An Orthologue of the Retinoic Acid Receptor (RAR) Is Present in the Ecdysozoa Phylum Priapulida
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. RNA Extraction
2.3. RXR and RAR Gene Isolation
2.4. Sequence and Phylogenetic Analysis
2.5. Construction of Plasmid Vectors
2.6. Chemicals and Solutions
2.7. Cell Culture and Transactivation Assays
2.8. Ligand Binding Assays
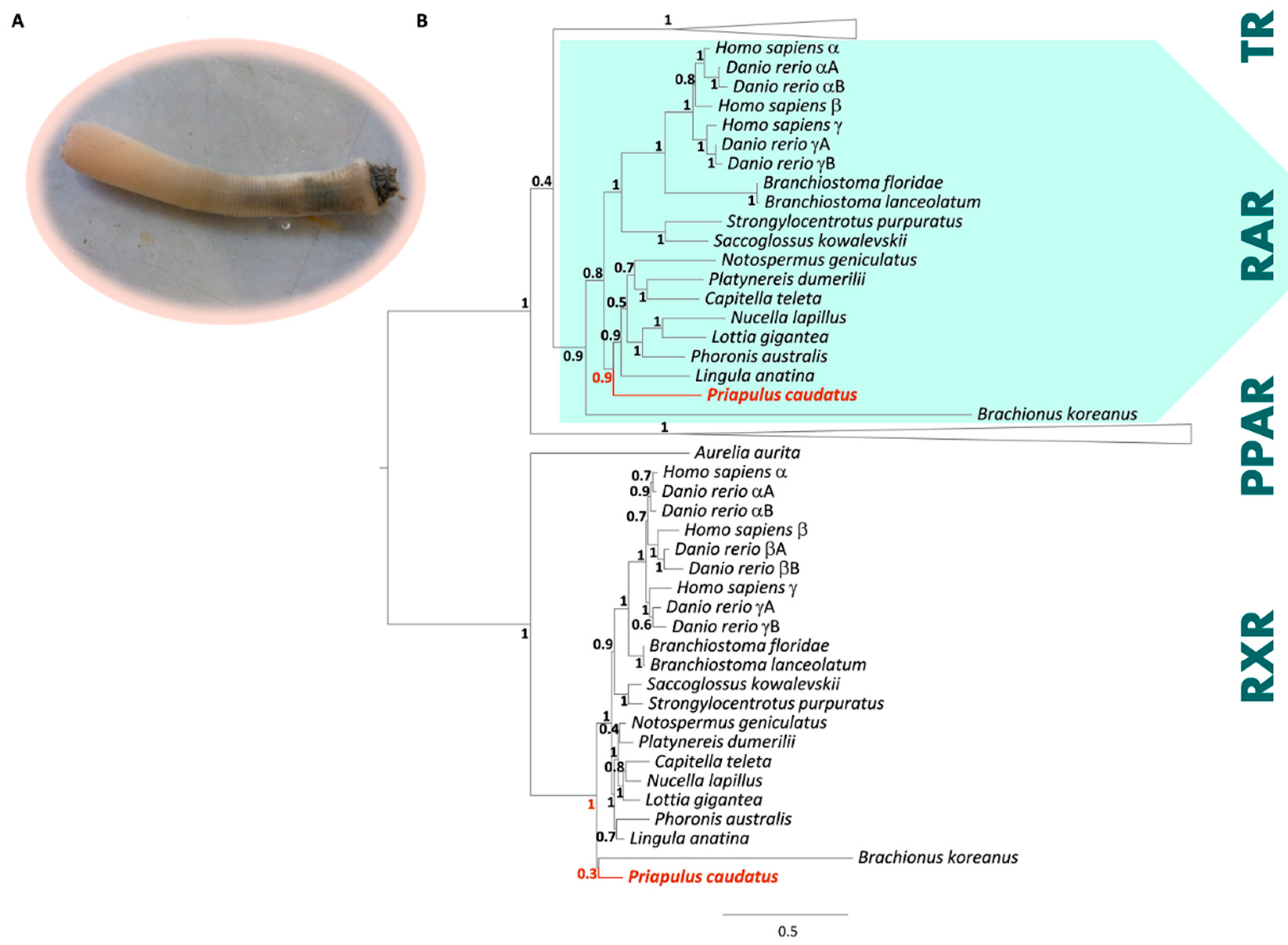
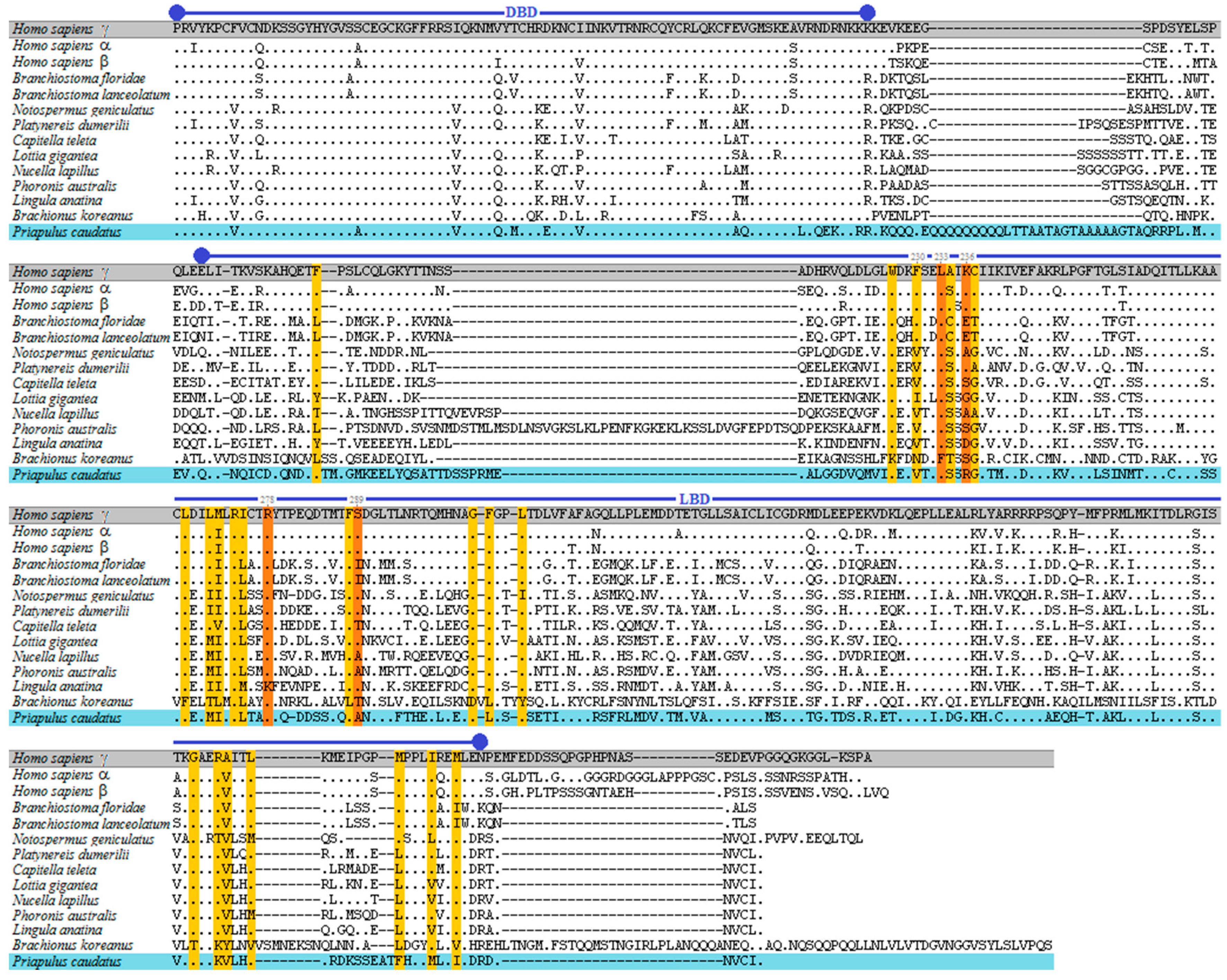
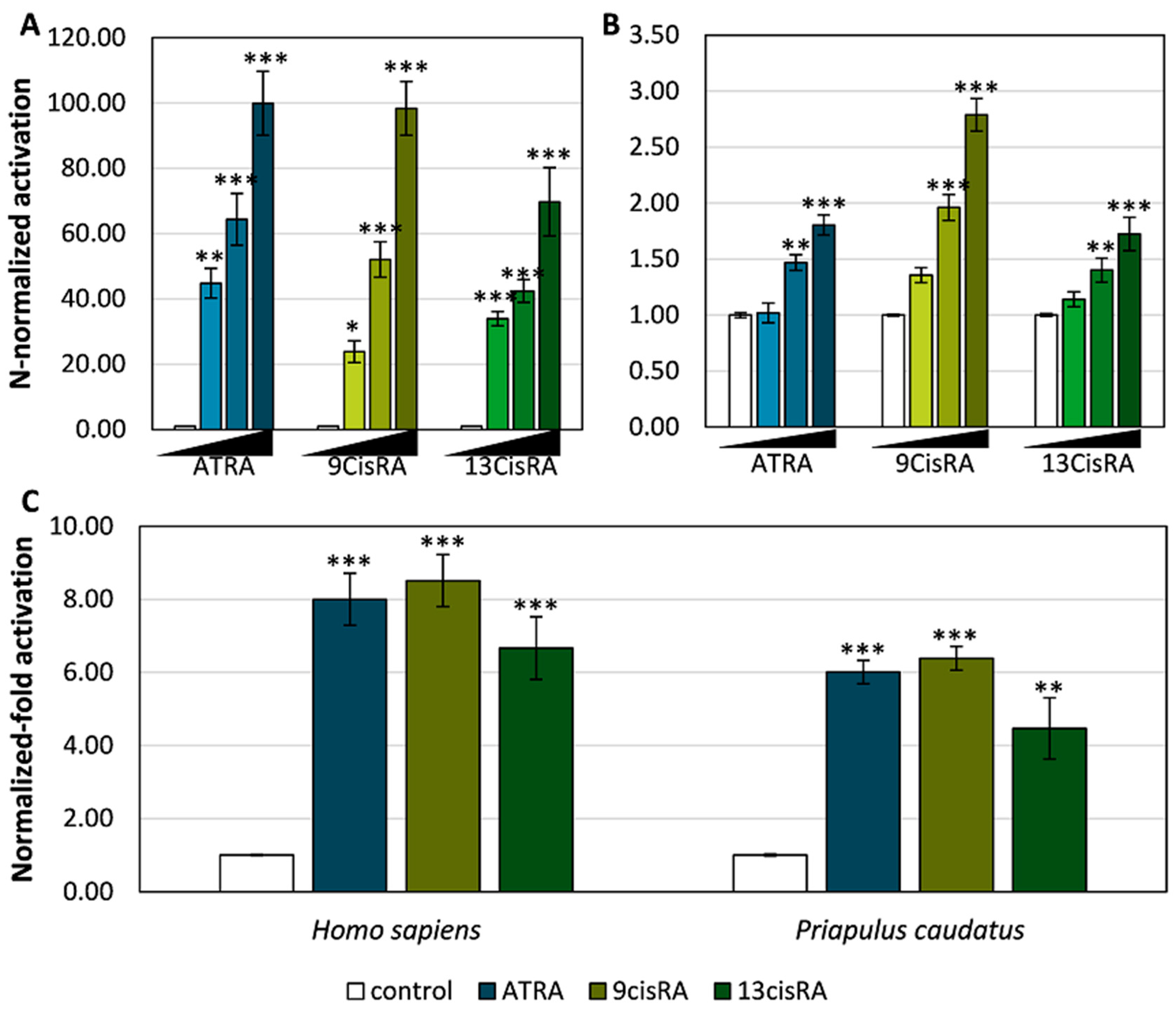
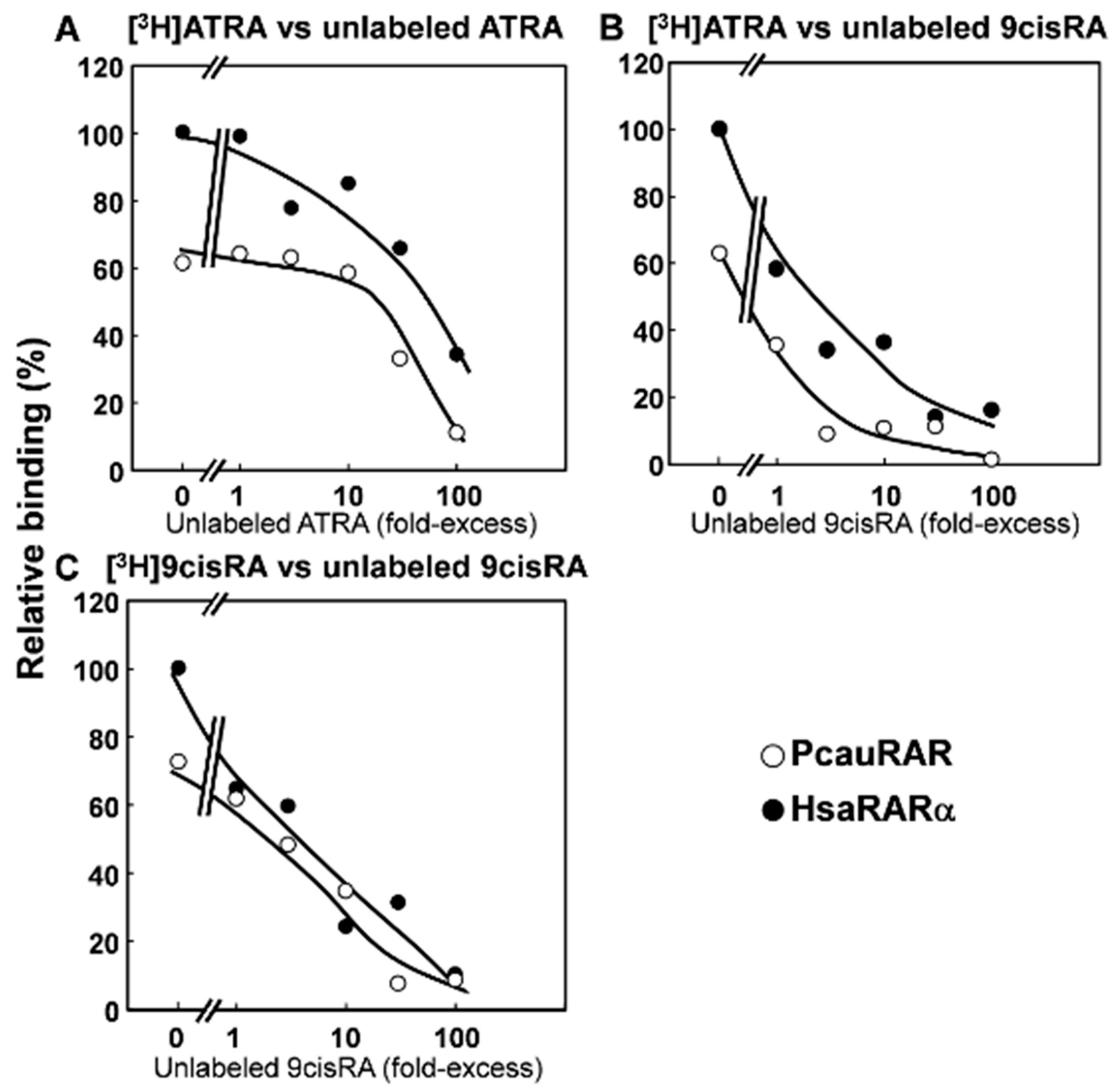
3. Results
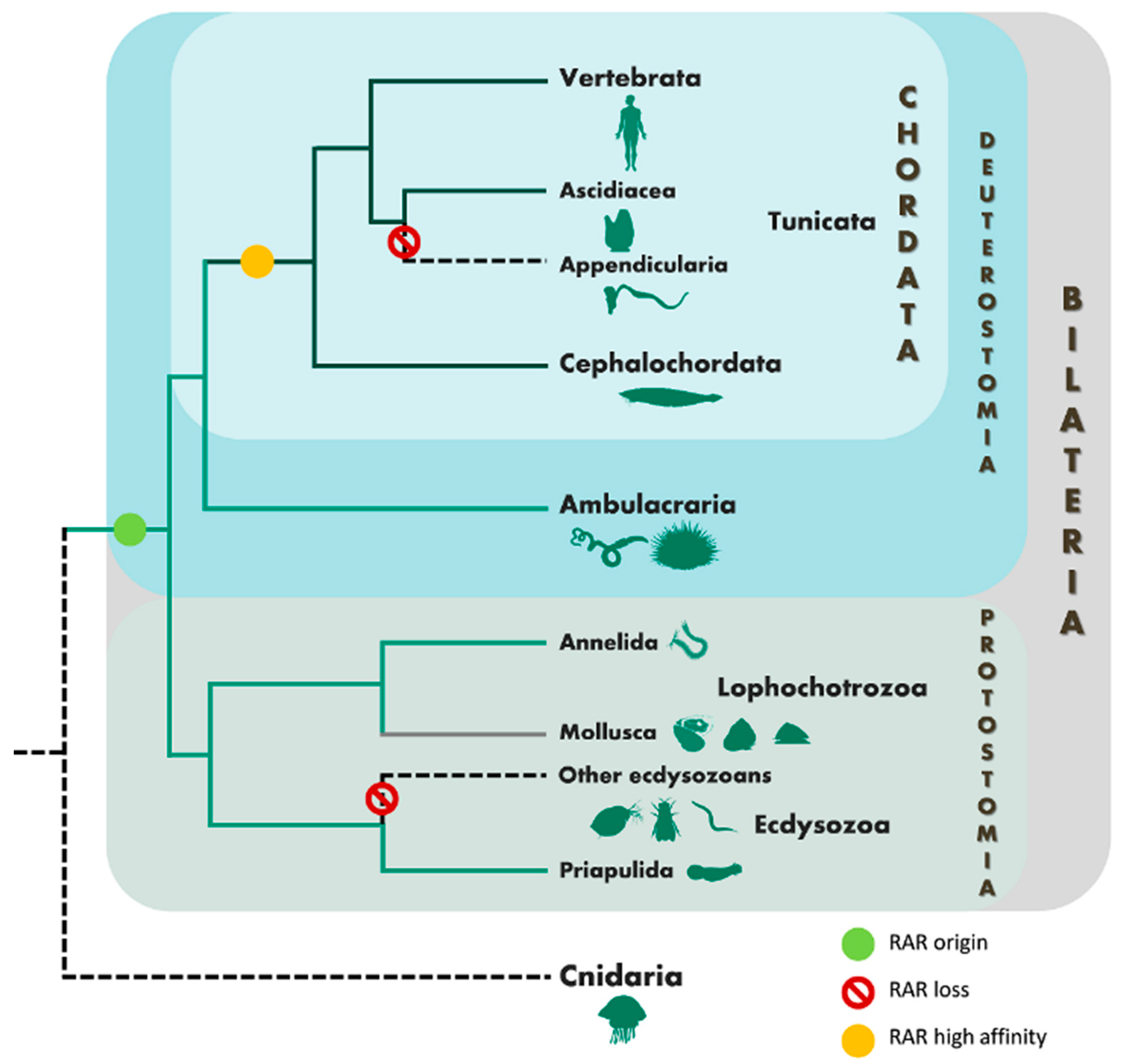
3.1. Phylogenetic and Sequence Analysis of Priapulid RAR
3.2. Functional Characterization of the Priapulid RAR Orthologue
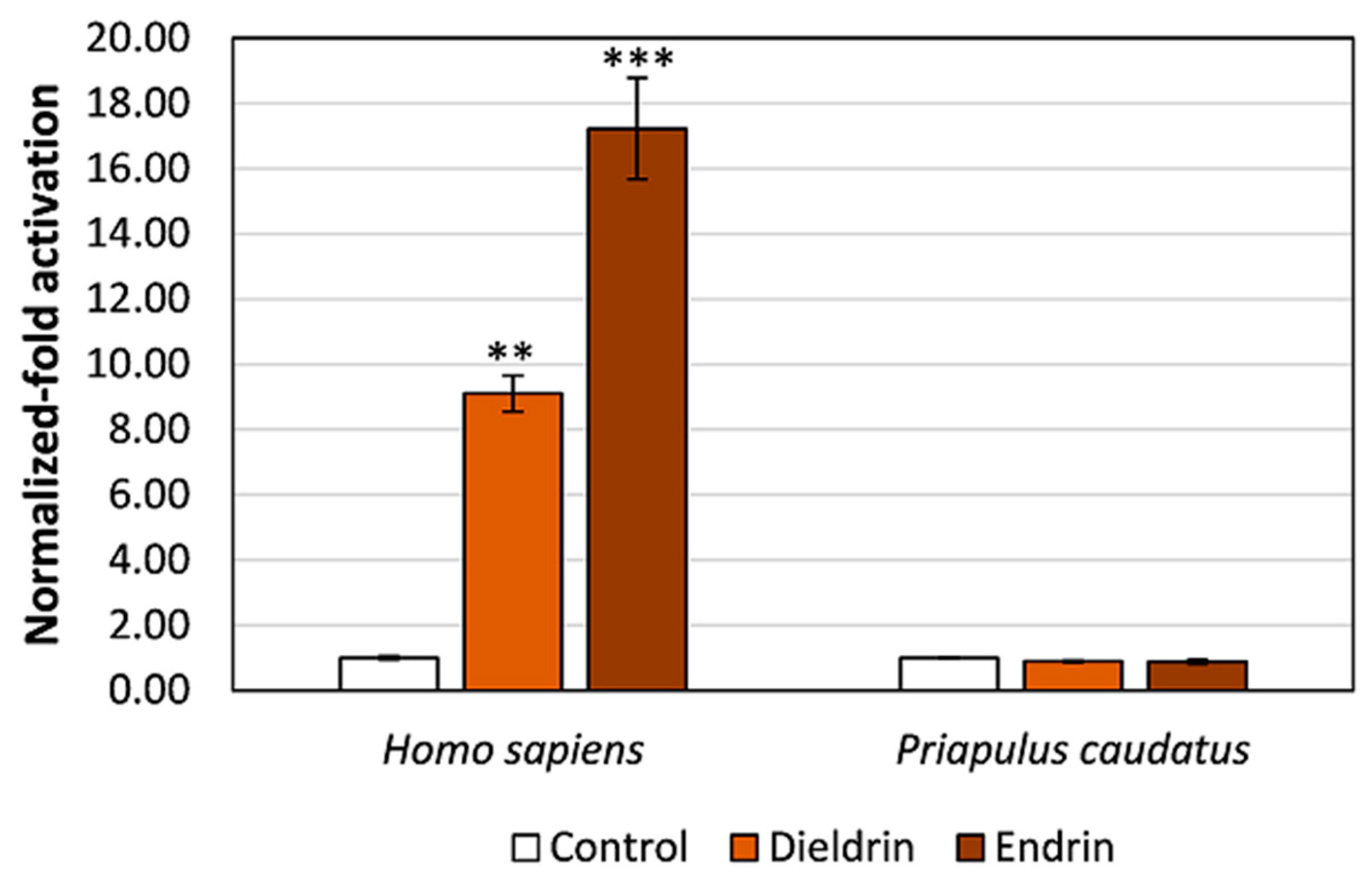
3.3. Pesticides Do Not Activate Transcription via the Priapulid RAR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ross, S.; McCaffery, P.; Drager, U.; De Luca, L. Retinoids in embryonal development. Physiol. Rev. 2000, 80, 1021–1054. [Google Scholar] [CrossRef] [PubMed]
- Samarut, E.; Rochette-Egly, C. Nuclear retinoic acid receptors: Conductors of the retinoic acid symphony during development. Mol. Cell Endocrinol. 2012, 348, 348–360. [Google Scholar] [CrossRef] [PubMed]
- Niederreither, K.; Dollé, P. Retinoic acid in development: Towards an integrated view. Nat. Rev. Genet. 2008, 9, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Escriva, H.; Holland, N.; Gronemeyer, H.; Laudet, V.; Holland, L. The retinoic acid signaling pathway regulates anterior/posterior patterning in the nerve cord and pharynx of amphioxus, a chordate lacking neural crest. Development 2002, 129, 2905–2916. [Google Scholar] [PubMed]
- Schubert, M.; Holland, N.; Escriva, H.; Holland, L.; Laudet, V. Retinoic acid influences anteroposterior positioning of epidermal sensory neurons and their gene expression in a developing chordate (amphioxus). Proc. Natl. Acad. Sci. USA 2004, 101, 10320–10325. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, T.; Duester, G. Mechanisms of retinoic acid signalling and its roles in organ and limb development. Nat. Rev. Mol. Cell Biol. 2015, 16, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Maden, M.; Hind, M. Retinoic acid, a regeneration-inducing molecule. Dev. Dyn. 2003, 226, 237–244. [Google Scholar] [CrossRef]
- Bertrand, S.; Thisse, B.; Tavares, R.; Sachs, L.; Chaumot, A.; Bardet, P.; Escriva, H.; Duffraisse, M.; Marchand, O.; Safi, R.; et al. Unexpected novel relational links uncovered by extensive developmental profiling of nuclear receptor expression. PLoS Genet. 2007, 3, e188. [Google Scholar] [CrossRef]
- Cvekl, A.; Wang, W. Retinoic acid signaling in mammalian eye development. Exp. Eye Res. 2009, 89, 280–291. [Google Scholar] [CrossRef]
- Albalat, R. The retinoic acid machinery in invertebrates: Ancestral elements and vertebrate innovations. Mol. Cell Endocrinol. 2009, 313, 23–35. [Google Scholar] [CrossRef]
- Rochette-Egly, C.; Germain, P. Dynamic and combinatorial control of gene expression by nuclear retinoic acid receptors (RARs). Nucl. Recep. Signal. 2009, 7, e005. [Google Scholar] [CrossRef] [PubMed]
- Germain, P.; Staels, B.; Dacquet, C.; Spedding, M.; Laudet, V. Overview of nomenclature of nuclear receptors. Pharm. Rev. 2006, 58, 685–704. [Google Scholar] [CrossRef] [PubMed]
- Germain, P.; Altucci, L.; Bourguet, W.; Rochette-Egly, C.; Gronemeyer, H. Nuclear receptor superfamily: Principles of signaling. Pure Appl. Chem. 2003, 75, 1619–1664. [Google Scholar] [CrossRef]
- Chatagnon, A.; Veber, P.; Morin, V.; Bedo, J.; Triqueneaux, G.; Sémon, M.; Laudet, V.; D’Alché-Buc, F.; Benoit, G. RAR/RXR binding dynamics distinguish pluripotency from differentiation associated cis-regulatory elements. Nucleic Acids Res. 2015, 43, 4833–4854. [Google Scholar] [CrossRef] [PubMed]
- Albalat, R.; Cañestro, C. Identification of Aldh1a, Cyp26 and RAR orthologs in protostomes pushes back the retinoic acid genetic machinery in evolutionary time to the bilaterian ancestor. Chem. Biol. Interact. 2009, 178, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Cañestro, C.; Postlethwait, J.; Gonzalez-Duarte, R.; Albalat, R. Is retinoic acid genetic machinery a chordate innovation? Evol. Dev. 2006, 8, 394–406. [Google Scholar] [CrossRef] [PubMed]
- Howard-Ashby, M.; Materna, S.; Brown, C.; Chen, L.; Cameron, R.; Davidson, E. Gene families encoding transcription factors expressed in early development of Strongylocentrotus purpuratus. Dev. Biol. 2006, 300, 90–107. [Google Scholar] [CrossRef]
- Marlétaz, F.; Holland, L.; Laudet, V.; Schubert, M. Retinoic acid signaling and the evolution of chordates. Int. J. Biol. Sci. 2006, 2, 38–47. [Google Scholar] [CrossRef]
- Ollikainen, N.; Chansawangbhuwana, C.; Baker, M. Evolution of the thyroid hormone, retinoic acid, ecdysone and liver X receptors. Integr. Comp. Biol. 2006, 46, 815–826. [Google Scholar] [CrossRef]
- Simões-Costa, M.; Azambuja, A.; Xavier-Neto, J. The search for non-chordate retinoic acid signaling: Lessons from chordates. J. Exp. Zool. B Mol. Dev. Evol. 2008, 310, 54–72. [Google Scholar] [CrossRef]
- Campo-Paysaa, F.; Marlétaz, F.; Laudet, V.; Schubert, M. Retinoic acid signaling in development: Tissue-specific functions and evolutionary origins. Genesis 2008, 46, 640–656. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Mazariegos, J.; Nadendla, E.; Lima, D.; Pierzchalski, K.; Jones, J.; Kane, M.; Nishikawa, J.-I.; Hiromori, Y.; Nakanishi, T.; Santos, M.; et al. A mollusk retinoic acid receptor (RAR) ortholog sheds light on the evolution of ligand binding. Endocrinology 2014, 155, 4275–4286. [Google Scholar] [CrossRef] [PubMed]
- Urushitani, H.; Katsu, Y.; Ohta, Y.; Shiraishi, H.; Iguchi, T.; Horiguchi, T. Cloning and characterization of the retinoic acid receptor-like protein in the rock shell, Thais clavigera. Aquat. Toxicol. 2013, 142–143, 403–413. [Google Scholar] [CrossRef] [PubMed]
- André, A.; Ruivo, R.; Fonseca, E.; Froufe, E.; Castro, L.; Santos, M. The retinoic acid receptor (RAR) in molluscs: Function, evolution and endocrine disruption insights. Aquat. Toxicol. 2019, 208, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Handberg-Thorsager, M.; Gutierrez-Mazariegos, J.; Arold, S.; Kumar Nadendla, E.; Bertucci, P.; Germain, P.; Tomançak, P.; Pierzchalski, K.; Jones, J.; Albalat, R.; et al. The ancestral retinoic acid receptor was a low-affinity sensor triggering neuronal differentiation. Sci. Adv. 2018, 4, eaao1261. [Google Scholar] [CrossRef] [PubMed]
- Janesick, A.; Wu, S.; Blumberg, B. Retinoic acid signaling and neuronal differentiation. Cell Mol. Life Sci. 2015, 72, 1559–1576. [Google Scholar] [CrossRef]
- Castro, L.; Santos, M. To bind or not to bind: The taxonomic scope of nuclear receptor mediated endocrine disruption in invertebrate phyla. Environiron. Sci. Technol. 2014, 48, 5361–5363. [Google Scholar] [CrossRef]
- Capitão, A.; Lyssimachou, A.; Castro, L.; Santos, M. Obesogens in the aquatic Environironment: An evolutionary and toxicological perspective. Environ. Int. 2017, 106, 153–169. [Google Scholar]
- Balaguer, P.; Delfosse, V.; Grimaldi, M.; Bourguet, W. Structural and functional evidences for the interactions between nuclear hormone receptors and endocrine disruptors at low doses. C. R. Biol. 2017, 340, 414–420. [Google Scholar] [CrossRef]
- Keay, J.; Thornton, J. Hormone-activated estrogen receptors in annelid invertebrates: Implications for evolution and endocrine disruption. Endocrinology 2009, 150, 1731–1738. [Google Scholar] [CrossRef]
- Vogeler, S.; Galloway, T.; Isupov, M.; Bean, T. Cloning retinoid and peroxisome proliferator-activated nuclear receptors of the Pacific oyster and in silico binding to Environironmental chemicals. PLoS ONE 2017, 12, e0176024. [Google Scholar] [CrossRef] [PubMed]
- Katsiadaki, I. Are marine invertebrates really at risk from endocrine-disrupting chemicals? Curr. Opin. Environ. Sci. Health 2019, 11, 37–42. [Google Scholar] [CrossRef]
- Scott, A. Is there any value in measuring vertebrate steroids in invertebrates? Gen. Comp. Endocrinol. 2018, 265, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Lemaire, G.; Balaguer, P.; Michel, S.; Rahmani, R. Activation of retinoic acid receptor-dependent transcription by organochlorine pesticides. Toxicol. Appl. Pharm. 2005, 202, 38–49. [Google Scholar] [CrossRef]
- Sansom, R. Preservation and phylogeny of Cambrian ecdysozoans tested by experimental decay of Priapulus. Sci. Rep. 2016, 6, 32817. [Google Scholar] [CrossRef]
- Webster, B.; Copley, R.; Jenner, R.; Mackenzie-Dodds, J.; Bourlat, S.; Rota-Stabelli, O.; Littlewood, D.; Telford, M. Mitogenomics and phylogenomics reveal priapulid worms as extant models of the ancestral Ecdysozoan. Evol. Dev. 2006, 8, 502–510. [Google Scholar] [CrossRef]
- Wills, M.; Gerber, S.; Ruta, M.; Hughes, M. The disparity of priapulid, archaeopriapulid and palaeoscolecid worms in the light of new data. J. Evol. Biol. 2012, 25, 2056–2076. [Google Scholar] [CrossRef]
- Katoh, K.; Toh, H. Parallelization of the MAFFT multiple sequence alignment program. Bioinformatics 2010, 26, 1899–1900. [Google Scholar] [CrossRef]
- Renaud, J.; Rochel, N.; Ruff, M.; Vivat, V.; Chambon, P.; Gronemeyer, H.; Moras, D. Crystal structure of the RAR-γ ligand-binding domain bound to all-trans retinoic acid. Nature 1995, 378, 681–689. [Google Scholar] [CrossRef]
- Hisata, K.; Fujiwara, S.; Tsuchida, Y.; Ohashi, M.; Kawamura, K. Expression and function of a retinoic acid receptor in budding ascidians. Dev. Genes Evol. 1998, 208, 537–546. [Google Scholar] [CrossRef]
- Gesto, M.; Ruivo, R.; Páscoa, I.; André, A.; Castro, L.; Santos, M. Retinoid level dynamics during gonad recycling in the limpet Patella vulgata. Gen. Comp. Endocrinol. 2016, 225, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Escriva, H.; Bertrand, S.; Germain, P.; Robinson-Rechavi, M.; Umbhauer, M.; Cartry, J.; Duffraisse, M.; Holland, L.; Gronemeyer, H.; Laudet, V. Neofunctionalization in vertebrates: The example of retinoic acid receptors. PLoS Genet. 2006, 2, e102. [Google Scholar] [CrossRef] [PubMed]
- Guindon, S.; Dufayard, J.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New Algorithms and Methods to Estimate Maximum-Likelihood Phylogenies: Assessing the Performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Duffy, J. GAL4 system in Drosophila: A fly geneticist’s Swiss army knife. Genesis 2002, 34, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Hagmann, M.; Georgiev, O.; Schaffner, W. The VP16 paradox: Herpes simplex virus VP16 contains a long-range activation domain but within the natural multiprotein complex activates only from promoter-proximal positions. J. Virol. 1997, 71, 5952–5962. [Google Scholar] [PubMed]
- Nakanishi, T.; Nishikawa, J.; Hiromori, Y.; Yokoyama, H.; Koyanagi, M.; Takasuga, S.; Ishizaki, J.; Watanabe, M.; Isa, S.; Utoguchi, N.; et al. Trialkyltin compounds bind retinoid X receptor to alter human placental endocrine functions. Mol. Endocrinol. 2005, 19, 2502–2516. [Google Scholar] [CrossRef]
- Hiromori, Y.; Nishikawa, J.; Yoshida, I.; Nagase, H.; Nakanishi, T. Structure-dependent activation of peroxisome proliferator-activated receptor (PPAR) γ by organotin compounds. Chem. Biol. Interact. 2009, 180, 238–244. [Google Scholar] [CrossRef]
- Hiromori, Y.; Ido, A.; Aoki, A.; Kimura, T.; Nagase, H.; Nakanishi, T. Ligand activity of group 15 compounds possessing triphenyl substituent for the RXR and PPARγ nuclear receptors. Biol. Pharm. Bull. 2016, 39, 1596–1603. [Google Scholar] [CrossRef]
- Aranda, A.; Pascual, A. Nuclear hormone receptors and gene expression. Physiol. Rev. 2001, 81, 1269–1304. [Google Scholar] [CrossRef]
- Chambon, P. The nuclear receptor superfamily: A personal retrospect on the first two decades. Mol. Endocrinol. 2005, 19, 1418–1428. [Google Scholar] [CrossRef]
- Bertrand, S.; Brunet, F.; Escriva, H.; Parmentier, G.; Laudet, V.; Robinson-Rechavi, M. Evolutionary genomics of nuclear receptors: From twenty-five ancestral genes to derived endocrine systems. Mol. Biol. Evol. 2004, 21, 1923–1937. [Google Scholar] [PubMed]
- Darbre, P. Endocrine Disruption and Human Health. Overview of EDCs and Human Health Which Sets the Bigger Picture; Academic: New York, NY, USA, 2015. [Google Scholar]
- Laudet, V.; Gronemeyer, H. The Nuclear Receptors Factsbook; Academic Press: London, UK, 2002; ISBN 0-12-437735-1. [Google Scholar]
- Kamata, R.; Shiraishi, F.; Nishikawa, J.; Yonemoto, J.; Shiraishi, H. Screening and detection of the in vitro agonistic activity of xenobiotics on the retinoic acid receptor. Toxicol. Vitr. 2008, 22, 1050–1061. [Google Scholar]
- Strandberg, B.; Strandberg, L.; van Bavel, B.; Bergqvist, P.; Broman, D.; Falandysz, J.; Näf, C.; Papakosta, O.; Rolff, C.; Rappe, C. Concentrations and spatial variations of cyclodienes and other organochlorines in herring and perch from the Baltic Sea. Sci. Total Environ. 1998, 215, 69–83. [Google Scholar] [PubMed]
- Strandberg, B.; Bandh, C.; van Bavel, B.; Bergqvist, P.; Broman, D.; Näf, C.; Pettersen, H.; Rappe, C. Concentrations, biomagnification and spatial variation of organochlorine compounds in a pelagic food web in the northern part of the Baltic Sea. Sci. Total Environ. 1998, 217, 143–154. [Google Scholar] [CrossRef]
- Falandysz, J.; Strandberg, B. Persistent Organochlorine Compounds in Sludge and Sediments from the Gdańsk Region, Baltic Sea. Pol. J. Environ. Stud. 2004, 13, 133–138. [Google Scholar]
- Schubert, S.; Keddig, N.; Gerwinski, W.; Neukirchen, J.; Kammann, U.; Haarich, M.; Hanel, R.; Theobald, N. Persistent organic pollutants in Baltic herring (Clupea harengus)-an aspect of gender. Environ. Monit. Assess. 2016, 188, 368. [Google Scholar] [CrossRef]
- Telford, M.; Bourlat, S.; Economou, A.; Papillon, D.; Rota-Stabelli, O. The evolution of the Ecdysozoa. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2008, 363, 1529–1537. [Google Scholar] [CrossRef]
- Garnaga-Budrė, G. Integrated assessment of pollution in the Baltic Sea. Ekologija 2012, 58, 331–355. [Google Scholar]
- Hosamani, N.; Reddy, S.; Reddy, R. Crustacean Molting: Regulation and Effects of Environironmental Toxicants. J. Mar. Sci. Res. Dev. 2017, 7, 236. [Google Scholar] [CrossRef]
- Lagadic, L.; Katsiadaki, I.; Biever, R.; Guiney, P.; Karouna-Renier, N.; Schwarz, T.; Meador, J. Tributyltin: Advancing the Science on Assessing Endocrine Disruption with an Unconventional Endocrine-Disrupting Compound. Rev. Environ. Contam. Toxicol. 2018, 245, 65–127. [Google Scholar]
- Vogt, É.; Model, J.; Vinagre, A. Effects of Organotins on Crustaceans: Update and Perspectives. Front. Endocrinol. 2018, 9, 65. [Google Scholar] [CrossRef] [PubMed]
- Höss, S.; Weltje, L. Endocrine disruption in nematodes: Effects and mechanisms. Ecotoxicology 2007, 16, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, C.; Li, H.; Ma, R.; Yu, Z.; Li, L.; Xiang, M.; Chen, X.; Hua, X.; Yu, Y. A review of toxicity induced by persistent organic pollutants (POPs) and endocrine-disrupting chemicals (EDCs) in the nematode Caenorhabditis elegans. J. Environ. Manag. 2019, 237, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Castro, L.; Lima, D.; Machado, A.; Melo, C.; Hiromori, Y.; Nishikawa, J.; Nakanishi, T.; Reis-Henriques, M.; Santos, M. Imposex induction is mediated through the Retinoid X receptor signalling pathway in the neogastropod Nucella lapillus. Aquat. Toxicol. 2007, 85, 57–66. [Google Scholar] [CrossRef]
- Lima, D.; Reis-Henriques, M.; Silva, R.; Santos, A.; Castro, L.; Santos, M. Tributyltin-induced imposex in marine gastropods involves tissue-specific modulation of the retinoid X receptor. Aquat. Toxicol. 2011, 101, 221–227. [Google Scholar] [CrossRef]
- Stange, D.; Sieratowicz, A.; Oehlmann, J. Imposex development in Nucella lapillus—Evidence for the involvement of retinoid X receptor and androgen signalling pathways in vivo. Aquat. Toxicol. 2012, 106–107, 20–24. [Google Scholar] [CrossRef]
- Capitão, A.; Lopes-Marques, M.; Ishii, Y.; Ruivo, R.; Fonseca, E.; Páscoa, I.; Jorge, R.; Barbosa, M.; Hiromori, Y.; Miyagi, T.; et al. Evolutionary Exploitation of Vertebrate Peroxisome Proliferator-Activated Receptor γ by Organotins. Environ. Sci. Technol. 2018, 52, 13951–13959. [Google Scholar]
- Fonseca, E.; Ruv, R.; Machado, A.M.; Conrado, F.; Tay, B.H.; Venkatesh, B.; Santos, M.M.; Castro, L.F.C. Evolutionary Plasticity in Detoxification Gene Modules: The Preservation and Loss of the Pregnane X Receptor in Chondrichthyes Lineages. Int. J. Mol. Sci. 2019, 10, 2331. [Google Scholar] [CrossRef]
- Paris, M.; Pettersson, K.; Schubert, M.; Bertrand, S.; Pongratz, I.; Escriva, H.; Laudet, V. An amphioxus orthologue of the estrogen receptor that does not bind estradiol: Insights into estrogen receptor evolution. BMC Evol. Biol. 2008, 8, 219. [Google Scholar] [CrossRef]
- Santos, M.M.; Ruivo, R.; Capitão, A.; Fonseca, E.; Castro, L.F.C. Identifying the gaps: Resources and perspectives on the use of nuclear receptor based-assays to improve hazard assessment of emerging contaminants. J. Hazard. Mater. 2018, 358, 508–511. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fonseca, E.S.S.; Hiromori, Y.; Kaite, Y.; Ruivo, R.; Franco, J.N.; Nakanishi, T.; Santos, M.M.; Castro, L.F.C. An Orthologue of the Retinoic Acid Receptor (RAR) Is Present in the Ecdysozoa Phylum Priapulida. Genes 2019, 10, 985. https://doi.org/10.3390/genes10120985
Fonseca ESS, Hiromori Y, Kaite Y, Ruivo R, Franco JN, Nakanishi T, Santos MM, Castro LFC. An Orthologue of the Retinoic Acid Receptor (RAR) Is Present in the Ecdysozoa Phylum Priapulida. Genes. 2019; 10(12):985. https://doi.org/10.3390/genes10120985
Chicago/Turabian StyleFonseca, Elza S. S., Youhei Hiromori, Yoshifumi Kaite, Raquel Ruivo, João N. Franco, Tsuyoshi Nakanishi, Miguel M. Santos, and L. Filipe C. Castro. 2019. "An Orthologue of the Retinoic Acid Receptor (RAR) Is Present in the Ecdysozoa Phylum Priapulida" Genes 10, no. 12: 985. https://doi.org/10.3390/genes10120985
APA StyleFonseca, E. S. S., Hiromori, Y., Kaite, Y., Ruivo, R., Franco, J. N., Nakanishi, T., Santos, M. M., & Castro, L. F. C. (2019). An Orthologue of the Retinoic Acid Receptor (RAR) Is Present in the Ecdysozoa Phylum Priapulida. Genes, 10(12), 985. https://doi.org/10.3390/genes10120985





