Rescue of Sly Expression Is Not Sufficient to Rescue Spermiogenic Phenotype of Mice with Deletions of Y Chromosome Long Arm
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Mice
2.3. Production of Anti-SLY Antibody
2.4. Production of SLY1, SLY2, SLX, and SLXL1 Proteins
2.5. Dot-Blot
2.6. Immunofluorescence
2.7. Western Blotting
2.8. Production of Mice Transgenic for Sly
2.9. Sperm Analyses
2.10. Assisted Reproduction
2.11. Real-Time RT-PCR
2.12. Development and Analyses of SP4 Transgenic Mice
2.13. Statistics
3. Results
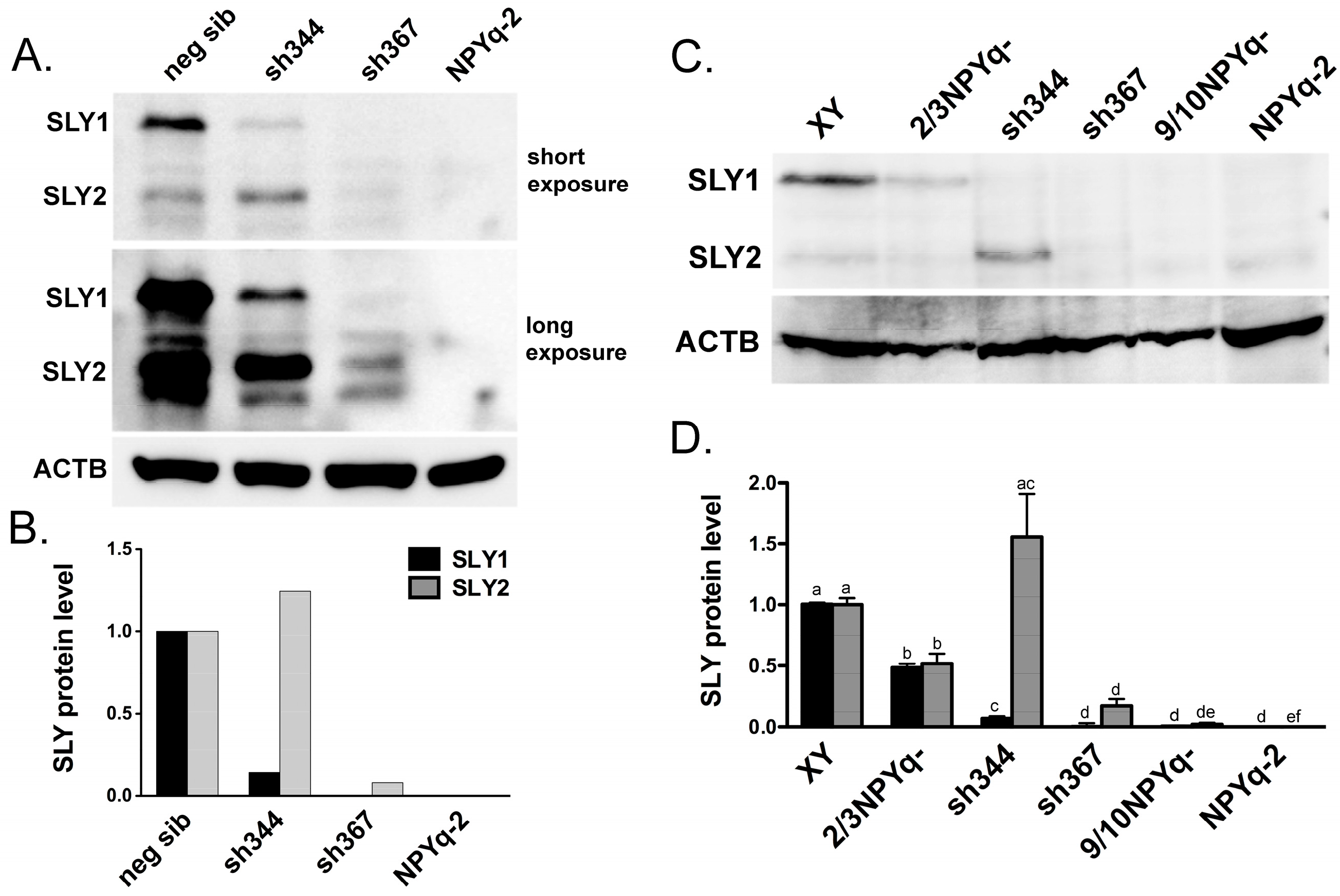
3.1. New Anti-SLY Antibody Specifically Recognizes SLY1 and SLY2 Proteins
3.2. SLY Protein Expression in Mice with NPYq and Sly Deficiencies Matches Previously Reported Transcript Expression Data
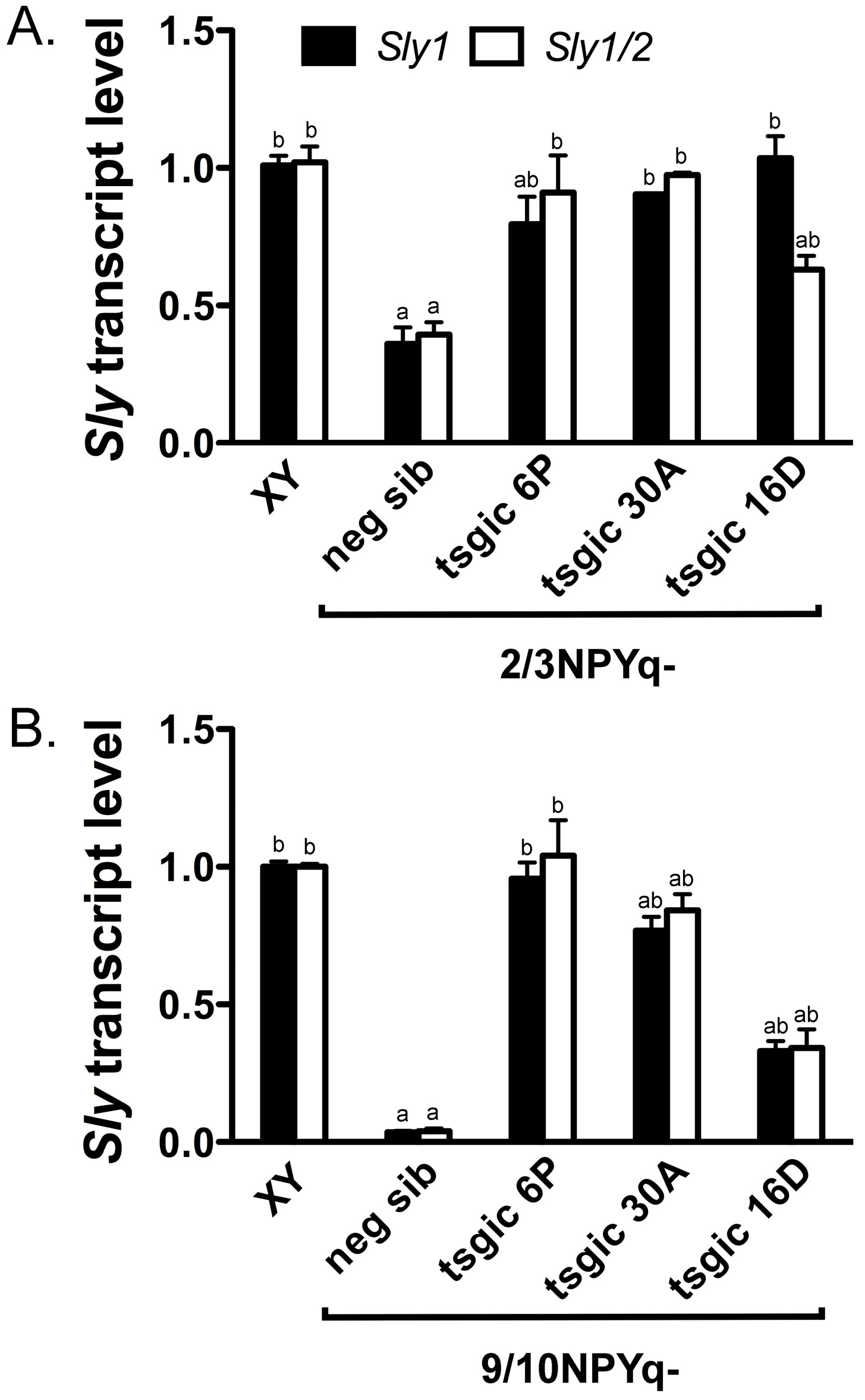
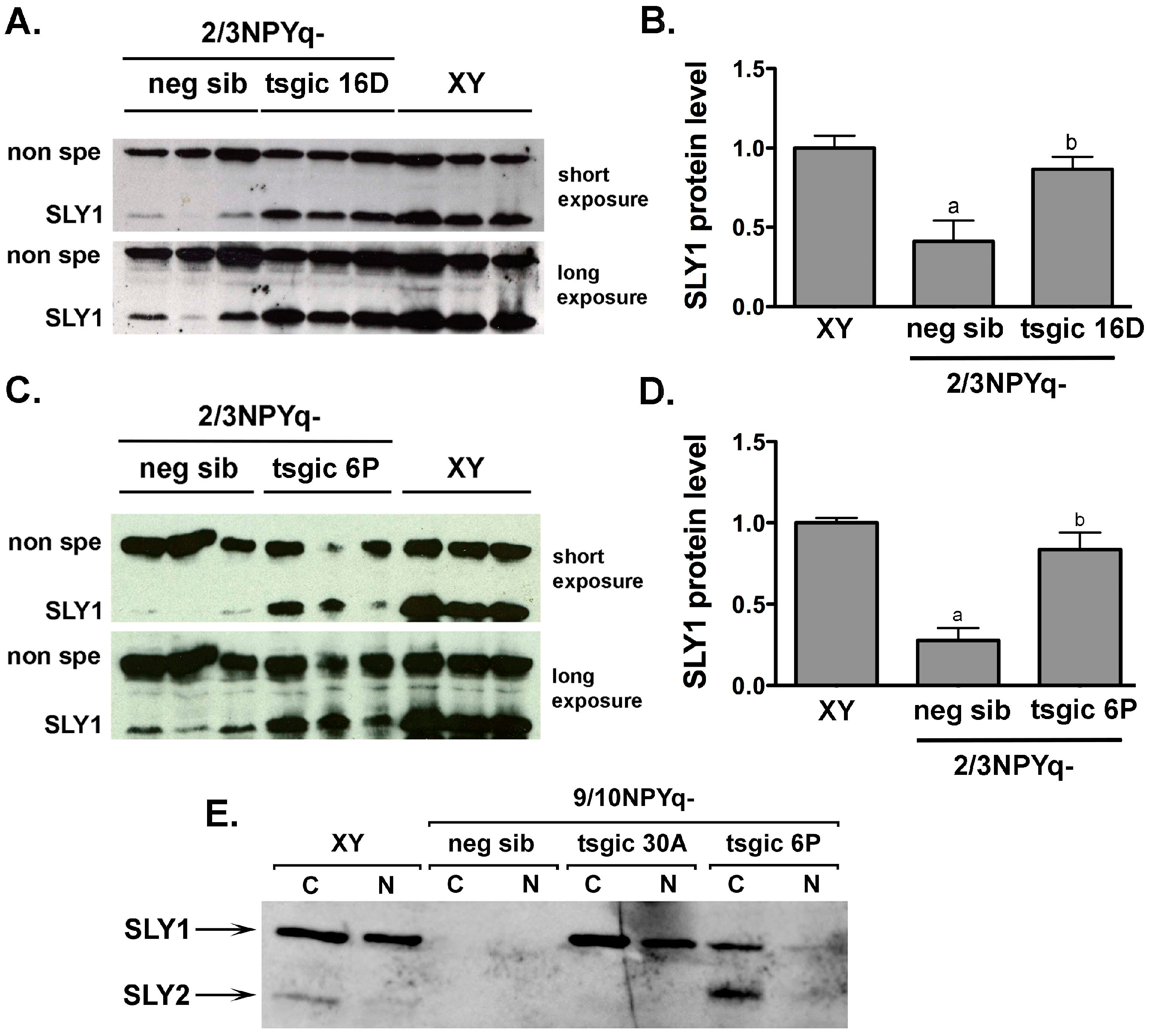
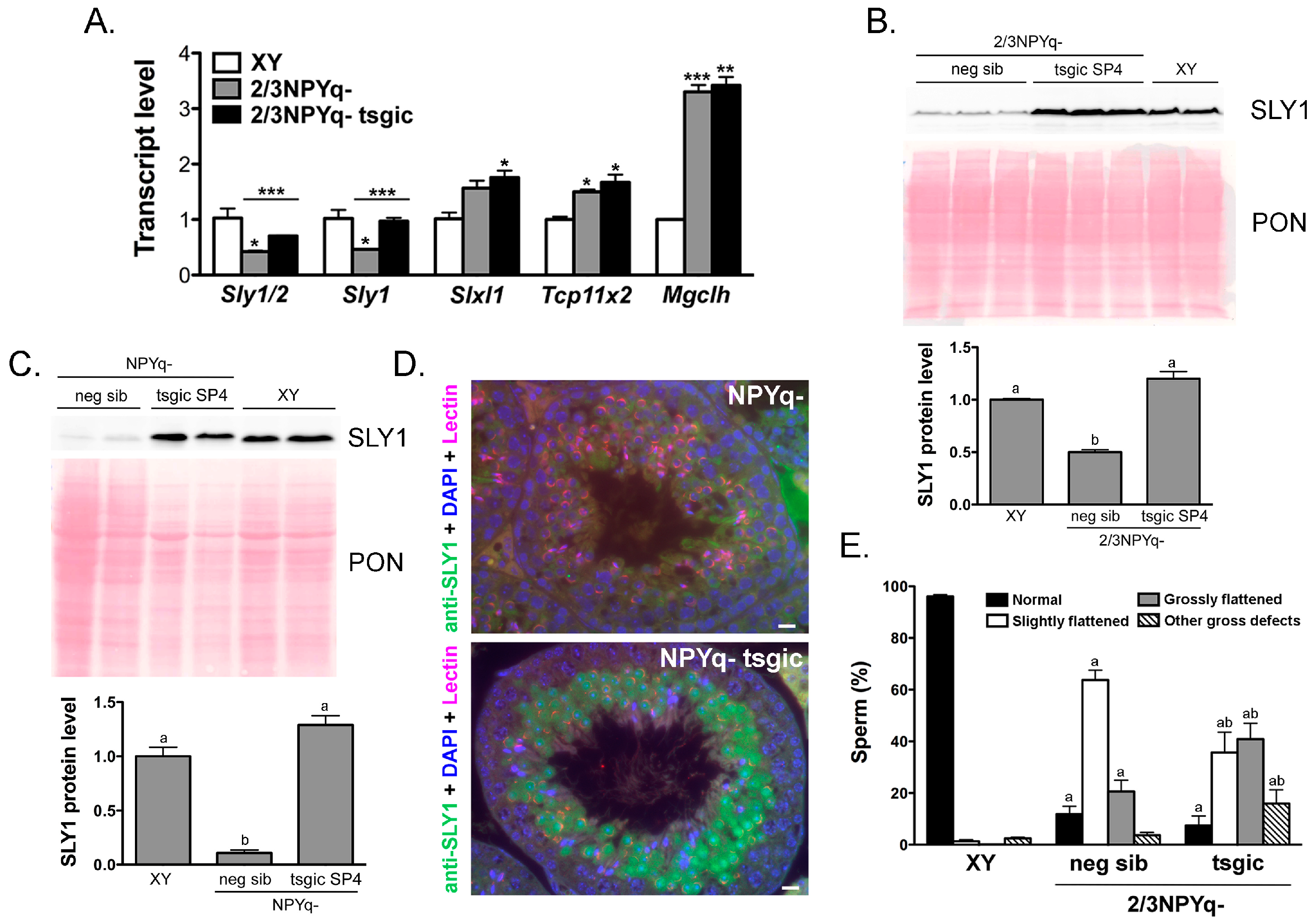
3.3. Addition of Flag-Sly Transgenes Rescues Sly/SLY Expression Deficiency in 2/3NPYq- and 9/10NPYq-Mice
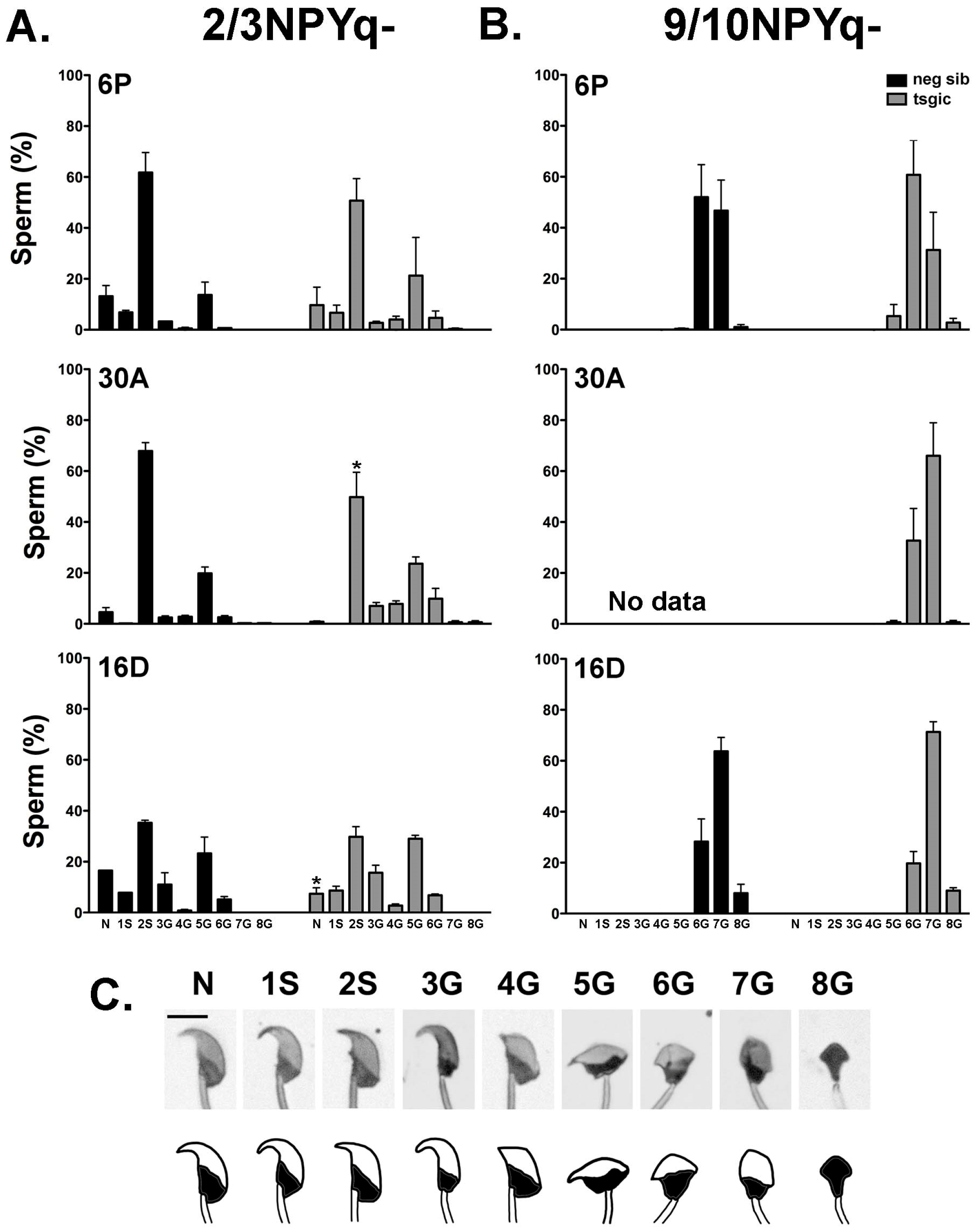
3.4. Addition of Flag-Sly Transgenes Does Not Rescue Spermiogenic Phenotype of 2/3NPYq- and 9/10NPYq-Mice
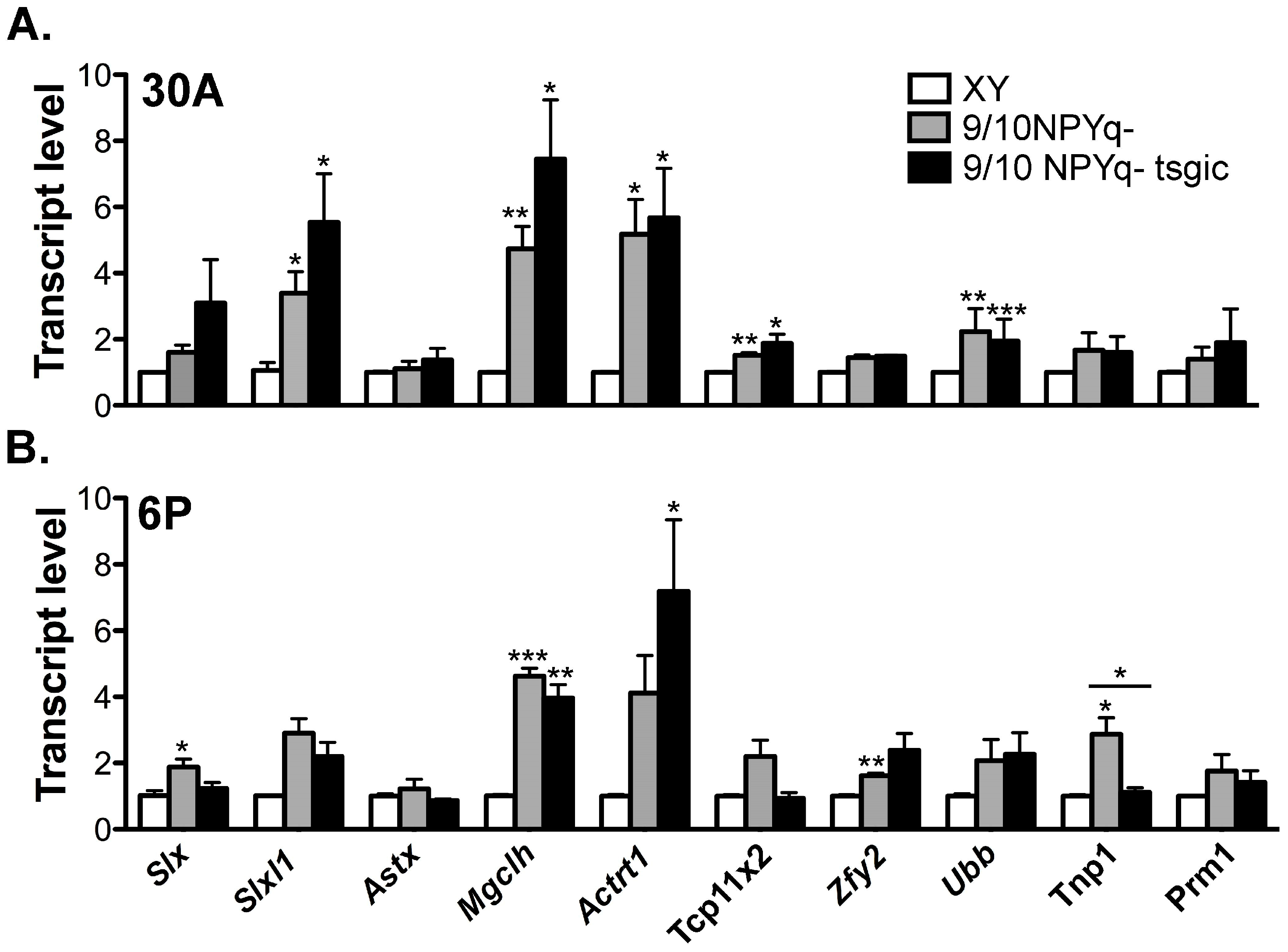
3.5. Addition of Flag-Sly Transgenes Does Not Rescue Gene Upregulation Associated with 2/3NPYq- and 9/10NPYq-Deficiency
3.6. Flag-Sly Transgenic Line Generated and Analyzed Independently Confirms that Addition of the Sly1 Transgene Does Not Rescue Spermiogenic Phenotype of Mice with NPYq Deficiency
4. Discussion
4.1. New anti-SLY Antibody Confirms that Some SLY Protein is Retained in sh367 Sly-KD Mice
4.2. Transgenic SLY Rescues Sly/SLY Expression But Not the NPYq Specific Spermiogenic Phenotype, Suggesting the Involvement of Another NPYq Gene in the Same Pathway
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Soh, Y.Q.; Alfoldi, J.; Pyntikova, T.; Brown, L.G.; Graves, T.; Minx, P.J.; Fulton, R.S.; Kremitzki, C.; Koutseva, N.; Mueller, J.L.; et al. Sequencing the mouse y chromosome reveals convergent gene acquisition and amplification on both sex chromosomes. Cell 2014, 159, 800–813. [Google Scholar] [CrossRef] [PubMed]
- Toure, A.; Clemente, E.J.; Ellis, P.; Mahadevaiah, S.K.; Ojarikre, O.A.; Ball, P.A.; Reynard, L.; Loveland, K.L.; Burgoyne, P.S.; Affara, N.A. Identification of novel Y chromosome encoded transcripts by testis transcriptome analysis of mice with deletions of the Y chromosome long arm. Genome Biol. 2005, 6, R102. [Google Scholar] [CrossRef]
- Burgoyne, P.S.; Mahadevaiah, S.K.; Sutcliffe, M.J.; Palmer, S.J. Fertility in mice requires X-Y pairing and a Y-chromosomal “spermiogenesis” gene mapping to the long arm. Cell 1992, 71, 391–398. [Google Scholar] [CrossRef]
- Moriwaki, K.; Suh, D.S. Genetic factors affecting sperm morphology in the mouse. Mouse Newsl. 1988, 82, 138. [Google Scholar]
- Styrna, J.; Imai, H.T.; Moriwaki, K. An increased level of sperm abnormalities in mice with a partial deletion of the Y chromosome. Genet. Res. 1991, 57, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Styrna, J.; Klag, J.; Moriwaki, K. Influence of partial deletion of the Y chromosome on mouse sperm phenotype. J. Reprod. Fertil. 1991, 92, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Toure, A.; Szot, M.; Mahadevaiah, S.K.; Rattigan, A.; Ojarikre, O.A.; Burgoyne, P.S. A new deletion of the mouse Y chromosome long arm associated with the loss of Ssty expression, abnormal sperm development and sterility. Genetics 2004, 166, 901–912. [Google Scholar] [CrossRef]
- Yamauchi, Y.; Riel, J.M.; Wong, S.J.; Ojarikre, O.A.; Burgoyne, P.S.; Ward, M.A. Live offspring from mice lacking the Y chromosome long arm gene complement. Biol. Reprod. 2009, 81, 353–361. [Google Scholar] [CrossRef]
- Ward, M.A.; Burgoyne, P.S. The effects of deletions of the mouse Y chromosome long arm on sperm function—Intracytoplasmic sperm injection (ICSI)-based analysis. Biol. Reprod. 2006, 74, 652–658. [Google Scholar] [CrossRef]
- Yamauchi, Y.; Riel, J.M.; Stoytcheva, Z.; Burgoyne, P.S.; Ward, M.A. Deficiency in mouse Y chromosome long arm gene complement is associated with sperm DNA damage. Genome Biol. 2010, 11, R66. [Google Scholar] [CrossRef]
- Cocquet, J.; Ellis, P.J.; Yamauchi, Y.; Mahadevaiah, S.K.; Affara, N.A.; Ward, M.A.; Burgoyne, P.S. The multicopy gene Sly represses the sex chromosomes in the male mouse germline after meiosis. PLoS Biol. 2009, 7, e1000244. [Google Scholar] [CrossRef] [PubMed]
- Riel, J.M.; Yamauchi, Y.; Sugawara, A.; Li, H.Y.; Ruthig, V.; Stoytcheva, Z.; Ellis, P.J.; Cocquet, J.; Ward, M.A. Deficiency of the multi-copy mouse Y gene Sly causes sperm DNA damage and abnormal chromatin packaging. J. Cell Sci. 2013, 126, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Ellis, P.J.; Clemente, E.J.; Ball, P.; Toure, A.; Ferguson, L.; Turner, J.M.; Loveland, K.L.; Affara, N.A.; Burgoyne, P.S. Deletions on mouse Yq lead to upregulation of multiple X- and Y-linked transcripts in spermatids. Hum. Mol. Genet. 2005, 14, 2705–2715. [Google Scholar] [CrossRef] [PubMed]
- Moretti, C.; Serrentino, M.E.; Ialy-Radio, C.; Delessard, M.; Soboleva, T.A.; Tores, F.; Leduc, M.; Nitschke, P.; Drevet, J.R.; Tremethick, D.J.; et al. SLY regulates genes involved in chromatin remodeling and interacts with TBL1XR1 during sperm differentiation. Cell Death Differ. 2017, 24, 1029–1044. [Google Scholar] [CrossRef] [PubMed]
- Reynard, L.N.; Turner, J.M. Increased sex chromosome expression and epigenetic abnormalities in spermatids from male mice with Y chromosome deletions. J. Cell Sci. 2009, 122, 4239–4248. [Google Scholar] [CrossRef] [PubMed]
- Lavery, R.; Chassot, A.A.; Pauper, E.; Gregoire, E.P.; Klopfenstein, M.; de Rooij, D.G.; Mark, M.; Schedl, A.; Ghyselinck, N.B.; Chaboissier, M.C. Testicular differentiation occurs in absence of R-spondin1 and Sox9 in mouse sex reversals. PLoS Genet. 2012, 8, e1003170. [Google Scholar] [CrossRef] [PubMed]
- Reynard, L.N.; Cocquet, J.; Burgoyne, P.S. The multi-copy mouse gene Sycp3-like Y-linked (Sly) encodes an abundant spermatid protein that interacts with a histone acetyltransferase and an acrosomal protein. Biol. Reprod. 2009, 81, 250–257. [Google Scholar] [CrossRef]
- Akerfelt, M.; Henriksson, E.; Laiho, A.; Vihervaara, A.; Rautoma, K.; Kotaja, N.; Sistonen, L. Promoter ChIP-chip analysis in mouse testis reveals Y chromosome occupancy by HSF2. Proc. Natl. Acad. Sci. USA 2008, 105, 11224–11229. [Google Scholar] [CrossRef]
- Garcia, M.A.; Collado, M.; Munoz-Fontela, C.; Matheu, A.; Marcos-Villar, L.; Arroyo, J.; Esteban, M.; Serrano, M.; Rivas, C. Antiviral action of the tumor suppressor ARF. EMBO J. 2006, 25, 4284–4292. [Google Scholar] [CrossRef]
- Kimura, Y.; Yanagimachi, R. Intracytoplasmic sperm injection in the mouse. Biol. Reprod. 1995, 52, 709–720. [Google Scholar] [CrossRef]
- Chatot, C.L.; Ziomek, C.A.; Bavister, B.D.; Lewis, J.L.; Torres, I. An improved culture medium supports development of random-bred 1-cell mouse embryos in vitro. J. Reprod. Fertil. 1989, 86, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Quinn, P.; Barros, C.; Whittingham, D.G. Preservation of hamster oocytes to assay the fertilizing capacity of human spermatozoa. J. Reprod. Fertil. 1982, 66, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Ajduk, A.; Yamauchi, Y.; Ward, M.A. Sperm chromatin remodeling after intracytoplasmic sperm injection differs from that of in vitro fertilization. Biol. Reprod. 2006, 75, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.A.; Yanagimachi, R. Intracytoplasmic Sperm Injection in Mice. Cold Spring Harb. Protoc. 2018, 2018, pdb.prot094482. [Google Scholar] [CrossRef] [PubMed]
- Burgoyne, P.S.; Mahadevaiah, S.K.; Perry, J.; Palmer, S.J.; Ashworth, A. The Y* rearrangement in mice: New insights into a perplexing PAR. Cytogenet. Cell Genet. 1998, 80, 37–40. [Google Scholar] [CrossRef]
- Cattanach, B.M. Sex-reversed mice and sex determination. Ann. N. Y. Acad. Sci. 1987, 513, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Cattanach, B.M.; Pollard, C.E.; Hawker, S.G. Sex-reversed mice: XX and XO males. Cytogenetics 1971, 10, 318–337. [Google Scholar] [CrossRef]
- Eicher, E.M.; Hale, D.W.; Hunt, P.A.; Lee, B.K.; Tucker, P.K.; King, T.R.; Eppig, J.T.; Washburn, L.L. The mouse Y* chromosome involves a complex rearrangement, including interstitial positioning of the pseudoautosomal region. Cytogenet. Cell Genet. 1991, 57, 221–230. [Google Scholar] [CrossRef]
- Comptour, A.; Moretti, C.; Serrentino, M.E.; Auer, J.; Ialy-Radio, C.; Ward, M.A.; Toure, A.; Vaiman, D.; Cocquet, J. SSTY proteins co-localize with the post-meiotic sex chromatin and interact with regulators of its expression. FEBS J. 2014, 281, 1571–1584. [Google Scholar] [CrossRef]
- Cocquet, J.; Ellis, P.J.; Mahadevaiah, S.K.; Affara, N.A.; Vaiman, D.; Burgoyne, P.S. A genetic basis for a postmeiotic X versus Y chromosome intragenomic conflict in the mouse. PLoS Genet. 2012, 8, e1002900. [Google Scholar] [CrossRef]
- Syrjanen, J.L.; Pellegrini, L.; Davies, O.R. A molecular model for the role of SYCP3 in meiotic chromosome organisation. eLife 2014, 3. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Liu, J.G.; Zhao, J.; Brundell, E.; Daneholt, B.; Hoog, C. The murine SCP3 gene is required for synaptonemal complex assembly, chromosome synapsis, and male fertility. Mol. Cell 2000, 5, 73–83. [Google Scholar] [CrossRef]
- Mazeyrat, S.; Saut, N.; Grigoriev, V.; Mahadevaiah, S.K.; Ojarikre, O.A.; Rattigan, A.; Bishop, C.; Eicher, E.M.; Mitchell, M.J.; Burgoyne, P.S. A Y-encoded subunit of the translation initiation factor Eif2 is essential for mouse spermatogenesis. Nat. Genet. 2001, 29, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Vernet, N.; Mahadevaiah, S.K.; Ellis, P.J.; de Rooij, D.G.; Burgoyne, P.S. Spermatid development in XO male mice with varying Y chromosome short-arm gene content: evidence for a Y gene controlling the initiation of sperm morphogenesis. Reproduction 2012, 144, 433–445. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, Y.; Riel, J.M.; Ruthig, V.A.; Ortega, E.A.; Mitchell, M.J.; Ward, M.A. Two genes substitute for the mouse Y chromosome for spermatogenesis and reproduction. Science 2016, 351, 514–516. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, Y.; Riel, J.M.; Stoytcheva, Z.; Ward, M.A. Two Y genes can replace the entire Y chromosome for assisted reproduction in the mouse. Science 2014, 343, 69–72. [Google Scholar] [CrossRef]
- Reddi, P.P.; Shore, A.N.; Shapiro, J.A.; Anderson, A.; Stoler, M.H.; Acharya, K.K. Spermatid-specific promoter of the SP-10 gene functions as an insulator in somatic cells. Dev. Biol. 2003, 262, 173–182. [Google Scholar] [CrossRef]
- Reddi, P.P.; Flickinger, C.J.; Herr, J.C. Round spermatid-specific transcription of the mouse SP-10 gene is mediated by a 294-base pair proximal promoter. Biol. Reprod. 1999, 61, 1256–1266. [Google Scholar] [CrossRef]
- Laval, S.H.; Reed, V.; Blair, H.J.; Boyd, Y. The structure of DXF34, a human X-linked sequence family with homology to a transcribed mouse Y-linked repeat. Mamm. Genome 1997, 8, 689–691. [Google Scholar] [CrossRef]
- Oh, B.; Hwang, S.Y.; Solter, D.; Knowles, B.B. Spindlin, a major maternal transcript expressed in the mouse during the transition from oocyte to embryo. Development 1997, 124, 493–503. [Google Scholar]
- Su, X.; Zhu, G.; Ding, X.; Lee, S.Y.; Dou, Y.; Zhu, B.; Wu, W.; Li, H. Molecular basis underlying histone H3 lysine-arginine methylation pattern readout by Spin/Ssty repeats of Spindlin1. Genes Dev. 2014, 28, 622–636. [Google Scholar] [CrossRef] [PubMed]
- Toure, A.; Grigoriev, V.; Mahadevaiah, S.K.; Rattigan, A.; Ojarikre, O.A.; Burgoyne, P.S. A protein encoded by a member of the multicopy Ssty gene family located on the long arm of the mouse Y chromosome is expressed during sperm development. Genomics 2004, 83, 140–147. [Google Scholar] [CrossRef]
- Eberl, H.C.; Spruijt, C.G.; Kelstrup, C.D.; Vermeulen, M.; Mann, M. A map of general and specialized chromatin readers in mouse tissues generated by label-free interaction proteomics. Mol. Cell 2013, 49, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Ellis, P.J.; Ferguson, L.; Clemente, E.J.; Affara, N.A. Bidirectional transcription of a novel chimeric gene mapping to mouse chromosome Yq. BMC Evol. Biol. 2007, 7, 171. [Google Scholar] [CrossRef] [PubMed]
- Ellis, P.J.; Bacon, J.; Affara, N.A. Association of Sly with sex-linked gene amplification during mouse evolution: A side effect of genomic conflict in spermatids? Hum. Mol. Genet. 2011, 20, 3010–3021. [Google Scholar] [CrossRef] [PubMed]
| Tg Line | Genotype | No Males | Age (Weeks) | Average ± SEM | Eggs Fertilized/Eggs Inseminated (%) | ||
|---|---|---|---|---|---|---|---|
| Body Weight (g) | Testis Weight (mg) | Sperm Number (1CE, ×106) | |||||
| 6P | 9/10NPYq-Sly1/2 | 4 | 10 | 27 ± 1 | 58 ± 4 | 0.1 ± 0.1 | 0/236 (0) |
| 9/10NPYq- | 3 | 10 | 26 ± 2 | 60 ± 3 | 1.9 ± 0.9 | 0/167 (0) | |
| WT IVF control | 2 | 16 | 34 ± 0 | 92 ± 3 | n/a | 75/121 (62) | |
| 16D | 9/10NPYq-Sly1 | 4 | 12 | 32 ± 4 | 79 ± 10 | 2.5 ± 1.2 | 1/236 (0) |
| 9/10NPYq- | 3 | 12 | 27 ± 1 | 81 ± 2 | 4.2 ± 2.4 | 0/166 (0) | |
| WT IVF control | 2 | 14 | 29 ± 1 | 94 ± 3 | n/a | 114/122 (93) | |
| 30A | 9/10NPYq-Sly1 | 3 | 10 | 26 ± 1 | 64 ± 4 | 0.4 ± 0.03 | 0/129 (0) |
| WT IVF control | 1 | 16 | 38 | 99, 100 | n/a | 39/49 (80) | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riel, J.M.; Yamauchi, Y.; Ruthig, V.A.; Malinta, Q.U.; Blanco, M.; Moretti, C.; Cocquet, J.; Ward, M.A. Rescue of Sly Expression Is Not Sufficient to Rescue Spermiogenic Phenotype of Mice with Deletions of Y Chromosome Long Arm. Genes 2019, 10, 133. https://doi.org/10.3390/genes10020133
Riel JM, Yamauchi Y, Ruthig VA, Malinta QU, Blanco M, Moretti C, Cocquet J, Ward MA. Rescue of Sly Expression Is Not Sufficient to Rescue Spermiogenic Phenotype of Mice with Deletions of Y Chromosome Long Arm. Genes. 2019; 10(2):133. https://doi.org/10.3390/genes10020133
Chicago/Turabian StyleRiel, Jonathan M., Yasuhiro Yamauchi, Victor A. Ruthig, Qushay U. Malinta, Mélina Blanco, Charlotte Moretti, Julie Cocquet, and Monika A. Ward. 2019. "Rescue of Sly Expression Is Not Sufficient to Rescue Spermiogenic Phenotype of Mice with Deletions of Y Chromosome Long Arm" Genes 10, no. 2: 133. https://doi.org/10.3390/genes10020133
APA StyleRiel, J. M., Yamauchi, Y., Ruthig, V. A., Malinta, Q. U., Blanco, M., Moretti, C., Cocquet, J., & Ward, M. A. (2019). Rescue of Sly Expression Is Not Sufficient to Rescue Spermiogenic Phenotype of Mice with Deletions of Y Chromosome Long Arm. Genes, 10(2), 133. https://doi.org/10.3390/genes10020133





