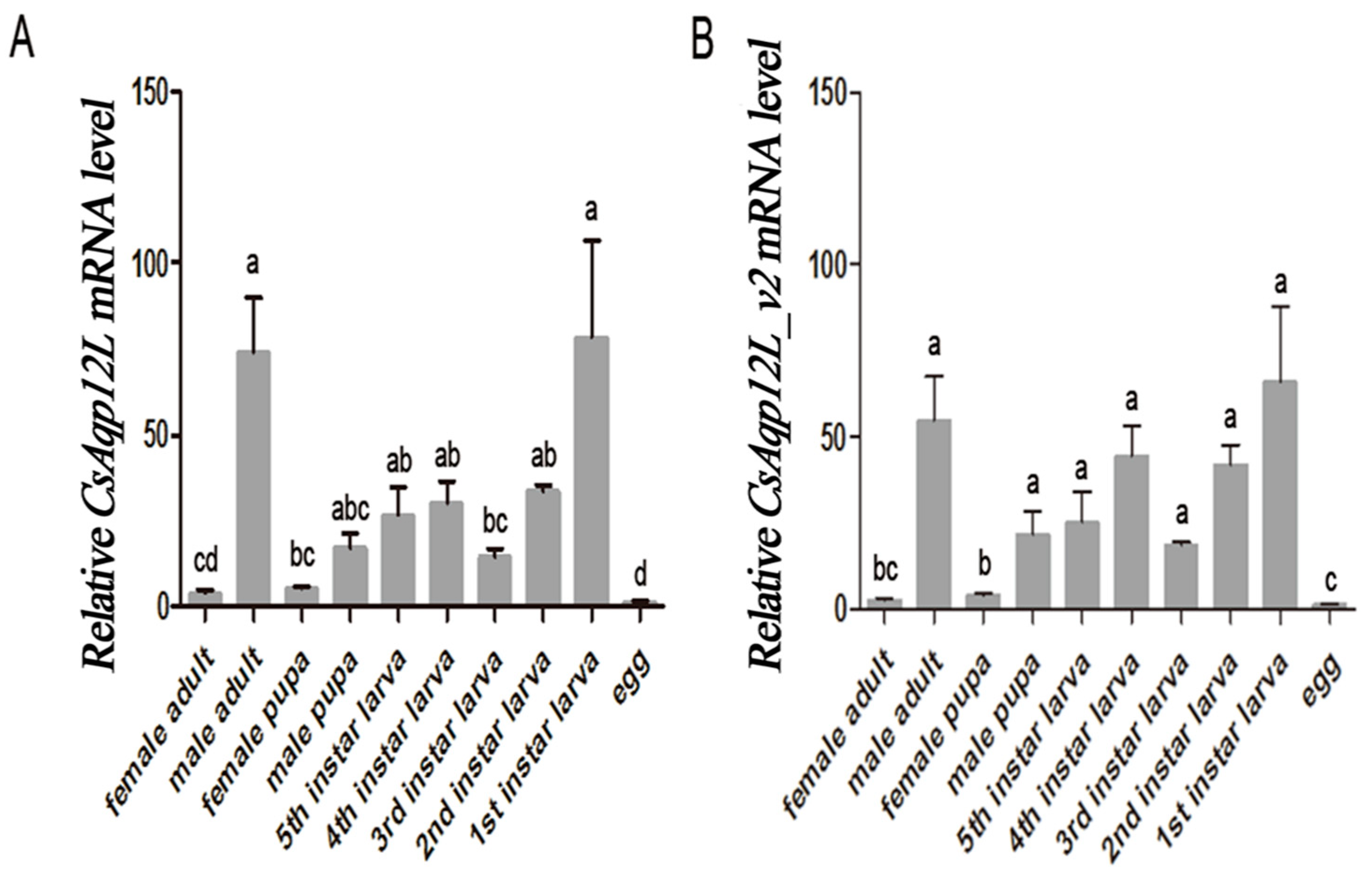
3.3. CsAqp12L Expression in Different Developmental Stages of C. Suppressalis
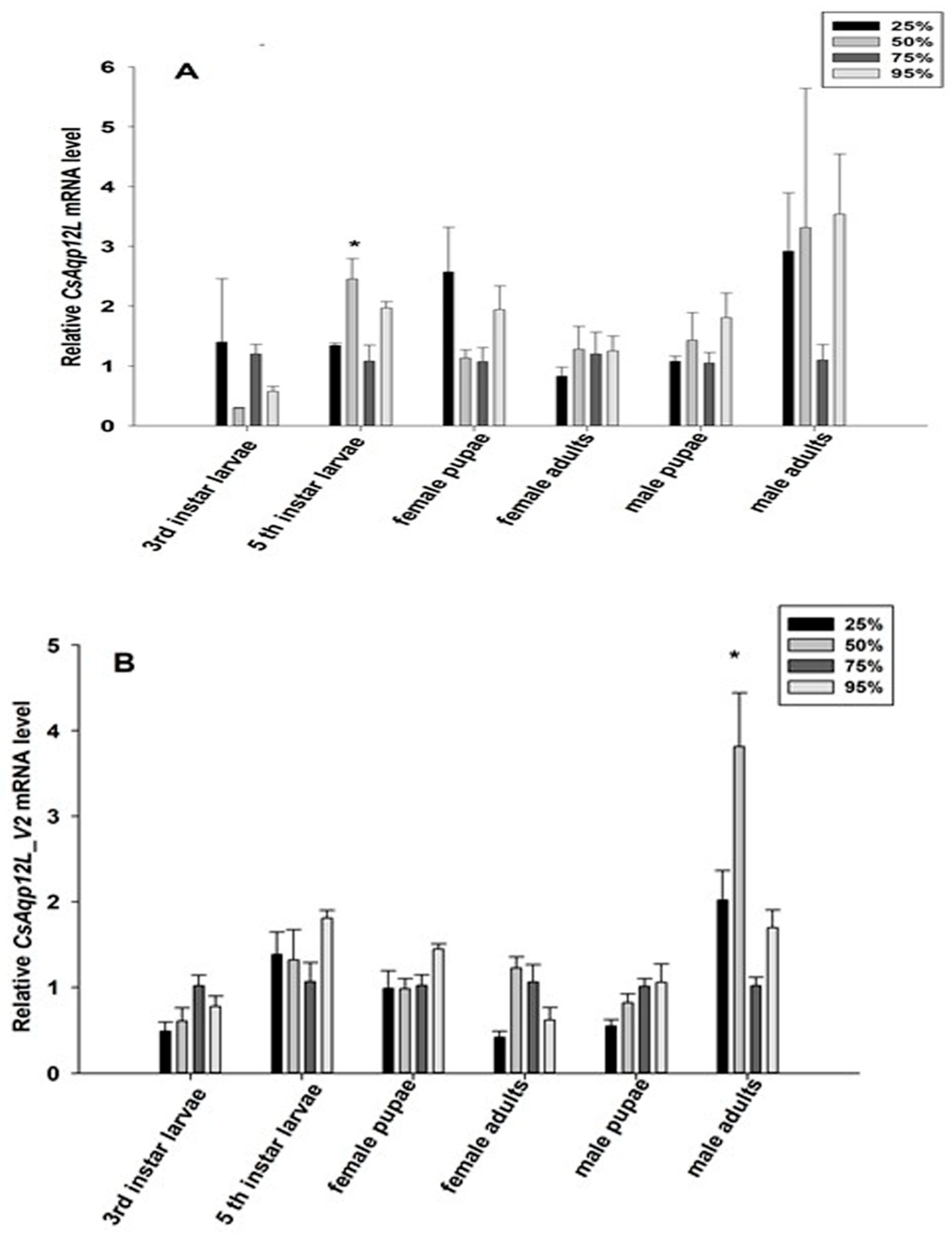
3.4. CsAqp12L Expression in Response to Different RH Levels and Temperatures
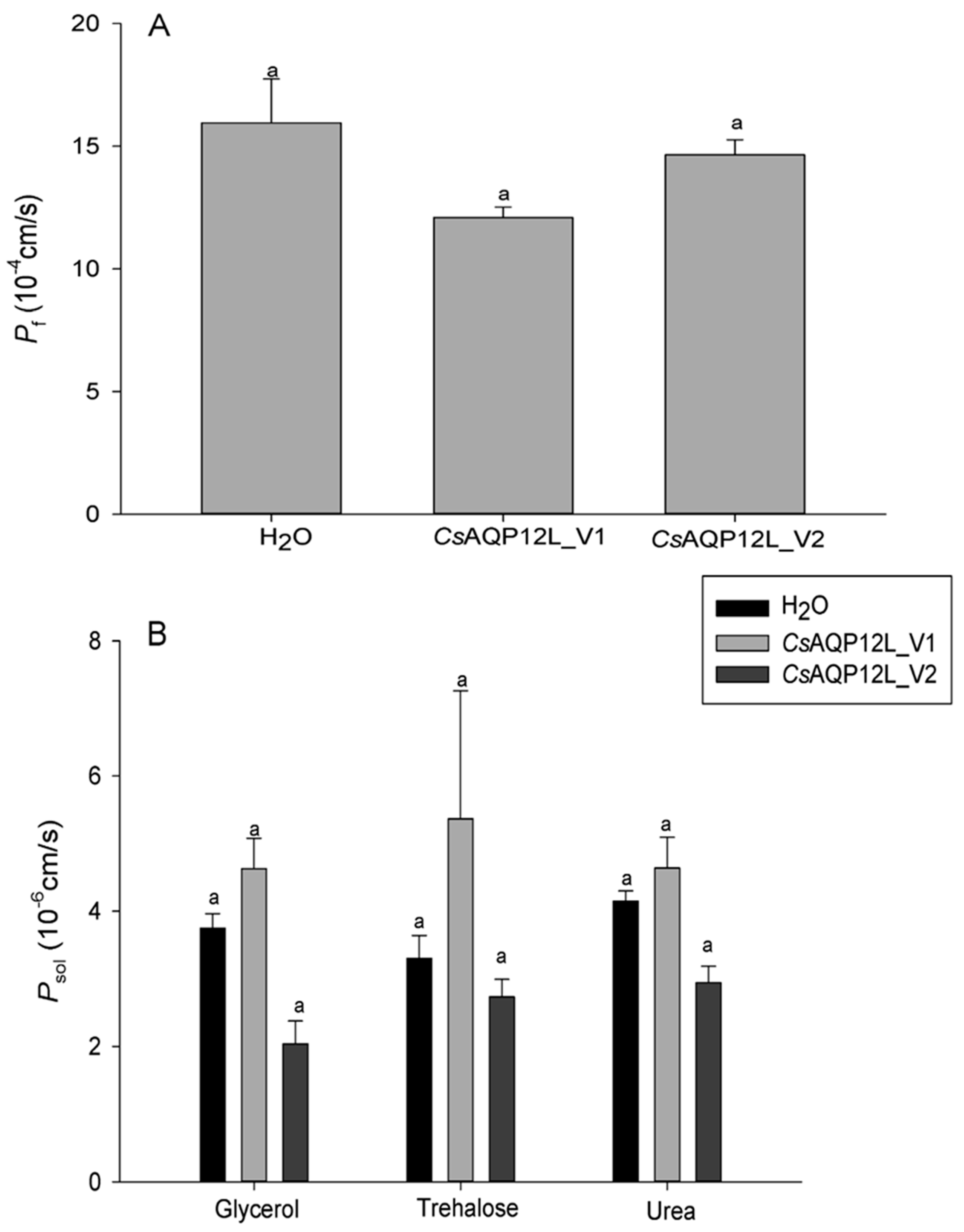
3.5. Water and Solute Permeability Assays in Xenopus Oocytes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- King, L.S.; Kozono, D.; Agre, P. From structure to disease: The evolving tale of aquaporin biology. Nat. Rev. Mol. Cell Biol. 2004, 5, 687–698. [Google Scholar] [CrossRef]
- Gomes, D.; Agasse, A.; Thiébaud, P.; Delrot, S.; Gerós, H.; Chaumont, F. Aquaporins are multifunctional water and solute transporters highly divergent in living organisms. Biochim. Biophys. Acta 2009, 1788, 1213–1228. [Google Scholar] [CrossRef] [PubMed]
- Shakesby, A.J.; Wallace, I.S.; Isaacs, H.V.; Pritchard, J.; Roberts, D.M.; Douglas, A.E. A water-specific aquaporin involved in aphid osmoregulation. Insect Biochem. Mol. Biol. 2009, 39, 1–10. [Google Scholar] [CrossRef]
- Liu, K.; Tsujimoto, H.; Cha, S.J.; Agre, P.; Rasgon, J.L. Aquaporin water channel AgAQP1 in the malaria vector mosquito Anopheles gambiae during blood feeding and humidity adaptation. Proc. Natl. Acad. Sci. USA 2011, 108, 6062–6066. [Google Scholar] [CrossRef] [PubMed]
- Spring, J.H.; Robichaux, S.R.; Hamlin, J.A. The role of aquaporins in excretion in insects. J. Exp. Biol. 2009, 212, 358–362. [Google Scholar] [CrossRef]
- Philip, B.N.; Kiss, A.J.; Lee, R.E. The protective role of aquaporins in the freeze-tolerant insect Eurosta solidaginis: Functional characterization and tissue abundance of EsAQP1. J. Exp. Biol. 2011, 214, 848–857. [Google Scholar] [CrossRef]
- Benoit, J.B.; Hansen, I.A.; Attardo, G.M.; Mireji, P.O.; Bargul, J.L.; Drake, L.L.; Masiga, D.K.; Aksoy, S. Aquaporins are critical for provision of water during lactation and intrauterine progeny hydration to maintain tsetse fly reproductive success. PLoS Neg. Trop Dis. 2014, 8, e2517. [Google Scholar] [CrossRef] [PubMed]
- Benoit, J.B.; Hansen, I.A.; Szuter, E.M.; Drake, L.L.; Burnett, D.L.; Attardo, G.M. Emerging roles of aquaporins in relation to the physiology of blood-feeding arthropods. J. Comp. Physiol. B 2014, 18, 4811–4825. [Google Scholar] [CrossRef] [PubMed]
- Engel, A.; Stahlberg, H. Aquaglyceroporins: channel proteins with a conserved core, multiple functions, and variable surfaces. Int. Rev. Cytol. 2002, 215, 75–104. [Google Scholar] [PubMed]
- Finn, R.N.; Cerdà, J. Evolution and functional diversity of aquaporins. Biol. Bull. 2015, 229, 6–23. [Google Scholar] [CrossRef] [PubMed]
- Mathai, J.C.; Agre, P. Hourglass pore-forming domains restrict aquaporin-1 tetramer assembly. Biochemistry 1999, 38, 923–928. [Google Scholar] [CrossRef]
- Jung, J.S.; Preston, G.M.; Smith, B.L.; Guggino, W.B.; Agre, P. Molecular structure of the water channel through aquaporin CHIP. The hourglass model. J. Biol. Chem. 1994, 269, 14648–14654. [Google Scholar] [PubMed]
- Fu, D.; Libson, A.; Miercke, L.J.; Weitzman, C.; Nollert, P.; Krucinski, J.; Stroud, R.M. Structure of a glycerol-conducting channel and the basis for its selectivity. Science 2009, 290, 481–486. [Google Scholar] [CrossRef]
- Stavang, J.A.; Chauvigné, F.; Kongshaug, H.; Cerdà, J.; Nilsen, F.; Finn, R.N. Phylogenomic and functional analyses of salmon lice aquaporins uncover the molecular diversity of the superfamily in Arthropoda. BMC Genom. 2015, 16, 618. [Google Scholar] [CrossRef] [PubMed]
- Fabrick, J.A.; Pei, J.; Hull, J.J.; Yool, A.J. Molecular and functional characterization of multiple aquaporin water channel proteins from the western tarnished plant bug, Lygus Hesperus. Insect Biochem. Mol. Biol. 2014, 45, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Van Ekert, E.; Chauvigné, F.; Finn, R.N.; Mathew, L.G.; Hull, J.J.; Cerdà, J.; Fabrick, J.A. Molecular and functional characterization of Bemisia tabaci aquaporins reveals the water channel diversity of hemipteran insects. Insect Biochem. Mol. Biol. 2016, 77, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Denlinger, D.L.; Piermarini, P.M. The diapause program impacts renal excretion and molecular expression of aquaporins in the northern house mosquito, Culex pipiens. J. Insect Physiol. 2017, 98, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Itoh, T.; Rai, T.; Kuwahara, M.; Ko, S.B.; Uchida, S.; Sasaki, S.; Ishibashi, K. Identification of a novel aquaporin, AQP12, expressed in pancreatic acinar cells. Biochem. Biophys. Res. Commun. 2005, 330, 832–838. [Google Scholar] [CrossRef] [PubMed]
- Shang, Z.Z.; Wang, Y.S.; Zou, Y.H. Study on rearing method of rice stem borer Chilo suppressalis Walker. Acta Entomol. Sin. 1979, 2, 164–167. (In Chinese) [Google Scholar]
- Yin, C.; Liu, Y.; Liu, J.; Xiao, H.; Huang, S.; Lin, Y.; Han, Z.; Li, F. ChiloDB: A genomic and transcriptome database for an important rice insect pest Chilo suppressalis. Database 2014, 15, 92–108. [Google Scholar] [CrossRef]
- Xu, J.; Lu, M.X.; Cui, Y.D.; Du, Y.Z. Selection and evaluation of reference genes forexpression analysis using qRT-PCR in Chilo suppressalis (Lepidoptera: Pyralidae). J. Econ. Entomol. 2017, 110, 683–691. [Google Scholar] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.B.; Logee, K.A.; Verkman, A.S. Expression of mRNA coding for kidney and red cell water channels in Xenopus oocytes. J. Biol. Chem. 1990, 265, 15375–15378. [Google Scholar] [PubMed]
- Kataoka, N.; Miyake, S.; Azuma, M. Molecular characterization of aquaporin and aquaglyceroporin in the alimentary canal of Grapholita molesta (the oriental fruit moth)-comparison with Bombyx mori aquaporins. J. Insect Biotechnol. 2009, 78, 81–90. [Google Scholar]
- Chang, H.; Liu, Y.; Yang, T.; Pelosi, P.; Dong, S.; Wang, G. Pheromone binding proteins enhance the sensitivity of olfactory receptors to sex pheromones in Chilo suppressalis. Sci. Rep. 2015, 5, 13093. [Google Scholar] [CrossRef]
- Le Cahérec, F.; Bron, P.; Verbavatz, J.M.; Garret, A.; Morel, G.; Cavalier, A.; Hubert, J.F. Incorporation of proteins into (Xenopus) oocytes by proteoliposome microinjection: Functional characterization of a novel aquaporin. J. Cell Sci. 1996, 109, 1285–1295. [Google Scholar] [PubMed]
- Duchesne, L.; Hubert, J.F.; Verbavatz, J.M.; Thomas, D.; Pietrantonio, P.V. Mosquito (Aedes aegypti) aquaporin, present in tracheolar cells, transports water, not glycerol, and forms orthogonal arrays in Xenopus oocyte membranes. Eur. J. Biochem. 2003, 270, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Hohna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Marusalin, J.; Matier, B.J.; Rheault, M.R.; Donini, A. Aquaporin homologs and water transport in the anal papillae of the larval mosquito, Aedes aegypti. J. Comp. Physiol. B 2012, 182, 1047–1056. [Google Scholar] [CrossRef]
- Lu, M.X.; Pan, D.D.; Xu, J.; Liu, Y.; Wang, G.R.; Du, Y.Z. Identification and functional analysis of the first aquaporin from striped stem borer, Chilo suppressalis. Front. Physiol. 2018, 9, 57. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.N.; Chauvigné, F.; Stavang, J.A.; Belles, X.; Cerdà, J. Insect glycerol transporters evolved by functional co-option and gene replacement. Nat. Commun. 2015, 6, 7814. [Google Scholar] [CrossRef] [PubMed]
- Kikawada, T.; Saito, A.; Kanamori, Y.; Fujita, M.; Śnigórska, K.; Watanabe, M.; Okuda, T. Dehydration-inducible changes in expression of two aquaporins in the sleeping chironomid, Polypedilum vanderplanki. Biochim. Biophys. Acta 2008, 1778, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Ohta, E.; Itoh, T.; Nemoto, T.; Kumagai, J.; Ko, S.B.; Ishibashi, K.; Uchida, S. Pancreas-specific aquaporin 12 null mice showed increased susceptibility to caerulein-induced acute pancreatitis. Am. J. Physiol. Cell Physiol. 2009, 297, 1368–1378. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, K.; Tanaka, Y.; Morishita, Y. The role of mammalian superaquaporins inside the cell. Biochim. Biophys. Acta 2014, 1840, 1507–1512. [Google Scholar] [CrossRef]
- Tsujimoto, H.; Liu, K.; Linser, P.J.; Agre, P.; Rasgon, J.L. Organ-specific splice variants of aquaporin water channel AgAQP1 in the malaria vector Anopheles gambiae. PLoS ONE 2013, 8, e75888. [Google Scholar] [CrossRef]


| Primer Name | Primer Sequences (5′ → 3′) | Tm (°C) | Length (bp) |
|---|---|---|---|
| Cloning Aqp12L fragment | |||
| Aqp12L-F | GCCTTCATCGGGACTTCTTTA | 65–45 | 325 |
| Aqp12L-R | CGTTGACTTCTGTAGTGCCCC | ||
| 5′- and 3′-Race PCR | |||
| Aqp12L 5′ | TAGCAACCTGCCTATGTAGACTAGCCTC | 68 | 941 |
| Aqp12L 3′ | AACTGGGGTGATGCGACAGCTTGTC | 1225 | |
| Vector construction | |||
| V-AQP12L-F | TCAACTAGTGCCACCATGAAGTTCACAATCGATGTATTG | 68 | 873 + 975 |
| V-AQP12L-R | TCAGCGGCCGCTTAATCTTCTTTGTCCGCCC | ||
| qRT-PCR | |||
| qPCR- Aqp12L-F | GGGGACTTGAATCCTCGGTT | 57 | 125 + 227 |
| qPCR- Aqp12L-R | CCTGCTTTCAGTGAGGTGGC | ||
| qPCR- Aqp12L_v2-F | CGGTATCAGGACAATAGTGCCA | 56.5 | 158 |
| qPCR- Aqp12L_v2-R | GCGTGTTCCAACAGCGAGT | ||
| Ef1-F | AAAATGGACTCGACTGAACCCC | 56.6 | 137 |
| Ef1-R | TCTCCGTGCCAACCAGAAATA | ||
| H3-F | TGACGAAACCCCTTCGCTT | 56 | 184 |
| H3-R | CCCAGGTCGGTCTTGAAATCT | ||
| 18S-F | GTGATGGGACGAGTGCTTTTATT | 62.5 | 258 |
| 18S-R | GCTGCCTTCCTTGGATGTGG | ||
| Actin-F | AAAGAAACAGCAAAAGTCGGGG | 56 | 243 |
| Actin-R | GTTCAATGGAGGTTCGGTAAGTAAA | ||
| Ubi-F | TCACCGACAGCAAACCAGACT | 60.2 | 219 |
| Ubi-R | GGAAGAAAACACCCCCCTCATATA | ||
| Tub-F | GAGGGCATGGACGAGATGGA | 60.4 | 178 |
| Tub-R | ACGACGGTACGAGTATGACGGG | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, M.-X.; Song, J.; Xu, J.; Wang, G.; Liu, Y.; Du, Y.-Z. A Novel Aquaporin 12-like Protein from Chilo suppressalis: Characterization and Functional Analysis. Genes 2019, 10, 311. https://doi.org/10.3390/genes10040311
Lu M-X, Song J, Xu J, Wang G, Liu Y, Du Y-Z. A Novel Aquaporin 12-like Protein from Chilo suppressalis: Characterization and Functional Analysis. Genes. 2019; 10(4):311. https://doi.org/10.3390/genes10040311
Chicago/Turabian StyleLu, Ming-Xing, Jie Song, Jing Xu, Guirong Wang, Yang Liu, and Yu-Zhou Du. 2019. "A Novel Aquaporin 12-like Protein from Chilo suppressalis: Characterization and Functional Analysis" Genes 10, no. 4: 311. https://doi.org/10.3390/genes10040311
APA StyleLu, M.-X., Song, J., Xu, J., Wang, G., Liu, Y., & Du, Y.-Z. (2019). A Novel Aquaporin 12-like Protein from Chilo suppressalis: Characterization and Functional Analysis. Genes, 10(4), 311. https://doi.org/10.3390/genes10040311




