Genome-Wide Analysis of the Zn(II)2Cys6 Zinc Cluster-Encoding Gene Family in Tolypocladium guangdongense and Its Light-Induced Expression
Abstract
1. Introduction
2. Materials and Methods
2.1. Identification of C6 Zinc Genes and C6-TFs
2.2. Sequence Analysis of C6 Zinc Proteins
2.3. Chromosomal Mapping and Protein Motif Analysis of C6 Zinc Genes
2.4. Sequence Alignment and Phylogenetic Tree Construction
2.5. Expression Analysis of C6 Zinc Cluster Genes under Different Light Conditions
3. Results
3.1. Identification of C6 Zinc Cluster Genes in Tolypocladium guangdongense
3.2. Sub-Grouping of C6 Zinc Proteins of Tolypocladium guangdongense
3.3. Characteristics and Classification of C6 Zinc Proteins in Tolypocladium guangdongense
3.4. Chromosomal Distribution of C6 Zinc Genes in Tolypocladium guangdongense Genome
3.5. Functional Analysis of C6 Zinc Proteins Associated with Metabolic Process in Tolypocladium guangdongense
3.6. Function Analysis of C6 Zinc Proteins Associated with Fruiting Body Development in Tolypocladium guangdongense
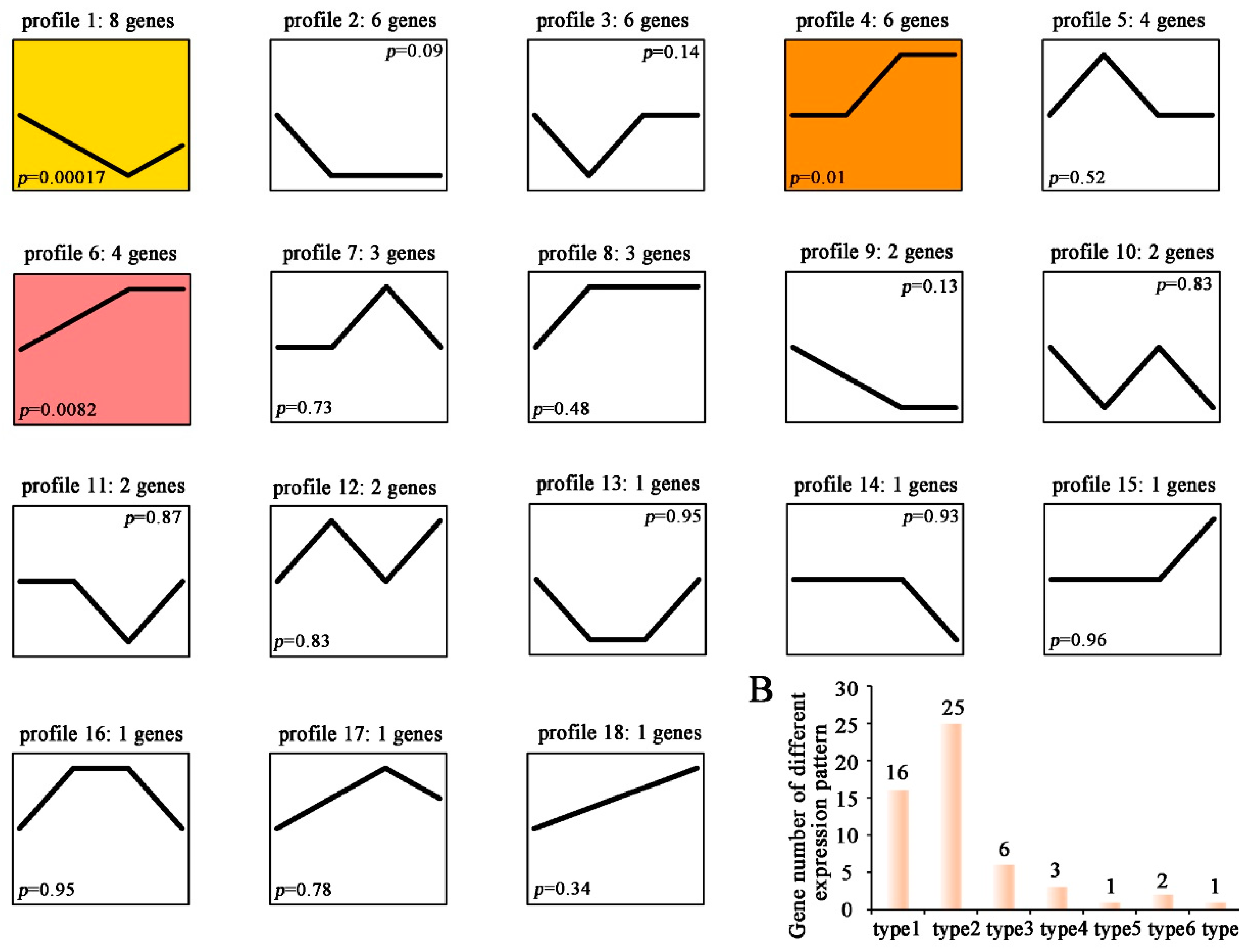
3.7. Expression Profile of C6 Zinc Proteins in Tolypocladium guangdongense under Light Conditions
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- MacPherson, S.; Larochelle, M.; Turcotte, B. A fungal family of transcriptional regulators: The zinc cluster proteins. Microbiol. Mol. Biol. Rev. 2006, 70, 583–604. [Google Scholar] [CrossRef] [PubMed]
- Vallee, B.L.; Coleman, J.E.; Auld, D.S. Zinc fingers, zinc clusters, and zinc twists in DNA-binding protein domains. Proc. Natl. Acad. Sci. USA 1991, 88, 999–1003. [Google Scholar] [CrossRef] [PubMed]
- Marmorstein, R.; Carey, M.; Ptashne, M.; Harrison, S.C. DNA recognition by GAL4: Structure of a protein-DNA complex. Nature 1992, 356, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.; Ehrlich, K.C. Genome-wide analysis of the Zn(II)(2)Cys(6) zinc cluster-encoding gene family in Aspergillus flavus. Appl. Microbiol. Biotechnol. 2013, 97, 4289–4300. [Google Scholar] [CrossRef] [PubMed]
- Hedges, D.; Proft, M.; Entian, K. CAT8, a new zinc cluster-encoding gene necessary for derepression of gluconeogenic enzymes in the yeast Saccharomyces cerevisiae. Mol. Cell. Biol. 1995, 15, 1915–1922. [Google Scholar] [CrossRef] [PubMed]
- Vincent, O.; Carlson, M. Sip4, a Snf1 kinase-dependent transcriptional activator, binds to the carbon source-responsive element of gluconeogenic genes. EMBO J. 1998, 17, 7002–7008. [Google Scholar] [CrossRef] [PubMed]
- Creusot, F.; Verdiere, J.; Gaisne, M.; Slonimski, P.P. CYP1 (HAP1) regulator of oxygen-dependent gene expression in yeast. I. Overall organization of the protein sequence displays several novel structural domains. J. Mol. Biol. 1988, 204, 263–276. [Google Scholar] [CrossRef]
- Pfeifer, K.; Kim, K.S.; Kogan, S.; Guarente, L. Functional dis-section and sequence of yeast HAP1 activator. Cell 1989, 56, 291–301. [Google Scholar] [CrossRef]
- Nishimura, H.; Kawasaki, Y.; Kaneko, Y.; Nosaka, K.; Iwashima, A. Cloning and characteristics of a positive regulatory gene, THI2 (PHO6), of thiamin biosynthesis in Saccharomyces cerevisiae. FEBS Lett. 1992, 297, 155–158. [Google Scholar] [CrossRef]
- Jackson, J.C.; Lopes, J.M. The yeast UME6 gene is required for both negative and positive transcriptional regulation of phospholipid biosynthetic gene expression. Nucleic Acids Res. 1996, 24, 1322–1329. [Google Scholar] [CrossRef] [PubMed]
- Ness, F.; Bourot, S.; Regnacq, M.; Spagnoli, R.; Berges, T.; Karst, F. SUT1 is a putative Zn[II]2Cys6-transcription factor whose upregulation enhances both sterol uptake and synthesis in aerobically growing Saccharomyces cerevisiae cells. Eur. J. Biochem. 2001, 268, 1585–1595. [Google Scholar] [CrossRef] [PubMed]
- Vik, A.; Rine, J. Upc2p and Ecm22p, dual regulators of sterol biosynthesis in Saccharomyces cerevisiae. Mol. Cell. Biol. 2001, 21, 6395–6405. [Google Scholar] [CrossRef] [PubMed]
- Larochelle, M.; Drouin, S.; Robert, F.; Turcotte, B. Oxidative stress-activated zinc cluster protein Stb5 has dual activator/repressor functions required for pentose phosphate pathway regulation and NADPH production. Mol. Cell. Biol. 2006, 26, 6690–6701. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.Y.; Han, S.Y.; Choi, H.G.; Kim, J.H.; Han, K.H.; Han, D.M. Screening of Growth- or Development-Related Genes by Using Genomic Library with Inducible Promoter in Aspergillus nidulans. J. Microbiol. 2005, 43, 523–528. [Google Scholar] [PubMed]
- Seo, J.A.; Guan, Y.J.; Yu, J.H. FluG-dependent asexual development in Aspergillus nidulans occurs via derepression. Genetics 2006, 172, 1535–1544. [Google Scholar] [CrossRef] [PubMed]
- Vienken, K.; Scherer, M.; Fischer, R. The Zn(II)(2)Cys(6) putative Aspergillus nidulans transcription factor repressor of sexual development inhibits sexual development under low-carbon conditions and in submersed culture. Genetics 2005, 169, 619–630. [Google Scholar] [CrossRef] [PubMed]
- Vienken, K.; Fischer, R. The Zn(II)(2)Cys(6) putative transcription factor NosA controls fruiting body formation in Aspergillus nidulans. Mol. Microbiol. 2006, 61, 544–554. [Google Scholar] [CrossRef] [PubMed]
- Masloff, S.; Jacobsen, S.; Poggeler, S.; Kuck, U. Functional analysis of the C-6 zinc finger gene pro1 involved in fungal sexual development. Fungal Genet. Biol. 2002, 36, 107–116. [Google Scholar] [CrossRef]
- Gautier, V.; Tong, L.C.H.; Tinh-Suong, N.; Debuchy, R.; Silar, P. PaPro1 and IDC4, two genes controlling stationary phase, sexual development and cell degeneration in Podospora anserina. J. Fungi 2018, 4, 85–112. [Google Scholar] [CrossRef] [PubMed]
- Sung, G.H.; Hywel-Jones, N.L.; Sung, J.M.; Luangsa-Ard, J.J.; Shrestha, B.; Spatafora, J.W. Phylogenetic classification of Cordyceps and the clavicipitaceous fungi. Stud. Mycol. 2007, 57, 5–59. [Google Scholar] [CrossRef] [PubMed]
- Bushley, K.E.; Raja, R.; Jaiswal, P.; Cumbie, J.S.; Nonogaki, M.; Boyd, A.E.; Owensby, C.A.; Knaus, B.J.; Elser, J.; Miller, D.; et al. The genome of Tolypocladium inflatum: Evolution, organization, and expression of the cyclosporin biosynthetic gene cluster. PLoS Genet. 2013, 9, e1003496. [Google Scholar] [CrossRef] [PubMed]
- Quandt, C.A.; Kepler, R.M.; Gams, W.; Araújo, J.P.M.; Ban, S.; Evans, H.C.; Hughes, D.; Humber, R.; Hywel-Jones, N.; Li, Z.; et al. Phylogenetic-based nomenclatural proposals for Ophiocordycipitaceae (Hypocreales) with new combinations in Tolypocladium. IMA Fungus. 2014, 5, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.J.; Li, T.H. Antiviral Activity of Cordyceps guangdongensis against Influenza Virus Infections in Mice. Acta Edulis Fungi 2010, 17, 64–66. [Google Scholar]
- Yan, W.J.; Li, T.H.; Jiang, Z.D. Anti-Fatigue and Life-Prolonging Effects of Cordyceps guangdongensis. Food R & D 2011, 32, 164–167. [Google Scholar]
- Yan, W.J.; Li, T.H.; Jiang, Z.D. Therapeutic Effects of Cordyceps guangdongensis on Chronic Renal Failure Rats Induced by Adenine. Junwu Xuebao 2012, 31, 432–442. [Google Scholar]
- Yan, W.J.; Li, T.H.; Lao, J.H.; Song, B.; Shen, Y.H. Anti-fatigue property of Cordyceps guangdongensis and the underlying mechanisms. Pharm. Biol. 2013, 51, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.J.; Li, T.H.; Zhong, Z.Y. Anti-inflammatory effect of a novel food Cordyceps guangdongensis on experimental rats with chronic bronchitis induced by tobacco smoking. Food Funct. 2014, 5, 2552–2557. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.Y.; Li, T.H.; Song, B.; Huang, H. Comparison of Selected Chemical Component Levels in Cordyceps guangdongensis, C. sinensis and C. militaris. Acta Edulis Fungi. 2009, 16, 54–57. [Google Scholar]
- Quandt, C.A.; Bushley, K.E.; Spatafora, J.W. The genome of the truffle-parasite Tolypocladium ophioglossoides and the evolution of antifungal Peptaibiotics. BMC Genom. 2015, 16, 553. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Deng, W.; Yan, W.; Li, T. Whole genome sequence of an edible and potential medicinal fungus, Cordyceps guangdongensis. G3-Genes Genom. Genet. 2018, 8, 1863–1870. [Google Scholar] [CrossRef] [PubMed]
- De Castro, E.; Sigrist, C.J.A.; Gattiker, A.; Bulliard, V.; Langendijk-Genevaux, P.S.; Gasteiger, E.; Bairoch, A.; Hulo, N. ScanProsite: Detection of PROSITE signature matches and ProRule-associated functional and structural residues in proteins. Nucleic Acids. Res. 2006, 34, 362–365. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.D.; Bateman, A.; Clements, J.; Coggill, P.; Eberhardt, R.Y.; Eddy, S.R.; Heger, A.; Hetherington, K.; Holm, L.; Mistry, J.; et al. Pfam: The protein families database. Nucleic Acids Res. 2014, 42, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Doerks, T.; Bork, P. SMART 7: Recent updates to the protein domain annotation resource. Nucleic Acids Res. 2012, 40, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Fan, K.; Ma, F.; Yue, E.; Bibi, N.; Wang, M.; Shen, H.; Hasan, M.M.; Wang, X. Genomic identification and comparative expansion analysis of the non-specific lipid transfer protein gene family in Gossypium. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The Clustal X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997, 25, 4876–4882. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, Y.; Sano, M.; Kanamaru, K.; Ko, T.; Takeuchi, M.; Kato, M.; Kobayashi, T. Genes regulated by AoXlnR, the xylanolytic and cellulolytic transcriptional regulator, in Aspergillus oryzae. Appl. Microbiol. Biotechnol. 2009, 85, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.; Kwon-Chung, K.J. A zinc finger protein from Candida albicans is involved in sucrose utilization. J. Bacteriol. 1992, 174, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Li, D.X.; Sirakova, T.; Rogers, L.; Ettinger, W.F.; Kolattukudy, P.E. Regulation of constitutively expressed and induced cutinase genes by different zinc finger transcription factors in Fusarium solani f. sp. pisi (Nectria haematococca). J. Biol. Chem. 2002, 277, 7905–7912. [Google Scholar] [CrossRef] [PubMed]
- Crowley, J.H.; Leak, F.W.; Shianna, K.V.; Tove, S.; Parks, L.W. A mutation in a purported regulatory gene affects control of sterol uptake in Saccharomyces cerevisiae. J. Bacteriol. 1998, 180, 4177–4183. [Google Scholar] [PubMed]
- Feng, B.; Marzluf, G.A. The regulatory protein NIT4 that mediates nitrate induction in Neurospora crassa contains a complex tripartite activation domain with a novel leucine-rich, acidic motif. Curr. Genet. 1996, 29, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Narendja, F.; Goller, S.P.; Wolschek, M.; Strauss, J. Nitrate and the GATA factor AreA are necessary for in vivo binding of NirA, the pathway-specific transcriptional activator of Aspergillus nidulans. Mol. Microbiol. 2002, 44, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Small, A.J.; Todd, R.B.; Zanker, M.C.; Delimitrou, S.; Hynes, M.J.; Davis, M.A. Functional analysis of TamA, a coactivator of nitrogen-regulated gene expression in Aspergillus nidulans. Mol. Genet. Genom. 2001, 265, 636–646. [Google Scholar] [CrossRef]
- Bricmont, P.A.; Daugherty, J.R.; Cooper, T.G. The DAL81 gene product is required for induced expression of two differently regulated nitrogen catabolic genes in Saccharomyces cerevisiae. Mol. Cell. Biol. 1991, 11, 1161–1166. [Google Scholar] [CrossRef] [PubMed]
- Bibbins, M.; Crepin, V.F.; Cummings, N.J.; Mizote, T.; Baker, K.; Mellits, K.H.; Connerton, I.F. A regulator gene for acetate utilisation from Neurospora crassa. Mol. Genet. Genom. 2002, 267, 498–505. [Google Scholar] [CrossRef]
- Kohlhaw, G.B. Leucine biosynthesis in fungi: Entering metabolism through the back door. Microbiol. Mol. Biol. Rev. 2003, 67, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, E.; Ben-Ari, G.; Wildenhain, J.; Tyers, M.; Grammentz, D.; Lee, T.A. Characterizing the roles of Met31 and Met32 in coordinating Met4-activated transcription in the absence of Met30. Mol. Biol. Cell 2012, 23, 1928–1942. [Google Scholar] [CrossRef] [PubMed]
- Krogan, N.J.; Cagney, G.; Yu, H.; Zhong, G.; Guo, X.; Ignatchenko, A.; Li, J.; Pu, S.; Datta, N.; Tikuisis, A.P.; et al. Global landscape of protein complexes in the yeast Saccharomyces cerevisiae. Nature 2006, 440, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Rempola, B.; Karkusiewicz, I.; Piekarska, I.; Rytka, J. Fcf1p and Fcf2p are novel nucleolar Saccharomyces cerevisiae proteins involved in pre-rRNA processing. Biochem. Biophys. Res. Commun. 2006, 346, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Chooi, Y.H.; Cacho, R.; Tang, Y. Identification of the viridicatumtoxin and griseofulvin gene clusters from Penicillium aethiopicum. Chem. Biol. 2010, 17, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Akache, B.; Wu, K.; Turcotte, B. Phenotypic analysis of genes encoding yeast zinc cluster proteins. Nucleic Acids Res. 2001, 29, 2181–2190. [Google Scholar] [CrossRef] [PubMed]
- Todd, R.B.; Zhou, M.; Ohm, R.A.; Leeggangers, H.A.C.F.; Visser, L.; de Vries, R.P. Prevalence of transcription factors in ascomycete and basidiomycete fungi. BMC Genom. 2014, 15, 214–225. [Google Scholar] [CrossRef] [PubMed]
- Endo, H.; Kajiwara, S.; Tsunoka, O.; Shishido, K. A novel cDNA, priBc, encoding a protein with a Zn(II)2Cys6 zinc cluster DNA-binding motif, derived from the basidiomycete Lentinus edodes. Gene 1994, 139, 117–121. [Google Scholar] [CrossRef]
- Joshua, I.M.; Hofken, T. From lipid homeostasis to differentiation: Old and new functions of the zinc cluster proteins Ecm22, Upc2, Sut1 and Sut2. Int. J. Mol. Sci. 2017, 18, 772–788. [Google Scholar] [CrossRef] [PubMed]
- Braun, B.R.; Hoog, M.V.; D’Enfert, C.; Martchenko, M.; Dungan, J.; Kuo, A.; Inglis, D.O.; Uhl, M.A.; Hogues, H.; Berriman, M.; et al. A human-curated annotation of the Candida albicans genome. PLoS Gent. 2005, 1, 36–57. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Cao, H.; Zhang, L.; Huang, P.; Lin, F. Systematic analysis of Zn(2)Cys(6) transcription factors required for development and pathogenicity by high-throughput gene knockout in the rice blast fungus. PLoS Pathog. 2014, 10, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Xia, Y.; Xiao, G.; Xiong, C.; Hu, X.; Zhang, S.; Zheng, H.; Huang, Y.; Zhou, Y.; Wang, S.; et al. Genome sequence of the insect pathogenic fungus Cordyceps militaris, a valued traditional chinese medicine. Genome Biol. 2011, 12, R116. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Guo, M.; Yang, H.; Guo, S.; Dong, C. The blue-light receptor CmWC-1 mediates fruit body development and secondary metabolism in Cordyceps militaris. Appl. Microbiol. Biotechnol. 2016, 100, 743–755. [Google Scholar] [CrossRef] [PubMed]
- Steffens, E.K.; Becker, K.; Krevet, S.; Teichert, I.; Kueck, U. Transcription factor PRO1 targets genes encoding conserved components of fungal developmental signaling pathways. Mol. Microbiol. 2016, 102, 792–809. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, M.; Kobayashi, T.; Koyama, Y. ManR, a novel Zn(II)(2)Cys(6) transcriptional activator, controls the β-mannan utilization system in Aspergillus oryzae. Fungal Genet. Biol. 2012, 49, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Masloff, S.; Poggeler, S.; Kuck, U. The pro1(+) gene from Sordaria macrospora encodes a C-6 zinc finger transcription factor required for fruiting body development. Genetics 1999, 152, 191–199. [Google Scholar] [PubMed]
- Fuller, K.K.; Loros, J.J.; Dunlap, J.C. Fungal photobiology: Visible light as a signal for stress, space and time. Curr. Genet. 2015, 61, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, A.; Fuller, K.K.; Dunlap, J.C.; Loros, J.J. Seeing the world differently: Variability in the photosensory mechanisms of two model fungi. Environ. Microbiol. 2016, 18, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Fischer, R. Light sensing and responses in fungi. Nat. Rev. Microbiol. 2019, 17, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Idnurm, A.; Heitman, J. Light controls growth and development via a conserved pathway in the fungal kingdom. PLoS Biol. 2005, 3, 615–626. [Google Scholar] [CrossRef] [PubMed]
- Bayram, O.; Krappmann, S.; Ni, M.; Bok, J.W.; Helmstaedt, K.; Valerius, O.; Braus-Stromeyer, S.; Kwon, N.; Keller, N.P.; Yu, J.; et al. VelB/VeA/LaeA complex coordinates light signal with fungal development and secondary metabolism. Science 2008, 320, 1504–1506. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Ridenour, J.B.; Dunkle, L.D.; Bluhm, B.H. Regulation of stomatal tropism and infection by light in Cercospora zeaemaydis: Evidence for coordinated host/pathogen responses to photoperiod? PLoS Pathog. 2011, 7, e1002113. [Google Scholar] [CrossRef] [PubMed]
- Fuller, K.K.; Ringelberg, C.S.; Loros, J.J.; Dunlap, J.C. The fungal pathogen Aspergillus fumigatus regulates growth, metabolism, and stress resistance in response to light. MBIO 2013, 4, e00142. [Google Scholar] [CrossRef] [PubMed]
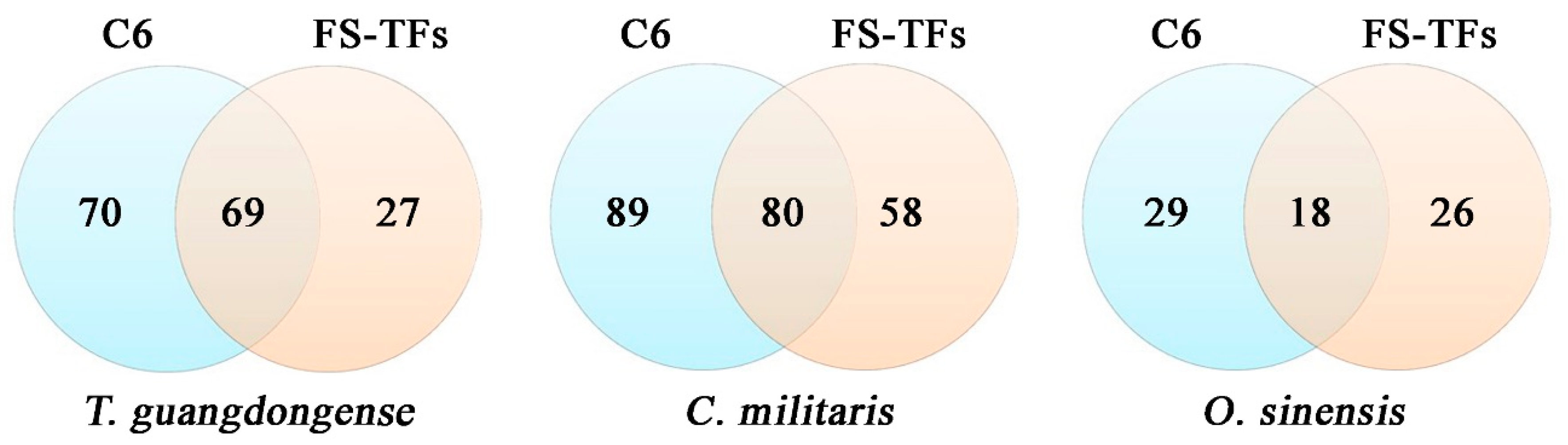
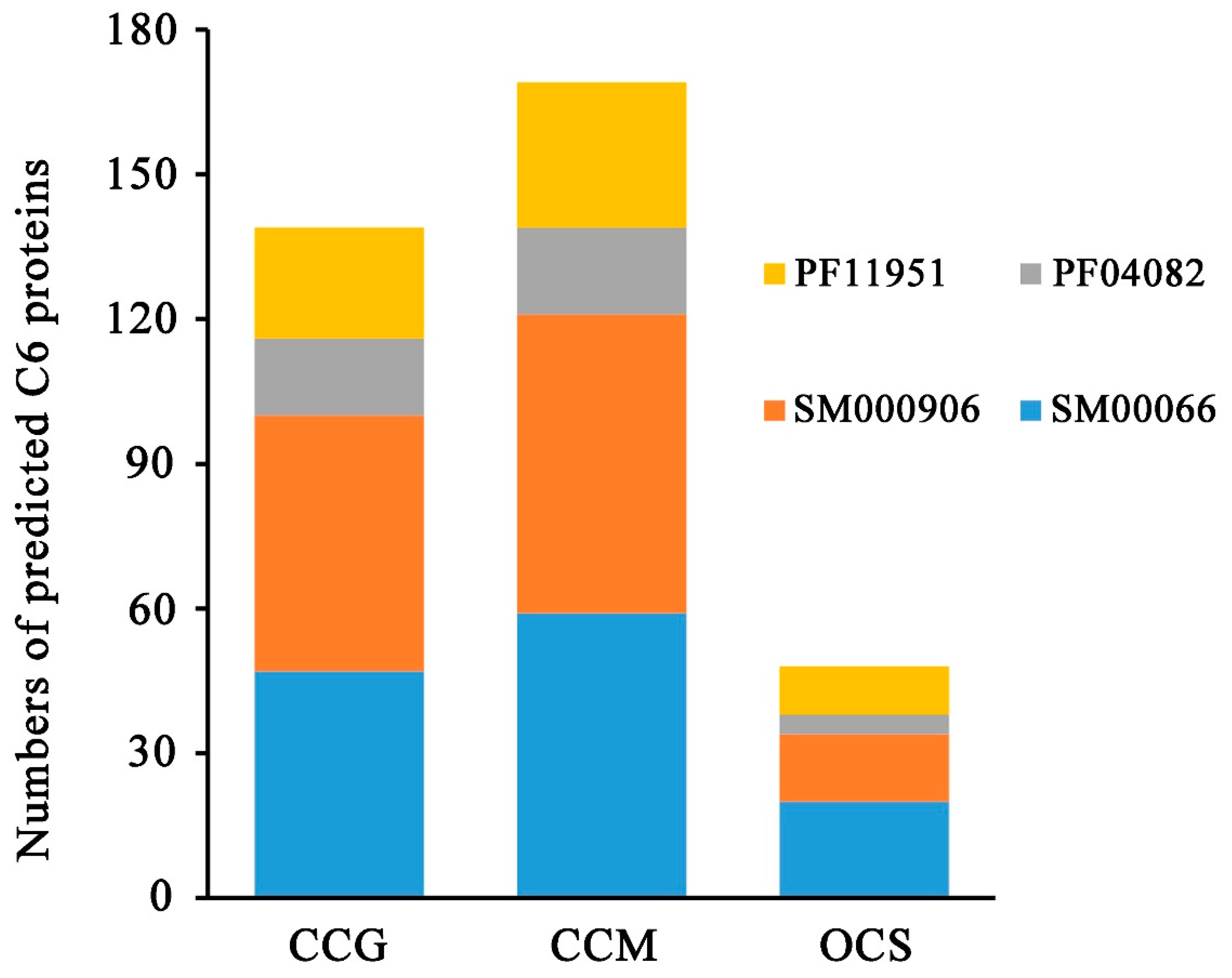
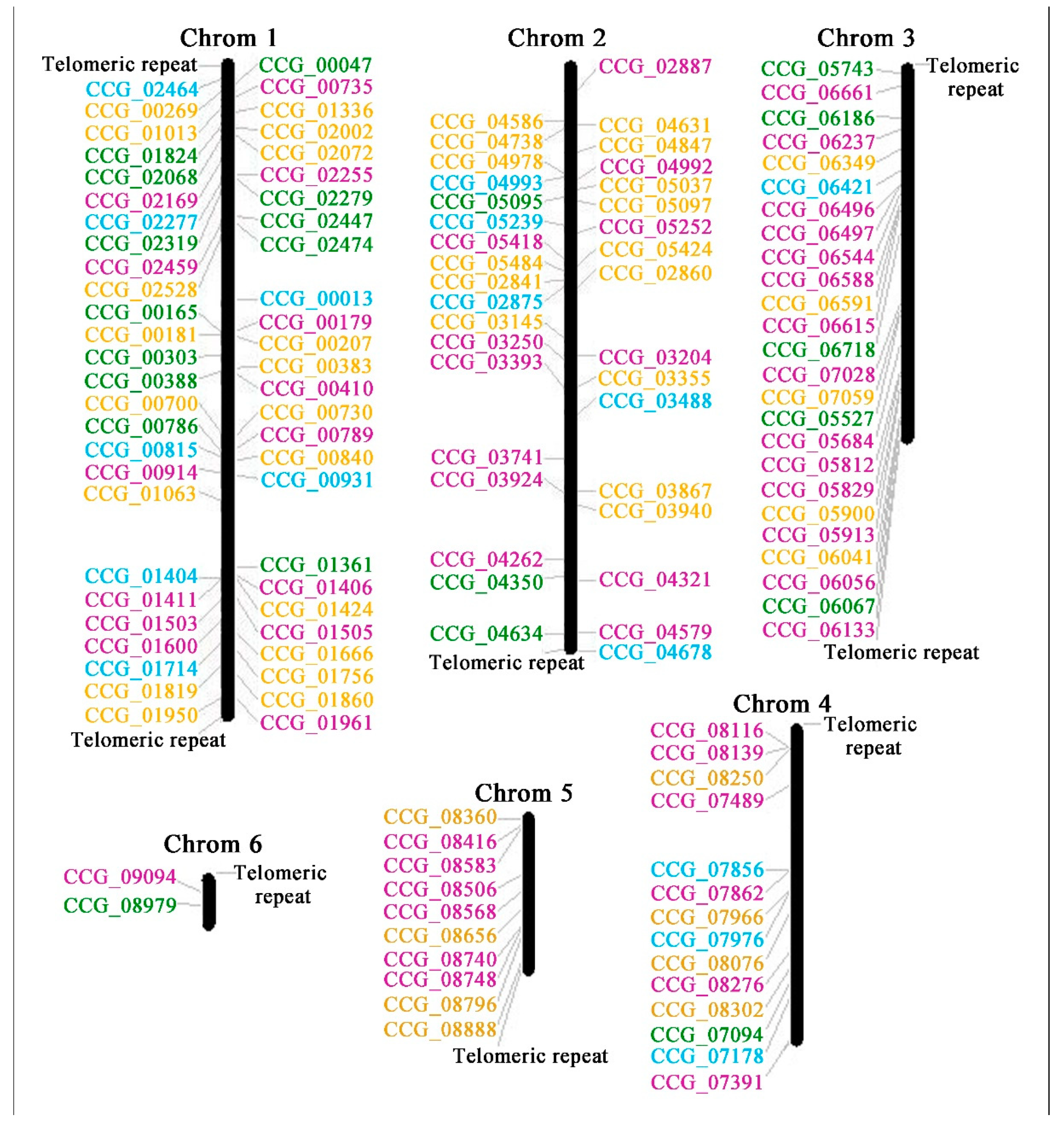

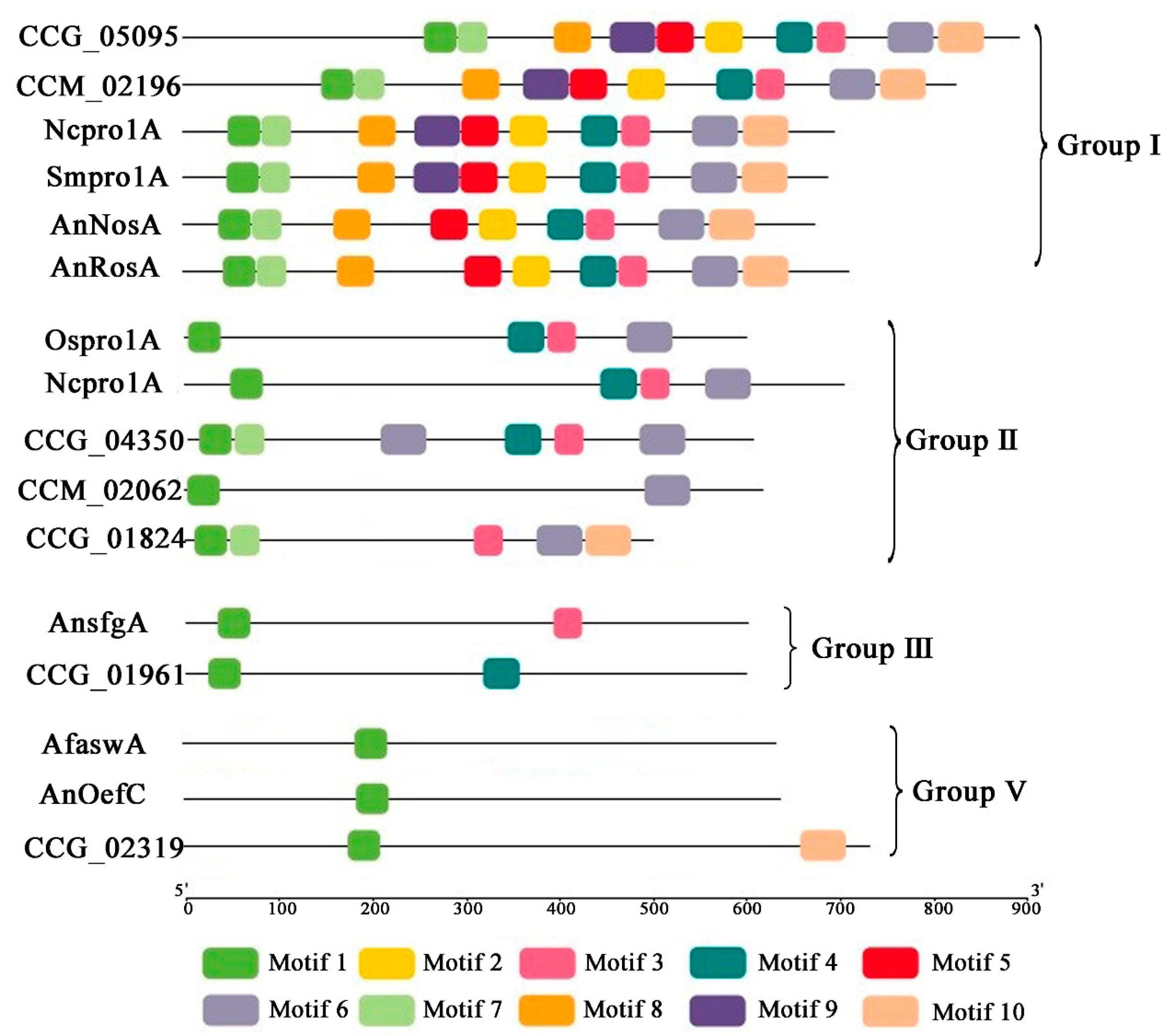

| Subgroup | Tolypocladium guangdongense | Cordyceps ilitaris | Ophiocordyceps sinensis |
|---|---|---|---|
| C-2-C-6-C-6-C-2-C-6-C | 65 | 83 | 24 |
| C-2-C-6-C-5-C-2-C-6-C | 26 | 26 | 4 |
| C-2-C-6-C-5-C-2-C-8-C | 15 | 10 | 7 |
| C-2-C-6-C-8-C-2-C-6-C | 11 | 14 | 3 |
| C-2-C-6-C-9-C-2-C-6-C | 6 | 8 | 1 |
| C-2-C-6-C-7-C-2-C-6-C | 5 | 7 | 0 |
| C-2-C-6-C-12-C-2-C-6-C | 2 | 2 | 0 |
| C-2-C-6-C-5-C-2-C-7-C | 2 | 2 | 0 |
| C-2-C-6-C-10-C-2-C-12-C | 1 | 0 | 1 |
| C-2-C-6-C-10-C-2-C-6-C | 1 | 2 | 2 |
| C-2-C-6-C-14-C-2-C-6-C | 1 | 0 | 0 |
| C-2-C-6-C-17-C-2-C-6-C | 1 | 0 | 0 |
| C-2-C-6-C-5-C-2-C-11-C | 1 | 4 | 2 |
| C-2-C-6-C-5-C-2-C-5-C | 1 | 0 | 0 |
| C-2-C-6-C-5-C-2-C-9-C | 1 | 1 | 0 |
| C-2-C-6-C-6-C-2-C-7-C | 1 | 1 | 1 |
| C-2-C-6-C-6-C-2-C-8-C | 1 | 5 | 0 |
| C-2-C-6-C-9-C-2-C-7-C | 1 | 1 | 0 |
| C-2-C-6-C-11-C-2-C-7-C | 0 | 1 | 0 |
| C-2-C-6-C-12-C-2-C-6-C | 0 | 1 | 0 |
| C-2-C-6-C-6-C-2-C-7-C | 0 | 1 | 0 |
| C-2-C-6-C-8-C-2-C-8-C | 0 | 7 | 0 |
| C-2-C-6-C-5-C-2-C-12-C | 0 | 2 | 1 |
| C-2-C-6-C-6-C-2-C-11-C | 0 | 1 | 0 |
| C-2-C-6-C-6-C-2-C-9-C | 0 | 1 | 0 |
| Gene_ID | Blast Result | Putative Gene Product Function | Role | References |
|---|---|---|---|---|
| CCG_08139 | xlnR | Controls expression of xylanolytic enzymes genes | xylanolytic and cellulolytic utilization | [37] |
| CCG_06056 | SUC1 | Regulates sucrose metabolic genes | sucrose utilization | [38] |
| CCG_06421 | SUC1 | Regulates sucrose metabolic genes | sucrose utilization | [38] |
| CCG_02169 | SUC1 | Regulates sucrose metabolic genes | sucrose utilization | [38] |
| CCG_00181 | - | maltose O-acetyltransferase | maltose utilization | / |
| CCG_08276 | - | chitinase 1 precursor | Chitin degradation | / |
| CCG_05812 | ctf1β | Activator of cutinase genes | Cutin degradation | [39] |
| CCG_01013 | UPC2 | activator of ergosterol biosynthetic genes | Ergosterol metabolism | [40] |
| CCG_04586 | UPC2 | activator of ergosterol biosynthetic genes | Ergosterol metabolism | [40] |
| CCG_01961 | ECM22 | Activator of ergosterol biosynthetic genes | Ergosterol metabolism | [12] |
| CCG_02464 | nit-4 | Activator of the nitrate assimilatory pathway | nitrate assimilation | [41] |
| CCG_08116 | nit-4 | Activator of the nitrate assimilatory pathway | nitrate assimilation | [41] |
| CCG_09094 | nirA | Regulator of nitrate assimilation | nitrate assimilation | [42] |
| CCG_02875 | OTam/TamA | Involved in nitrogen regulation | nitrogen utilization | [43] |
| CCG_02255 | DAL81 | Activator of nitrogen catabolic genes | nitrogen utilization | [44] |
| CCG_04992 | acu-15 | Transcriptional activator protein | acetate utilization | [45] |
| CCG_00410 | acu-15 | Transcriptional activator protein | acetate utilization | [45] |
| CCG_03393 | acu-15 | Transcriptional activator protein | acetate utilization | [45] |
| CCG_04678 | acu-15/FacB | Activator of acetate regulatory genes | acetate utilization | [45] |
| CCG_04262 | LEU3 | Activator/repressor of leucine biosynthesis genes | Amino acid metabolism | [46] |
| CCG_06591 | MET32 | Transcriptional regulator involved in sulfate assimilation and sulfonate metabolism | sulfate assimilation and sulfonate metabolism | [47] |
| CCG_02459 | - | Positive regulator of purine utilization | purine utilization | / |
| CCG_04993 | - | Pyrimidine pathway regulatory protein 1 | Pyrimidine utilization | / |
| CCG_02072 | - | Quinic acid utilization activator | Quinic acid utilization | / |
| CCG_00730 | Trz1 | tRNA processing endoribonuclease Trz1 | RNase Z activity | [48] |
| CCG_05829 | fcf1 | rRNA-processing protein fcf1 | pre-rRNA processing | [49] |
| CCG_06615 | - | stress gene activator | stress response | [29] |
| CCG_07862 | vrtR1 | Viridicatumtoxin synthesis protein R1 | viridicatumtoxin biosynthesis | [50] |
| CCG_07856 | vrtR2 | Viridicatumtoxin synthesis protein R2 | viridicatumtoxin biosynthesis | [50] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Huang, H.; Deng, W.; Li, T. Genome-Wide Analysis of the Zn(II)2Cys6 Zinc Cluster-Encoding Gene Family in Tolypocladium guangdongense and Its Light-Induced Expression. Genes 2019, 10, 179. https://doi.org/10.3390/genes10030179
Zhang C, Huang H, Deng W, Li T. Genome-Wide Analysis of the Zn(II)2Cys6 Zinc Cluster-Encoding Gene Family in Tolypocladium guangdongense and Its Light-Induced Expression. Genes. 2019; 10(3):179. https://doi.org/10.3390/genes10030179
Chicago/Turabian StyleZhang, Chenghua, Hong Huang, Wangqiu Deng, and Taihui Li. 2019. "Genome-Wide Analysis of the Zn(II)2Cys6 Zinc Cluster-Encoding Gene Family in Tolypocladium guangdongense and Its Light-Induced Expression" Genes 10, no. 3: 179. https://doi.org/10.3390/genes10030179
APA StyleZhang, C., Huang, H., Deng, W., & Li, T. (2019). Genome-Wide Analysis of the Zn(II)2Cys6 Zinc Cluster-Encoding Gene Family in Tolypocladium guangdongense and Its Light-Induced Expression. Genes, 10(3), 179. https://doi.org/10.3390/genes10030179




