A TAC3 Missense Variant in a Domestic Shorthair Cat with Testicular Hypoplasia and Persistent Primary Dentition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animal Selection
2.3. Hormone Measurement
2.4. DNA Extraction
2.5. Whole Genome Sequencing
2.6. Variant Calling
2.7. Gene Analysis
2.8. Sanger Sequencing
3. Results
3.1. Clinical Examination
3.2. Laboratory Findings
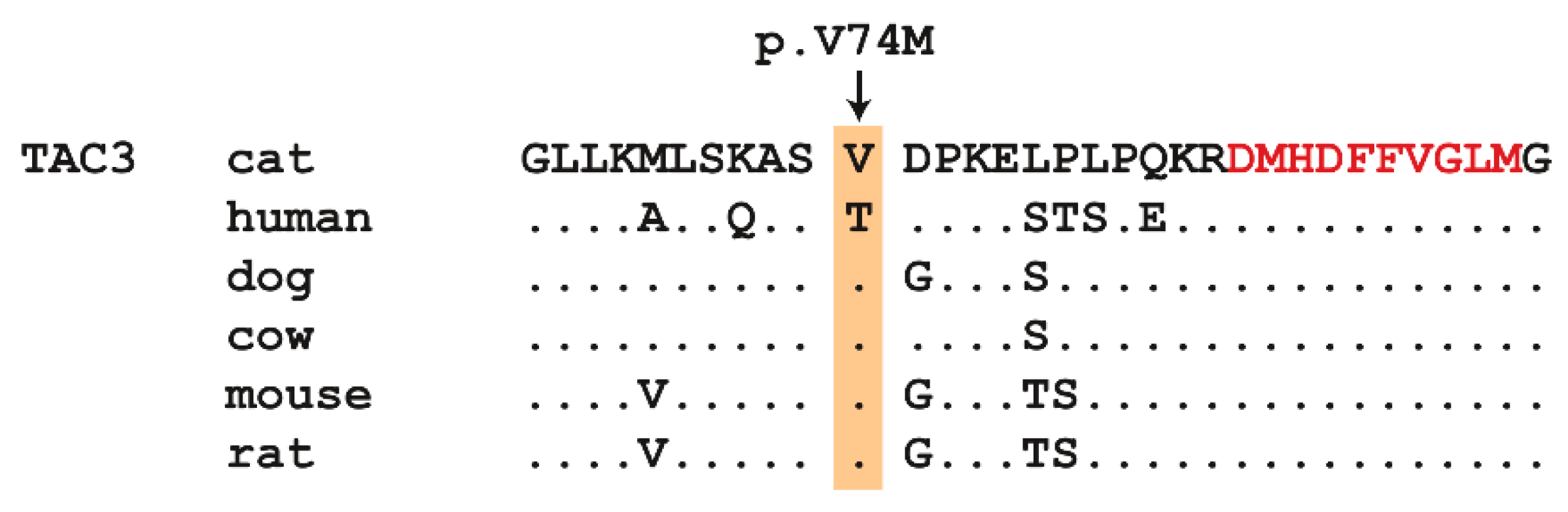
3.3. Genetic Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Marques, P.; Skorupskaite, K.; George, J.T.; Anderson, R.A. Physiology of GNRH and Gonadotropin Secretion. In Endotext; Feingold, K.R., Anawalt, B., Boyce, A., et al., Eds.; South Dartmouth (MA): MDText.com. Available online: https://www.ncbi.nlm.nih.gov/books/NBK279070/ (accessed on 27 August 2019).
- Ikegami, K.; Minabe, S.; Ieda, N.; Goto, T.; Sugimoto, A.; Nakamura, S.; Inoue, N.; Oishi, S.; Maturana, A.D.; Sanbo, M.; et al. Evidence of involvement of neurone-glia/neurone-neurone communications via gap junctions in synchronised activity of KNDy neurones. J. Neuroendocrinol. 2017, 29. [Google Scholar] [CrossRef]
- Skorupskaite, K.; George, J.T.; Anderson, R.A. The kisspeptin-GnRH pathway in human reproductive health and disease. Hum. Reprod. Update 2014, 20, 485–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fraietta, R.; Zylbergstejn, D.S.; Eseves, S.C. Hypogonadotropic Hypogonadism Revisited. Clinics 2013, 68, 81–88. [Google Scholar] [CrossRef]
- Kim, H.-G.; Kurth, I.; Lan, F.; Meliciani, I.; Wenzel, W.; Eom, S.H.; Kang, G.B.; Rosenberger, G.; Tekin, M.; Ozata, M.; et al. Mutations in CHD7, encoding a chromatin-remodeling protein, cause idiopathic hypogonadotropic hypogonadism and Kallmann syndrome. Am. J. Hum. Genet 2008, 83, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Achermann, J.C.; Gu, W.X.; Kotlar, T.J.; Meeks, J.J.; Sabacan, L.P.; Seminara, S.B.; Habiby, R.L.; Hindmarsh, P.C.; Bick, D.P.; Sherins, R.J.; et al. Mutational analysis of DAX1 in patients with hypogonadotropic hypogonadism or pubertal delay. J. Clin. Endocr. Metab. 1999, 84, 4497–4500. [Google Scholar] [CrossRef] [PubMed]
- Miraoui, H.; Dwyer, A.A.; Sykiotis, G.P.; Plummer, L.; Chung, W.; Feng, B.; Beenken, A.; Clarke, J.; Pers, T.H.; Dworzynski, P.; et al. Mutations in FGF17, IL17RD, DUPS6, SPRY4, and FLRT3 are identified in individuals with congenital hypogonadotropic hypogonadism. Am. J. Hum. Genet. 2013, 92, 725–743. [Google Scholar] [CrossRef] [PubMed]
- Kotan, L.D.; Hutchins, B.I.; Ozkan, Y.; Demirel, F.; Stoner, H.; Cheng, P.J.; Esen, I.; Gurbuz, F.; Bicakci, Y.K.; Mengen, E.; et al. Mutations in FEZF1 cause Kallmann syndrome. Am. J. Hum. Genet. 2014, 95, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Falardeau, J.; Chung, W.C.J.; Beenken, A.; Raivio, T.; Plummer, L.; Sidis, Y.; Jacobson-Dickman, E.E.; Eliseenkova, A.V.; Ma, J.; Dwyer, A.; et al. Decreased FGF8 signaling causes deficiency of gonadotropin-releasing hormone in humans and mice. J. Clin. Investig. 2008, 118, 2822–2831. [Google Scholar] [CrossRef] [Green Version]
- Dode, C.; Levilliers, J.; Dupont, J.M.; De Paepe, A.; Le Du, N.; Soussi-Yanicostas, N.; Coimbra, R.S.; Delmaghani, S.; Compain-Nouaille, S.; Baverel, F. Loss-of-function mutations in FGFR1 cause autosomal dominant Kallmann syndrome. Nat. Genet. 2003, 33, 463–465. [Google Scholar] [CrossRef] [Green Version]
- Layman, L.C.; Lee, E.J.; Peak, D.B.; Namnoum, A.B.; Vu, K.V.; van Lingen, B.L.; Gray, M.R.; McDonough, P.G.; Reindollar, R.H.; Jameson, J.L. Delayed puberty and hypogonadism caused by mutations in the follicle-stimulating hormone beta-subunit gene. N. Eng. J. Med. 1997, 337, 607–611. [Google Scholar] [CrossRef]
- Bouligand, J.; Ghervan, C.; Tello, J.A.; Brailly-Tabard, S.; Salenave, S.; Chanson, P.; Lombes, M.; Millar, R.P.; Guiochon-Mantel, A.; Young, J. Isolated familial hypogonadotropic hypogonadism and a GNRH1 mutation. N. Eng. J. Med. 2009, 360, 2742–2748. [Google Scholar] [CrossRef] [PubMed]
- Pralong, F.P.; Gomez, F.; Castillo, E.; Cotecchia, S.; Abuin, L.; Aubert, M.L.; Portmann, L.; Gaillard, R.C. Complete hypogonadotropic hypogonadism associated with a novel inactivating mutation of the gonadotropin-releasing hormone receptor. J. Clin. Endocr. Metab. 1999, 84, 3811–3816. [Google Scholar] [CrossRef] [PubMed]
- Tajima, T.; Hattorri, T.; Nakajima, T.; Okuhara, K.; Sato, K.; Abe, S.; Nakae, J.; Fujieda, K. Sporadic heterozygous frameshift mutation of HESX1 causing pituitary and optic nerve hypoplasia and combined pituitary hormone deficiency in a Japanese patient. J. Clin. Endocr. Metab. 2008, 88, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Bick, D.; Franco, B.; Sherins, R.J.; Heye, B.; Pike, L.; Crawford, J.; Maddalena, A.; Incerti, B.; Pragliola, A.; Meitinger, T.; et al. Brief report: intragenic deletion of the KALIG-1 gene in Kallmann’s syndrome. N. Engl. J. Med. 1992, 326, 1752–1755. [Google Scholar] [CrossRef] [PubMed]
- Topaloglu, A.K.; Tello, J.A.; Kotan, L.D.; Ozbek, M.N.; Yilmaz, M.B.; Erdogan, S.; Gurbuz, F.; Temiz, F.; Millar, R.P.; Yuksel, B. Inactivating KISS1 mutation and hypogonadotropic hypogonadism. N. Eng. J. Med. 2012, 366, 629–635. [Google Scholar] [CrossRef]
- Brioude, F.; Bouligand, J.; Francou, B.; Fagart, J.; Roussel, R.; Viengchareun, S.; Combettes, L.; Brailly-Tabard, S.; Lombes, M.; Young, J.; et al. Two families with normosmic congenital hypogonadotropic hypogonadism and biallelic mutations in KISS1R (KISS1 receptor): clinical evaluation and molecular characterization of a novel mutation. PLoS ONE 2013, 8, e53896. [Google Scholar] [CrossRef]
- Licinio, J.; Caglayan, S.; Ozata, M.; Yildiz, B.O.; de Miranda, P.B.; O’Kirwan, F.; Whitby, R.; Liang, L.; Cohen, P.; Bhasin, S.; et al. Phenotypic effects of leptin replacement on morbid obesity, diabetes mellitus, hypogonadism, and behavior in leptin-deficient adults. Proc. Natl. Acad. Sci. USA 2004, 101, 4531–4536. [Google Scholar] [CrossRef] [Green Version]
- Basciani, S.; Watanabe, M.; Mariani, S.; Passeri, M.; Persichetti, A.; Fiore, D.; Scotto d’Abusco, A.; Caprio, M.; Lenzi, A.; Fabbri, A.; et al. Hypogonadism in a patient with two novel mutations of the luteinizing hormone beta-subunit gene expressed in a compound heterozygous form. J. Clin. Endocr. Metab. 2012, 97, 3031–3038. [Google Scholar] [CrossRef]
- Rajab, A.; Kelberman, D.; de Castro, S.C.P.; Biebermann, H.; Shaikh, H.; Pearce, K.; Hall, C.M.; Shaikh, G.; Gerrelli, D.; Grueters, A. Novel mutations in LHX3 are associated with hypopituitarism and sensorineural hearing loss. Hum. Mol. Genet. 2008, 17, 2150–2159. [Google Scholar] [CrossRef] [Green Version]
- Miura, K.; Acierno, J.S.; Seminara, S.B. Characterization of the human nasal embryonic LHRH factor gene, NELF, and a mutation screening among 65 patients with idiopathic hypogonadotropic hypogonadism (IHH). J. Hum. Genet. 2004, 49, 265–268. [Google Scholar] [CrossRef] [Green Version]
- Margolin, D.H.; Kousi, M.; Chan, Y.M.; Lim, E.T.; Schmahmann, J.D.; Hadjivassiliou, M.; Hall, J.E.; Adam, I.; Dwyer, A.; Plummer, L. Ataxia, dementia, and hypogonadotropism caused by disordered ubiquitination. N. Eng. J. Med. 2013, 368, 1992–2003. [Google Scholar] [CrossRef] [PubMed]
- Synofzik, M.; Gonzalez, M.A.; Lourenco, C.M.; Coutelier, M.; Haack, T.B.; Rebelo, A.; Hannequin, D.; Strom, T.M.; Prokisch, H.; Kernstock, C.; et al. PNPLA6 mutations cause Boucher-Neuhauser and Gordon Holmes syndromes as part of a broad neurodegenerative spectrum. Brain 2014, 137, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Saitsu, H.; Osaka, H.; Sasaki, M.; Takanashi, J.; Hamada, K.; Yamashita, A.; Shibayama, H.; Shiina, M.; Kondo, Y.; Nishiyama, K.; et al. Mutations in POLR3A and POLR3B encoding RNA polymerase III subunits cause an autosomal-recessive hypomyelinating leukoencephalopathy. Am. J. Hum. Genet. 2011, 89, 644–651. [Google Scholar] [CrossRef]
- Dode, C.; Teixeira, L.; Levilliers, J.; Fouveaut, C.; Bouchard, P.; Kottler, M.L.; Lespinasse, J.; Lienhardt-Roussie, A.; Mathieu, M.; Moerman, A. Kallmann syndrome: mutations in the genes encoding prokineticin-2 and prokineticin receptor-2. PLoS Genet. 2006, 2, e175. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, G.; Bhatia, V.; Cook, S.; Thomas, P.Q. Adrenocorticotropin deficiency in combined pituitary hormone deficiency patients homozygous for a novel PROP1 deletion. J. Clin. Endocr. Metab. 2000, 85, 4556–4561. [Google Scholar] [CrossRef] [PubMed]
- Handley, M.T.; Morris-Rosendahl, D.J.; Brown, S.; Macdonald, F.; Hardy, C.; Bem, D.; Carpanini, S.M.; Borck, G.; Martorell, L.; Izzi, C.; et al. Mutation spectrum in RAB3GAP1, RAB3GAP2, and RAB18 and genotype-phenotype correlations in Warburg Micro syndrome and Martsolf syndrome. Hum. Mutat. 2013, 34, 686–696. [Google Scholar] [CrossRef] [PubMed]
- Seminara, S.B.; Acierno, J.S.; Abdulwahid, N.A.; Crowley, W.F.; Margolin, D.H. Hypogonadotropic hypogonadism and cerebellar ataxia: detailed phenotypic characterization of a large, extended kindred. J. Clin. Endocr. Metab. 2002, 87, 1607–1612. [Google Scholar] [CrossRef]
- Young, J.; Metay, C.; Bouligand, J.; Tou, B.; Francou, B.; Maione, L.; Tosca, L.; Sarfati, J.; Brioude, F.; Esteva, B.; et al. SEMA3A deletion in a family with Kallmann syndrome validates the role of semaphorin 3A in human puberty and olfactory system development. Hum. Reprod. 2012, 27, 1460–1465. [Google Scholar] [CrossRef] [Green Version]
- Kelberman, D.; Rizzoti, K.; Avilion, A.; Bitner-Glindicz, M.; Cianfarani, S.; Collins, J.; Chong, W.K.; Kirk, J.M.W.; Achermann, J.C.; Ross, R. Mutations within Sox2/SOX2 are associated with abnormalities in the hypothalamo-pituitary-gonadal axis in mice and humans. J. Clin. Investig. 2006, 116, 2442–2455. [Google Scholar] [CrossRef]
- Shi, C.H.; Schisler, J.C.; Rubel, C.E.; Tan, S.; Song, B.; McDonough, H.; Xu, L.; Portbury, A.L.; Mao, C.Y.; True, C.; et al. Ataxia and hypogonadism caused by the loss of ubiquitin ligase activity of the U box protein CHIP. Hum. Mol. Genet. 2014, 23, 1013–1024. [Google Scholar] [CrossRef]
- Topaloglu, A.K.; Reimann, F.; Guclu, M.; Yalin, A.S.; Kotan, L.D.; Porter, K.M.; Serin, A.; Mungan, N.O.; Cook, J.R.; Ozbek, M.N.; et al. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for neurokinin B in the central control of reproduction. Nature Genet. 2009, 41, 354–358. [Google Scholar] [CrossRef] [PubMed]
- Liegel, R.P.; Handley, M.T.; Ronchetti, A.; Brown, S.; Langemeyer, L.; Linford, A.; Chang, B.; Morris-Rosendahl, D.J.; Carpanini, S.; Posmyk, R.; et al. Loss-of-function mutations in TBC1D20 cause cataracts and male infertility in blind sterile mice and Warburg Micro syndrome in humans. Am. J. Hum. Genet. 2013, 93, 1001–1014. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.G.; Ahn, J.W.; Kurth, I.; Ullmann, R.; Kim, H.T.; Kulharya, A.; Ha, K.S.; Itokawa, Y.; Meliciani, I.; Wenzel, W.; et al. WDR11, a WD protein that interacts with transcription factor EMX1, is mutated in idiopathic hypogonadotropic hypogonadism and Kallmann syndrome. Am. J. Hum. Genet. 2010, 87, 465–479. [Google Scholar] [CrossRef] [PubMed]
- Jagannathan, V.; Drögemüller, C.; Leeb, T.; Dog Biomedical Variant Database Consortium (DBVDC). A comprehensive biomedical variant catalogue based on whole genome sequences of 582 dogs and 8 wolves. Anim Genet. 2019. [Google Scholar] [CrossRef]
- Cingolani, P.; Platts, A.; Wang le, L.; Coon, M.; Nguyen, T.; Wang, L.; Land, S.J.; Lu, X.; Ruden, D.M. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly 2012, 6, 80–92. [Google Scholar] [CrossRef]
- Adzhubei, I.A.; Schmidt, S.; Peshkin, L.; Ramensky, V.E.; Gerasimova, A.; Bork, P.; Kondrashov, A.S.; Sunyaev, S.R. A method and server for predicting damaging missense mutations. Nat. Methods 2010, 4, 248–249. [Google Scholar] [CrossRef]
- Vaser, R.; Adusumalli, S.; Leng, S.N.; Sikic, M.; Ng, P.C. SIFT missense predictions for genomes. Nat Protoc. 2016, 11, 1–9. [Google Scholar] [CrossRef]
- Fukami, M.; Maruyama, T.; Dateki, S.; Sato, N.; Yoshimura, Y.; Ogata, T. Hypothalamic dysfunction in a female with isolated hypogonadotropic hypogonadism and compound heterozygous TACR3 mutations and clinical manifestation in her heterozygous mother. Horm. Res. Paediatr. 2010, 73, 477–481. [Google Scholar] [CrossRef]
- Guran, T.; Tolhurst, G.; Bereket, A.; Rocha, N.; Porter, K.; Turan, S.; Gribble, F.M.; Kotan, L.D.; Akcay, T.; Atay, Z. Hypogonadotropic hypogonadism due to a novel missense mutation in the first extracellular loop of the neurokinin B receptor. J. Clin. Endocrinol. Metab. 2009, 94, 3633–3639. [Google Scholar] [CrossRef]
- Francou, B.; Bouligand, J.; Voican, A.; Amazit, L.; Trabado, S.; Fagart, J.; Meduri, G.; Brailly-Tabard, S.; Chanson, P.; Lecomte, P. Normosmic congenital hypogonadotropic hypogonadism due to TAC3/TACR3 mutations: characterization of neuroendocrine phenotypes and novel mutations. PLoS ONE 2011, 10, e25614. [Google Scholar]
- Young, J.; Bouligand, J.; Francou, B.; Raffin-Sanson, M.L.; Gaillez, S.; Jeanpierre, M.; Grynberg, M.; Kamenicky, P.; Chanson, P.; Brailly-Tabard, S.; et al. TAC3 and TACR3 defects cause hypothalamic congenital hypogonadotropic hypogonadism in humans. J. Clin. Endocrinol. Metab. 2010, 95, 2287–2295. [Google Scholar] [CrossRef] [PubMed]
- Gianetti, E.; Tusset, C.; Noel, S.D.; Au, M.G.; Dwyer, A.A.; Hughes, V.A.; Abreu, A.P.; Carroll, J.; Trarbach, E.; Silveira, L.F.; et al. TAC3/TACR3 mutations reveal preferential activation of gonadotropin-releasing hormone release by neurokinin B in neonatal life followed by reversal in adulthood. J. Clin. Endocrinol. Metab. 2010, 95, 2857–2867. [Google Scholar] [CrossRef] [PubMed]
- Dissen, G.A.; Adachi, K.; Lomniczi, A.; Chatkupt, T.; Davidson, B.L.; Nakai, H.; Ojeda, S.R. Engineering a Gene Silencing Viral Construct that Targets the Cat Hypothalamus to Induce Permanent Sterility: An Update. Reprod. Domest. Anim. 2017, 52, 354–358. [Google Scholar] [CrossRef] [PubMed]


| Gene | Phenotype | Inheritance | Ref. |
|---|---|---|---|
| CHD7 | Hypogonadotropic hypogonadism 5 with or without anosmia | AD | [5] |
| DAX1 | Adrenal hypoplasia, congenital | XLR | [6] |
| DUSP6 | Hypogonadotropic hypogonadism 19 with or without anosmia | AD | [7] |
| FEZF1 | Hypogonadotropic hypogonadism 22 with or without anosmia | AR | [8] |
| FGF8 | Hypogonadotropic hypogonadism 6 with or without anosmia | AD | [9] |
| FGF17 | Hypogonadotropic hypogonadism 20 with or without anosmia | AD | [7] |
| FGFR1 | Hypogonadotropic hypogonadism 2 with or without anosmia | AD | [10] |
| FLRT3 | Hypogonadotropic hypogonadism 21 with anosmia | AD | [7] |
| FSHB | Hypogonadotropic hypogonadism 24 without anosmia | AR | [11] |
| GNRH1 | Hypogonadotropic hypogonadism 12 with or without anosmia | AR | [12] |
| GNRHR | Hypogonadotropic hypogonadism 7 without anosmia | AR | [13] |
| HESX-1 | Growth hormone deficiency with pituitary anomalies | AR, AD | [14] |
| HS6ST1 | Hypogonadotropic hypogonadism 15 with or without anosmia | AD | [7] |
| IL17RD | Hypogonadotropic hypogonadism 18 with or without anosmia | AR, AD | [7] |
| KAL1 | Hypogonadotropic hypogonadism 1 with or without anosmia | XLR | [15] |
| KISS1 | Hypogonadotropic hypogonadism 13 with or without anosmia | AR | [16] |
| KISS1R | Hypogonadotropic hypogonadism 8 with or without anosmia | AR | [17] |
| LEP | Obesity, morbid, due to leptin deficiency | AR | [18] |
| LEPR | Obesity, morbid, due to leptin deficiency | AR | [18] |
| LHB | Hypogonadotropic hypogonadism 23 with or without anosmia | AR | [19] |
| LHX3 | Pituitary hormone deficiency, combined, 3 | AR | [20] |
| NELF | Hypogonadotropic hypogonadism 9 with or without anosmia | AD | [21] |
| OTUD4 | Gordon Holmes syndrome | AR | [22] |
| PNPLA6 | Boucher–Neuhauser Syndrome | AR | [23] |
| POLR3B | hypogonadotropic hypogonadism | AR | [24] |
| PROK2 | Hypogonadotropic hypogonadism 4 with or without anosmia | AD | [25] |
| PROKR2 | Hypogonadotropic hypogonadism 3 with or without anosmia | AD | [25] |
| PROP-1 | Pituitary hormone deficiency, combined, 2 | AR | [26] |
| RAB18 | Warburg micro syndrome 3 | AR | [27] |
| RAB3GAP1 | Warburg micro syndrome 1 | AR | [27] |
| RAB3GAP2 | Warburg micro syndrome 2 | AR | [27] |
| RNF216 | Cerebellar ataxia and hypogonadotropic hypogonadism | AR | [28] |
| SEMA3A | Hypogonadotropic hypogonadism 16 with or without anosmia | AD | [29] |
| SOX2 | Abnormalities of the central nervous system | AD | [30] |
| SPRY4 | Hypogonadotropic hypogonadism 17 with or without anosmia | AD | [7] |
| STUB1 | Spinocerebellar ataxia, autosomal recessive 16 | AR | [31] |
| TAC3 | Hypogonadotropic hypogonadism 10 with or without anosmia | AR | [32] |
| TACR3 | Hypogonadotropic hypogonadism 11 with or without anosmia | AR | [32] |
| TBC1D20 | Warburg micro syndrome 4 | AR | [33] |
| WDR11 | Hypogonadotropic hypogonadism 14 with or without anosmia | AD | [34] |
| Filtering Step | Homozygous Variants | Heterozygous Variant |
|---|---|---|
| Private variants | 25,355 | 209,967 |
| Protein-changing private variants | 111 | 756 |
| Private variants in known candidate genes | 1 | 0 |
| Cats | G/G | G/A | A/A |
|---|---|---|---|
| Cases (n = 1) | 0 | 0 | 1 |
| Control cats (n = 171) | 169 | 2 | 0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hug, P.; Kern, P.; Jagannathan, V.; Leeb, T. A TAC3 Missense Variant in a Domestic Shorthair Cat with Testicular Hypoplasia and Persistent Primary Dentition. Genes 2019, 10, 806. https://doi.org/10.3390/genes10100806
Hug P, Kern P, Jagannathan V, Leeb T. A TAC3 Missense Variant in a Domestic Shorthair Cat with Testicular Hypoplasia and Persistent Primary Dentition. Genes. 2019; 10(10):806. https://doi.org/10.3390/genes10100806
Chicago/Turabian StyleHug, Petra, Patricia Kern, Vidhya Jagannathan, and Tosso Leeb. 2019. "A TAC3 Missense Variant in a Domestic Shorthair Cat with Testicular Hypoplasia and Persistent Primary Dentition" Genes 10, no. 10: 806. https://doi.org/10.3390/genes10100806





