Telomere Length Changes during Critical Illness: A Prospective, Observational Study
Abstract
:1. Introduction
2. Materials and Methods
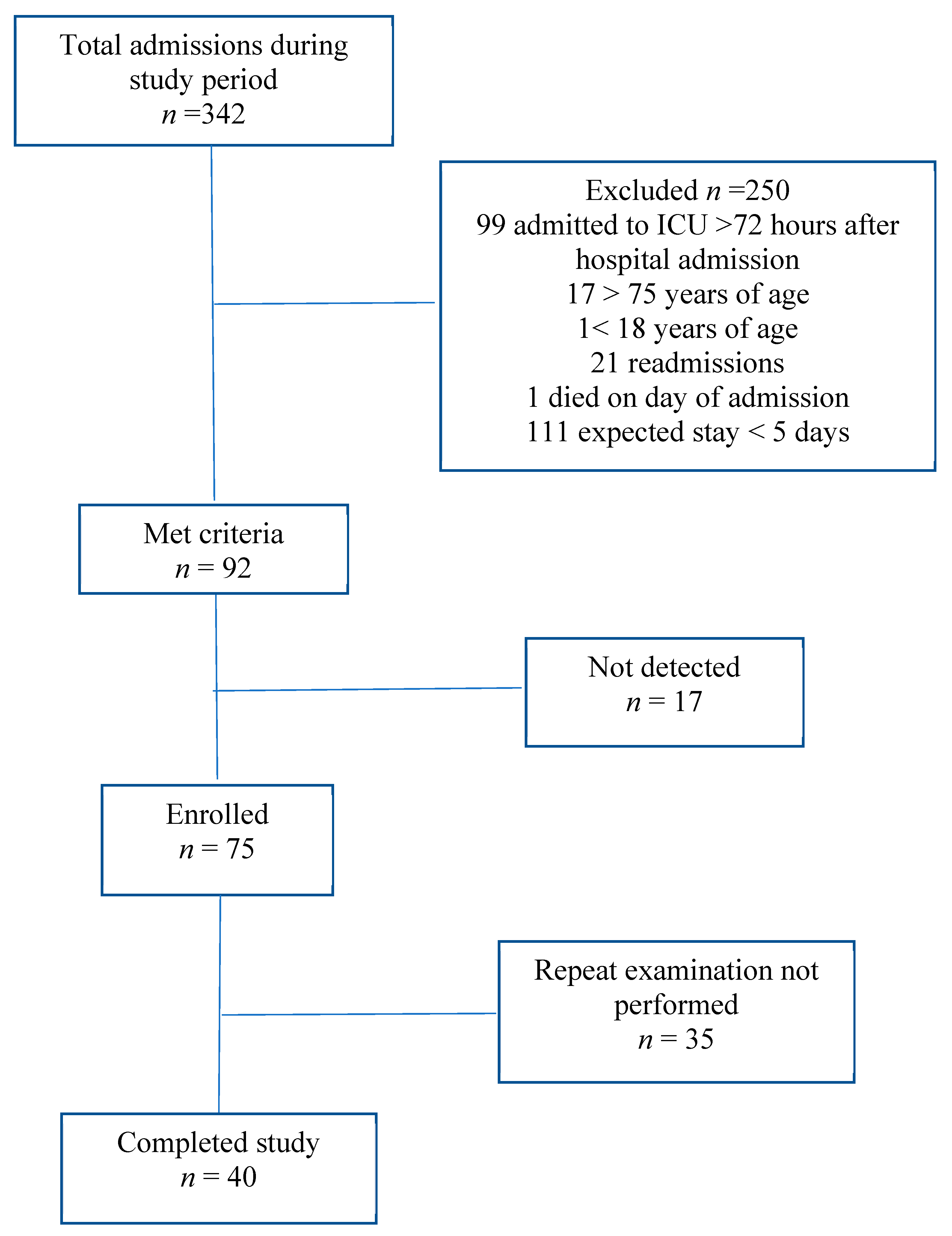
2.1. Study Population
2.2. Data Collection
2.3. Telomere Length Analysis
2.4. Statistical Analysis
3. Results
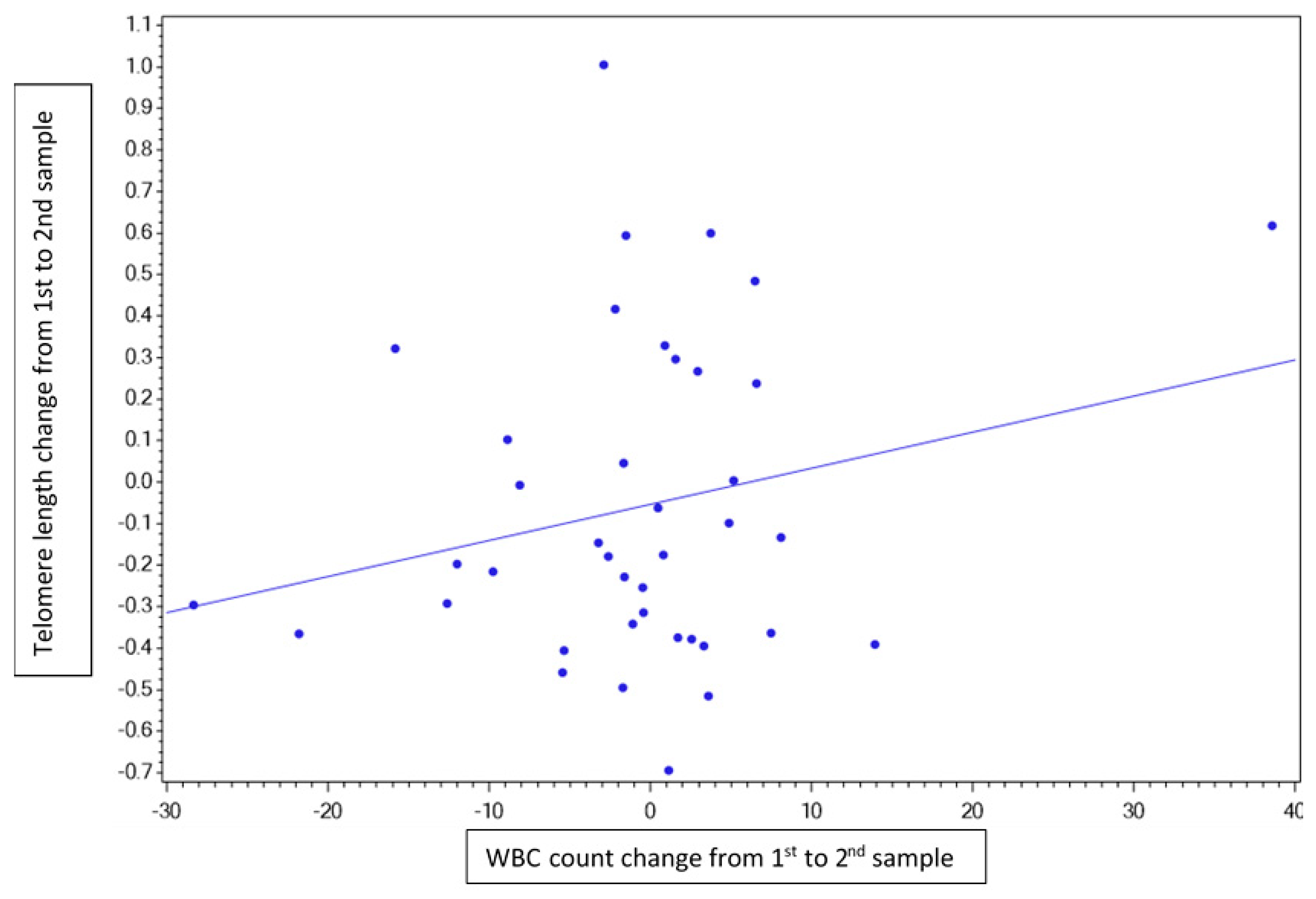
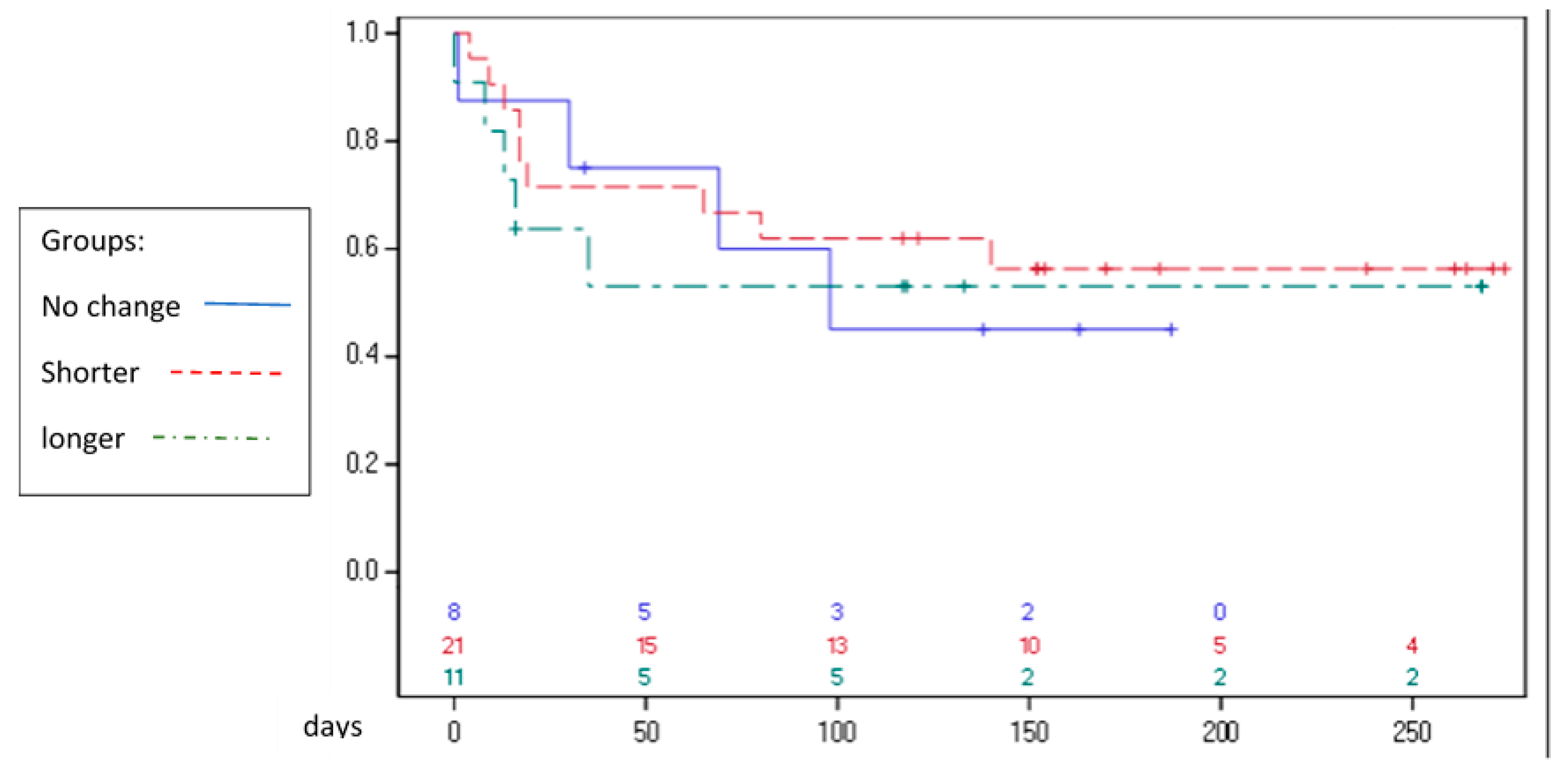
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Explanation |
| ICU | intensive care unit |
| PCR | polymerase chain reaction |
| SIRS | systemic inflammatory response syndrome |
| DNA | deoxyribonucleic acid |
| CRP | c reactive protein |
| WBC | white blood cells |
| SOFA | sequential organ failure assessment |
| APACHE | Acute Physiology and Chronic Health Evaluation |
| RBC | red blood cells |
| USA | United States of America |
| SD | standard deviation |
| BMI | body mass index |
| COPD | chronic obstructive pulmonary disease |
| CVA | cerebrovascular accident |
| CPR | cardiopulmonary resuscitation |
| GI | gastrointestinal |
| PLT | platelets |
| FA | Fanconi anemia |
| UVA | ultraviolet A |
| ALT | alternative lengthening of telomeres |
References
- Darville, T.; Jacobs, R.; Giroir, B. The systemic inflammatory response syndrome (SIRS): Immunology and potential immunotherapy. Infection 1993, 21, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Shankar-Hari, M.; Phillips, G.S.; Levy, M.L.; Levy, M.; Seymour, C.W.; Liu, V.X.; Deutschman, C.S.; Angus, D.C.; Rubenfeld, G.D.; Singer, M. Developing a New Definition and Assessing New Clinical Criteria for Septic Shock. JAMA 2016, 315, 775. [Google Scholar] [CrossRef] [PubMed]
- Fry, D.E.; Pearlstein, L.; Fulton, R.L.; Polk, H.C. Multiple system organ failure: The role of uncontrolled infection. Arch. Surg. 1980, 115, 136. [Google Scholar] [CrossRef] [PubMed]
- Deitch, E. Multiple Organ Failure Pathophysiology and Potential Future Therapy. Ann. Surg. 1992, 216, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Salvo, I.; de Cian, W.; Musicco, M.; Langer, M.; Piadena, R.; Wölfler, A.; Montani, C.; Magni, E. The Italian SEPSIS study: Preliminary results on the incidence and evolution of SIRS, sepsis, severe sepsis and septic shock. Intensive Care Med. 1995, 21, S244–S249. [Google Scholar] [CrossRef] [PubMed]
- Brun-Buisson, C. Incidence, risk factors, and outcome of severe sepsis and septic shock in adults. A multicenter prospective study in intensive care units. French ICU Group for Severe Sepsis. JAMA 1995, 274, 968–974. [Google Scholar] [CrossRef] [PubMed]
- Galley, H. Oxidative stress and mitochondrial dysfunction in sepsis. BJA 2011, 107, 57–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dröge, W. Free Radicals in the Physiological Control of Cell Function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef]
- Zhou, S. Telomeres and Telomerase: Methods and Protocols (Methods in Molecular Biology), 3rd ed.; Humana: New jersey, NJ, USA, 2017; pp. 1–13. [Google Scholar]
- Risques, R.; Arbeev, K.; Yashin, A.; Ukraintseva, S.V.; Martin, G.M.; Rabinovitch, P.S.; Oshima, J. Leukocyte Telomere Length Is Associated with Disability in Older, U.S. Population. J. Am. Geriatr. Soc. 2010, 58, 1289–1298. [Google Scholar] [CrossRef]
- Strub, G.; Depcrynski, A.; Elmore, L.; Holt, S.E. Recovery from stress is a function of age and telomere length. Cell Stress Chaperones 2008, 13, 475–482. [Google Scholar] [CrossRef] [Green Version]
- Adelfalk, C.; Lorenz, M.; Serra, V.; Von Zglinicki, T.; Hirsch-Kauffmann, M.; Schweiger, M. Accelerated telomere shortening in Fanconi anemia fibroblasts—A longitudinal study. FEBS Lett. 2001, 506, 22–26. [Google Scholar] [CrossRef]
- Sekoguchi, S.; Nakajima, T.; Moriguchi, M.; Jo, M.; Nishikawa, T.; Katagishi, T.; Kimura, H.; Minami, M.; Itoh, Y.; Kagawa, K.; et al. Role of cell-cycle turnover and oxidative stress in telomere shortening and cellular senescence in patients with chronic hepatitis C. J. Gastroenterol. Hepatol. 2007, 22, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Hochstrasser, T.; Marksteiner, J.; Humpel, C. Telomere length is age-dependent and reduced in monocytes of Alzheimer patients. Exp. Gerontol. 2012, 47, 160–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotrschal, A.; Ilmonen, P.; Penn, D. Stress Impacts Telomere Dynamics. Biol. Lett. 2007, 3, 128–130. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.Y.; Vivo, I.D.; Lin, X.; Fang, S.C.; Christiani, D.C. The Relationship between Inflammatory Biomarkers and Telomere Length in an Occupational Prospective Cohort Study. PLoS ONE 2014, 9, e87348. [Google Scholar] [CrossRef] [PubMed]
- Zglinicki, T.V. Oxidative stress shortens telomeres. TIBS 2002, 27, 339–344. [Google Scholar] [CrossRef]
- Verstraete, S.; Vanhorebeek, I.; Puffelen, E.V.; Derese, I.; Ingels, C.; Verbruggen, S.C.; Wouters, P.J.; Joosten, K.F.; Hanot, J.; Guerra, G.G.; et al. Leukocyte telomere length in paediatric critical illness: Effect of early parenteral nutrition. Crit. Care 2018, 22, 28. [Google Scholar] [CrossRef]
- Lustig, A.; Liu, H.B.; Metter, E.J.; An, Y.; Swaby, M.A.; Elango, P.; Ferrucci, L.; Hodes, R.J.; Weng, N.-P. Telomere Shortening, Inflammatory Cytokines, and Anti-Cytomegalovirus Antibody Follow Distinct Age-Associated Trajectories in Humans. Front. Immunol. 2017, 8, 1027. [Google Scholar] [CrossRef]
- Oliveira, N.M.; Rios, E.C. Sepsis Induces Telomere Shortening: A Potential Mechanism Responsible for Delayed Pathophysiological Events in Sepsis Survivors? Mol. Med. 2016, 22, 1. [Google Scholar] [CrossRef]
- Cawthon, R.M. Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic Acids Res. 2009, 37, e21. [Google Scholar] [CrossRef]
- Montpetit, A.J.; Alhareeri, A.A.; Montpetit, M.; Starkweather, A.R.; Elmore, L.W.; Filler, K.; Mohanraj, L.; Burton, C.W.; Menzies, V.S.; Lyon, D.E.; et al. Telomere Length: A Review of Methods for Measurement. Nurs. Res. 2014, 63, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Stewart, S.A.; Ben-Porath, I.; Carey, V.J.; O’Connor, B.F.; Hahn, W.C.; Weinberg, R.A. Erosion of the telomeric single-strand overhang at replicative senescence. Nat. Genet. 2003, 33, 492–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uziel, O.; Reshef, H.; Ravid, A.; Fabian, I.; Halperin, D.; Ram, R.; Bakhanashvili, M.; Nordenberg, J.; Lahav, M. Oxidative stress causes telomere damage in Fanconi anaemia cells—A possible predisposition for malignant transformation. Br. J. Haematol. 2008, 142, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Tchirkov, A. Role of oxidative stress in telomere shortening in cultured fibroblasts from normal individuals and patients with ataxia-telangiectasia. Hum. Mol. Genet. 2003, 12, 227–232. [Google Scholar] [CrossRef]
- Raeisi, S.; Ghorbanihaghjo, A.; Argani, H.; Dastmalchi, S.; Seifi, M.; Ghasemi, B.; Ghazizadeh, T.; Abbasi, M.M.; Karimi, P. Oxidative stress-induced renal telomere shortening as a mechanism of cyclosporine-induced nephrotoxicity. J. Biochem. Mol. Toxicol. 2018, 32, e22166. [Google Scholar] [CrossRef]
- Yokoo, S.; Furumoto, K.; Hiyama, E.; Miwa, N. Slow-down of age-dependent telomere shortening is executed in human skin keratinocytes by hormesis-like-effects of trace hydrogen peroxide or by anti-oxidative effects of pro-vitamin C in common concurrently with reduction of intracellular oxidative stress. J. Cell Biochem. 2004, 93, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Moritoh, Y.; Miwa, N. Age-dependent telomere-shortening is repressed by phosphorylated α-tocopherol together with cellular longevity and intracellular oxidative-stress reduction in human brain microvascular endotheliocytes. J. Cell Biochem. 2007, 102, 689–703. [Google Scholar] [CrossRef]
- Kawanishi, S.; Oikawa, S. Mechanism of telomere shortening by oxidative stress. Ann. N. Y. Acad. Sci. 2004, 1019, 278–284. [Google Scholar] [CrossRef]
- Von Zglinicki, T.; Pilger, R.; Sitte, N. Accumulation of single-strand breaks is the major cause of telomere shortening in human fibroblasts. Free Radic. Biol. Med. 2000, 28, 64–74. [Google Scholar] [CrossRef]
- Callen, E.; Samper, E.; Ramirez, M.J.; Creus, A.; Marcos, R.; Ortega, J.J.; Olivé, T.; Badell, I.; Blasco, M.A.; Surrallés, J. Breaks at telomeres and TRF2-independent end fusions in Fanconi anemia. Hum. Mol. Genet. 2002, 11, 439–444. [Google Scholar] [CrossRef]
- Diker-Cohen, T.; Uziel, O.; Szyper-Kravitz, M.; Shapira, H.; Natur, A.; Lahav, M. The effect of chemotherapy on telomere dynamics: Clinical results and possible mechanisms. Leuk. Lymphoma 2013, 54, 2023–2029. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | n = 40 |
|---|---|
| Age (years) | 50.6 (±18.8) |
| BMI (kg/m2) | 28 (±6.9) |
| Gender (male), n (%) | 31 (78%) |
| APACHE II score | 22.8 (±6.8) |
| Ventilated on admission, n (%) | 37 (93%) |
| WBC count on admission (K/mcL) | 12 (±5.7) |
| Platelets count on admission (K/mcL) | 190 (±96) |
| CRP on admission (mg/dL) | 14.7 (±12.6) |
| Main diagnosis | |
| Sepsis | 8 (20%) |
| Respiratory | 5 (12.5%) |
| Trauma | 14 (35%) |
| Organ Transplant | 3 (7.5%) |
| Other | 10 (25%) |
| Medical (renal failure, COPD, sepsis, CVA, encephalitis, liver failure, hyponatremia, post-CPR) | 22 (55%) |
| Surgical (trauma, upper GI bleeding, liver transplant, bowel perforation, necrotizing fasciitis) | 18 (45%) |
| Patient Number | T/S Ratio of First Sampling | T/S Ratio of Second Sampling | Delta between First and Second Samplings |
|---|---|---|---|
| 22 | 1 | 0.109 | −0.891 |
| 24 | 1 | 1.855 | 0.855 |
| 25 | 1 | 0.305 | −0.695 |
| 26 | 1 | 0.746 | −0.254 |
| 27 | 1 | 0.505 | −0.495 |
| 28 | 1 | 0.605 | −0.395 |
| 29 | 1 | 1.485 | 0.485 |
| 30 | 1 | 1.237 | 0.237 |
| 32 | 1 | 0.704 | -0.296 |
| 34 | 1 | 0.707 | −0.293 |
| 35 | 1 | 1.618 | 0.618 |
| 37 | 1 | 0.785 | −0.215 |
| 38 | 1 | 1.296 | 0.296 |
| 39 | 1 | 1.416 | 0.416 |
| 41 | 1 | 0.992 | −0.008 |
| 45 | 1 | 0.626 | −0.374 |
| 46 | 1 | 1.329 | 0.329 |
| 47 | 1 | 2.005 | 1.005 |
| 49 | 1 | 0.658 | −0.342 |
| 50 | 1 | 1.6 | 0.6 |
| 51 | 1 | 0.902 | −0.098 |
| 52 | 1 | 0.803 | −0.197 |
| 53 | 1 | 0.937 | −0.063 |
| 55 | 1 | 0.541 | −0.459 |
| 56 | 1 | 0.608 | −0.392 |
| 57 | 1 | 1.103 | 0.103 |
| 59 | 1 | 0.854 | −0.146 |
| 60 | 1 | 0.82 | −0.18 |
| 61 | 1 | 1.046 | 0.046 |
| 62 | 1 | 1.003 | 0.003 |
| 64 | 1 | 0.634 | −0.366 |
| 65 | 1 | 0.867 | −0.133 |
| 66 | 1 | 0.636 | −0.364 |
| 68 | 1 | 0.622 | −0.378 |
| 70 | 1 | 0.772 | −0.228 |
| 71 | 1 | 0.594 | −0.406 |
| 72 | 1 | 1.321 | 0.321 |
| 73 | 1 | 0.484 | −0.516 |
| 74 | 1 | 1.5943 | 0.5943 |
| 75 | 1 | 0.824 | −0.176 |
| Patient Characteristic | Delta Telomere Length (>15% Baseline) | |||
|---|---|---|---|---|
| Shorter | Unchanged | Longer | p-Value | |
| Number of patients | 21 | 8 | 11 | |
| Age (years ± SD) | 45.38 *(±20.84) | 49.50 (±15.63) | 61.45 (±13.53) | 0.0712 |
| BMI (kg/m2 ± SD) | 29.04 (±7.98) | 28.35 (±8.12) | 25.74 (±2.98) | 0.4523 |
| Male/Female | 18/3 | 6/2 | 7/4 | 0.35813 |
| APACHE-II score (±SD) | 21 (±8) | 24 (±5) | 25 (±7) | 0.2562 |
| SOFA score | 9 (±3) | 10 (±3) | 11 (±4) | 0.3959 |
| WBC count (K/mcL ± SD) | 12.2 (±6.5) | 11.6 (±5) | 11.8 (±5.3) | 0.9557 |
| Platelet count (K/mcL ± SD) | 203 (±94) | 201 (±131) | 156 (±76) | 0.4128 |
| CRP | 16 (±13.3) | 15.5 (±12.4) | 11.6 (±13.1) | 0.7066 |
| Albumin | 3 (±0.9) | 2.8 (±0.9) | 3 (±0.6) | 0.8334 |
| pH | 7.3 (±0.11) | 7.25 (±0.12) | 7.31 (±0.11) | 0.4361 |
| Ventilated on admission | 21/21 | 7/8 | 9/11 | 0.14956 |
| Admission diagnosis | 0.48791 | |||
| trauma | 9 | 2 | 3 | |
| sepsis | 6 | 1 | 1 | |
| respiratory | 3 | 1 | 1 | |
| transplant | 1 | 1 | 1 | |
| other | 2 | 3 | 5 | |
| Medical/Surgical | 10/11 | 5/3 | 7/4 | 0.61399 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zribi, B.; Uziel, O.; Lahav, M.; Mesilati Stahy, R.; Singer, P. Telomere Length Changes during Critical Illness: A Prospective, Observational Study. Genes 2019, 10, 761. https://doi.org/10.3390/genes10100761
Zribi B, Uziel O, Lahav M, Mesilati Stahy R, Singer P. Telomere Length Changes during Critical Illness: A Prospective, Observational Study. Genes. 2019; 10(10):761. https://doi.org/10.3390/genes10100761
Chicago/Turabian StyleZribi, Benjamin, Orit Uziel, Meir Lahav, Ronit Mesilati Stahy, and Pierre Singer. 2019. "Telomere Length Changes during Critical Illness: A Prospective, Observational Study" Genes 10, no. 10: 761. https://doi.org/10.3390/genes10100761





