Long Noncoding RNA from PVT1 Exon 9 Is Overexpressed in Prostate Cancer and Induces Malignant Transformation and Castration Resistance in Prostate Epithelial Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tissue Samples
2.2. Cell Culture
2.3. Transfections
2.4. Cloning of PVT1 Exon 9 to Make Stable Cell Line
2.5. RNA Extractions
2.6. Quantitative Reverse Transcriptase Polymerase Chain Reaction (qPCR)
2.7. Migration Assays
2.8. Cell Proliferation Assays
2.9. Western Blotting
2.10. Xenograft Tumor Studies
2.11. Assessment of Castration Resistance
2.12. Statistical Analysis
3. Results
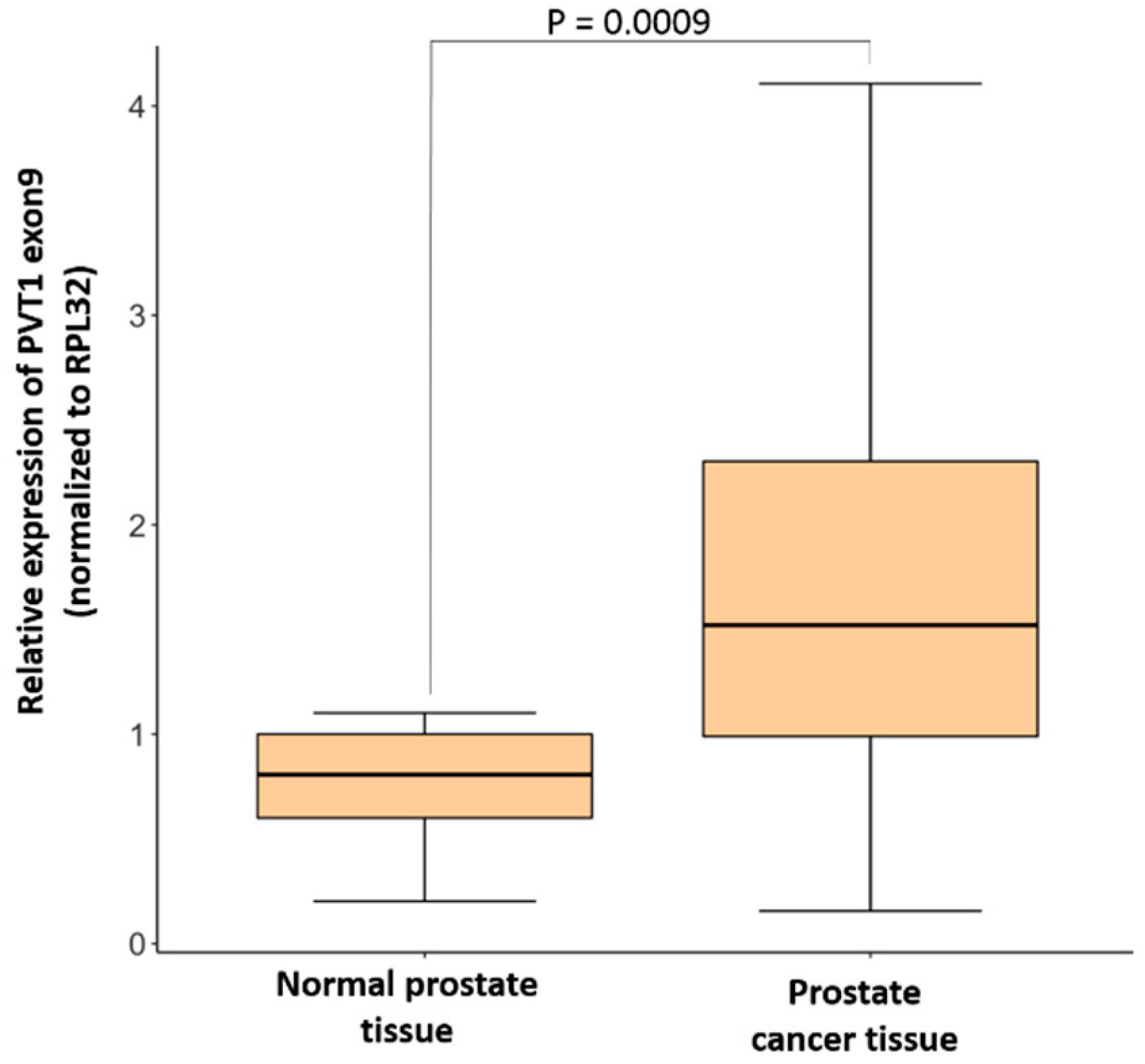
3.1. Expression of PVT1 Exon 9 Is Upregulated in PCa Tissues
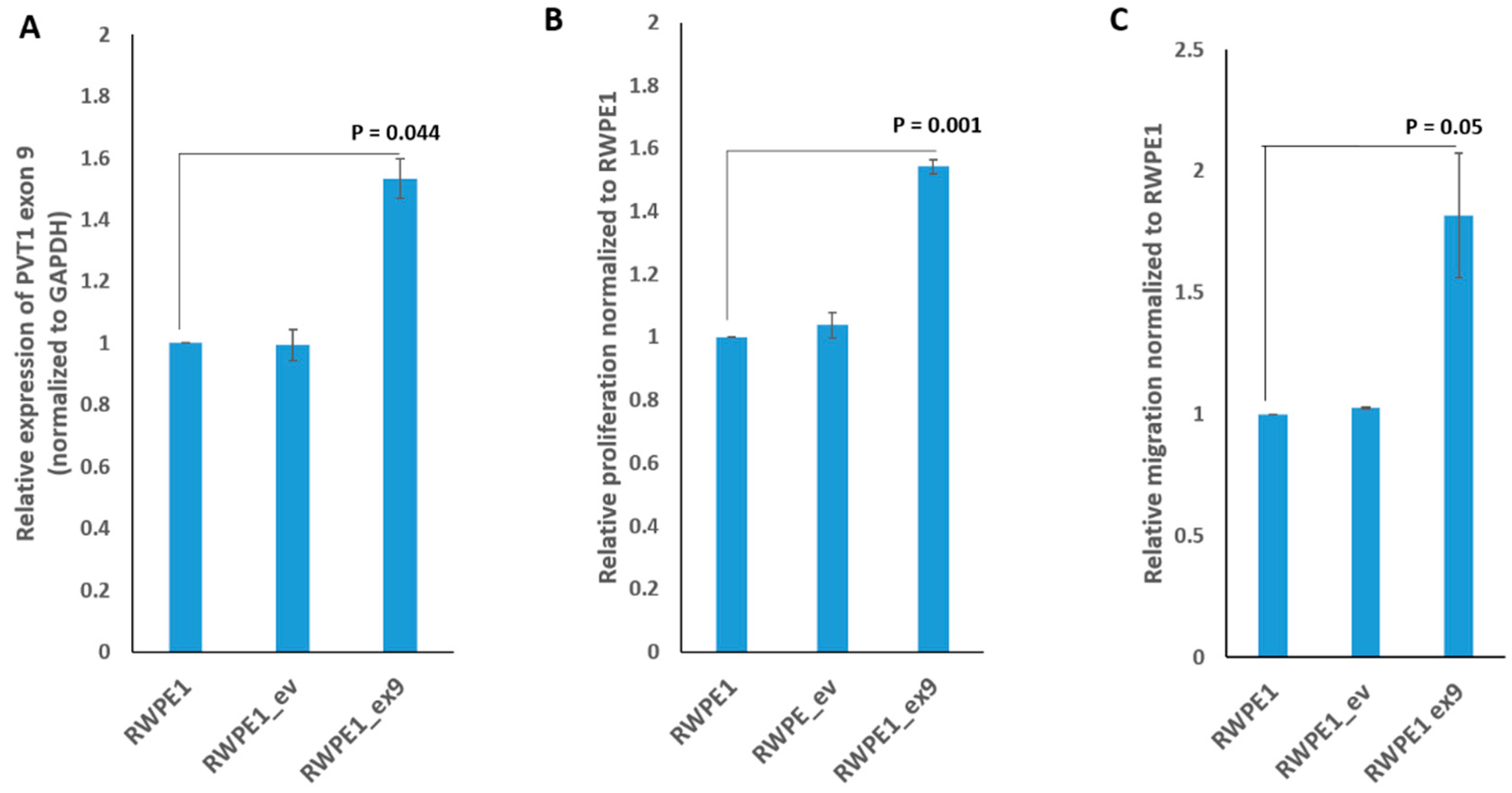
3.2. PVT1 Exon 9 Promotes Increased Cell Proliferation and Migration
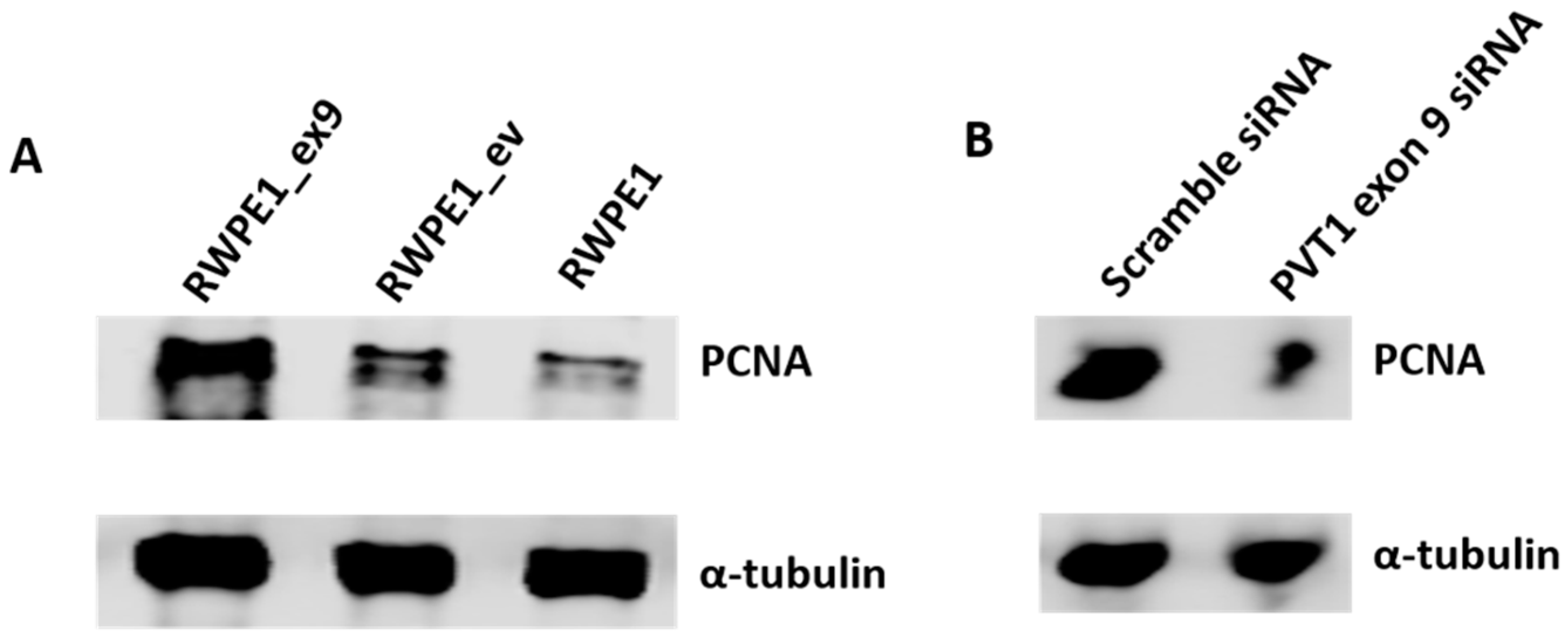
3.3. PVT1 Exon 9 Regulates Proliferating Cell Nuclear Antigen (PCNA) Expression
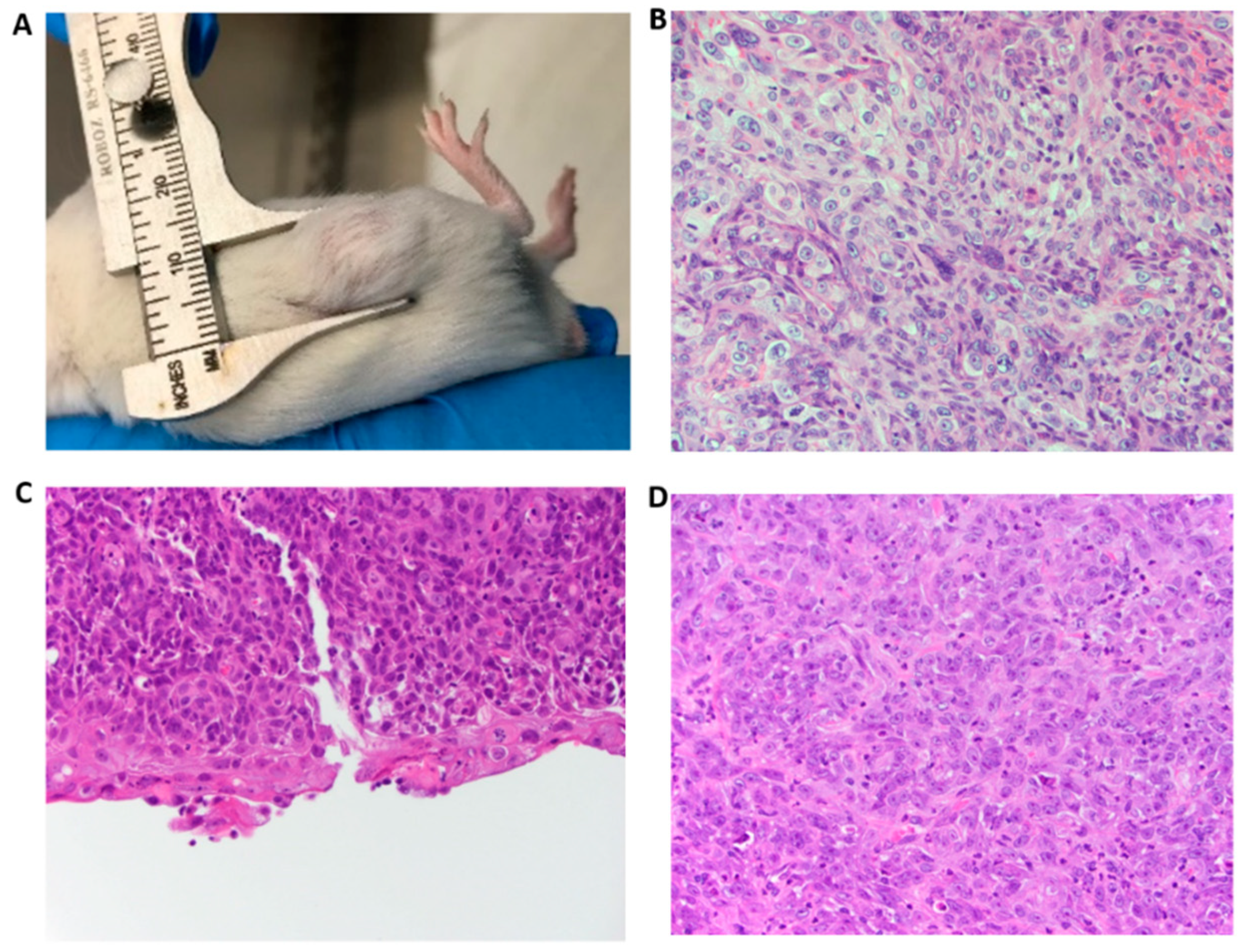
3.4. PVT1 Exon 9 Initiates Carcinogenesis In Vivo
3.5. PVT1 Exon 9 Induces Castration Resistance
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xu, J.; Sun, J.; Lilly Zheng, S. Prostate cancer risk-associated genetic markers and their potential clinical utility. Asian J. Androl. 2013, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Global Burden of Disease Cancer Collaboration; Fitzmaurice, C.; Allen, C.; Barber, R.M.; Barregard, L.; Bhutta, Z.A.; Brenner, H.; Dicker, D.J.; Chimed-Orchir, O.; Dandona, R.; et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived with Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2017, 3, 524–548. [Google Scholar] [PubMed]
- Yu, C.; Wang, Y.; Li, G.; She, L.; Zhang, D.; Chen, X.; Zhang, X.; Qin, Z.; Cao, H.; Liu, Y. LncRNA PVT1 promotes malignant progression in squamous cell carcinoma of the head and neck. J. Cancer 2018, 9, 3593–3602. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.T.; Fang, L.; Cheng, Y.X.; Sun, Q. LncRNA PVT1 regulates prostate cancer cell growth by inducing the methylation of miR-146a. Cancer Med. 2016, 3512–3519. [Google Scholar] [CrossRef] [PubMed]
- Matejcic, M.; Saunders, E.J.; Dadaev, T.; Brook, M.N.; Wang, K.; Sheng, X.; Al Olama, A.A.; Schumacher, F.R.; Ingles, S.A.; Govindasami, K.; et al. Author Correction: Germline variation at 8q24 and prostate cancer risk in men of European ancestry. Nat. Commun. 2019, 10, 382. [Google Scholar] [CrossRef]
- Ilboudo, A.; Chouhan, J.; McNeil, B.K.; Osborne, J.R.; Ogunwobi, O.O. PVT1 Exon 9, A Potential Biomarker of Aggressive Prostate Cancer? Int. J. Environ. Res. Public. Health 2015, 13, 12. [Google Scholar] [CrossRef]
- Huang, C.; Liu, S.; Wang, H.; Zhang, Z.; Yang, Q.; Gao, F. LncRNA PVT1 overexpression is a poor prognostic biomarker and regulates migration and invasion in small cell lung cancer. Am. J. Transl. Res. 2016, 8, 5025–5034. [Google Scholar]
- Chang, Z.; Cui, J.; Song, Y. Long noncoding RNA PVT1 promotes EMT via mediating microRNA-186 targeting of Twist1 in prostate cancer. Gene 2018, 654, 36–42. [Google Scholar] [CrossRef]
- Kong, R.; Zhang, E.B.; Yin, D.D.; You, L.H.; Xu, T.P.; Chen, W.M.; Xia, R.; Wan, L.; Sun, M.; Wang, Z.X.; et al. Long noncoding RNA PVT1 indicates a poor prognosis of gastric cancer and promotes cell proliferation through epigenetically regulating p15 and p16. Mol. Cancer 2015, 14, 82. [Google Scholar] [CrossRef]
- Garzon, R.; Volinia, S.; Papaioannou, D.; Nicolet, D.; Kohlschmidt, J.; Yan, P.S.; Mrózek, K.; Bucci, D.; Carroll, A.J.; Baer, M.R.; et al. Expression and prognostic impact of lncRNAs in acute myeloid leukemia. Proc. Natl. Acad. Sci. USA 2014, 111, 18679–18684. [Google Scholar] [CrossRef] [PubMed]
- Iden, M.; Fye, S.; Li, K.; Chowdhury, T.; Ramchandran, R.; Rader, J.S. The lncRNA PVT1 Contributes to the Cervical Cancer Phenotype and Associates with Poor Patient Prognosis. PLoS ONE 2016, 11, e0156274. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.W.; Xu, J.; Sun, R.; Mumbach, M.R.; Carter, A.C.; Chen, Y.G.; Yost, K.E.; Kim, J.; He, J.; Nevins, S.A.; et al. Promoter of lncRNA Gene PVT1 Is a Tumor-Suppressor DNA Boundary Element. Cell 2018, 173, 1398–1412. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yang, Y.; Cao, Y.; Wu, C.; Wu, S.; Su, Z.; Jin, H.; Wang, D.; Zhang, G.; Fan, W.; et al. lncRNA PVT1 identified as an independent biomarker for prognosis surveillance of solid tumors based on transcriptome data and meta-analysis. Cancer Manag. Res. 2018, 10, 2711–2727. [Google Scholar] [CrossRef]
- Tseng, Y.-Y.; Bagchi, A. The PVT1-MYC duet in cancer. Mol. Cell Oncol. 2015, 2, e974467. [Google Scholar] [CrossRef]
- Riquelme, E.; Suraokar, M.B.; Rodriguez, J.; Mino, B.; Lin, H.Y.; Rice, D.C.; Tsao, A.; Wistuba, I.I. Frequent coamplification and cooperation between C-MYC and PVT1 oncogenes promote malignant pleural mesothelioma. J. Thorac. Oncol. 2014, 9, 998–1007. [Google Scholar] [CrossRef]
- Guan, Y.; Kuo, W.L.; Stilwell, J.L.; Takano, H.; Lapuk, A.V.; Fridlyand, J.; Mao, J.H.; Yu, M.; Miller, M.A.; Santos, J.L.; et al. Amplification of PVT1 Contributes to the Pathophysiology of Ovarian and Breast Cancer. Clin. Cancer Res. 2007, 13, 5745–5755. [Google Scholar] [CrossRef]
- Carramusa, L.; Contino, F.; Ferro, A.; Minafra, L.; Perconti, G.; Giallongo, A.; Feo, S. The PVT-1 oncogene is a Myc protein target that is overexpressed in transformed cells. J. Cell Physiol. 2007, 213, 511–518. [Google Scholar] [CrossRef]
- Wan, B.; Wu, H.Y.; Lv, D.J.; Zhou, X.M.; Zhong, L.R.; Lei, B.; Zhang, S.B.; Mao, X.M. Downregulation of lncRNA PVT1 expression inhibits proliferation and migration by regulating p38 expression in prostate cancer. Oncol. Lett. 2018, 16, 5160–5166. [Google Scholar] [CrossRef]
- Reid, G. Molecular Cloning: A Laboratory Manual, 2nd ed.; Sambrook, J., Fritsch, E.F., Maniatis, T., Eds.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1989; Volume 3, p. 1659. ISBN 0-87969-309-6. [Google Scholar]
- Ogunwobi, O.O.; Liu, C. Hepatocyte growth factor upregulation promotes carcinogenesis and epithelial-mesenchymal transition in hepatocellular carcinoma via Akt and COX-2 pathways. Clin. Exp. Metastasis 2011, 28, 721–731. [Google Scholar] [CrossRef]
- Wunderlich, M.; Chou, F.S.; Link, K.; Mizukawa, B.; Perry, R.L.; Carroll, M.; Mulloy, J.C. AML xenograft efficiency is significantly improved in NOD/SCID-IL2RG mice constitutively expressing human SCF, GM-CSF and IL-3. Leukemia 2010, 24, 1785–1788. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Jing, Y.; Wei, F.; Tang, Y.; Yang, L.; Luo, J.; Yang, P.; Ni, Q.; Pang, J.; Liao, Q.; et al. Long non-coding RNA PVT1 predicts poor prognosis and induces radioresistance by regulating DNA repair and cell apoptosis in nasopharyngeal carcinoma. Cell Death Dis. 2018, 9, 235. [Google Scholar] [CrossRef] [PubMed]
- Cui, D.; Yu, C.H.; Liu, M.; Xia, Q.Q.; Zhang, Y.F.; Jiang, W.L. Long non-coding RNA PVT1 as a novel biomarker for diagnosis and prognosis of non-small cell lung cancer. Tumour Biol. 2016, 37, 4127–4134. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhu, Z.; Zhang, B.; Li, W.; Li, X.; Wu, X.; Wang, L.; Fu, L.; Fu, L.; Dong, J.T. Frequent mutation of rs13281615 and its association with PVT1 expression and cell proliferation in breast cancer. J. Genet. Genom. 2014, 41, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.R.; Zang, S.Z.; Zhong, C.L.; Li, Y.X.; Zhao, S.S.; Feng, X.J. Increased expression of the lncRNA PVT1 promotes tumorigenesis in non-small cell lung cancer. Int. J. Clin. Exp. Pathol. 2014, 7, 6929–6935. [Google Scholar]
- Ding, C.; Yang, Z.; Lv, Z.; Du, C.; Xiao, H.; Peng, C.; Cheng, S.; Xie, H.; Zhou, L.; Wu, J.; et al. Long non-coding RNA PVT1 is associated with tumor progression and predicts recurrence in hepatocellular carcinoma patients. Oncol. Lett. 2015, 9, 955–963. [Google Scholar] [CrossRef]
- Takahashi, Y.; Sawada, G.; Kurashige, J.; Uchi, R.; Matsumura, T.; Ueo, H.; Takano, Y.; Eguchi, H.; Sudo, T.; Sugimachi, K.; et al. Amplification of PVT-1 is involved in poor prognosis via apoptosis inhibition in colorectal cancers. Br. J. Cancer 2014, 110, 164–171. [Google Scholar] [CrossRef]
- Bronson, P.G.; Chang, D.; Bhangale, T.; Seldin, M.F.; Ortmann, W.; Ferreira, R.C.; Urcelay, E.; Pereira, L.F.; Martin, J.; Plebani, A.; et al. Common variants at PVT1, ATG13-AMBRA1, AHI1 and CLEC16A are associated with selective IgA deficiency. Nat Genet. 2016, 48, 1425–1429. [Google Scholar] [CrossRef]
- Huang, C.; Yu, W.; Wang, Q.; Cui, H.; Wang, Y.; Zhang, L.; Han, F.; Huang, T. Increased expression of the lncRNA PVT1 is associated with poor prognosis in pancreatic cancer patients. Minerva Med. 2015, 106, 143–149. [Google Scholar]
- Yang, J.; Li, C.; Mudd, A.; Gu, X. LncRNA PVT1 predicts prognosis and regulates tumor growth in prostate cancer. Biosci. Biotechnol. Biochem. 2017, 81, 2301–2306. [Google Scholar] [CrossRef]
- Lu-Yao, G.L. Survival Following Primary Androgen Deprivation Therapy among Men with Localized Prostate Cancer. JAMA 2008, 173. [Google Scholar] [CrossRef]
- Karantanos, T.; Corn, P.G.; Thompson, T.C. Prostate cancer progression after androgen deprivation therapy: Mechanisms of castrate resistance and novel therapeutic approaches. Oncogene 2013, 32, 5501–5511. [Google Scholar] [CrossRef]
- De Bono, J.S.; Logothetis, C.J.; Molina, A.; Fizazi, K.; North, S.; Chu, L.; Chi, K.N.; Jones, R.J.; Goodman, O.B., Jr.; Saad, F.; et al. Abiraterone and Increased Survival in Metastatic Prostate Cancer. New Engl. J. Med. 2011, 364, 1995–2005. [Google Scholar] [CrossRef]
- Fizazi, K.; Scher, H.I.; Molina, A.; Logothetis, C.J.; Chi, K.N.; Jones, R.J.; Staffurth, J.N.; North, S.; Vogelzang, N.J.; Saad, F.; et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: Final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2012, 13, 983–992. [Google Scholar] [CrossRef]

| Name | Sequence (5′ to 3′) |
|---|---|
| Scamble siRNA Forward | CUCACUACCGUCGACCCCAUU |
| Scamble siRNA Reverse | UGGGGUCGACGGUAGUGAGUU |
| PVT1 exon 9 siRNA Forward | ACCUAUGAGCUUUGAAUAA |
| PVT1 exon 9 siRNA Reverse | UUAUUCAAAGCUCAUAGGU |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pal, G.; Huaman, J.; Levine, F.; Orunmuyi, A.; Olapade-Olaopa, E.O.; Onagoruwa, O.T.; Ogunwobi, O.O. Long Noncoding RNA from PVT1 Exon 9 Is Overexpressed in Prostate Cancer and Induces Malignant Transformation and Castration Resistance in Prostate Epithelial Cells. Genes 2019, 10, 964. https://doi.org/10.3390/genes10120964
Pal G, Huaman J, Levine F, Orunmuyi A, Olapade-Olaopa EO, Onagoruwa OT, Ogunwobi OO. Long Noncoding RNA from PVT1 Exon 9 Is Overexpressed in Prostate Cancer and Induces Malignant Transformation and Castration Resistance in Prostate Epithelial Cells. Genes. 2019; 10(12):964. https://doi.org/10.3390/genes10120964
Chicago/Turabian StylePal, Gargi, Jeannette Huaman, Fayola Levine, Akintunde Orunmuyi, E. Oluwabunmi Olapade-Olaopa, Onayemi T. Onagoruwa, and Olorunseun O. Ogunwobi. 2019. "Long Noncoding RNA from PVT1 Exon 9 Is Overexpressed in Prostate Cancer and Induces Malignant Transformation and Castration Resistance in Prostate Epithelial Cells" Genes 10, no. 12: 964. https://doi.org/10.3390/genes10120964
APA StylePal, G., Huaman, J., Levine, F., Orunmuyi, A., Olapade-Olaopa, E. O., Onagoruwa, O. T., & Ogunwobi, O. O. (2019). Long Noncoding RNA from PVT1 Exon 9 Is Overexpressed in Prostate Cancer and Induces Malignant Transformation and Castration Resistance in Prostate Epithelial Cells. Genes, 10(12), 964. https://doi.org/10.3390/genes10120964






