Wnt-11 Expression Promotes Invasiveness and Correlates with Survival in Human Pancreatic Ductal Adeno Carcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Patient Samples
2.3. Isolation of RNA, cDNA and qRT-PCR
2.4. RNA Sequencing
2.5. siRNA
2.6. Invasion and Proliferation Assays
2.7. Immunostaining
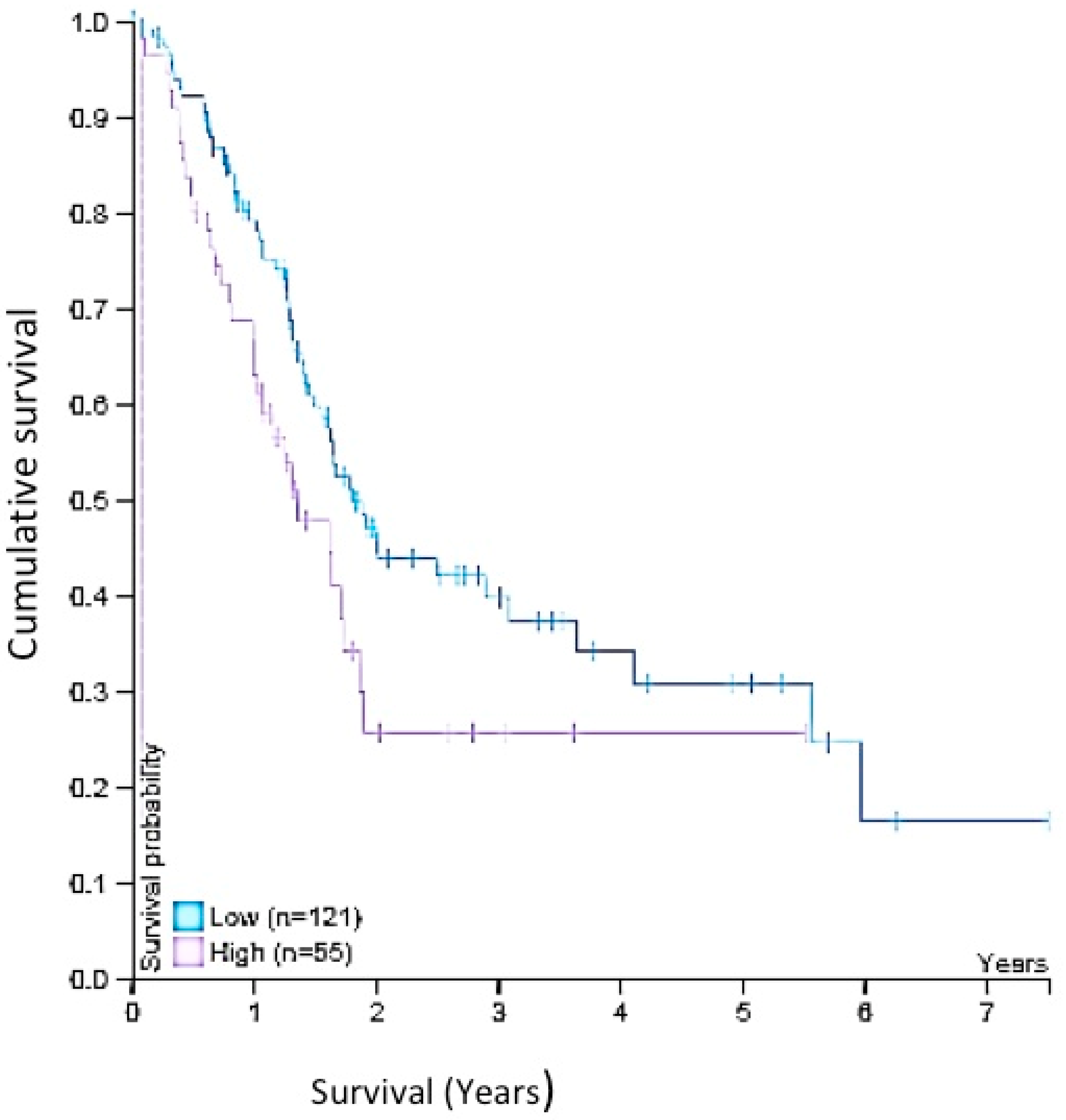
2.8. Survival Analysis
2.9. Data Analysis
3. Results
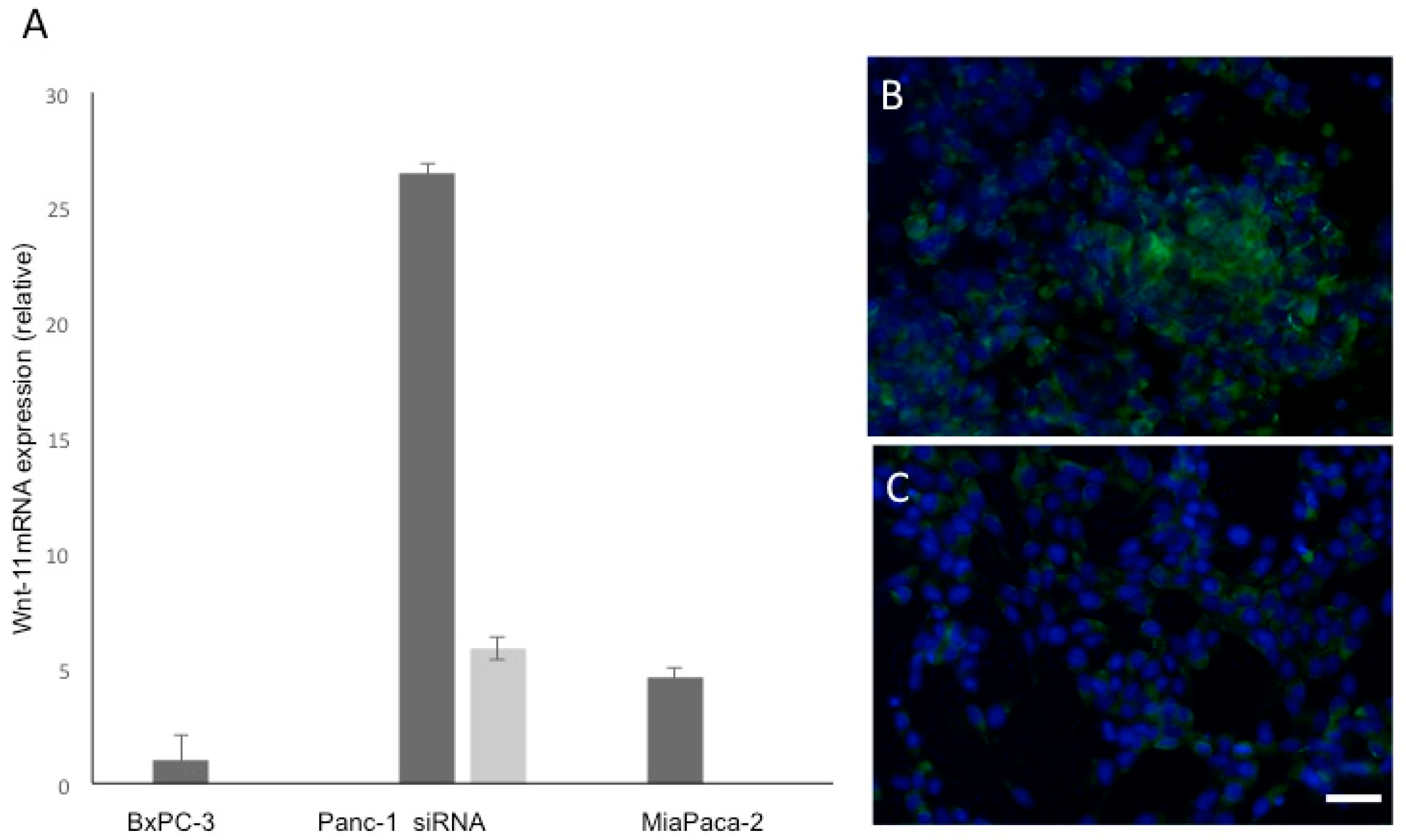
3.1. Wnt-11 mRNA Expression Profiling
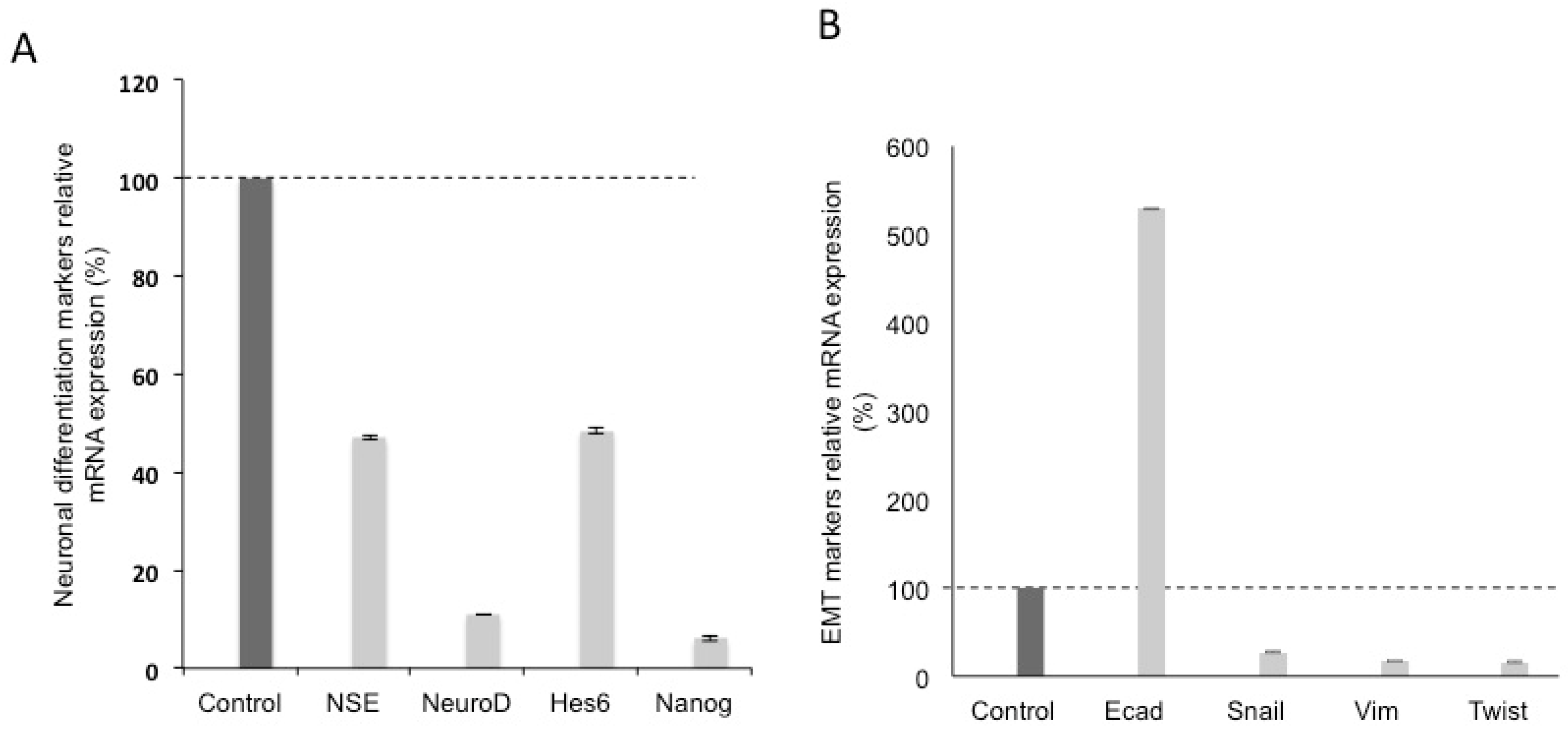
3.2. Effects of Silencing Wnt-11 on Biomarkers Associated with Metastasis
3.3. Effect of Silencing Wnt-11 on Cellular Invasiveness
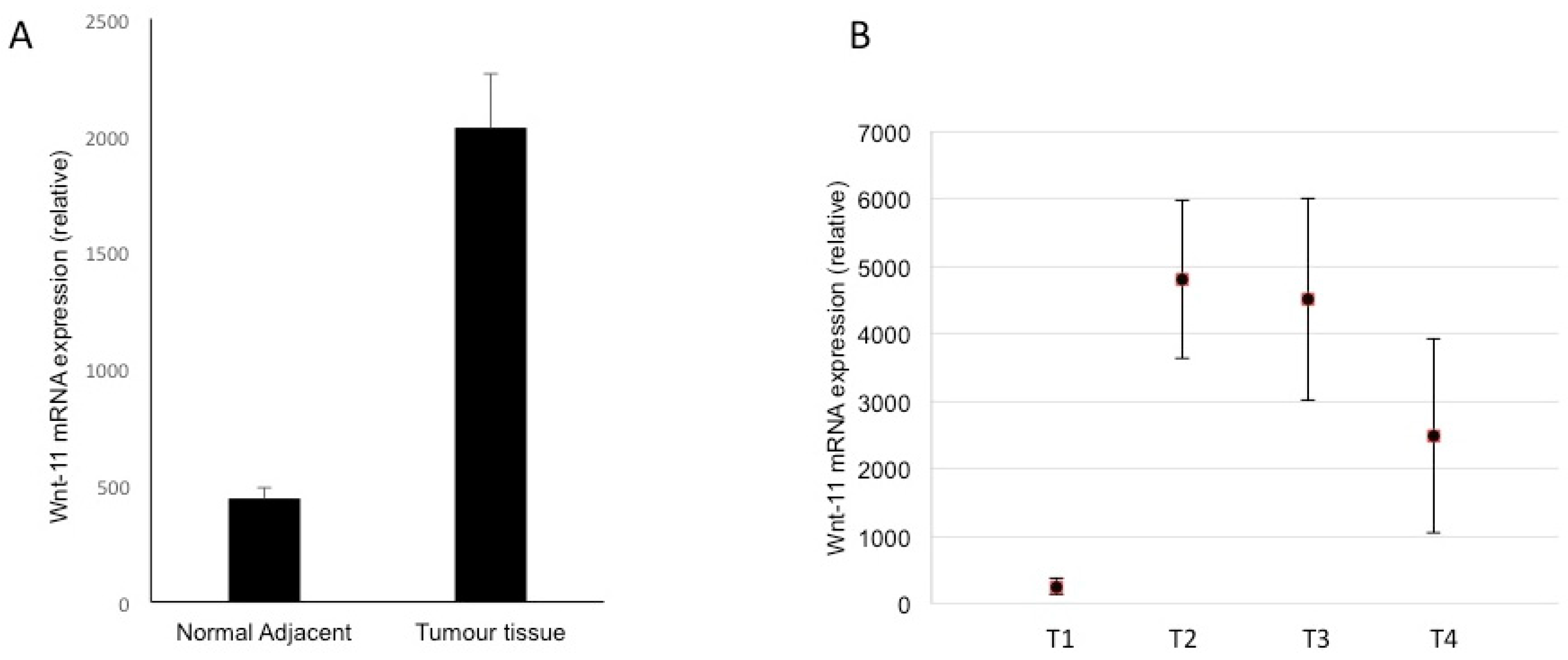
3.4. Expression in Biopsies
4. Discussion
4.1. Wnt-11 Expression in PDAC Cells and Tissues: Control of NEMs, EMT and Invasiveness
4.2. Mechanistic Aspects
4.3. Wnt-11 Expression in PDAC and Survival
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Pai, M.; Spalding, D. Pancreatic cancer. Medicine 2015, 43, 329–333. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, L.; Lang, J.; Cheng, K.; Wang, Y.; Li, X.; Shi, J.; Wang, Y.; Nie, G. A CRISPR-Cas13a system for efficient and specific therapeutic targeting of mutant KRAS for pancreatic cancer treatment. Cancer Lett. 2018, 431, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Strobel, O.; Neoptolemos, J.; Jäger, D.; Büchler, M.W. Optimizing the outcomes of pancreatic cancer surgery. Nat. Rev. Clin. Oncol. 2019, 16, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.; Raymond, V.M.; Geis, J.A.; Collisson, E.A.; Jensen, B.V.; Hermann, K.L.; Erlander, M.G.; Tempero, M.; Johansen, J.S. Ultrasensitive plasma ctDNA KRAS assay for detection, prognosis, and assessment of therapeutic response in patients with unresectable pancreatic ductal adenocarcinoma. Oncotarget 2017, 8, 97769–97786. [Google Scholar] [CrossRef] [PubMed]
- Zhan, J.; Song, J.; Wang, P.; Chi, X.; Wang, Y.; Guo, Y.; Fang, W.; Zhang, H. Kindlin-2 induced by TGF-β signaling promotes pancreatic ductal adenocarcinoma progression through downregulation of transcriptional factor HOXB9. Cancer Lett. 2015, 361, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.L.; Lan, C.; Pei, H.; Yang, S.N.; Liu, Y.F.; Xiao, L.L. Applicative Value of Serum CA19-9, CEA, CA125 and CA242 in Diagnosis and Prognosis for Patients with Pancreatic Cancer Treated by Concurrent Chemoradiotherapy. Asian Pac. J. Cancer Prev. 2015, 16, 6569–6573. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Shi, S.; Liang, C.; Liang, D.; Xu, W.; Ji, S.; Zhang, B.; Ni, Q.; Xu, J.; Yu, X. Diagnostic and prognostic value of carcinoembryonic antigen in pancreatic cancer: A systematic review and meta-analysis. OncoTargets Ther. 2017, 10, 4591–4598. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Kumar, S.; Momi, N.; Sasson, A.R.; Batra, S.K. Mucins in pancreatic cancer and its microenvironment. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 607–620. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Paris, P.L.; Chen, J.; Ngo, V.; Yao, H.; Frazier, M.L.; Killary, A.M.; Liu, C.G.; Liang, H.; Mathy, C.; et al. Next generation sequencing of pancreatic cyst fluid microRNAs from low grade-benign and high grade-invasive lesions. Cancer Lett. 2015, 356 Pt B, 404–409. [Google Scholar] [CrossRef]
- Neoptolemos, J.P.; Kleeff, J.; Michl, P.; Costello, E.; Greenhalf, W.; Palmer, D.H. Therapeutic developments in pancreatic cancer: Current and future perspectives. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 333–348. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.B.; Yang, Y.; Zhao, Y.P.; Zhang, T.P.; Liao, Q.; Shu, H. Recent studies of 5-fluorouracil resistance in pancreatic cancer. World J. Gastroenterol. 2014, 20, 15682–15690. [Google Scholar] [CrossRef] [PubMed]
- Philip, P.A.; Lutz, M.P. Targeting Epidermal Growth Factor Receptor-Related Signaling Pathways in Pancreatic Cancer. Pancreas 2015, 44, 1046–1052. [Google Scholar] [CrossRef] [PubMed]
- Troiani, T.; Martinelli, E.; Capasso, A.; Morgillo, F.; Orditura, M.; De Vita, F.; Ciardiello, F. Targeting EGFR in pancreatic cancer treatment. Curr. Drug Targets 2012, 13, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Faller, B.A.; Burtness, B. Treatment of pancreatic cancer with epidermal growth factor receptor-targeted therapy. Biologics 2009, 3, 419–428. [Google Scholar] [PubMed]
- Uysal-Onganer, P.; Kawano, Y.; Caro, M.; Walker, M.M.; Diez, S.; Darrington, R.S.; Waxman, J.; Kypta, R.M. Wnt-11 promotes neuroendocrine-like differentiation, survival and migration of prostate cancer cells. Mol. Cancer 2010, 9, 55. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Wang, N.; Zhang, Y.; Wang, S.; Pang, X.; Zhang, J.; Luo, Q.; Su, Y.; Zhang, S. Clinical significance of Wnt-11 and squamous cell carcinoma antigen expression in cervical cancer. Med. Oncol. 2014, 31, 933. [Google Scholar] [CrossRef] [PubMed]
- Jannesari-Ladani, F.; Hossein, G.; Izadi-Mood, N. Differential Wnt-11 expression related to Wnt5a in high- and low-grade serous ovarian cancer: Implications for migration, adhesion and survival. Asian Pac. J. Cancer Prev. 2014, 15, 1489–1495. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nishioka, M.; Ueno, K.; Hazama, S.; Okada, T.; Sakai, K.; Suehiro, Y.; Okayama, N.; Hirata, H.; Oka, M.; Imai, K.; et al. Possible involvement of Wnt-11 in colorectal cancer progression. Mol. Carcinog. 2013, 52, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Murillo-Garzón, V.; Gorroño-Etxebarria, I.; Åkerfelt, M.; Puustinen, M.C.; Sistonen, L.; Nees, M.; Carton, J.; Waxman, J.; Kypta, R.M. Frizzled-8 integrates Wnt-11 and transforming growth factor-β signaling in prostate cancer. Nat. Commun. 2018, 9, 1747. [Google Scholar] [CrossRef] [PubMed]
- Kumawat, K.; Koopmans, T.; Menzen, M.H.; Prins, A.; Smit, M.; Halayko, A.J.; Gosens, R. Cooperative signaling by TGF-β1 and WNT-11 drives sm-α-actin expression in smooth muscle via Rho kinase-actin-MRTF-A signaling. Am. J. Physiol. Lung Cell. Mol. Physiol. 2016, 311, L529–L537. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Lu, Y.; Hu, H.; Zhang, J.; Qin, B.; Wang, Y.; Xing, S.; Xi, Q.; Wang, S. The Wnt-11 Signalling Pathway in Potential Cellular EMT and Osteochondral Differentiation Progression in Nephrolithiasis Formation. Int. J. Mol. Sci. 2015, 16, 16313–16329. [Google Scholar] [CrossRef] [PubMed]
- Melzer, C.; Hass, R.; von der Ohe, J.; Lehnert, H.; Ungefroren, H. The role of TGF-β and its crosstalk with RAC1/RAC1b signaling in breast and pancreas carcinoma. Cell Commun. Signal. 2017, 15, 19. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Tao, G.Q.; Zhang, Y.; Cai, B.; Sun, J.; Tian, Z.Q. TGF-β in pancreatic cancer initiation and progression: Two sides of the same coin. Cell Biosci. 2017, 7, 39. [Google Scholar] [CrossRef] [PubMed]
- Kano, M.R.; Bae, Y.; Iwata, C.; Morishita, Y.; Yashiro, M.; Oka, M.; Fujii, T.; Komuro, A.; Kiyono, K.; Kaminishi, M.; et al. Improvement of cancer-targeting therapy, using nanocarriers for intractable solid tumors by inhibition of TGF-beta signaling. Proc. Natl. Acad. Sci. USA 2007, 104, 3460–3465. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.H.; Yang, J. Epithelial-mesenchymal plasticity in carcinoma metastasis. Genes Dev. 2013, 27, 2192–2206. [Google Scholar] [CrossRef] [PubMed]
- Onganer, P.U.; Seckl, M.J.; Djamgoz, M.B. Neuronal characteristics of small-cell lung cancer. Br. J. Cancer 2005, 93, 1197–1201. [Google Scholar] [CrossRef] [PubMed]
- Mancino, M.; Ametller, E.; Gascón, P.; Almendro, V. The neuronal influence on tumor progression. Biochim. Biophys. Acta 2011, 1816, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Farach, A.; Ding, Y.; Lee, M.; Creighton, C.; Delk, N.A.; Ittmann, M.; Miles, B.; Rowley, D.; Farach-Carson, M.C.; Ayala, G.E. Neuronal Trans-Differentiation in Prostate Cancer Cells. Prostate 2016, 76, 1312–1325. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.J.; Jang, G.B.; Lee, H.Y.; Park, S.R.; Kim, J.Y.; Nam, J.S.; Hong, I.S. The Wnt/β-catenin signaling/Id2 cascade mediates the effects of hypoxia on the hierarchy of colorectal-cancer stem cells. Sci. Rep. 2016, 6, 22966. [Google Scholar] [CrossRef] [PubMed]
- Nozato, M.; Kaneko, S.; Nakagawara, A.; Komuro, H. Epithelial-mesenchymal transition-related gene expression as a new prognostic marker for neuroblastoma. Int. J. Oncol. 2013, 42, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Radonić, A.; Thulke, S.; Mackay, I.M.; Landt, O.; Siegert, W.; Nitsche, A. Guideline to reference gene selection for quantitative real-time PCR. Biochem. Biophys. Res. Commun. 2004, 313, 856–862. [Google Scholar] [CrossRef] [PubMed]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef] [PubMed]
- Forte, E.; Chimenti, I.; Rosa, P.; Angelini, F.; Pagano, F.; Calogero, A.; Giacomello, A.; Messina, E. EMT/MET at the Crossroad of Stemness, Regeneration and Oncogenesis: The Ying-Yang Equilibrium Recapitulated in Cell Spheroids. Cancers 2017, 9, 98. [Google Scholar] [CrossRef] [PubMed]
- Malta, T.M.; Sokolov, A.; Gentles, A.J.; Burzykowski, T.; Poisson, L.; Weinstein, J.N.; Kamińska, B.; Huelsken, J.; Omberg, L.; Gevaert, O.; et al. Machine Learning Identifies Stemness Features Associated with Oncogenic Dedifferentiation. Cell 2018, 173, 338–354. [Google Scholar] [CrossRef] [PubMed]
- Zhan, T.; Rindtorff, N.; Boutros, M. Wnt signaling in cancer. Oncogene 2017, 36, 1461–1473. [Google Scholar] [CrossRef] [PubMed]
- Elizalde, C.; Campa, V.M.; Caro, M.; Schlangen, K.; Aransay, A.M.; Vivanco, M.D.; Kypta, R.M. Distinct roles for Wnt-4 and Wnt-11 during retinoic acid-induced neuronal differentiation. Stem Cells 2011, 29, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, M.A.; Joseph, J.D.; Wade, H.E.; Eaton, M.L.; Kunder, R.S.; Kazmin, D.; Chang, C.Y.; McDonnell, D.P. WNT-11 expression is induced by estrogen-related receptor alpha and beta-catenin and acts in an autocrine manner to increase cancer cell migration. Cancer Res. 2010, 70, 9298–9308. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Yao, Y.; Learman, B.S.; Kurozumi, K.; Ishida, J.; Ramakrishnan, S.K.; Overmyer, K.A.; Xue, X.; Cawthorn, W.P.; Reid, M.A.; et al. Induction of WNT-11 by hypoxia and hypoxia-inducible factor-1α regulates cell proliferation, migration and invasion. Sci. Rep. 2016, 6, 21520. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Wang, N.; Zhang, Y.; Wang, S.; Pang, X.; Zhang, S. Wnt-11 overexpression promoting the invasion of cervical cancer cells. Tumour. Biol. 2016, 37, 11789–11798. [Google Scholar] [CrossRef] [PubMed]
- Kühl, M. The WNT/calcium pathway: Biochemical mediators, tools and future requirements. Front. Biosci. 2004, 9, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Cai, Y.; Soofi, A.; Dressler, G.R. Activation of Wnt-11 by transforming growth factor-β drives mesenchymal gene expression through non-canonical Wnt protein signaling in renal epithelial cells. J. Biol. Chem. 2012, 287, 21290–21302. [Google Scholar] [CrossRef] [PubMed]
- Van Amerongen, R.; Nusse, R. Towards an integrated view of Wnt signaling in development. Development 2009, 136, 3205–3214. [Google Scholar] [CrossRef] [PubMed]
- Bonito, B.; Sauter, D.R.; Schwab, A.; Djamgoz, M.B.; Novak, I. KCa3.1 (IK) modulates pancreatic cancer cell migration, invasion and proliferation: Anomalous effects on TRAM-34. Pflugers Arch. 2016, 468, 1865–1875. [Google Scholar] [CrossRef] [PubMed]
- Kohn, A.D.; Moon, R.T. Wnt and calcium signaling: Beta-catenin-independent pathways. Cell Calcium. 2005, 38, 439–446. [Google Scholar] [CrossRef] [PubMed]
- De, A. Wnt/Ca2+ signaling pathway: A brief overview. Acta Biochim. Biophys. Sin. (Shanghai) 2011, 43, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Qu, Q.; Sun, G.; Murai, K.; Ye, P.; Li, W.; Asuelime, G.; Cheung, Y.T.; Shi, Y. Wnt7a regulates multiple steps of neurogenesis. Mol. Cell. Biol. 2013, 33, 2551–2559. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Estellés, M.; González-Gómez, P.; Hortigüela, R.; Díaz-Moreno, M.; San Emeterio, J.; Carvalho, A.L.; Fariñas, I.; Mira, H. Symmetric expansion of neural stem cells from the adult olfactory bulb is driven by astrocytes via WNT7A. Stem Cells 2012, 30, 2796–2809. [Google Scholar] [CrossRef] [PubMed]
- Many, A.M.; Brown, A.M. Both canonical and non-canonical Wnt signaling independently promote stem cell growth in mammospheres. PLoS ONE 2014, 9, e101800. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.H.; Bonaguidi, M.A.; Kitabatake, Y.; Sun, J.; Song, J.; Kang, E.; Jun, H.; Zhong, C.; Su, Y.; Guo, J.U.; et al. Secreted frizzled-related protein 3 regulates activity-dependent adult hippocampal neurogenesis. Cell Stem Cell 2013, 12, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Wexler, E.M.; Paucer, A.; Kornblum, H.I.; Palmer, T.D.; Geschwind, D.H. Endogenous Wnt signaling maintains neural progenitor cell potency. Stem Cells 2009, 27, 1130–1141. [Google Scholar] [CrossRef] [PubMed]
- Munshi, H.G.; Stack, M.S. Reciprocal interactions between adhesion receptor signaling and MMP regulation. Cancer Metastasis Rev. 2006, 25, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Zhou, H.Y.; Deng, S.C.; Deng, S.J.; He, C.; Li, X.; Chen, J.Y.; Jin, Y.; Hu, Z.L.; Wang, F.; et al. ASIC1 and ASIC3 contribute to acidity-induced EMT of pancreatic cancer through activating Ca2+/RhoA pathway. Cell Death Dis. 2017, 8, e2806. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yao, M.; Fang, M.; Zheng, W.J.; Dong, Z.Z.; Pan, L.H.; Zhang, H.J.; Yao, D.F. Expression of hepatic Wnt5a and its clinicopathological features in patients with hepatocellular carcinoma. Hepatobiliary Pancreat Dis. Int. 2018, 17, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, D.; Zhou, K.; Wang, B.; Liu, Q.; Deng, F.; Li, Q.; Ma, Y. Expression of Wnt-11 and Rock2 in esophageal squamous cell carcinoma by activation of the WNT/PCP pathway and its clinical significance. Pathol. Res. Pract. 2016, 212, 880–885. [Google Scholar] [CrossRef] [PubMed]
- Gorroño-Etxebarria, I.; Aguirre, U.; Sanchez, S.; González, N.; Escobar, A.; Zabalza, I.; Quintana, J.M.; Vivanco, M.D.; Waxman, J.; Kypta, R.M. Wnt-11 as a Potential Prognostic Biomarker and Therapeutic Target in Colorectal Cancer. Cancers 2019, 11, 908. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, C.; Pebre Pereira, L. Wnt Signalling-Targeted Therapy in the CMS2 Tumour Subtype: A New Paradigm in CRC Treatment? Adv. Exp. Med. Biol. 2018, 1110, 75–100. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dart, D.A.; Arisan, D.E.; Owen, S.; Hao, C.; Jiang, W.G.; Uysal-Onganer, P. Wnt-11 Expression Promotes Invasiveness and Correlates with Survival in Human Pancreatic Ductal Adeno Carcinoma. Genes 2019, 10, 921. https://doi.org/10.3390/genes10110921
Dart DA, Arisan DE, Owen S, Hao C, Jiang WG, Uysal-Onganer P. Wnt-11 Expression Promotes Invasiveness and Correlates with Survival in Human Pancreatic Ductal Adeno Carcinoma. Genes. 2019; 10(11):921. https://doi.org/10.3390/genes10110921
Chicago/Turabian StyleDart, Dafydd A., Damla E Arisan, Sioned Owen, Chunyi Hao, Wen G. Jiang, and Pinar Uysal-Onganer. 2019. "Wnt-11 Expression Promotes Invasiveness and Correlates with Survival in Human Pancreatic Ductal Adeno Carcinoma" Genes 10, no. 11: 921. https://doi.org/10.3390/genes10110921
APA StyleDart, D. A., Arisan, D. E., Owen, S., Hao, C., Jiang, W. G., & Uysal-Onganer, P. (2019). Wnt-11 Expression Promotes Invasiveness and Correlates with Survival in Human Pancreatic Ductal Adeno Carcinoma. Genes, 10(11), 921. https://doi.org/10.3390/genes10110921






