Macrophages from Susceptible and Resistant Chicken Lines have Different Transcriptomes following Marek’s Disease Virus Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Marek’s Disease Virus
2.3. Cell Cultures
2.4. Co-Culture Infection Experiments and Fluorescence Activated Cell Sorting (FACS)
2.5. Flow Cytometry
2.6. Real-Time Quantitative RT-PCR
2.7. Cells and Sample Preparation for RNA-sequencing (RNA-seq)
2.8. RNA-seq and Analysis
2.9. Functional Analysis of DE Genes
2.10. Determining MDV Quantitative Trait Loci (QTL) Candidate Genes
3. Results
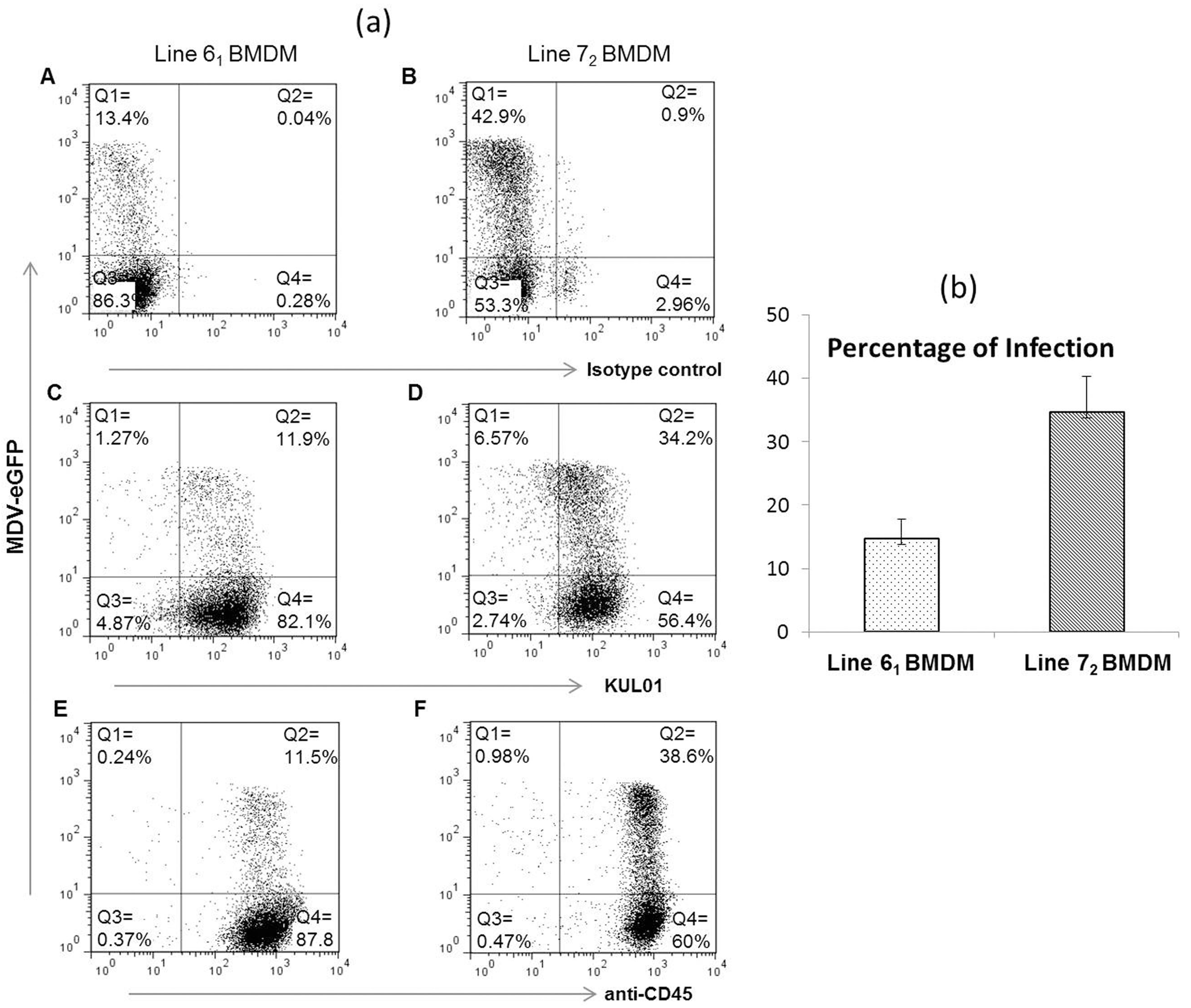
3.1. Infection of Macrophages from Susceptible and Resistant Birds
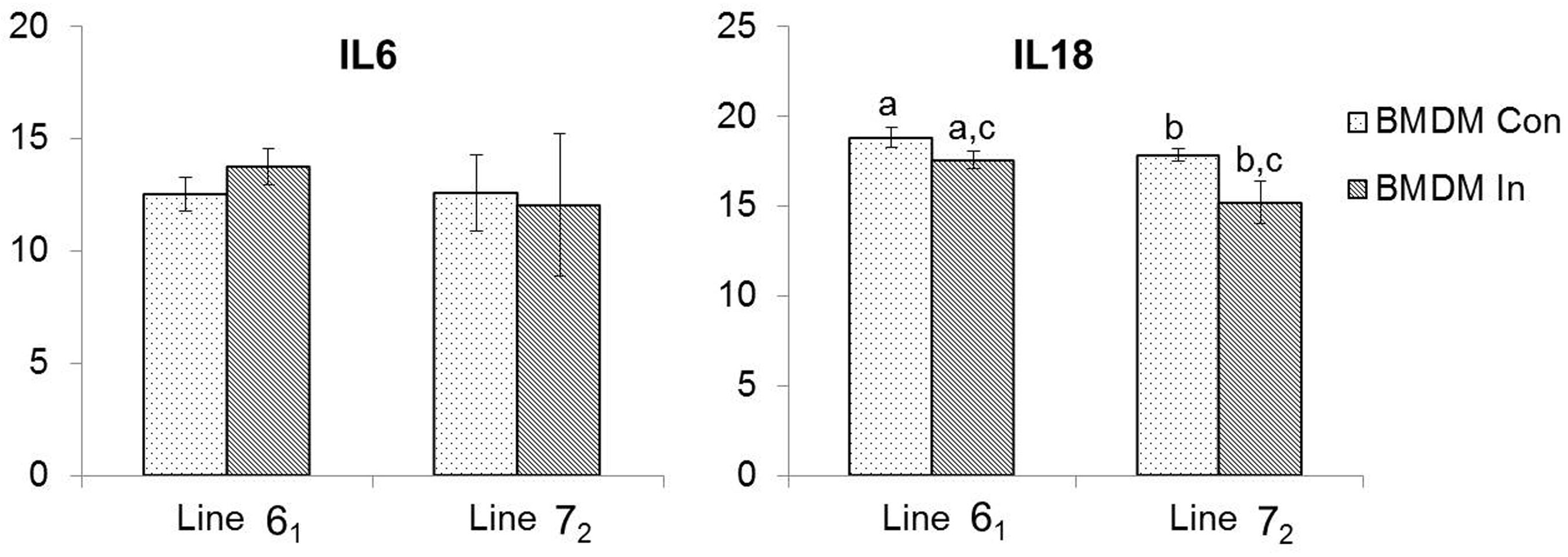
3.2. Pro-Inflammatory Cytokine Expression
3.3. Analysis of Gene Expression in Susceptible and Resistant Lines
3.4. Inherent Differences in Gene Expression between the Two Lines
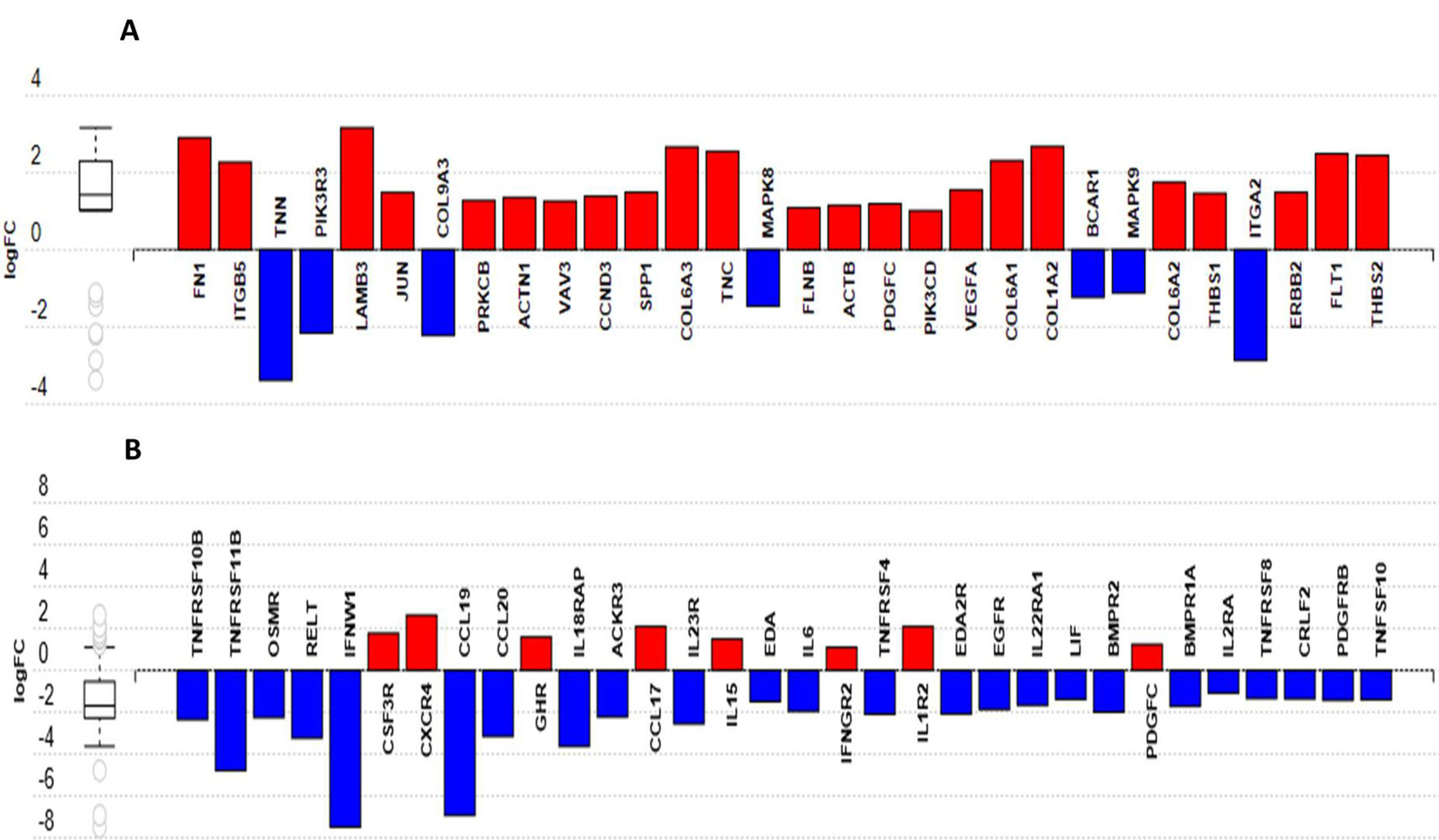
3.5. Host Response to MDV Infection in Resistant and Susceptible Lines
3.6. Differences in Host Response between the Two Lines Following MDV Infection
3.7. Viral Gene Expression
3.8. Gene Ontology Analysis of Differentially Expressed (DE) Genes
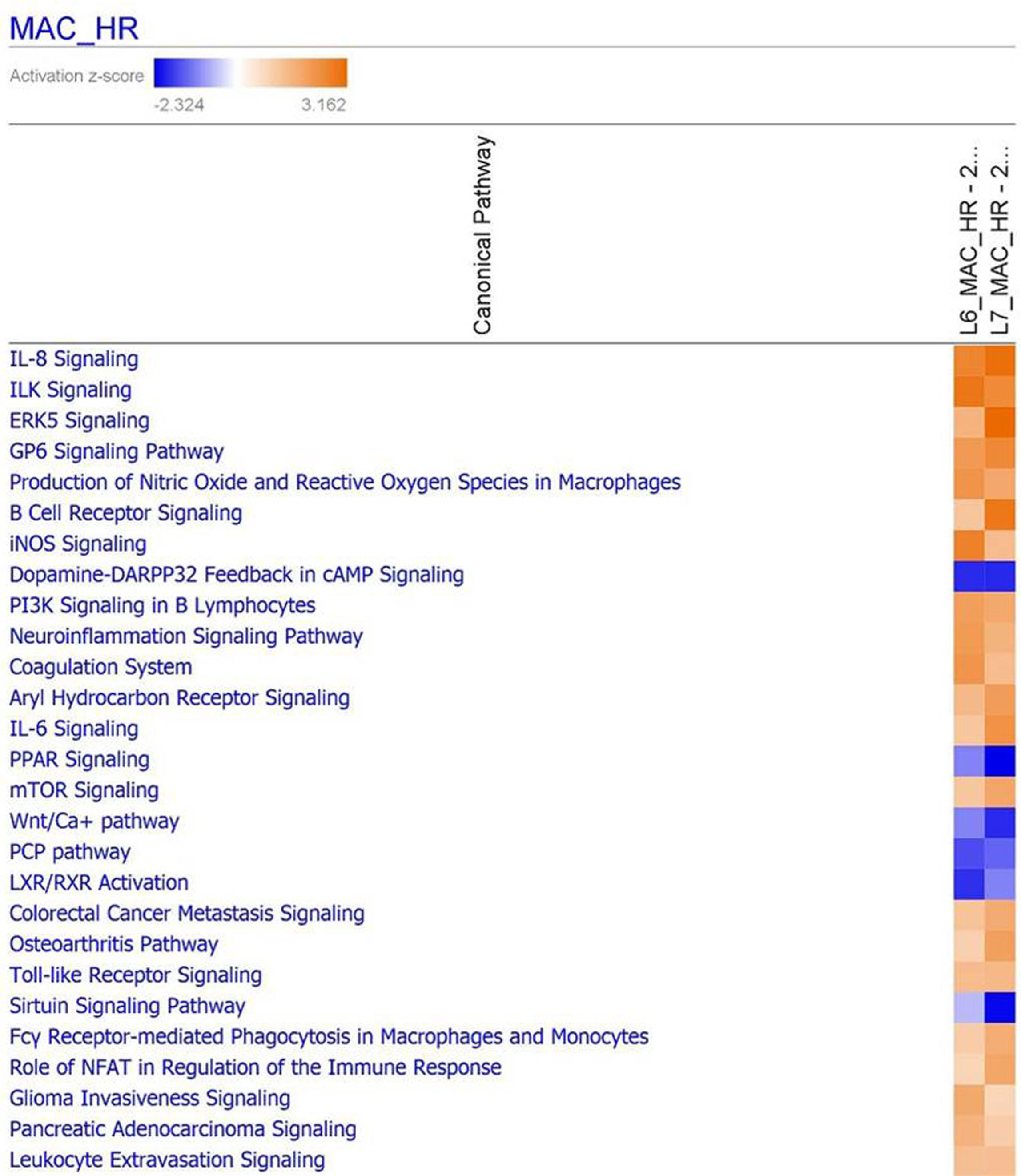
3.9. Functional Analysis Reveals Significantly Regulated Biological Pathways
3.10. Identification of Putative Candidate Genes for Resistance to MDV
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Ethical Approval
Availability of RNA-seq Data and Materials
Conflicts of Interest
References
- Gimeno, I.M. Marek’s disease vaccines: A solution for today but a worry for tomorrow? Vaccine 2008, 26, C31–C41. [Google Scholar] [CrossRef] [PubMed]
- Gavora, J.S.; Spencer, J.L. Studies on genetic resistance of chickens to Marek’s disease—A review. Comp. Immunol. Microbiol. Infect. Dis. 1979, 2, 359–371. [Google Scholar] [CrossRef]
- Von Krosigk, C.M.; McClary, C.F.; Vielitz, E.; Zander, D.V. Selection for resistance to Marek’s disease and its expected effects on other important traits in White Leghorn strain crosses. Avian Dis. 1972, 16, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Rogers, S.L.; Kaufman, J. High allelic polymorphism, moderate sequence diversity and diversifying selection for B-NK but not B-lec, the pair of lectin-like receptor genes in the chicken MHC. Immunogenetics 2008, 60, 461–475. [Google Scholar] [CrossRef]
- Lee, S.H.; Gitas, J.; Zafer, A.; Lepage, P.; Hudson, T.J.; Belouchi, A.; Vidal, S.M. Haplotype mapping indicates two independent origins for the Cmv1s susceptibility allele to cytomegalovirus infection and refines its localization within the Ly49 cluster. Immunogenetics 2001, 53, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Iizuka, K.; Naidenko, O.V.; Plougastel, B.F.; Fremont, D.H.; Yokoyama, W.M. Genetically linked C-type lectin-related ligands for the NKRP1 family of natural killer cell receptors. Nat. Immunol. 2003, 4, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Vallejo, R.L.; Bacon, L.D.; Liu, H.C.; Witter, R.L.; Groenen, M.A.; Hillel, J.; Cheng, H.H. Genetic mapping of quantitative trait loci affecting susceptibility to Marek’s disease virus induced tumours in F2 intercross chickens. Genetics 1998, 148, 349–360. [Google Scholar] [PubMed]
- Yonash, N.; Bacon, L.D.; Witter, R.L.; Cheng, H.H. High resolution mapping and identification of new quantitative trait loci (QTL) affecting susceptibility to Marek’s disease. Anim. Genet. 1999, 30, 126–135. [Google Scholar] [CrossRef]
- McElroy, J.P.; Dekkers, J.C.; Fulton, J.E.; O’Sullivan, N.P.; Soller, M.; Lipkin, E.; Zhang, W.; Koehler, K.J.; Lamont, S.J.; Cheng, H.H. Microsatellite markers associated with resistance to Marek’s disease in commercial layer chickens. Poult. Sci. 2005, 84, 1678–1688. [Google Scholar] [CrossRef] [PubMed]
- Cole, R.K. Studies on genetic resistance to Marek’s disease. Avian Dis. 1968, 12, 9–28. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.F.; Powell, P.C.; Rennie, M.; Ross, L.J.; Payne, L.N. Nature of genetic resistance to Marek’s disease in chickens. J. Natl. Cancer Inst. 1981, 66, 789–796. [Google Scholar] [PubMed]
- Bumstead, N.; Sillibourne, J.; Rennie, M.; Ross, N.; Davison, F. Quantification of Marek’s disease virus in chicken lymphocytes using the polymerase chain reaction with fluorescence detection. J. Virol. Methods 1997, 65, 75–81. [Google Scholar] [CrossRef]
- Haunshi, S.; Cheng, H.H. Differential expression of Toll-like receptor pathway genes in chicken embryo fibroblasts from chickens resistant and susceptible to Marek’s disease. Poult. Sci. 2014, 93, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.C.; Kung, H.J.; Fulton, J.E.; Morgan, R.W.; Cheng, H.H. Growth hormone interacts with the Marek’s disease virus SORF2 protein and is associated with disease resistance in chicken. Proc. Natl. Acad. Sci. USA 2001, 98, 9203–9208. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.C.; Niikura, M.; Fulton, J.E.; Cheng, H.H. Identification of chicken lymphocyte antigen 6 complex, locus E (LY6E, alias SCA2) as a putative Marek’s disease resistance gene via a virus-host protein interaction screen. Cytogenet. Genome Res. 2003, 102, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.; Sadeyen, J.R.; Paton, I.R.; Hocking, P.M.; Salmon, N.; Fife, M.; Nair, V.; Burt, D.W.; Kaiser, P. Systems analysis of immune responses in Marek’s disease virus-infected chickens identifies a gene involved in susceptibility and highlights a possible novel pathogenicity mechanism. J. Virol. 2011, 85, 11146–11158. [Google Scholar] [CrossRef]
- Calnek, B.W. Pathogenesis of Marek’s disease virus infection. Curr. Top. Microbiol. Immunol. 2001, 255, 25–55. [Google Scholar]
- Chakraborty, P.; Vervelde, L.; Dalziel, R.G.; Wasson, P.S.; Nair, V.; Dutia, B.M.; Kaiser, P. Marek’s disease virus infection of phagocytes: A de novo in vitro infection model. J. Gen. Virol. 2017, 98, 1080–1088. [Google Scholar] [CrossRef]
- Jeurissen, S.H.; Scholten, R.; Hilgers, L.A.; Pol, J.M.; De Boer, G.F. In situ detection by monoclonal antibody D-35.1 of cells infected with Marek’s disease virus that interact with splenic ellipsoid-associated reticulum cells. Avian Dis. 1989, 33, 657–663. [Google Scholar] [CrossRef]
- Burgess, S.C.; Davison, T.F. Identification of the neoplastically transformed cells in Marek’s disease herpesvirus-induced lymphomas: Recognition by the monoclonal antibody AV37. J. Virol. 2002, 76, 7276–7292. [Google Scholar] [CrossRef]
- Bumstead, N.; Kaufman, J. Genetic resistance to Marek’s disease. In Marek’s Disease: An Evolving Problem; Davison, F., Nair, V., Eds.; Elsevier Academic Press: London, UK, 2004; pp. 112–125. [Google Scholar]
- Cheng, H.H.; Perumbakkam, S.; Black Pyrkosz, A.; Subramaniam, S.; Preeyanon, L.; Dunn, J.; van Sambeek, F.; Ansah, G.; Muir, W.M. The genetic architecture of genetic resistance to Marek’s Disease. In Proceedings of the 8th European Symposium on Poultry Genetics, Venice, Italy, 25–27 September 2013. [Google Scholar]
- Wasson, P. Development of Novel Virus Vectors for Influenza Vaccination. Ph.D. Thesis, University of Edinburgh, Edinburgh, UK, July 2011. [Google Scholar]
- Gimeno, I.; Silva, R.F. Deletion of the Marek’s disease virus UL41 gene (vhs) has no measurable effect on latency or pathogenesis. Virus Genes 2008, 36, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Petherbridge, L.; Howes, K.; Baigent, S.J.; Sacco, M.A.; Evans, S.; Osterrieder, N.; Nair, V. Replication-competent bacterial artificial chromosomes of Marek’s disease virus: Novel tools for generation of molecularly defined herpesvirus vaccines. J. Virol. 2003, 77, 8712–8718. [Google Scholar] [CrossRef] [PubMed]
- Garceau, V.; Smith, J.; Paton, I.R.; Davey, M.; Fares, M.A.; Sester, D.P.; Burt, D.W.; Hume, D.A. Pivotal Advance: Avian colony-stimulating factor 1 (CSF-1), interleukin-34 (IL-34), and CSF-1 receptor genes and gene products. J. Leukoc. Biol. 2010, 87, 753–764. [Google Scholar] [CrossRef] [PubMed]
- Balic, A.; Garcia-Morales, C.; Vervelde, L.; Gilhooley, H.; Sherman, A.; Garceau, V.; Gutowska, M.W.; Burt, D.W.; Kaiser, P.; Hume, D.A.; et al. Visualization of chicken macrophages using transgenic reporter genes: Insights into the development of the avian macrophage lineage. Development 2014, 141, 3255–3265. [Google Scholar] [CrossRef] [PubMed]
- Staines, K.; Hunt, L.G.; Young, J.R.; Butter, C. Evolution of an expanded mannose receptor gene family. PLoS ONE 2014, 9, e110330. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, P.; Underwood, G.; Davison, F. Differential cytokine responses following Marek’s disease virus infection of chickens differing in resistance to Marek’s disease. J. Virol. 2003, 77, 762–768. [Google Scholar] [CrossRef]
- Wu, Z.; Rothwell, L.; Young, J.R.; Kaufman, J.; Butter, C.; Kaiser, P. Generation and characterization of chicken bone marrow-derived dendritic cells. Immunology 2010, 129, 133–145. [Google Scholar] [CrossRef]
- Borowska, D.; Rothwell, L.; Bailey, R.A.; Watson, K.; Kaiser, P. Identification of stable reference genes for quantitative PCR in cells derived from chicken lymphoid organs. Vet. Immunol. Immunopathol. 2016, 170, 20–24. [Google Scholar] [CrossRef]
- FastQC: A Quality Control Tool for High Throughput Sequence Data. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc (accessed on 22 January 2019).
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet.journal 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. 1000 Genome Project Data Processing Subgroup. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq-a Python framework to work with high-throughput sequencing data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; Smyth, G.K. Moderated statistical tests for assessing differences in tag abundance. Bioinformatics 2007, 23, 2881–2887. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; Smyth, G.K. Small-sample estimation of negative binomial dispersion, with applications to SAGE data. Biostatistics 2008, 9, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Huang da, W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Draghici, S.; Khatri, P.; Tarca, A.L.; Amin, K.; Done, A.; Voichita, C.; Georgescu, C.; Romero, R. A systems biology approach for pathway level analysis. Genome Res. 2007, 17, 1537–1545. [Google Scholar] [CrossRef] [PubMed]
- Mudduluru, G.; Vajkoczy, P.; Allgayer, H. Myeloid zinc finger 1 induces migration, invasion, and in vivo metastasis through Axl gene expression in solid cancer. Mol. Cancer Res. 2010, 8, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Kodama, H.; Sugimoto, C.; Inage, F.; Mikami, T. Anti-viral immunity against Marek’s disease virus infected chicken kidney cells. Avian Pathol. 1979, 8, 33–44. [Google Scholar] [CrossRef]
- Powell, P.C.; Hartley, K.J.; Mustill, B.M.; Rennie, M. Studies on the role of macrophages in Marek’s disease of the chicken. J. Reticuloendothel. Soc. 1983, 34, 289–297. [Google Scholar]
- Loudovaris, T.; Yoo, B.H.; Fahey, K.J. Genetic resistance to infectious laryngotracheitis in inbred lines of White Leghorn chickens. Avian Pathol. 1991, 20, 357–361. [Google Scholar] [CrossRef]
- Xing, Z.; Schat, K.A. Inhibitory effects of nitric oxide and gamma interferon on in vitro and in vivo replication of Marek’s disease virus. J. Virol. 2000, 74, 3605–3612. [Google Scholar] [CrossRef]
- Heidari, M.; Fitzgerald, S.D.; Zhang, H. Marek’s disease virus-induced transient caecal tonsil atrophy. Avian Dis. 2014, 58, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, H.; Nakanishi, K.; Matsui, K.; Higashino, K.; Okamura, H.; Miyazawa, Y.; Kaneda, K. IFN-gamma-inducing factor up-regulates Fas ligand-mediated cytotoxic activity of murine natural killer cell clones. J. Immunol. 1996, 157, 3967–3973. [Google Scholar] [PubMed]
- Garcia-Camacho, L.; Schat, K.A.; Brooks, R., Jr.; Bounous, D.I. Early cell-mediated immune responses to Marek’s disease virus in two chicken lines with defined major histocompatibility complex antigens. Vet. Immunol. Immunopathol. 2003, 95, 145–153. [Google Scholar] [CrossRef]
- Sharma, J. Natural killer cells activity in chickens exposed to Marek’s disease virus: Inhibition of activity in susceptible chickens and enhancement of activity in resistant and vaccinated chickens. Avian Dis. 1981, 25, 882–893. [Google Scholar] [CrossRef] [PubMed]
- MacEachern, S.; Muir, W.M.; Crosby, S.D.; Cheng, H.H. Genome-wide identification and quantification of cis- and trans-regulated genes responding to Marek’s disease virus infection via analysis of allele specific expression. Front. Genet. 2012, 2, 113. [Google Scholar] [CrossRef] [PubMed]
- Perumbakkam, S.; Muir, W.M.; Black-Pyrkosz, A.; Okimoto, R.; Cheng, H.H. Comparison and contrast of genes and biological pathways responding to Marek’s disease virus infection using allele-specific expression and differential expression in broiler and layer chickens. BMC Genom. 2013, 14, 64. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, S.; Preeyanon, L.; Cheng, H.H. Transcriptional profiling of Meq-dependent genes in Marek’s disease resistant and susceptible inbred chicken lines. PLoS ONE 2013, 8, e78171. [Google Scholar] [CrossRef]
- Boudny, V.; Kovarik, J. JAK/STAT signalling pathways and cancer. Janus kinases/signal transducers and activators of transcription. Neoplasma 2002, 49, 349–355. [Google Scholar]
- Denesvre, C. Marek’s disease virus morphogenesis. Avian Dis. 2013, 57, 340–350. [Google Scholar] [CrossRef]
- Richerioux, N.; Blondeau, C.; Wiedemann, A.; Rémy, S.; Vautherot, J.F.; Denesvre, C. Rho-ROCK and Rac-PAK signalling pathways have opposing effects on the cell-to-cell spread of Marek’s disease virus. PLoS ONE 2012, 7, e44072. [Google Scholar] [CrossRef]
- Lee, J.J.; Kim, D.H.; Kim, D.G.; Lee, H.J.; Min, W.; Rhee, M.H.; Cho, J.Y.; Watarai, M.; Kim, S. Toll-like receptor 4-linked Janus kinase 2 signalling contributes to internalization of Brucella abortus by macrophages. Infect. Immun. 2013, 81, 2448–2458. [Google Scholar] [CrossRef] [PubMed]
- Bavisotto, L.; Kaushansky, K.; Lin, N.; Hromas, R. Antisense oligonucleotides from the stage-specific myeloid zinc finger gene MZF-1 inhibit granulopoiesis in vitro. J. Exp. Med. 1991, 174, 1097–1101. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.F.; Rauscher, F.J.; Davis, B.; Klemsz, M.; Xu, D.; Tenen, D.; Hromas, R. The myeloid zinc finger gene, MZF-1, regulates the CD34 promoter in vitro. Blood 1995, 86, 3640–3647. [Google Scholar] [PubMed]
- Hsieh, Y.H.; Wu, T.T.; Tsai, J.H.; Huang, C.Y.; Hsieh, Y.S.; Liu, J.Y. PKC alpha expression regulated by Elk-1 and MZF-1 in human HCC cells. Biochem. Biophys. Res. Commun. 2006, 339, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Matsumoto, C.; Ra, C. FHL3 negatively regulates human high-affinity IgE receptor beta-chain gene expression by acting as a transcriptional co-repressor of MZF-1. Biochem. J. 2005, 386, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.; Nicholas, H.; Bishop, D.; Matthews, J.M.; Crossley, M. The LIM protein FHL3 binds basic Krüppel-like factor/Krüppel-like factor 3 and its co-repressor C-terminal-binding protein 2. J. Biol. Chem. 2003, 278, 12786–12795. [Google Scholar] [CrossRef]
- Brown, A.C.; Baigent, S.J.; Smith, L.P.; Chattoo, J.P.; Petherbridge, L.J.; Hawes, P.; Allday, M.J.; Nair, V. Interaction of Meq protein and C-terminal-binding protein is critical for induction of lymphomas by Marek’s disease virus. Proc. Natl. Acad. Sci. USA 2006, 103, 1687–1692. [Google Scholar] [CrossRef]
- Ajithdoss, D.K.; Reddy, S.M.; Suchodolski, P.F.; Lee, L.F.; Kung, H.J.; Lupiani, B. In vitro characterization of the Meq proteins of Marek’s disease virus vaccine strain CVI988. Virus Res. 2009, 142, 57–67. [Google Scholar] [CrossRef]
- Maat, W.; Beiboer, S.H.; Jager, M.J.; Luyten, G.P.; Gruis, N.A.; van der Velden, P.A. Epigenetic regulation identifies RASEF as a tumor-suppressor gene in uveal melanoma. Investig. Ophthalmol. Vis. Sci. 2008, 49, 1291–1298. [Google Scholar] [CrossRef]
- Kaplon, J.; Hömig-Hölzel, C.; Gao, L.; Meissl, K.; Verdegaal, E.M.; van der Burg, S.H.; van Doorn, R.; Peeper, D.S. Near-genomewide RNAi screening for regulators of BRAF(V600E)-induced senescence identifies RASEF, a gene epigenetically silenced in melanoma. Pigment Cell Melanoma Res. 2014, 27, 640–652. [Google Scholar] [CrossRef]
- Koirala, P.; Roth, M.E.; Gill, J.; Chinai, J.M.; Ewart, M.R.; Piperdi, S.; Geller, D.S.; Hoang, B.H.; Fatakhova, Y.V.; Ghorpade, M.; et al. HHLA2, a member of the B7 family, is expressed in human osteosarcoma and is associated with metastases and worse survival. Sci. Rep. 2016, 6, 31154. [Google Scholar] [CrossRef] [PubMed]

| RNA Target | Probe/Primer Sequence (5′-3′) |
|---|---|
| 28S | Probe (FAM)-AGGACCGCTACGGACCTCCACCA-(TAMRA) F GGCGAAGCCAGAGGAAACT R GACGACCGATTTGCACGTC |
| IL6 | Probe (FAM)-AGGAGAAATGCCTGACGAAGCTCTCCA-(TAMRA) F GCTCGCCGGCTTCGA R GGTAGGTCTGAAAGGCGAACAG |
| IL18 | Probe (FAM)-CCGCGCCTTCAGCACGGATG-(TAMRA) F AGGTGAAATCTGGCAGTGGAAT R ACCTGGACGCTGAATGCAA |
| Category | Genes More Highly Expressed in Line 61 Compared to Line 72 | Genes More Highly Expressed in line 72 Compared to Line 61 |
|---|---|---|
| Host response in line 72 | / | 1394↓ and 1133↑ |
| Host response in line 61 | 786↓ and 479↑ | / |
| Inherent difference between lines | 360 | 729 |
| Difference between lines upon infection | 401 | 555 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chakraborty, P.; Kuo, R.; Vervelde, L.; Dutia, B.M.; Kaiser, P.; Smith, J. Macrophages from Susceptible and Resistant Chicken Lines have Different Transcriptomes following Marek’s Disease Virus Infection. Genes 2019, 10, 74. https://doi.org/10.3390/genes10020074
Chakraborty P, Kuo R, Vervelde L, Dutia BM, Kaiser P, Smith J. Macrophages from Susceptible and Resistant Chicken Lines have Different Transcriptomes following Marek’s Disease Virus Infection. Genes. 2019; 10(2):74. https://doi.org/10.3390/genes10020074
Chicago/Turabian StyleChakraborty, Pankaj, Richard Kuo, Lonneke Vervelde, Bernadette M. Dutia, Pete Kaiser, and Jacqueline Smith. 2019. "Macrophages from Susceptible and Resistant Chicken Lines have Different Transcriptomes following Marek’s Disease Virus Infection" Genes 10, no. 2: 74. https://doi.org/10.3390/genes10020074
APA StyleChakraborty, P., Kuo, R., Vervelde, L., Dutia, B. M., Kaiser, P., & Smith, J. (2019). Macrophages from Susceptible and Resistant Chicken Lines have Different Transcriptomes following Marek’s Disease Virus Infection. Genes, 10(2), 74. https://doi.org/10.3390/genes10020074






