Tyrosyl-DNA Phosphodiesterase I N-Terminal Domain Modifications and Interactions Regulate Cellular Function
Abstract
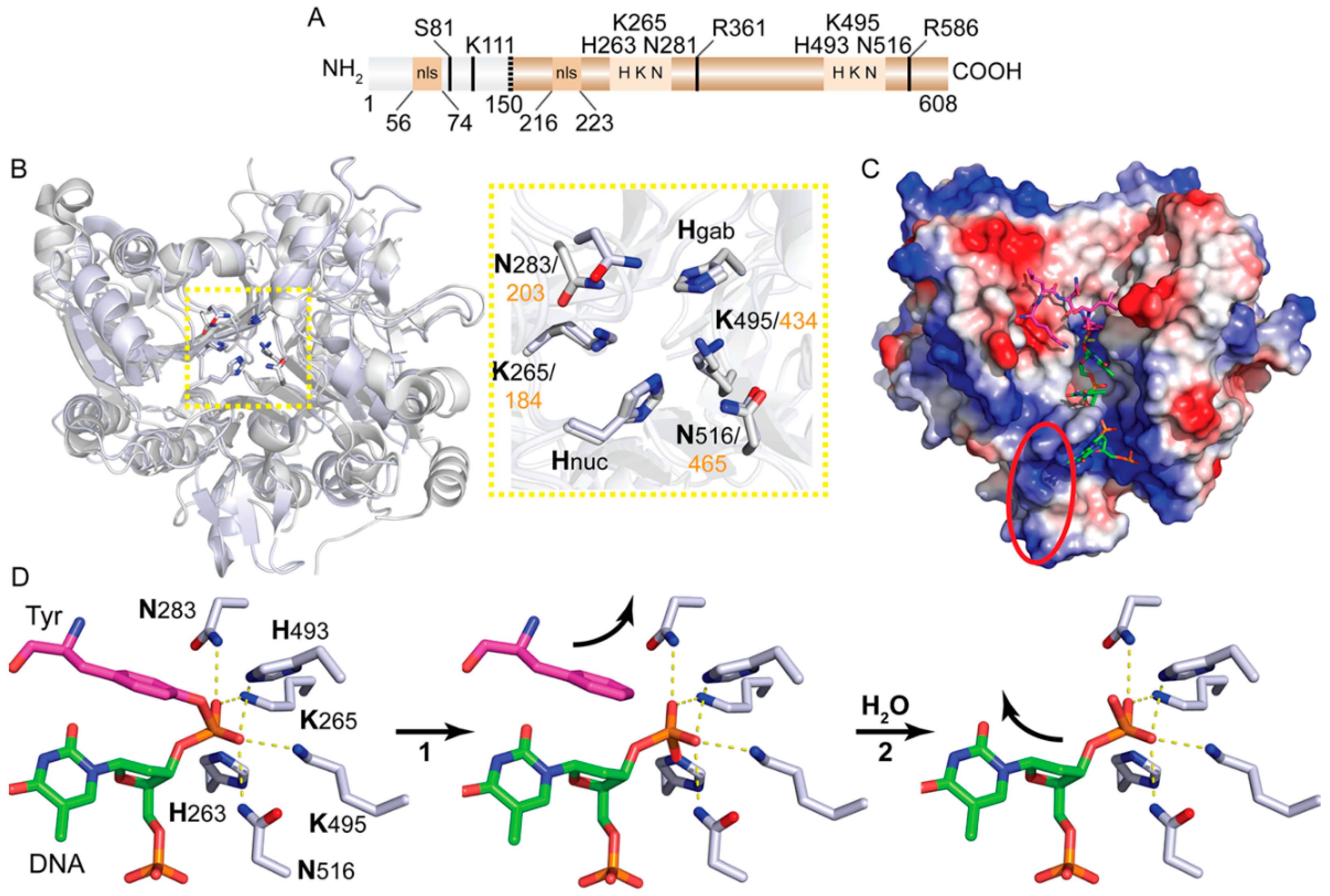
1. Introduction
2. Tdp1 Catalytic Mechanism
3. Diversity of Tdp1 Substrates
4. N-terminus Domain as the “Social” Mediator for Tdp1
4.1. Phosphorylation
4.2. PARylation
4.3. SUMOylation
4.4. Ubiquitylation/Deubiquitylation
4.5. Methylation
5. In Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yang, S.W.; Burgin, A.B., Jr.; Huizenga, B.N.; Robertson, C.A.; Yao, K.C.; Nash, H.A. A eukaryotic enzyme that can disjoin dead-end covalent complexes between DNA and type i topoisomerases. Proc. Natl. Acad. Sci. USA 1996, 93, 11534–11539. [Google Scholar] [CrossRef]
- Pouliot, J.J.; Robertson, C.A.; Nash, H.A. Pathways for repair of topoisomerase i covalent complexes in saccharomyces cerevisiae. Genes Cells 2001, 6, 677–687. [Google Scholar] [CrossRef]
- Pouliot, J.J.; Yao, K.C.; Robertson, C.A.; Nash, H.A. Yeast gene for a tyr-DNA phosphodiesterase that repairs topoisomerase i complexes. Science 1999, 286, 552–555. [Google Scholar] [CrossRef] [PubMed]
- Esposito, D.; Scocca, J.J. The integrase family of tyrosine recombinases: Evolution of a conserved active site domain. Nucleic Acids Res. 1997, 25, 3605–3614. [Google Scholar] [CrossRef] [PubMed]
- Interthal, H.; Pouliot, J.J.; Champoux, J.J. The tyrosyl-DNA phosphodiesterase tdp1 is a member of the phospholipase d superfamily. Proc. Natl. Acad. Sci. USA 2001, 98, 12009–12014. [Google Scholar] [CrossRef] [PubMed]
- Ponting, C.P.; Kerr, I.D. A novel family of phospholipase d homologues that includes phospholipid synthases and putative endonucleases: Identification of duplicated repeats and potential active site residues. Protein Sci. 1996, 5, 914–922. [Google Scholar] [CrossRef] [PubMed]
- Stuckey, J.A.; Dixon, J.E. Crystal structure of a phospholipase d family member. Nat. Struct. Biol. 1999, 6, 278–284. [Google Scholar]
- Davies, D.R.; Interthal, H.; Champoux, J.J.; Hol, W.G. The crystal structure of human tyrosyl-DNA phosphodiesterase, tdp1. Structure 2002, 10, 237–248. [Google Scholar] [CrossRef]
- He, X.; van Waardenburg, R.C.; Babaoglu, K.; Price, A.C.; Nitiss, K.C.; Nitiss, J.L.; Bjornsti, M.A.; White, S.W. Mutation of a conserved active site residue converts tyrosyl-DNA phosphodiesterase i into a DNA topoisomerase i-dependent poison. J. Mol. Biol. 2007, 372, 1070–1081. [Google Scholar] [CrossRef]
- Comeaux, E.Q.; van Waardenburg, R.C. Tyrosyl-DNA phosphodiesterase i resolves both naturally and chemically induced DNA adducts and its potential as a therapeutic target. Drug Metab. Rev. 2014, 46, 494–507. [Google Scholar] [CrossRef]
- Pommier, Y.; Huang, S.Y.; Gao, R.; Das, B.B.; Murai, J.; Marchand, C. Tyrosyl-DNA-phosphodiesterases (tdp1 and tdp2). DNA Repair 2014, 19, 114–129. [Google Scholar] [CrossRef] [PubMed]
- Povirk, L.F. Processing of damaged DNA ends for double-strand break repair in mammalian cells. ISRN Mol. Biol. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, A.J.; Subler, M.A.; Akopiants, K.; Wiley, J.L.; Taylor, S.M.; Rice, A.C.; Windle, J.J.; Valerie, K.; Povirk, L.F. In vitro complementation of tdp1 deficiency indicates a stabilized enzyme-DNA adduct from tyrosyl but not glycolate lesions as a consequence of the scan1 mutation. DNA Repair 2009, 8, 654–663. [Google Scholar] [CrossRef] [PubMed]
- Hirano, R.; Interthal, H.; Huang, C.; Nakamura, T.; Deguchi, K.; Choi, K.; Bhattacharjee, M.B.; Arimura, K.; Umehara, F.; Izumo, S.; et al. Spinocerebellar ataxia with axonal neuropathy: Consequence of a tdp1 recessive neomorphic mutation? EMBO J. 2007, 26, 4732–4743. [Google Scholar] [CrossRef] [PubMed]
- Katyal, S.; el-Khamisy, S.F.; Russell, H.R.; Li, Y.; Ju, L.; Caldecott, K.W.; McKinnon, P.J. Tdp1 facilitates chromosomal single-strand break repair in neurons and is neuroprotective in vivo. EMBO J. 2007, 26, 4720–4731. [Google Scholar] [CrossRef]
- Katyal, S.; Lee, Y.; Nitiss, K.C.; Downing, S.M.; Li, Y.; Shimada, M.; Zhao, J.; Russell, H.R.; Petrini, J.H.; Nitiss, J.L.; et al. Aberrant topoisomerase-1 DNA lesions are pathogenic in neurodegenerative genome instability syndromes. Nat. Neurosci. 2014, 17, 813–821. [Google Scholar] [CrossRef]
- Barthelmes, H.U.; Habermeyer, M.; Christensen, M.O.; Mielke, C.; Interthal, H.; Pouliot, J.J.; Boege, F.; Marko, D. Tdp1 overexpression in human cells counteracts DNA damage mediated by topoisomerases i and ii. J. Biol. Chem. 2004, 279, 55618–55625. [Google Scholar] [CrossRef]
- Das, B.B.; Dexheimer, T.S.; Maddali, K.; Pommier, Y. Role of tyrosyl-DNA phosphodiesterase (tdp1) in mitochondria. Proc. Natl. Acad. Sci. USA 2010, 107, 19790–19795. [Google Scholar] [CrossRef]
- Fam, H.K.; Choi, K.; Fougner, L.; Lim, C.J.; Boerkoel, C.F. Reactive oxygen species stress increases accumulation of tyrosyl-DNA phsosphodiesterase 1 within mitochondria. Sci. Rep. 2018, 8, 4304. [Google Scholar] [CrossRef]
- Fam, H.K.; Chowdhury, M.K.; Walton, C.; Choi, K.; Boerkoel, C.F.; Hendson, G. Expression profile and mitochondrial colocalization of tdp1 in peripheral human tissues. J. Mol. Histol. 2013, 44, 481–494. [Google Scholar] [CrossRef]
- Dean, R.A.; Fam, H.K.; An, J.; Choi, K.; Shimizu, Y.; Jones, S.J.; Boerkoel, C.F.; Interthal, H.; Pfeifer, T.A. Identification of a putative tdp1 inhibitor (cd00509) by in vitro and cell-based assays. J. Biomol. Screen. 2014, 19, 1372–1382. [Google Scholar] [CrossRef] [PubMed]
- Fam, H.K.; Walton, C.; Mitra, S.A.; Chowdhury, M.; Osborne, N.; Choi, K.; Sun, G.; Wong, P.C.; O’Sullivan, M.J.; Turashvili, G.; et al. Tdp1 and parp1 deficiency are cytotoxic to rhabdomyosarcoma cells. Mol. Cancer Res. 2013, 11, 1179–1192. [Google Scholar] [CrossRef] [PubMed]
- Meisenberg, C.; Gilbert, D.C.; Chalmers, A.; Haley, V.; Gollins, S.; Ward, S.E.; El-Khamisy, S.F. Clinical and cellular roles for tdp1 and top1 in modulating colorectal cancer response to irinotecan. Mol. Cancer Ther. 2015, 14, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Meisenberg, C.; Ward, S.E.; Schmid, P.; El-Khamisy, S.F. Tdp1/top1 ratio as a promising indicator for the response of small cell lung cancer to topotecan. J. Cancer Sci. 2014, 6, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Comeaux, E.Q.; Cuya, S.M.; Kojima, K.; Jafari, N.; Wanzeck, K.C.; Mobley, J.A.; Bjornsti, M.A.; van Waardenburg, R.C. Tyrosyl-DNA phosphodiesterase i catalytic mutants reveal an alternative nucleophile that can catalyze substrate cleavage. J. Biol. Chem. 2015, 290, 6203–6214. [Google Scholar] [CrossRef] [PubMed]
- Cuya, S.M.; Comeaux, E.Q.; Wanzeck, K.; Yoon, K.J.; van Waardenburg, R.C. Dysregulated human tyrosyl-DNA phosphodiesterase i acts as cellular toxin. Oncotarget 2016, 7, 86660. [Google Scholar] [CrossRef] [PubMed]
- Duffy, S.; Fam, H.K.; Wang, Y.K.; Styles, E.B.; Kim, J.H.; Ang, J.S.; Singh, T.; Larionov, V.; Shah, S.P.; Andrews, B.; et al. Overexpression screens identify conserved dosage chromosome instability genes in yeast and human cancer. Proc. Natl. Acad. Sci. USA 2016, 113, 9967–9976. [Google Scholar] [CrossRef] [PubMed]
- Gajewski, S.; Comeaux, E.Q.; Jafari, N.; Bharatham, N.; Bashford, D.; White, S.W.; van Waardenburg, R.C. Analysis of the active-site mechanism of tyrosyl-DNA phosphodiesterase i: A member of the phospholipase d superfamily. J. Mol. Biol. 2012, 415, 741–758. [Google Scholar] [CrossRef]
- DeYonker, N.J.; Webster, C.E. Phosphoryl transfers of the phospholipase d superfamily: A quantum mechanical theoretical study. J. Am. Chem. Soc. 2013, 135, 13764–13774. [Google Scholar] [CrossRef]
- DeYonker, N.J.; Webster, C.E. A theoretical study of phosphoryl transfers of tyrosyl-DNA phosphodiesterase i (tdp1) and the possibility of a “dead-end” phosphohistidine intermediate. Biochemistry 2015, 54, 4236–4247. [Google Scholar] [CrossRef]
- Davies, D.R.; Interthal, H.; Champoux, J.J.; Hol, W.G. Crystal structure of a transition state mimic for tdp1 assembled from vanadate, DNA, and a topoisomerase i-derived peptide. Chem. Biol. 2003, 10, 139–147. [Google Scholar] [CrossRef]
- Flett, F.J.; Ruksenaite, E.; Armstrong, L.A.; Bharati, S.; Carloni, R.; Morris, E.R.; Mackay, C.L.; Interthal, H.; Richardson, J.M. Structural basis for DNA 3′-end processing by human tyrosyl-DNA phosphodiesterase 1. Nat. Commun. 2018, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- Davies, D.R.; Interthal, H.; Champoux, J.J.; Hol, W.G. Explorations of peptide and oligonucleotide binding sites of tyrosyl-DNA phosphodiesterase using vanadate complexes. J. Med. Chem. 2004, 47, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Raymond, A.C.; Rideout, M.C.; Staker, B.; Hjerrild, K.; Burgin, A.B., Jr. Analysis of human tyrosyl-DNA phosphodiesterase i catalytic residues. J. Mol. Biol. 2004, 338, 895–906. [Google Scholar] [CrossRef]
- Interthal, H.; Chen, H.J.; Champoux, J.J. Human tdp1 cleaves a broad spectrum of substrates, including phosphoamide linkages. J. Biol. Chem. 2005, 280, 36518–36528. [Google Scholar] [CrossRef]
- Interthal, H.; Chen, H.J.; Kehl-Fie, T.E.; Zotzmann, J.; Leppard, J.B.; Champoux, J.J. Scan1 mutant tdp1 accumulates the enzyme--DNA intermediate and causes camptothecin hypersensitivity. EMBO J. 2005, 24, 2224–2233. [Google Scholar] [CrossRef]
- Caldecott, K.W. DNA single-strand break repair and spinocerebellar ataxia. Cell 2003, 112, 7–10. [Google Scholar] [CrossRef]
- Chiang, S.C.; Carroll, J.; El-Khamisy, S.F. Tdp1 serine 81 promotes interaction with DNA ligase iiialpha and facilitates cell survival following DNA damage. Cell Cycle 2010, 9, 588–595. [Google Scholar] [CrossRef]
- El-Khamisy, S.F.; Saifi, G.M.; Weinfeld, M.; Johansson, F.; Helleday, T.; Lupski, J.R.; Caldecott, K.W. Defective DNA single-strand break repair in spinocerebellar ataxia with axonal neuropathy-1. Nature 2005, 434, 108–113. [Google Scholar] [CrossRef]
- Jilani, A.; Ramotar, D.; Slack, C.; Ong, C.; Yang, X.M.; Scherer, S.W.; Lasko, D.D. Molecular cloning of the human gene, pnkp, encoding a polynucleotide kinase 3′-phosphatase and evidence for its role in repair of DNA strand breaks caused by oxidative damage. J. Biol. Chem. 1999, 274, 24176–24186. [Google Scholar] [CrossRef]
- Karimi-Busheri, F.; Daly, G.; Robins, P.; Canas, B.; Pappin, D.J.; Sgouros, J.; Miller, G.G.; Fakhrai, H.; Davis, E.M.; Le Beau, M.M.; et al. Molecular characterization of a human DNA kinase. J. Biol. Chem. 1999, 274, 24187–24194. [Google Scholar] [CrossRef] [PubMed]
- Whitehouse, C.J.; Taylor, R.M.; Thistlethwaite, A.; Zhang, H.; Karimi-Busheri, F.; Lasko, D.D.; Weinfeld, M.; Caldecott, K.W. Xrcc1 stimulates human polynucleotide kinase activity at damaged DNA termini and accelerates DNA single-strand break repair. Cell 2001, 104, 107–117. [Google Scholar] [CrossRef]
- Champoux, J.J. Strand breakage by the DNA untwisting enzyme results in covalent attachment of the enzyme to DNA. Proc. Natl. Acad. Sci. USA 1977, 74, 3800–3804. [Google Scholar] [CrossRef] [PubMed]
- Schoeffler, A.J.; Berger, J.M. DNA topoisomerases: Harnessing and constraining energy to govern chromosome topology. Q. Rev. Biophys. 2008, 41, 41–101. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.C. Cellular roles of DNA topoisomerases: A molecular perspective. Nat. Rev. Mol. Cell Biol. 2002, 3, 430–440. [Google Scholar] [CrossRef]
- Cuya, S.M.; Bjornsti, M.A.; van Waardenburg, R. DNA topoisomerase-targeting chemotherapeutics: What’s new? Cancer Chemother. Pharmacol. 2017, 80, 1–14. [Google Scholar] [CrossRef]
- Hsiang, Y.H.; Hertzberg, R.; Hecht, S.; Liu, L.F. Camptothecin induces protein-linked DNA breaks via mammalian DNA topoisomerase i. J. Biol. Chem. 1985, 260, 14873–14878. [Google Scholar]
- Hsiang, Y.H.; Lihou, M.G.; Liu, L.F. Arrest of replication forks by drug-stabilized topoisomerase i-DNA cleavable complexes as a mechanism of cell killing by camptothecin. Cancer Res. 1989, 49, 5077–5082. [Google Scholar]
- Hsiang, Y.H.; Liu, L.F.; Wall, M.E.; Wani, M.C.; Nicholas, A.W.; Manikumar, G.; Kirschenbaum, S.; Silber, R.; Potmesil, M. DNA topoisomerase i-mediated DNA cleavage and cytotoxicity of camptothecin analogues. Cancer Res. 1989, 49, 4385–4389. [Google Scholar]
- Jaxel, C.; Capranico, G.; Kerrigan, D.; Kohn, K.W.; Pommier, Y. Effect of local DNA sequence on topoisomerase i cleavage in the presence or absence of camptothecin. J. Biol. Chem. 1991, 266, 20418–20423. [Google Scholar]
- Pourquier, P.; Gioffre, C.; Kohlhagen, G.; Urasaki, Y.; Goldwasser, F.; Hertel, L.W.; Yu, S.; Pon, R.T.; Gmeiner, W.H.; Pommier, Y. Gemcitabine (2′,2′-difluoro-2′-deoxycytidine), an antimetabolite that poisons topoisomerase i. Clin. Cancer Res. 2002, 8, 2499–2504. [Google Scholar] [PubMed]
- Pourquier, P.; Pilon, A.A.; Kohlhagen, G.; Mazumder, A.; Sharma, A.; Pommier, Y. Trapping of mammalian topoisomerase i and recombinations induced by damaged DNA containing nicks or gaps. Importance of DNA end phosphorylation and camptothecin effects. J. Biol. Chem. 1997, 272, 26441–26447. [Google Scholar] [CrossRef] [PubMed]
- Pourquier, P.; Takebayashi, Y.; Urasaki, Y.; Gioffre, C.; Kohlhagen, G.; Pommier, Y. Induction of topoisomerase i cleavage complexes by 1-β -d-arabinofuranosylcytosine (ara-c) in vitro and in ara-c-treated cells. Proc. Natl. Acad. Sci. USA 2000, 97, 1885–1890. [Google Scholar] [CrossRef] [PubMed]
- Pourquier, P.; Ueng, L.M.; Fertala, J.; Wang, D.; Park, H.J.; Essigmann, J.M.; Bjornsti, M.A.; Pommier, Y. Induction of reversible complexes between eukaryotic DNA topoisomerase i and DNA-containing oxidative base damages. 7, 8-dihydro-8-oxoguanine and 5-hydroxycytosine. J. Biol. Chem. 1999, 274, 8516–8523. [Google Scholar] [CrossRef]
- Pourquier, P.; Ueng, L.M.; Kohlhagen, G.; Mazumder, A.; Gupta, M.; Kohn, K.W.; Pommier, Y. Effects of uracil incorporation, DNA mismatches, and abasic sites on cleavage and religation activities of mammalian topoisomerase i. J. Biol. Chem. 1997, 272, 7792–7796. [Google Scholar] [CrossRef]
- Pourquier, P.; Waltman, J.L.; Urasaki, Y.; Loktionova, N.A.; Pegg, A.E.; Nitiss, J.L.; Pommier, Y. Topoisomerase i-mediated cytotoxicity of n-methyl-n’-nitro-n-nitrosoguanidine: Trapping of topoisomerase i by the o6-methylguanine. Cancer Res. 2001, 61, 53–58. [Google Scholar]
- van Waardenburg, R.C.; de Jong, L.A.; van Delft, F.; van Eijndhoven, M.A.; Bohlander, M.; Bjornsti, M.A.; Brouwer, J.; Schellens, J.H. Homologous recombination is a highly conserved determinant of the synergistic cytotoxicity between cisplatin and DNA topoisomerase i poisons. Mol. Cancer Ther. 2004, 3, 393–402. [Google Scholar]
- van Waardenburg, R.C.; de Jong, L.A.; van Eijndhoven, M.A.; Verseyden, C.; Pluim, D.; Jansen, L.E.; Bjornsti, M.A.; Schellens, J.H. Platinated DNA adducts enhance poisoning of DNA topoisomerase i by camptothecin. J. Biol. Chem. 2004, 279, 54502–54509. [Google Scholar] [CrossRef]
- Nivens, M.C.; Felder, T.; Galloway, A.H.; Pena, M.M.; Pouliot, J.J.; Spencer, H.T. Engineered resistance to camptothecin and antifolates by retroviral coexpression of tyrosyl DNA phosphodiesterase-i and thymidylate synthase. Cancer Chemother. Pharmacol. 2004, 53, 107–115. [Google Scholar] [CrossRef]
- Ben Hassine, S.; Arcangioli, B. Tdp1 protects against oxidative DNA damage in non-dividing fission yeast. EMBO J. 2009, 28, 632–640. [Google Scholar] [CrossRef]
- Li, J.; Summerlin, M.; Nitiss, K.C.; Nitiss, J.L.; Hanakahi, L.A. Tdp1 is required for efficient non-homologous end joining in human cells. DNA Repair 2017, 60, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Moriwaki, T.; Okamoto, S.; Sasanuma, H.; Nagasawa, H.; Takeda, S.; Masunaga, S.I.; Tano, K. Cytotoxicity of tirapazamine (3-amino-1,2,4-benzotriazine-1,4-dioxide)-induced DNA damage in chicken dt40 cells. Chem. Res. Toxicol. 2017, 30, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Murai, J.; Huang, S.Y.; Das, B.B.; Dexheimer, T.S.; Takeda, S.; Pommier, Y. Tyrosyl-DNA phosphodiesterase 1 (tdp1) repairs DNA damage induced by topoisomerases i and ii and base alkylation in vertebrate cells. J. Biol. Chem. 2012, 287, 12848–12857. [Google Scholar] [CrossRef] [PubMed]
- Scott, P.; Al Kindi, A.; Al Fahdi, A.; Al Yarubi, N.; Bruwer, Z.; Al Adawi, S.; Nandhagopal, R. Spinocerebellar ataxia with axonal neuropathy type 1 revisited. J. Clin. Neurosci. 2019, 67, 139–144. [Google Scholar] [CrossRef]
- Takashima, H.; Boerkoel, C.F.; John, J.; Saifi, G.M.; Salih, M.A.; Armstrong, D.; Mao, Y.; Quiocho, F.A.; Roa, B.B.; Nakagawa, M.; et al. Mutation of tdp1, encoding a topoisomerase i-dependent DNA damage repair enzyme, in spinocerebellar ataxia with axonal neuropathy. Nat. Genet. 2002, 32, 267–272. [Google Scholar] [CrossRef]
- Ross, W.; Rowe, T.; Glisson, B.; Yalowich, J.; Liu, L. Role of topoisomerase ii in mediating epipodophyllotoxin-induced DNA cleavage. Cancer Res. 1984, 44, 5857–5860. [Google Scholar]
- Wu, C.C.; Li, T.K.; Farh, L.; Lin, L.Y.; Lin, T.S.; Yu, Y.J.; Yen, T.J.; Chiang, C.W.; Chan, N.L. Structural basis of type ii topoisomerase inhibition by the anticancer drug etoposide. Science 2011, 333, 459–462. [Google Scholar] [CrossRef]
- Maede, Y.; Shimizu, H.; Fukushima, T.; Kogame, T.; Nakamura, T.; Miki, T.; Takeda, S.; Pommier, Y.; Murai, J. Differential and common DNA repair pathways for topoisomerase i- and ii-targeted drugs in a genetic dt40 repair cell screen panel. Mol. Cancer Ther. 2014, 13, 214–220. [Google Scholar] [CrossRef]
- Nitiss, K.C.; Malik, M.; He, X.; White, S.W.; Nitiss, J.L. Tyrosyl-DNA phosphodiesterase (tdp1) participates in the repair of top2-mediated DNA damage. Proc. Natl. Acad. Sci. USA 2006, 103, 8953–8958. [Google Scholar] [CrossRef]
- Curtin, N.J. DNA repair dysregulation from cancer driver to therapeutic target. Nat. Rev. Cancer 2012, 12, 801–817. [Google Scholar] [CrossRef]
- Tumbale, P.; Appel, C.D.; Kraehenbuehl, R.; Robertson, P.D.; Williams, J.S.; Krahn, J.; Ahel, I.; Williams, R.S. Structure of an aprataxin-DNA complex with insights into aoa1 neurodegenerative disease. Nat. Struct. Mol. Biol. 2011, 18, 1189–1195. [Google Scholar] [CrossRef] [PubMed]
- Cortes Ledesma, F.; El Khamisy, S.F.; Zuma, M.C.; Osborn, K.; Caldecott, K.W. A human 5′-tyrosyl DNA phosphodiesterase that repairs topoisomerase-mediated DNA damage. Nature 2009, 461, 674–678. [Google Scholar] [CrossRef] [PubMed]
- Caldecott, K.W. Tyrosyl DNA phosphodiesterase 2, an enzyme fit for purpose. Nat. Struct. Mol. Biol. 2012, 19, 1212–1213. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Sharma, A.; Ju, L.; Murai, J.; Umans, L.; Vermeire, L.; Pommier, Y.; Takeda, S.; Huylebroeck, D.; Caldecott, K.W.; et al. Tdp2 promotes repair of topoisomerase i-mediated DNA damage in the absence of tdp1. Nucleic Acids Res. 2012, 40, 8371–8380. [Google Scholar] [CrossRef]
- Prasad, R.; Horton, J.K.; Dai, D.P.; Wilson, S.H. Repair pathway for parp-1 DNA-protein crosslinks. DNA Repair 2019, 73, 71–77. [Google Scholar] [CrossRef]
- Prasad, R.; Dyrkheeva, N.; Williams, J.; Wilson, S.H. Mammalian base excision repair: Functional partnership between parp-1 and ape1 in ap-site repair. PLoS ONE 2015, 10, e0124269. [Google Scholar] [CrossRef]
- Murai, J.; Huang, S.Y.; Das, B.B.; Renaud, A.; Zhang, Y.; Doroshow, J.H.; Ji, J.; Takeda, S.; Pommier, Y. Trapping of parp1 and parp2 by clinical parp inhibitors. Cancer Res. 2012, 72, 5588–5599. [Google Scholar] [CrossRef]
- Kedar, P.S.; Stefanick, D.F.; Horton, J.K.; Wilson, S.H. Increased parp-1 association with DNA in alkylation damaged, parp-inhibited mouse fibroblasts. Mol. Cancer Res. 2012, 10, 360–368. [Google Scholar] [CrossRef]
- Quinones, J.L.; Demple, B. When DNA repair goes wrong: Ber-generated DNA-protein crosslinks to oxidative lesions. DNA Repair 2016, 44, 103–109. [Google Scholar] [CrossRef]
- Nakano, T.; Xu, X.; Salem, A.M.H.; Shoulkamy, M.I.; Ide, H. Radiation-induced DNA-protein cross-links: Mechanisms and biological significance. Free Radic. Biol. Med. 2017, 107, 136–145. [Google Scholar] [CrossRef]
- Ilina, E.S.; Khodyreva, S.N.; Berezhnoy, A.E.; Larin, S.S.; Lavrik, O.I. Tracking ku antigen levels in cell extracts with DNA containing abasic sites. Mutat. Res. 2010, 685, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Shao, H.; Han, Q.; Seiler, C.L.; Tretyakova, N.Y. Reversible DNA-protein cross-linking at epigenetic DNA marks. Angew. Chem. Int. Ed. Engl. 2017, 56, 14130–14134. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T.; Miyamoto-Matsubara, M.; Shoulkamy, M.I.; Salem, A.M.; Pack, S.P.; Ishimi, Y.; Ide, H. Translocation and stability of replicative DNA helicases upon encountering DNA-protein cross-links. J. Biol. Chem. 2013, 288, 4649–4658. [Google Scholar] [CrossRef] [PubMed]
- Al Abo, M.; Sasanuma, H.; Liu, X.; Rajapakse, V.N.; Huang, S.Y.; Kiselev, E.; Takeda, S.; Plunkett, W.; Pommier, Y. Tdp1 is critical for the repair of DNA breaks induced by sapacitabine, a nucleoside also targeting atm- and brca-deficient tumors. Mol. Cancer Ther. 2017, 16, 2543–2551. [Google Scholar] [CrossRef]
- Alagoz, M.; Chiang, S.C.; Sharma, A.; El-Khamisy, S.F. Atm deficiency results in accumulation of DNA-topoisomerase i covalent intermediates in neural cells. PLoS ONE 2013, 8, e58239. [Google Scholar] [CrossRef]
- Huang, S.Y.; Murai, J.; Dalla Rosa, I.; Dexheimer, T.S.; Naumova, A.; Gmeiner, W.H.; Pommier, Y. Tdp1 repairs nuclear and mitochondrial DNA damage induced by chain-terminating anticancer and antiviral nucleoside analogs. Nucleic Acids Res. 2013, 41, 7793–7803. [Google Scholar] [CrossRef]
- Lebedeva, N.A.; Rechkunova, N.I.; El-Khamisy, S.F.; Lavrik, O.I. Tyrosyl-DNA phosphodiesterase 1 initiates repair of apurinic/apyrimidinic sites. Biochimie 2012, 94, 1749–1753. [Google Scholar] [CrossRef]
- Lebedeva, N.A.; Rechkunova, N.I.; Ishchenko, A.A.; Saparbaev, M.; Lavrik, O.I. The mechanism of human tyrosyl-DNA phosphodiesterase 1 in the cleavage of ap site and its synthetic analogs. DNA Repair 2013, 12, 1037–1042. [Google Scholar] [CrossRef]
- Lebedeva, N.A.; Rechkunova, N.I.; Lavrik, O.I. Ap-site cleavage activity of tyrosyl-DNA phosphodiesterase 1. FEBS Lett. 2011, 585, 683–686. [Google Scholar] [CrossRef]
- Lebedeva, N.A.; Rechkunova, N.I.; Lavrik, O.I. Repair of apurinic/apyrimidinic sites in single-stranded DNA initiated by tyrosyl-DNA phosphodiesterase 1. Dokl. Biochem. Biophys. 2014, 455, 68–71. [Google Scholar] [CrossRef]
- Zhou, T.; Lee, J.W.; Tatavarthi, H.; Lupski, J.R.; Valerie, K.; Povirk, L.F. Deficiency in 3′-phosphoglycolate processing in human cells with a hereditary mutation in tyrosyl-DNA phosphodiesterase (tdp1). Nucleic Acids Res. 2005, 33, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Das, B.B.; Antony, S.; Gupta, S.; Dexheimer, T.S.; Redon, C.E.; Garfield, S.; Shiloh, Y.; Pommier, Y. Optimal function of the DNA repair enzyme tdp1 requires its phosphorylation by atm and/or DNA-pk. EMBO J. 2009, 28, 3667–3680. [Google Scholar] [CrossRef] [PubMed]
- Plo, I.; Liao, Z.Y.; Barcelo, J.M.; Kohlhagen, G.; Caldecott, K.W.; Weinfeld, M.; Pommier, Y. Association of xrcc1 and tyrosyl DNA phosphodiesterase (tdp1) for the repair of topoisomerase i-mediated DNA lesions. DNA Repair 2003, 2, 1087–1100. [Google Scholar] [CrossRef]
- Heo, J.; Li, J.; Summerlin, M.; Hays, A.; Katyal, S.; McKinnon, P.J.; Nitiss, K.C.; Nitiss, J.L.; Hanakahi, L.A. Tdp1 promotes assembly of non-homologous end joining protein complexes on DNA. DNA Repair 2015, 30, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Das, B.B.; Huang, S.Y.; Murai, J.; Rehman, I.; Ame, J.C.; Sengupta, S.; Das, S.K.; Majumdar, P.; Zhang, H.; Biard, D.; et al. Parp1-tdp1 coupling for the repair of topoisomerase i-induced DNA damage. Nucleic Acids Res. 2014, 42, 4435–4449. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Beveridge, R.; Hudson, J.J.R.; Parker, J.D.; Chiang, S.C.; Ray, S.; Ashour, M.E.; Sudbery, I.; Dickman, M.J.; El-Khamisy, S.F. Uchl3 regulates topoisomerase-induced chromosomal break repair by controlling tdp1 proteostasis. Cell Rep. 2018, 23, 3352–3365. [Google Scholar] [CrossRef]
- Rehman, I.; Basu, S.M.; Das, S.K.; Bhattacharjee, S.; Ghosh, A.; Pommier, Y.; Das, B.B. Prmt5-mediated arginine methylation of tdp1 for the repair of topoisomerase i covalent complexes. Nucleic Acids Res. 2018, 46, 5601–5617. [Google Scholar] [CrossRef]
- Hudson, J.J.; Chiang, S.C.; Wells, O.S.; Rookyard, C.; El-Khamisy, S.F. Sumo modification of the neuroprotective protein tdp1 facilitates chromosomal single-strand break repair. Nat. Commun. 2012, 3, 733. [Google Scholar] [CrossRef]
- Simsek, D.; Furda, A.; Gao, Y.; Artus, J.; Brunet, E.; Hadjantonakis, A.K.; Van Houten, B.; Shuman, S.; McKinnon, P.J.; Jasin, M. Crucial role for DNA ligase iii in mitochondria but not in xrcc1-dependent repair. Nature 2011, 471, 245–248. [Google Scholar] [CrossRef]
- Bahmed, K.; Nitiss, K.C.; Nitiss, J.L. Yeast tdp1 regulates the fidelity of nonhomologous end joining. Proc. Natl. Acad. Sci. USA 2010, 107, 4057–4062. [Google Scholar] [CrossRef]
- Li, M.; Lu, L.Y.; Yang, C.Y.; Wang, S.; Yu, X. The fha and brct domains recognize adp-ribosylation during DNA damage response. Genes Dev. 2013, 27, 1752–1768. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Rehman, I.; Ghosh, A.; Sengupta, S.; Majumdar, P.; Jana, B.; Das, B.B. Poly(adp-ribose) polymers regulate DNA topoisomerase i (top1) nuclear dynamics and camptothecin sensitivity in living cells. Nucleic Acids Res. 2016, 44, 8363–8375. [Google Scholar] [CrossRef] [PubMed]
- Murai, J.; Marchand, C.; Shahane, S.A.; Sun, H.; Huang, R.; Zhang, Y.; Chergui, A.; Ji, J.; Doroshow, J.H.; Jadhav, A.; et al. Identification of novel parp inhibitors using a cell-based tdp1 inhibitory assay in a quantitative high-throughput screening platform. DNA Repair 2014, 21, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Lorton, B.M.; Shechter, D. Cellular consequences of arginine methylation. Cell. Mol. Life Sci. 2019, 76, 2933–2956. [Google Scholar] [CrossRef] [PubMed]
- Hamard, P.J.; Santiago, G.E.; Liu, F.; Karl, D.L.; Martinez, C.; Man, N.; Mookhtiar, A.K.; Duffort, S.; Greenblatt, S.; Verdun, R.E.; et al. Prmt5 regulates DNA repair by controlling the alternative splicing of histone-modifying enzymes. Cell Rep. 2018, 24, 2643–2657. [Google Scholar] [CrossRef] [PubMed]
| Protein | Tdp1 domain | Response to | PTMA | Effector | Reference |
|---|---|---|---|---|---|
| ATM | N | CPT/IR | S81P | [92] | |
| DNA–PK | N | CPT/IR | S81P | [92] | |
| XRCC1 | N | CPT/IR | ↑S81P B | [39,92,93] | |
| Lig3α | N | CPT/IR | ↑S81P B | [38,39] | |
| XLF | Core | ↓S81P C | [61,94] | ||
| Ku70/80 | N | [94] | |||
| Ku70/80/DNAPkcsD | N | S81P | [94] | ||
| PARP1 | N | K?PAR E | [20,85,95] | ||
| UCHL3 | ? | Proteostasis F | deUb G | Ub | [96] |
| PRMT5 | N | diMeR361/586 | [97] | ||
| UBC9 H | N | ? | SUMO K111 | [98] | |
| E2/E3 Ub complex I | ? | ? | Ub | [96] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brettrager, E.J.; Segura, I.A.; van Waardenburg, R.C.A.M. Tyrosyl-DNA Phosphodiesterase I N-Terminal Domain Modifications and Interactions Regulate Cellular Function. Genes 2019, 10, 897. https://doi.org/10.3390/genes10110897
Brettrager EJ, Segura IA, van Waardenburg RCAM. Tyrosyl-DNA Phosphodiesterase I N-Terminal Domain Modifications and Interactions Regulate Cellular Function. Genes. 2019; 10(11):897. https://doi.org/10.3390/genes10110897
Chicago/Turabian StyleBrettrager, Evan J., Isaac A. Segura, and Robert C. A. M. van Waardenburg. 2019. "Tyrosyl-DNA Phosphodiesterase I N-Terminal Domain Modifications and Interactions Regulate Cellular Function" Genes 10, no. 11: 897. https://doi.org/10.3390/genes10110897
APA StyleBrettrager, E. J., Segura, I. A., & van Waardenburg, R. C. A. M. (2019). Tyrosyl-DNA Phosphodiesterase I N-Terminal Domain Modifications and Interactions Regulate Cellular Function. Genes, 10(11), 897. https://doi.org/10.3390/genes10110897





