Endothelial Basement Membrane Components and Their Products, Matrikines: Active Drivers of Pulmonary Hypertension?
Abstract
:1. Introduction
2. General Function of BM Components on the Vascular Endothelium
2.1. BM Type IV Collagens
2.2. BM-Laminin
2.3. BM-Nidogens
2.4. BM Heparan Sulfate Proteoglycans: Perlecan, Type XVIII Collagen α1, and Agrin
3. BM Matrikines
Regulation of EC Function by Matrikines
4. BM Components and Their Matrikines in PH
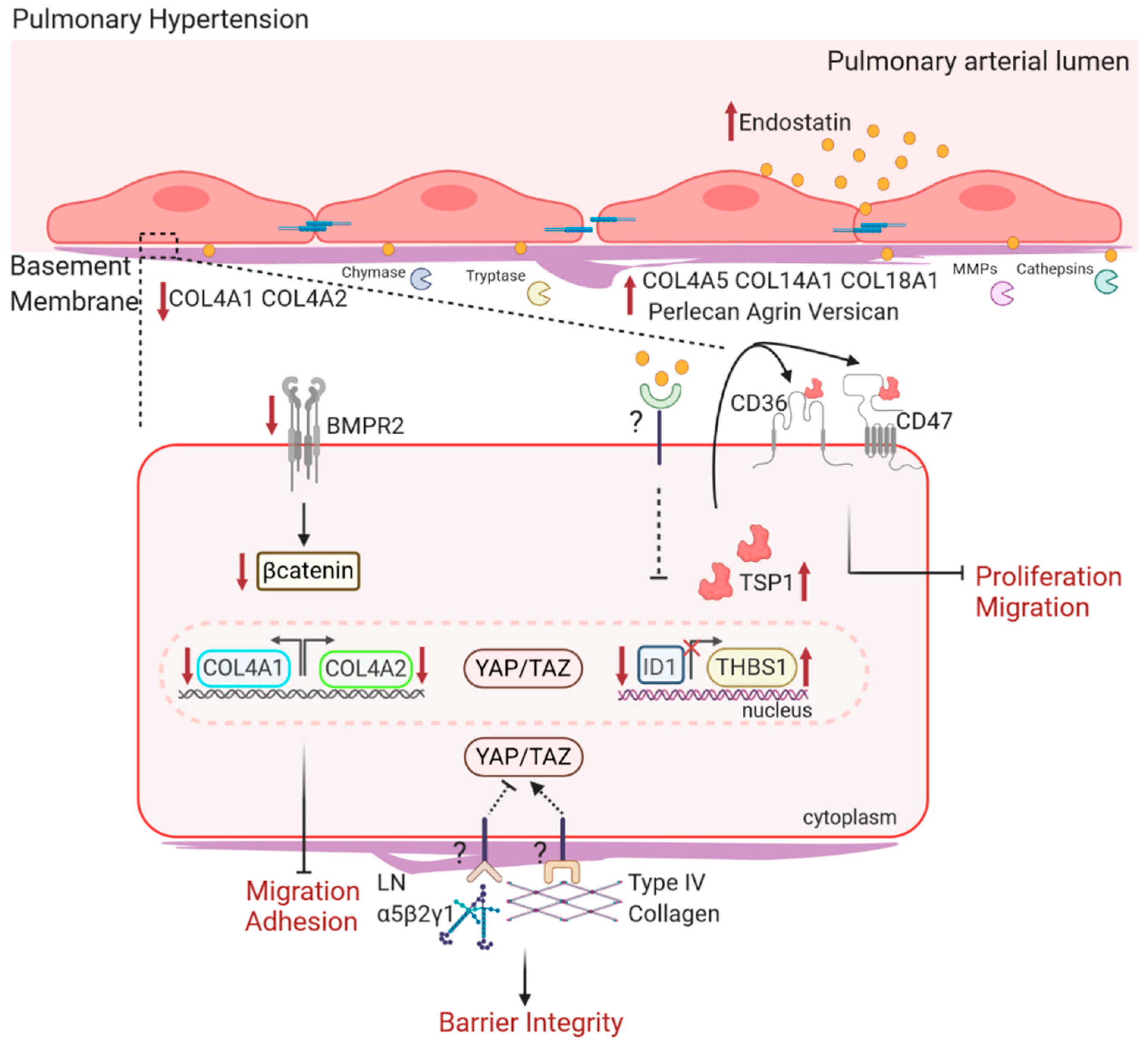
4.1. Changes in BM Ultrastructure and Composition in PH
4.2. BM Components on EC Function in PH
4.2.1. Type IV Collagen and Laminin in PH
4.2.2. Type XVIII Collagen and Endostatin in PH
4.2.3. BM HSPGs in PH
4.2.4. Inflammation in BM Degradation in PH
5. Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tuder, R.M.; Archer, S.L.; Dorfmüller, P.; Erzurum, S.C.; Guignabert, C.; Michelakis, E.; Rabinovitch, M.; Schermuly, R.; Stenmark, K.R.; Morrell, N.W. Relevant issues in the pathology and pathobiology of pulmonary hypertension. J. Am. Coll. Cardiol. 2013, 62, D4–D12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuder, R.M. Pulmonary vascular remodeling in pulmonary hypertension. Cell Tissue Res. 2017, 367, 643–649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guignabert, C.; Dorfmüller, P. Pathology and Pathobiology of Pulmonary Hypertension. Semin. Respir. Crit. Care Med. 2017, 38, 571–584. [Google Scholar] [CrossRef] [PubMed]
- Jandl, K.; Marsh, L.M.; Hoffmann, J.; Mutgan, A.C.; Baum, O.; Bloch, W.; Thekkekara-Puthenparampil, H.; Kolb, D.; Sinn, K.; Klepetko, W.; et al. Basement Membrane Remodelling Controls Endothelial Function in IPAH. Am. J. Respir. Cell Mol. Biol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, J.; Marsh, L.M.; Pieper, M.; Stacher, E.; Ghanim, B.; Kovacs, G.; König, P.; Wilkens, H.; Michael Haitchi, H.; Hoefler, G.; et al. Compartment-specific expression of collagens and their processing enzymes in intrapulmonary arteries of IPAH patients. Am. J. Physiol Lung Cell Mol. Physiol. 2015, 308, 1002–1013. [Google Scholar] [CrossRef]
- Biasin, V.; Crnkovic, S.; Sahu-Osen, A.; Birnhuber, A.; El Agha, E.; Sinn, K.; Klepetko, W.; Olschewski, A.; Bellusci, S.; Marsh, L.M.; et al. PDGFRα and αSMA mark two distinct mesenchymal cell populations involved in parenchymal and vascular remodeling in pulmonary fibrosis. Am. J. Physiol. Cell. Mol. Physiol. 2020, 318, L684–L697. [Google Scholar] [CrossRef]
- Takahashi, M. The role of endothelin-1 in vascular remodeling in vivo. Cardiovasc. Res. 2006, 71, 4–5. [Google Scholar] [CrossRef] [Green Version]
- Qi, Y.X.; Jiang, J.; Jiang, X.H.; Wang, X.D.; Ji, S.Y.; Han, Y.; Long, D.K.; Shen, B.R.; Yan, Z.Q.; Chien, S.; et al. PDGF-BB and TGF-β1 on cross-talk between endothelial and smooth muscle cells in vascular remodeling induced by low shear stress. Proc. Natl. Acad. Sci. USA 2011, 108, 1908–1913. [Google Scholar] [CrossRef] [Green Version]
- Biasin, V.; Chwalek, K.; Wilhelm, J.; Best, J.; Marsh, L.M.; Ghanim, B.; Klepetko, W.; Fink, L.; Schermuly, R.T.; Weissmann, N.; et al. Endothelin-1 driven proliferation of pulmonary arterial smooth muscle cells is c-fos dependent. Int. J. Biochem. Cell Biol. 2014, 54, 137–148. [Google Scholar] [CrossRef]
- Vestweber, D. How leukocytes cross the vascular endothelium. Nat. Rev. Immunol. 2015, 15, 692–704. [Google Scholar] [CrossRef]
- Ranchoux, B.; Harvey, L.D.; Ayon, R.J.; Babicheva, A.; Bonnet, S.; Chan, S.Y.; Yuan, J.X.J.; Perez, V.D.J. Endothelial dysfunction in pulmonary arterial hypertension: An evolving landscape (2017 Grover Conference Series). Pulm. Circ. 2018, 8, 2045893217752912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanhoutte, P.M.; Shimokawa, H.; Feletou, M.; Tang, E.H.C. Endothelial dysfunction and vascular disease—A 30th anniversary update. Acta Physiol. 2017, 219, 22–96. [Google Scholar] [CrossRef] [PubMed]
- Huertas, A.; Guignabert, C.; Barberà, J.A.; Bärtsch, P.; Bhattacharya, J.; Bhattacharya, S.; Bonsignore, M.R.; Dewachter, L.; Dinh-Xuan, A.T.; Dorfmüller, P.; et al. Pulmonary vascular endothelium: The orchestra conductor in respiratory diseases. Eur. Respir. J. 2018, 51. [Google Scholar] [CrossRef] [PubMed]
- Seimetz, M.; Parajuli, N.; Pichl, A.; Veit, F.; Kwapiszewska, G.; Weisel, F.C.; Milger, K.; Egemnazarov, B.; Turowska, A.; Fuchs, B.; et al. Inducible NOS inhibition reverses tobacco-smoke-induced emphysema and pulmonary hypertension in mice. Cell 2011, 147, 293–305. [Google Scholar] [CrossRef] [Green Version]
- Pozzi, A.; Yurchenco, P.D.; Iozzo, R.V. The nature and biology of basement membranes. Matrix Biol. 2017, 57–58, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Schlie-Wolter, S.; Ngezahayo, A.; Chichkov, B.N. The selective role of ECM components on cell adhesion, morphology, proliferation and communication in vitro. Exp. Cell Res. 2013, 319, 1553–1561. [Google Scholar] [CrossRef]
- Randles, M.J.; Lausecker, F.; Humphries, J.D.; Byron, A.; Clark, S.J.; Miner, J.H.; Zent, R.; Humphries, M.J.; Lennon, R. Basement membrane ligands initiate distinct signalling networks to direct cell shape. Matrix Biol. 2020. [Google Scholar] [CrossRef]
- Sarrazin, S.; Lamanna, W.C.; Esko, J.D. Heparan sulfate proteoglycans. Cold Spring Harb. Perspect. Biol. 2011, 3, 1–33. [Google Scholar] [CrossRef] [Green Version]
- Jayadev, R.; Sherwood, D.R. Basement membranes. Curr. Biol. 2017, 27, R207–R211. [Google Scholar] [CrossRef] [Green Version]
- Nurcombe, V.; Ford, M.D.; Wildschut, J.A.; Bartlett, P.F. Developmental regulation of neural response to FGF-1 and FGF-2 by heparan sulfate proteoglycan. Science 1993, 260, 103–106. [Google Scholar] [CrossRef]
- Rider, C.C.; Mulloy, B. Heparin, heparan sulphate and the TGF—Cytokine superfamily. Molecules 2017, 22, 713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitsi, M.; Hong, Z.; Costello, C.E.; Nugent, M.A. Heparin-mediated conformational changes in fibronectin expose vascular endothelial growth factor binding sites. Biochemistry 2006, 45, 10319–10328. [Google Scholar] [CrossRef] [PubMed]
- Ricard-Blum, S.; Salza, R. Matricryptins and matrikines: Biologically active fragments of the extracellular matrix. Exp. Dermatol. 2014, 23, 457–463. [Google Scholar] [CrossRef]
- Johnson, P.C.; Brendel, K.; Meezan, E. Thickened cerebral cortical capillary basement membranes in diabetes. Arch. Pathol. Lab. Med. 1982, 106, 214–217. [Google Scholar] [PubMed]
- Nielsen, S.H.; Tengryd, C.; Edsfeldt, A.; Brix, S.; Genovese, F.; Bengtsson, E.; Karsdal, M.; Leeming, D.J.; Nilsson, J.; Goncalves, I. Markers of basement membrane remodeling are associated with higher mortality in patients with known atherosclerosis. J. Am. Heart Assoc. 2018, 7, e009193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomsen, M.S.; Routhe, L.J.; Moos, T. The vascular basement membrane in the healthy and pathological brain. J. Cereb. Blood Flow Metab. 2017, 37, 3300–3317. [Google Scholar] [CrossRef]
- Zatz, R.; Brenner, B.M. Pathogenesis of diabetic microangiopathy. The hemodynamic view. Am. J. Med. 1986, 80, 443–453. [Google Scholar] [CrossRef]
- To, M.; Goz, A.; Camenzind, L.; Oertle, P.; Candiello, J.; Sullivan, M.; Henrich, P.B.; Loparic, M.; Safi, F.; Eller, A.; et al. Diabetes-induced morphological, biomechanical, and compositional changes in ocular basement membranes. Exp. Eye Res. 2013, 116, 298–307. [Google Scholar] [CrossRef] [Green Version]
- Lopes Pinheiro, M.A.; Kooij, G.; Mizee, M.R.; Kamermans, A.; Enzmann, G.; Lyck, R.; Schwaninger, M.; Engelhardt, B.; de Vries, H.E. Immune cell trafficking across the barriers of the central nervous system in multiple sclerosis and stroke. Biochim. Biophys. Acta Mol. Basis Dis. 2016, 1862, 461–471. [Google Scholar] [CrossRef]
- Brassart-Pasco, S.; Brézillon, S.; Brassart, B.; Ramont, L.; Oudart, J.B.; Monboisse, J.C. Tumor Microenvironment: Extracellular Matrix Alterations Influence Tumor Progression. Front. Oncol. 2020, 10, 397. [Google Scholar] [CrossRef] [Green Version]
- Xue, L.; Chen, H.; Zhang, T.; Chen, J.; Geng, Z.; Zhao, Y. Changes in serum vascular endothelial growth factor and endostatin concentrations associated with circulating endothelial progenitor cells after acute ischemic stroke. Metab. Brain Dis. 2017, 32, 641–648. [Google Scholar] [CrossRef]
- Brassart, B.; Da Silva, J.; Donet, M.; Seurat, E.; Hague, F.; Terryn, C.; Velard, F.; Michel, J.; Ouadid-Ahidouch, H.; Monboisse, J.C.; et al. Tumour cell blebbing and extracellular vesicle shedding: Key role of matrikines and ribosomal protein SA. Br. J. Cancer 2019, 120, 453–465. [Google Scholar] [CrossRef] [Green Version]
- Hope, C.; Emmerich, P.B.; Papadas, A.; Pagenkopf, A.; Matkowskyj, K.A.; Van De Hey, D.R.; Payne, S.N.; Clipson, L.; Callander, N.S.; Hematti, P.; et al. Versican-Derived Matrikines Regulate Batf3–Dendritic Cell Differentiation and Promote T Cell Infiltration in Colorectal Cancer. J. Immunol. 2017, 199, 1933–1941. [Google Scholar] [CrossRef]
- Szarvas, T.; László, V.; Vom Dorp, F.; Reis, H.; Szendröi, A.; Romics, I.; Tilki, D.; Rübben, H.; Ergün, S. Serum endostatin levels correlate with enhanced extracellular matrix degradation and poor patients’ prognosis in bladder cancer. Int. J. Cancer 2012, 130, 2922–2929. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, A.C.; Östgren, C.J.; Länne, T.; Larsson, A.; Nystrom, F.H.; Ärnlöv, J. The association between endostatin and kidney disease and mortality in patients with type 2 diabetes. Diabetes Metab. 2016, 42, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Kantola, T.; Väyrynen, J.P.; Klintrup, K.; Mäkelä, J.; Karppinen, S.M.; Pihlajaniemi, T.; Autio-Harmainen, H.; Karttunen, T.J.; Mäkinen, M.J.; Tuomisto, A. Serum endostatin levels are elevated in colorectal cancer and correlate with invasion and systemic inflammatory markers. Br. J. Cancer 2014, 111, 1605–1613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.H.; Zhu, Z.T.; Xiao, X.Y.; Sun, J. Correlation of serum levels of endostatin with tumor stage in gastric cancer: A systematic review and meta-analysis. Biomed. Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Qian, S.; Zhang, R.; Guo, D.; Wang, A.; Peng, Y.; Peng, H.; Li, Q.; Ju, Z.; Geng, D.; et al. Endostatin as a novel prognostic biomarker in acute ischemic stroke. Atherosclerosis 2020, 293, 42–48. [Google Scholar] [CrossRef]
- Qian, S.; Li, R.; Zhang, C.; Zhang, R.; Guo, D.; Bu, X.; Wang, A.; Peng, H.; Chen, J.; Zhang, Y.; et al. Plasma Endostatin Levels at Acute Phase of Ischemic Stroke Are Associated with Post-Stroke Cognitive Impairment. Neurotox. Res. 2020, 37, 956–964. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, H.S. Serial changes of serum endostatin and angiopoietin-1 levels in preterm infants with severe bronchopulmonary dysplasia and subsequent pulmonary artery hypertension. Neonatology 2014, 106, 55–61. [Google Scholar] [CrossRef]
- Damico, R.; Kolb, T.M.; Valera, L.; Wang, L.; Housten, T.; Tedford, R.J.; Kass, D.A.; Rafaels, N.; Gao, L.; Barnes, K.C.; et al. Serum endostatin is a genetically determined predictor of survival in pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2015, 191, 208–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glentis, A.; Gurchenkov, V.; Vignjevic, D.M. Assembly, heterogeneity, and breaching of the basement membranes. Cell Adh. Migr. 2014, 8, 236–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naba, A.; Clauser, K.R.; Ding, H.; Whittaker, C.A.; Carr, S.A.; Hynes, R.O. The extracellular matrix: Tools and insights for the omics era. Matrix Biol. 2016, 49, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Hohenester, E. Structural biology of laminins. Essays Biochem. 2019, 63, 285–295. [Google Scholar]
- Fujiwara, S.; Shinkai, H.; Deutzmann, R.; Paulsson, M.; Timpl, R. Structure and distribution of N-linked oligosaccharide chains on various domains of mouse tumour laminin. Biochem. J. 1988, 252, 453–461. [Google Scholar] [CrossRef] [Green Version]
- Basak, T.; Vega-Montoto, L.; Zimmerman, L.J.; Tabb, D.L.; Hudson, B.G.; Vanacore, R.M. Comprehensive Characterization of Glycosylation and Hydroxylation of Basement Membrane Collagen IV by High-Resolution Mass Spectrometry. J. Proteome Res. 2016, 15, 245–258. [Google Scholar] [CrossRef] [Green Version]
- Khoshnoodi, J.; Pedchenko, V.; Hudson, B.G. Mammalian collagen IV. Microsc. Res. Tech. 2008, 71, 357–370. [Google Scholar] [CrossRef] [Green Version]
- Parkin, J.D.; San Antonio, J.D.; Pedchenko, V.; Hudson, B.; Jensen, S.T.; Savige, J. Mapping structural landmarks, ligand binding sites, and missense mutations to the collagen IV heterotrimers predicts major functional domains, novel interactions, and variation in phenotypes in inherited diseases affecting basement membranes. Hum. Mutat. 2011, 32, 127–143. [Google Scholar] [CrossRef] [Green Version]
- Khoshnoodi, J.; Cartailler, J.P.; Alvares, K.; Veis, A.; Hudson, B.G. Molecular recognition in the assembly of collagens: Terminal noncollagenous domains are key recognition modules in the formation of triple helical protomers. J. Biol. Chem. 2006, 281, 38117–38121. [Google Scholar] [CrossRef] [Green Version]
- Pöschl, E.; Schlötzer-Schrehardt, U.; Brachvogel, B.; Saito, K.; Ninomiya, Y.; Mayer, U. Collagen IV is essential for basement membrane stability but dispensable for initiation of its assembly during early development. Development 2004, 131, 1619–1628. [Google Scholar] [CrossRef] [Green Version]
- Kuo, D.S.; Labelle-Dumais, C.; Gould, D.B. Col4a1 and col4a2 mutations and disease: Insights into pathogenic mechanisms and potential therapeutic targets. Hum. Mol. Genet. 2012, 21, R97–R110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gould, D.B.; Phalan, F.C.; Breedveld, G.J.; Van Mil, S.E.; Smith, R.S.; Schimenti, J.C.; Aguglia, U.; Van Der Knaap, M.S.; Heutink, P.; John, S.W.M. Mutations in Col4a1 cause perinatal cerebral hemorrhage and porencephaly. Science 2005, 308, 1167–1171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gould, D.B.; Phalan, F.C.; Van Mil, S.E.; Sundberg, J.P.; Vahedi, K.; Massin, P.; Bousser, M.G.; Heutink, P.; Miner, J.H.; Tournier-Lasserve, E.; et al. Role of COL4A1 in small-vessel disease and hemorrhagic stroke. N. Engl. J. Med. 2006, 354, 1489–1496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hudson, B.G.; Tryggvason, K.; Sundaramoorthy, M.; Neilson, E.G. Alport’s syndrome, Goodpasture’s syndrome, and type IV collagen. N. Engl. J. Med. 2003, 348, 2543–2556. [Google Scholar] [CrossRef]
- Gunwar, S.; Ballester, F.; Noelken, M.E.; Sado, Y.; Ninomiya, Y.; Hudson, B.G. Glomerular basement membrane. Identification of a novel disulfide- cross-linked network of α3, α4, and α5 chains of type IV collagen and its implications for the pathogenesis of Alport syndrome. J. Biol. Chem. 1998, 273, 8767–8775. [Google Scholar] [CrossRef] [Green Version]
- Pedchenko, V.; Zent, R.; Hudson, B.G. αvβ3 and αvβ 5 integrins bind both the proximal RGD site and non-RGD motifs within noncollagenous (NC1) domain of the α3 chain of type IV collagen: Implication for the mechanism of endothelial cell adhesion. J. Biol. Chem. 2004, 279, 2772–2780. [Google Scholar] [CrossRef] [Green Version]
- Smyth, N.; Vatansever, S.H.; Murray, P.; Meyer, M.; Frie, C.; Paulsson, M.; Edgar, D. Absence of basement membranes after targeting the LAMC1 gene results in embryonic lethality due to failure of endoderm differentiation. J. Cell Biol. 1999, 144, 151–160. [Google Scholar] [CrossRef] [Green Version]
- Thyboll, J.; Kortesmaa, J.; Cao, R.; Soininen, R.; Wang, L.; Iivanainen, A.; Sorokin, L.; Risling, M.; Cao, Y.; Tryggvason, K. Deletion of the Laminin 4 Chain Leads to Impaired Microvessel Maturation. Mol. Cell. Biol. 2002, 22, 1194–1202. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Gross, J.M. Laminin β1 and γ1 containing laminins are essential for basement membrane integrity in the zebrafish eye. Investig. Ophthalmol. Vis. Sci. 2007, 48, 2483–2490. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, N.M.; Senior, R.M. Laminin isoforms and lung development: All isoforms are not equal. Dev. Biol. 2006, 294, 271–279. [Google Scholar] [CrossRef] [Green Version]
- Di Russo, J.; Hannocks, M.J.; Luik, A.L.; Song, J.; Zhang, X.; Yousif, L.; Aspite, G.; Hallmann, R.; Sorokin, L. Vascular laminins in physiology and pathology. Matrix Biol. 2017, 57, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Sorokin, L.M.; Pausch, F.; Frieser, M.; Kröger, S.; Ohage, E.; Deutzmann, R. Developmental regulation of the laminin chain suggests a role in epithelial and endothelial cell maturation. Dev. Biol. 1997, 189, 285–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durbeej, M.; Fecker, L.; Hjalt, T.; Zhang, H.Y.; Salmivirta, K.; Klein, G.; Timpl, R.; Sorokin, L.; Ebendal, T.; Ekblom, P.; et al. Expression of laminin alpha 1, alpha 5 and beta 2 chains during embryogenesis of the kidney and vasculature. Matrix Biol. 1996, 15, 397–413. [Google Scholar] [CrossRef]
- Frieser, M.; Nöckel, H.; Pausch, F.; Röder, C.; Hahn, A.; Deutzmann, R.; Sorokin, L.M. Cloning of the mouse laminin α4 cDNA. Expression in a subset of endothelium. Eur. J. Biochem. 1997, 246, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Pierce, R.A.; Griffin, G.L.; Miner, J.H.; Senior, R.M. Expression patterns of laminin α1 and α5 in human lung during development. Am. J. Respir. Cell Mol. Biol. 2000, 23, 742–747. [Google Scholar] [CrossRef]
- Wu, C.; Ivars, F.; Anderson, P.; Hallmann, R.; Vestweber, D.; Nilsson, P.; Robenek, H.; Tryggvason, K.; Song, J.; Korpos, E.; et al. Endothelial basement membrane laminin α5 selectively inhibits T lymphocyte extravasation into the brain. Nat. Med. 2009, 15, 519–527. [Google Scholar] [CrossRef]
- Sixt, M.; Engelhardt, B.; Pausch, F.; Hallmann, R.; Wendler, O.; Sorokin, L.M. Endothelial cell laminin isoforms, laminins 8 and 10, play decisive roles in T cell recruitment across the blood-brain barrier in experimental autoimmune encephalomyelitis. J. Cell Biol. 2001, 153, 933–945. [Google Scholar] [CrossRef]
- Song, J.; Zhang, X.; Buscher, K.; Wang, Y.; Wang, H.; Di Russo, J.; Li, L.; Lütke-Enking, S.; Zarbock, A.; Stadtmann, A.; et al. Endothelial Basement Membrane Laminin 511 Contributes to Endothelial Junctional Tightness and Thereby Inhibits Leukocyte Transmigration. Cell Rep. 2017, 18, 1256–1269. [Google Scholar] [CrossRef] [Green Version]
- Di Russo, J.; Luik, A.; Yousif, L.; Budny, S.; Oberleithner, H.; Hofschröer, V.; Klingauf, J.; Bavel, E.; Bakker, E.N.; Hellstrand, P.; et al. Endothelial basement membrane laminin 511 is essential for shear stress response. EMBO J. 2017, 36, 183–201. [Google Scholar] [CrossRef]
- Wagner, J.U.G.; Chavakis, E.; Rogg, E.M.; Muhly-Reinholz, M.; Glaser, S.F.; Günther, S.; John, D.; Bonini, F.; Zeiher, A.M.; Schaefer, L.; et al. Switch in laminin β2 to laminin β1 isoforms during aging controls endothelial cell functions-brief report. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1170–1177. [Google Scholar] [CrossRef] [Green Version]
- Fox, J.W.; Mayer, U.; Nischt, R.; Aumailley, M.; Reinhardt, D.; Wiedemann, H.; Mann, K.; Timpl, R.; Krieg, T.; Engel, J. Recombinant nidogen consists of three globular domains and mediates binding of laminin to collagen type IV. EMBO J. 1991, 10, 3137–3146. [Google Scholar] [CrossRef]
- Aumailley, M.; Battaglia, C.; Mayer, U.; Reinhardt, D.; Nischt, R.; Timpl, R.; Fox, J.W. Nidogen mediates the formation of ternary complexes of basement membrane components. Kidney Int. 1993, 43, 7–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, J.C.; Sasaki, T.; Gohring, W.; Yamada, Y.; Timpl, R. The C-terminal domain V of perlecan promotes pl integrin-mediated cell adhesion, binds heparin, nidogen and fibulin-2 and can be modified by glycosaminoglycans. Eur. J. Biochem 1997, 250, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Kohfeldt, E.; Sasaki, T.; Göhring, W.; Timpl, R. Nidogen-2: A new basement membrane protein with diverse binding properties. J. Mol. Biol. 1998, 282, 99–109. [Google Scholar] [CrossRef]
- Miosge, N.; Köther, F.; Heinemann, S.; Kohfeldt, E.; Herken, R.; Timpl, R. Ultrastructural colocalization of nidogen-1 and nidogen-2 with laminin-1 in murine kidney basement membranes. Histochem. Cell Biol. 2000, 113, 115–124. [Google Scholar] [CrossRef]
- Schymeinsky, J.; Nedbal, S.; Miosge, N.; Poschl, E.; Rao, C.; Beier, D.R.; Skarnes, W.C.; Timpl, R.; Bader, B.L. Gene Structure and Functional Analysis of the Mouse Nidogen-2 Gene: Nidogen-2 Is Not Essential for Basement Membrane Formation in Mice. Mol. Cell. Biol. 2002, 22, 6820–6830. [Google Scholar] [CrossRef] [Green Version]
- Murshed, M.; Smyth, N.; Miosge, N.; Karolat, J.; Krieg, T.; Paulsson, M.; Nischt, R. The Absence of Nidogen 1 Does Not Affect Murine Basement Membrane Formation. Mol. Cell. Biol. 2000, 20, 7007–7012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miosge, N.; Sasaki, T.; Timpl, R. Evidence of nidogen-2 compensation for nidogen-1 deficiency in transgenic mice. Matrix Biol. 2002, 21, 611–621. [Google Scholar] [CrossRef]
- Semkova, I.; Kociok, N.; Karagiannis, D.; Nischt, R.; Smyth, N.; Paulsson, M.; Strauß, O.; Joussen, A.M. Anti-angiogenic effect of the basement membrane protein nidogen-1 in a mouse model of choroidal neovascularization. Exp. Eye Res. 2014, 118, 80–88. [Google Scholar] [CrossRef]
- Mokkapati, S.; Baranowsky, A.; Mirancea, N.; Smyth, N.; Breitkreutz, D.; Nischt, R. Basement membranes in skin are differently affected by lack of nidogen 1 and 2. J. Invest. Dermatol. 2008, 128, 2259–2267. [Google Scholar] [CrossRef]
- Bader, B.L.; Smyth, N.; Nedbal, S.; Miosge, N.; Baranowsky, A.; Mokkapati, S.; Murshed, M.; Nischt, R. Compound Genetic Ablation of Nidogen 1 and 2 Causes Basement Membrane Defects and Perinatal Lethality in Mice. Mol. Cell. Biol. 2005, 25, 6846–6856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prydz, K.; Dalen, K.T. Synthesis and sorting of proteoglycans. J. Cell Sci. 2000, 113, 193–205. [Google Scholar] [PubMed]
- Moon, J.J.; Matsumoto, M.; Patel, S.; Lee, L.; Guan, J.-L.; Li, S. Role of cell surface heparan sulfate proteoglycans in endothelial cell migration and mechanotransduction. J. Cell. Physiol. 2005, 203, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Collins, L.E.; Troeberg, L. Heparan sulfate as a regulator of inflammation and immunity. J. Leukoc. Biol. 2019, 105, 81–92. [Google Scholar] [CrossRef] [Green Version]
- Lanzi, C.; Cassinelli, G. Receptor tyrosine kinases and heparan sulfate proteoglycans: Interplay providing anticancer targeting strategies and new therapeutic opportunities. Biochem. Pharmacol. 2020, 178, 114084. [Google Scholar] [CrossRef] [PubMed]
- Reine, T.M.; Kusche-Gullberg, M.; Feta, A.; Jenssen, T.; Kolset, S.O. Heparan sulfate expression is affected by inflammatory stimuli in primary human endothelial cells. Glycoconj. J. 2012, 29, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, E.; Almonte-Becerril, M.; Bloch, W.; Costell, M. Perlecan Maintains Microvessel Integrity in Vivo and Modulates Their Formation In Vitro. PLoS ONE 2013, 8, e53715. [Google Scholar] [CrossRef] [Green Version]
- Maherally, Z.; Fillmore, H.L.; Tan, S.L.; Tan, S.F.; Jassam, S.A.; Quack, F.I.; Hatherell, K.E.; Pilkington, G.J. Real-time acquisition of transendothelial electrical resistance in an all-human, in vitro, 3-dimensional, blood–brain barrier model exemplifies tight-junction integrity. FASEB J. 2018, 32, 168–182. [Google Scholar] [CrossRef] [Green Version]
- Sakai, K.; Oka, K.; Matsumoto, K.; Nakamura, T. p27 Nuclear localization and growth arrest caused by perlecan knockdown in human endothelial cells. Biochem. Biophys. Res. Commun. 2010, 392, 403–408. [Google Scholar] [CrossRef]
- Lord, M.S.; Yu, W.; Cheng, B.; Simmons, A.; Poole-Warren, L.; Whitelock, J.M. The modulation of platelet and endothelial cell adhesion to vascular graft materials by perlecan. Biomaterials 2009, 30, 4898–4906. [Google Scholar] [CrossRef]
- Halfter, W.; Dong, S.; Schurer, B.; Cole, G.J. Collagen XVIII is a basement membrane heparan sulfate proteoglycan. J. Biol. Chem. 1998, 273, 25404–25412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muragaki, Y.; Timmons, S.; Griffith, C.M.; Oh, S.P.; Fadel, B.; Quertermous, T.; Olsen, B.R. Mouse Col18a1 is expressed in a tissue-specific manner as three alternative variants and is localized in basement membrane zones. Proc. Natl. Acad. Sci. USA 1995, 92, 8763–8767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saarela, J.; Rehn, M.; Oikarinen, A.; Autio-Harmainen, H.; Pihlajaniemi, T. The short and long forms of type XVIII collagen show clear tissue specificities in their expression and location in basement membrane zones in humans. Am. J. Pathol. 1998, 153, 611–626. [Google Scholar] [CrossRef] [Green Version]
- Heljasvaara, R.; Aikio, M.; Ruotsalainen, H.; Pihlajaniemi, T. Collagen XVIII in tissue homeostasis and dysregulation—Lessons learned from model organisms and human patients. Matrix Biol. 2017, 57, 55–75. [Google Scholar] [CrossRef] [Green Version]
- Moulton, K.S.; Olsen, B.R.; Sonn, S.; Fukai, N.; Zurakowski, D.; Zeng, X. Loss of collagen XVIII enhances neovascularization and vascular permeability in atherosclerosis. Circulation 2004, 110, 1330–1336. [Google Scholar] [CrossRef] [Green Version]
- Didangelos, A.; Yin, X.; Mandal, K.; Baumert, M.; Jahangiri, M.; Mayr, M. Proteomics characterization of extracellular space components in the human aorta. Mol. Cell. Proteom. 2010, 9, 2048–2062. [Google Scholar] [CrossRef] [Green Version]
- Steiner, E.; Enzmann, G.U.; Lyck, R.; Lin, S.; Rüegg, M.A.; Kröger, S.; Engelhardt, B. The heparan sulfate proteoglycan agrin contributes to barrier properties of mouse brain endothelial cells by stabilizing adherens junctions. Cell Tissue Res. 2014, 358, 465–479. [Google Scholar] [CrossRef] [Green Version]
- Kefalides, N.A. Basement Membranes: Current Concepts of Structure and Synthesis. Dermatology 1975, 150, 4–15. [Google Scholar] [CrossRef]
- Visse, R.; Nagase, H. Matrix Metalloproteinases and Tissue Inhibitors of Metalloproteinases. Circ. Res. 2003, 92, 827–839. [Google Scholar] [CrossRef] [Green Version]
- Thenappan, T.; Chan, S.Y.; Weir, E.K. Role of extracellular matrix in the pathogenesis of pulmonary arterial hypertension. Am. J. Physiol. Circ. Physiol. 2018, 315, H1322–H1331. [Google Scholar] [CrossRef]
- O’Reilly, M.S.; Boehm, T.; Shing, Y.; Fukai, N.; Vasios, G.; Lane, W.S.; Flynn, E.; Birkhead, J.R.; Olsen, B.R.; Folkman, J. Endostatin: An endogenous inhibitor of angiogenesis and tumor growth. Cell 1997, 88, 277–285. [Google Scholar] [CrossRef] [Green Version]
- Colorado, P.C.; Torre, A.; Kamphaus, G.; Maeshima, Y.; Hopfer, H.; Takahashi, K.; Volk, R.; Zamborsky, E.D.; Herman, S.; Sarkar, P.K.; et al. Anti-Angiogenic Cues from Vascular Basement Membrane. Cancer Res. 2000, 60, 2520–2526. [Google Scholar] [PubMed]
- Mundel, T.M.; Yliniemi, A.M.; Maeshima, Y.; Sugimoto, H.; Kieran, M.; Kalluri, R. Type IV collagen α6 chain-derived noncollagenous domain 1 (α6(IV)NC1) inhibits angiogenesis and tumor growth. Int. J. Cancer 2008, 122, 1738–1744. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.M.; Gaggar, A.; Blalock, J.E. MMP generated matrikines. Matrix Biol. 2015, 44–46, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Adair-Kirk, T.L.; Atkinson, J.J.; Broekelmann, T.J.; Doi, M.; Tryggvason, K.; Miner, J.H.; Mecham, R.P.; Senior, R.M. A Site on Laminin α5, AQARSAASKVKVSMKF, Induces Inflammatory Cell Production of Matrix Metalloproteinase-9 and Chemotaxis. J. Immunol. 2003, 171, 398–406. [Google Scholar] [CrossRef] [Green Version]
- Titz, B.; Dietrich, S.; Sadowski, T.; Beck, C.; Petersen, A.; Sedlacek, R. Activity of MMP-19 inhibits capillary-like formation due to processing of nidogen-1. Cell. Mol. Life Sci. 2004, 61, 1826–1833. [Google Scholar] [CrossRef]
- Sage, J.; Leblanc-Noblesse, E.; Nizard, C.; Sasaki, T.; Schnebert, S.; Perrier, E.; Kurfurst, R.; Brömme, D.; Lalmanach, G.; Lecaille, F. Cleavage of Nidogen-1 by Cathepsin S Impairs Its Binding to Basement Membrane Partners. PLoS ONE 2012, 7, e43494. [Google Scholar] [CrossRef]
- Walker, P.D.; Kaushal, G.P.; Shah, S.V. Meprin A, the major matrix degrading enzyme in renal tubules, produces a novel nidogen fragment in vitro and in vivo. Kidney Int. 1998, 53, 1673–1680. [Google Scholar] [CrossRef] [Green Version]
- Nyberg, P.; Xie, L.; Sugimoto, H.; Colorado, P.; Sund, M.; Holthaus, K.; Sudhakar, A.; Salo, T.; Kalluri, R. Characterization of the anti-angiogenic properties of arresten, an α1β1 integrin-dependent collagen-derived tumor suppressor. Exp. Cell Res. 2008, 314, 3292–3305. [Google Scholar] [CrossRef] [Green Version]
- Sugiyama, A.; Mitsui, A.; Okada, M.; Yamawaki, H. Cathepsin S degrades arresten and canstatin in infarcted area after myocardial infarction in rats. J. Vet. Med. Sci. 2019, 81, 522–531. [Google Scholar] [CrossRef] [Green Version]
- Rebustini, I.T.; Myers, C.; Lassiter, K.S.; Surmak, A.; Szabova, L.; Holmbeck, K.; Pedchenko, V.; Hudson, B.G.; Hoffman, M.P. MT2-MMP-Dependent Release of Collagen IV NC1 Domains Regulates Submandibular Gland Branching Morphogenesis. Dev. Cell 2009, 17, 482–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamphaus, G.D.; Colorado, P.C.; Panka, D.J.; Hopfer, H.; Ramchandran, R.; Torre, A.; Maeshima, Y.; Mier, J.W.; Sukhatme, V.P.; Kalluri, R. Canstatin, a novel matrix-derived inhibitor of angiogenesis and tumor growth. J. Biol. Chem. 2000, 275, 1209–1215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petitclerc, E.; Boutaud, A.; Prestayko, A.; Xu, J.; Sado, Y.; Ninomiya, Y.; Sarras, M.P.; Hudson, B.G.; Brooks, P.C. New functions for non-collagenous domains of human collagen type IV. Novel integrin ligands inhibiting angiogenesis and tumor growth in vivo. J. Biol. Chem. 2000, 275, 8051–8061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magnon, C.; Galaup, A.; Mullan, B.; Rouffiac, V.; Bidart, J.-M.; Griscelli, F.; Opolon, P.; Perricaudet, M. Canstatin Acts on Endothelial and Tumor Cells via Mitochondrial Damage Initiated through Interaction with A v B 3 and A v B 5 Integrins. Cancer Res. 2005, 65, 4353–4361. [Google Scholar] [CrossRef] [Green Version]
- Hamano, Y.; Kalluri, R. Tumstatin, the NC1 domain of α3 chain of type IV collagen, is an endogenous inhibitor of pathological angiogenesis and suppresses tumor growth. Biochem. Biophys. Res. Commun. 2005, 333, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Hamano, Y.; Zeisberg, M.; Sugimoto, H.; Lively, J.C.; Maeshima, Y.; Yang, C.; Hynes, R.O.; Werb, Z.; Sudhakar, A.; Kalluri, R. Physiological levels of tumstatin, a fragment of collagen IV α3 chain, are generated by MMP-9 proteolysis and suppress angiogenesis via αVβ3 integrin. Cancer Cell 2003, 3, 589–601. [Google Scholar] [CrossRef] [Green Version]
- Brassart-Pasco, S.; Sénéchal, K.; Thevenard, J.; Ramont, L.; Devy, J.; Di Stefano, L.; Dupont-Deshorgue, A.; Brézillon, S.; Feru, J.; Jazeron, J.F.; et al. Tetrastatin, the NC1 domain of the α4(IV) collagen chain: A novel potent anti-tumor matrikine. PLoS ONE 2012, 7, e29587. [Google Scholar] [CrossRef]
- Karagiannis, E.D.; Popel, A.S. Identification of novel short peptides derived from the α4, α5, and α6 fibrils of type IV collagen with anti-angiogenic properties. Biochem. Biophys. Res. Commun. 2007, 354, 434–439. [Google Scholar] [CrossRef] [Green Version]
- Weckmann, M.; Moir, L.M.; Heckman, C.A.; Black, J.L.; Oliver, B.G.; Burgess, J.K. Lamstatin—A novel inhibitor of lymphangiogenesis derived from collagen IV. J. Cell. Mol. Med. 2012, 16, 3062–3073. [Google Scholar] [CrossRef]
- Karagiannis, E.D.; Popel, A.S. A systematic methodology for proteome-wide identification of peptides inhibiting the proliferation and migration of endothelial cells. Proc. Natl. Acad. Sci. USA 2008, 105, 13775–13780. [Google Scholar] [CrossRef] [Green Version]
- Mongiat, M.; Sweeney, S.M.; San Antonio, J.D.; Fu, J.; Iozzo, R.V. Endorepellin, a novel inhibitor of angiogenesis derived from the C terminus of perlecan. J. Biol. Chem. 2003, 278, 4238–4249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cailhier, J.F.; Sirois, I.; Laplante, P.; Lepage, S.; Raymond, M.A.; Brassard, N.; Prat, A.; Iozzo, R.V.; Pshezhetsky, A.V.; Hébert, M.J. Caspase-3 activation triggers extracellular cathepsin L release and endorepellin proteolysis. J. Biol. Chem. 2008, 283, 27220–27229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woodall, B.P.; Nyström, A.; Iozzo, R.A.; Eble, J.A.; Niland, S.; Krieg, T.; Eckes, B.; Pozzi, A.; Iozzo, R.V. Integrin α2β1 is the required receptor for endorepellin angiostatic activity. J. Biol. Chem. 2008, 283, 2335–2343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goyal, A.; Pal, N.; Concannon, M.; Paul, M.; Doran, M.; Poluzzi, C.; Sekiguchi, K.; Whitelock, J.M.; Neill, T.; Iozzo, R.V. Endorepellin, the angiostatic module of perlecan, interacts with both the α2β1 integrin and vascular endothelial growth factor receptor 2 (VEGFR2): A dual receptor antagonism. J. Biol. Chem. 2011, 286, 25947–25962. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, E.M.; Reed, C.C.; Bix, G.; Fu, J.; Zhang, Y.; Gopalakrishnan, B.; Greenspan, D.S.; Iozzo, R.V. BMP-1/Tolloid-like metalloproteases process endorepellin, the angiostatic C-terminal fragment of perlecan. J. Biol. Chem. 2005, 280, 7080–7087. [Google Scholar] [CrossRef] [Green Version]
- Heljasvaara, R.; Nyberg, P.; Luostarinen, J.; Parikka, M.; Heikkilä, P.; Rehn, M.; Sorsa, T.; Salo, T.; Pihlajaniemi, T. Generation of biologically active endostatin fragments from human collagen XVIII by distinct matrix metalloproteases. Exp. Cell Res. 2005, 307, 292–304. [Google Scholar] [CrossRef]
- Wen, W.; Moses, M.A.; Wiederschain, D.; Arbiser, J.L.; Folkman, J. The generation of endostatin is mediated by elastase. Cancer Res. 1999, 59, 6052–6056. [Google Scholar]
- Veillard, F.; Saidi, A.; Burden, R.E.; Scott, C.J.; Gillet, L.; Lecaille, F.; Lalmanach, G. Cysteine cathepsins S and L modulate anti-angiogenic activities of human endostatin. J. Biol. Chem. 2011, 286, 37158–37167. [Google Scholar] [CrossRef] [Green Version]
- Wickström, S.A.; Alitalo, K.; Keski-Oja, J. Endostatin associates with integrin α5β1 and caveolin-1, and activates Src via a tyrosyl phosphatase-dependent pathway in human endothelial cells. Cancer Res. 2002, 62, 5580–5589. [Google Scholar]
- Kim, Y.M.; Hwang, S.; Kim, Y.M.; Pyun, B.J.; Kim, T.Y.; Lee, S.T.; Gho, Y.S.; Kwon, Y.G. Endostatin blocks vascular endothelial growth factor-mediated signaling via direct interaction with KDR/Flk-1. J. Biol. Chem. 2002, 277, 27872–27879. [Google Scholar] [CrossRef] [Green Version]
- Karumanchi, S.A.; Jha, V.; Ramchandran, R.; Karihaloo, A.; Tsiokas, L.; Chan, B.; Dhanabal, M.; Hanai, J.I.; Venkataraman, G.; Shriver, Z.; et al. Cell surface glypicans are low-affinity endostatin receptors. Mol. Cell 2001, 7, 811–822. [Google Scholar] [CrossRef] [Green Version]
- Shi, H.; Huang, Y.; Zhou, H.; Song, X.; Yuan, S.; Fu, Y.; Luo, Y. Nucleolin is a receptor that mediates antiangiogenic and antitumor activity of endostatin. Blood 2007, 110, 2899–2906. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.C.; Chang, J.H.; Jain, S.; Gabison, E.E.; Kure, T.; Kato, T.; Fukai, N.; Azar, D.T. Matrilysin cleavage of corneal collagen type XVIII NC1 domain and generation of a 28-kDa fragment. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2517–2524. [Google Scholar]
- Chang, J.H.; Javier, J.A.D.; Chang, G.Y.; Oliveira, H.B.; Azar, D.T. Functional characterization of neostatins, the MMP-derived, enzymatic cleavage products of type XVIII collagen. FEBS Lett. 2005, 579, 3601–3606. [Google Scholar] [CrossRef] [Green Version]
- Stephan, A.; Mateos, J.M.; Kozlov, S.V.; Cinelli, P.; Kistler, A.D.; Hettwer, S.; Rülicke, T.; Streit, P.; Kunz, B.; Sonderegger, P. Neurotrypsin cleaves agrin locally at the synapse. FASEB J. 2008, 22, 1861–1873. [Google Scholar] [CrossRef] [Green Version]
- McCulloch, D.R.; Nelson, C.M.; Dixon, L.J.; Silver, D.L.; Wylie, J.D.; Lindner, V.; Sasaki, T.; Cooley, M.A.; Argraves, W.S.; Apte, S.S. ADAMTS Metalloproteases Generate Active Versican Fragments that Regulate Interdigital Web Regression. Dev. Cell 2009, 17, 687–698. [Google Scholar] [CrossRef] [Green Version]
- Omura, J.; Satoh, K.; Kikuchi, N.; Satoh, T.; Kurosawa, R.; Nogi, M.; Ohtsuki, T.; Al-Mamun, M.E.; Siddique, M.A.H.; Yaoita, N.; et al. ADAMTS8 Promotes the Development of Pulmonary Arterial Hypertension and Right Ventricular Failure: A Possible Novel Therapeutic Target. Circ. Res. 2019, 125, 884–906. [Google Scholar] [CrossRef] [PubMed]
- Nowak-Sliwinska, P.; Alitalo, K.; Allen, E.; Anisimov, A.; Aplin, A.C.; Auerbach, R.; Augustin, H.G.; Bates, D.O.; van Beijnum, J.R.; Bender, R.H.F.; et al. Consensus guidelines for the use and interpretation of angiogenesis assays. Angiogenesis 2018, 21, 425–532. [Google Scholar] [CrossRef] [Green Version]
- Saman, H.; Raza, S.S.; Uddin, S.; Rasul, K. Inducing angiogenesis, a key step in cancer vascularization, and treatment approaches. Cancers 2020, 12, 1172. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [Green Version]
- Monboisse, J.C.; Oudart, J.B.; Ramont, L.; Brassart-Pasco, S.; Maquart, F.X. Matrikines from basement membrane collagens: A new anti-cancer strategy. Biochim. Biophys. Acta Gen. Subj. 2014, 1840, 2589–2598. [Google Scholar] [CrossRef] [PubMed]
- Smadja, D.M.; Nunes, H.; Juvin, K.; Bertil, S.; Valeyre, D.; Gaussem, P.; Israel-Biet, D. Increase in both angiogenic and angiostatic mediators in patients with idiopathic pulmonary fibrosis. Pathol. Biol. 2014, 62, 391–394. [Google Scholar] [CrossRef] [PubMed]
- García-Lucio, J.; Argemi, G.; Tura-Ceide, O.; Diez, M.; Paul, T.; Bonjoch, C.; Coll-Bonfill, N.; Blanco, I.; Barberà, J.A.; Musri, M.M.; et al. Gene expression profile of angiogenic factors in pulmonary arteries in COPD: Relationship with vascular remodeling. Am. J. Physiol. Lung Cell Mol. Physiol. 2016, 310, 583–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyoshi, T.; Hirohata, S.; Ogawa, H.; Doi, M.; Obika, M.; Yonezawa, T.; Sado, Y.; Kusachi, S.; Kyo, S.; Kondo, S.; et al. Tumor-specific expression of the RGD-α3(IV)NC1 domain suppresses endothelial tube formation and tumor growth in mice. FASEB J. 2006, 20, 1904–1906. [Google Scholar] [CrossRef]
- Boosani, C.S.; Nalabothula, N.; Munugalavadla, V.; Cosgrove, D.; Keshamoun, V.G.; Sheibani, N.; Sudhakar, A. FAK and p38-MAP kinase-dependent activation of apoptosis and caspase-3 in retinal endothelial cells by α1(IV)NC1. Investig. Ophthalmol. Vis. Sci. 2009, 50, 4567–4575. [Google Scholar] [CrossRef]
- Hwang-Bo, J.; Yoo, K.H.; Park, J.H.; Jeong, H.S.; Chung, I.S. Recombinant canstatin inhibits angiopoietin-1-induced angiogenesis and lymphangiogenesis. Int. J. Cancer 2012, 131, 298–309. [Google Scholar] [CrossRef]
- Koskimaki, J.E.; Karagiannis, E.D.; Rosca, E.V.; Vesuna, F.; Winnard, P.T.; Raman, V.; Bhujwalla, Z.M.; Popel, A.S. Peptides derived from type IV collagen, CXC chemokines, and thrombospondin-1 domain-containing proteins inhibit neovascularization and suppress tumor growth in MDA-MB-231 breast cancer xenografts. Neoplasia 2009, 11, 1285–1291. [Google Scholar] [CrossRef] [Green Version]
- Koskimaki, J.E.; Karagiannis, E.D.; Tang, B.C.; Hammers, H.; Watkins, D.N.; Pili, R.; Popel, A.S. Pentastatin-1, a collagen IV derived 20-mer peptide, suppresses tumor growth in a small cell lung cancer xenograft model. BMC Cancer 2010, 10, 29. [Google Scholar] [CrossRef] [Green Version]
- Ergün, S.; Kilic, N.; Wurmbach, J.H.; Ebrahimnejad, A.; Fernando, M.; Sevinc, S.; Kilic, E.; Chalajour, F.; Fiedler, W.; Lauke, H.; et al. Endostatin inhibits angiogenesis by stabilization of newly formed endothelial tubes. Angiogenesis 2001, 4, 193–206. [Google Scholar] [CrossRef]
- Goyal, A.; Poluzzi, C.; Willis, C.D.; Smythies, J.; Shellard, A.; Neill, T.; Iozzo, R.V. Endorepellin affects angiogenesis by antagonizing diverse vascular endothelial growth factor receptor 2 (VEGFR2)-evoked signaling pathways: Transcriptional repression of hypoxia-inducible factor 1α and VEGFA and concurrent inhibition of nuclear factor of activated T cell 1 (NFAT1) activation. J. Biol. Chem. 2012, 287, 43543–43556. [Google Scholar]
- Willis, C.D.; Poluzzi, C.; Mongiat, M.; Iozzo, R.V. Endorepellin laminin-like globular 1/2 domains bind Ig3-5 of vascular endothelial growth factor (VEGF) receptor 2 and block pro-angiogenic signaling by VEGFA in endothelial cells. FEBS J. 2013, 280, 2271–2284. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef]
- Maeshima, Y.; Sudhakar, A.; Lively, J.C.; Ueki, K.; Kharbanda, S.; Kahn, C.R.; Sonenberg, N.; Hynes, R.O.; Kalluri, R. Tumstatin, an Endothelial Cell-Specific Inhibitor of Protein Synthesis. Science 2002, 4, 140–143. [Google Scholar] [CrossRef] [Green Version]
- Panka, D.J.; Mier, J.W. Canstatin inhibits Akt activation and induces Fas-dependent apoptosis in endothelial cells. J. Biol. Chem. 2003, 278, 37632–37636. [Google Scholar] [CrossRef] [Green Version]
- Verma, R.K.; Gunda, V.; Pawar, S.C.; Sudhakar, Y.A. Extra cellular matrix derived metabolite regulates angiogenesis by FasL mediated apoptosis. PLoS ONE 2013, 8, e80555. [Google Scholar] [CrossRef]
- Zhao, X.; Guan, J.L. Focal adhesion kinase and its signaling pathways in cell migration and angiogenesis. Adv. Drug Deliv. Rev. 2011, 63, 610–615. [Google Scholar] [CrossRef] [Green Version]
- Lindqvist, L.M.; Tandoc, K.; Topisirovic, I.; Furic, L. Cross-talk between protein synthesis, energy metabolism and autophagy in cancer. Curr. Opin. Genet. Dev. 2018, 48, 104–111. [Google Scholar] [CrossRef]
- Nguyen, T.M.B.; Subramanian, I.V.; Xiao, X.; Ghosh, G.; Nguyen, P.; Kelekar, A.; Ramakrishnan, S. Endostatin induces autophagy in endothelial cells by modulating Beclin 1 and β-catenin levels. J. Cell. Mol. Med. 2009, 13, 3687–3698. [Google Scholar] [CrossRef] [Green Version]
- Poluzzi, C.; Casulli, J.; Goyal, A.; Mercer, T.J.; Neill, T.; Iozzo, R.V. Endorepellin evokes autophagy in endothelial cells. J. Biol. Chem. 2014, 289, 16114–16128. [Google Scholar] [CrossRef] [Green Version]
- Goyal, A.; Gubbiotti, M.A.; Chery, D.R.; Han, L.; Iozzo, R.V. Endorepellin-evoked autophagy contributes to angiostasis. J. Biol. Chem. 2016, 291, 19245–19256. [Google Scholar] [CrossRef] [Green Version]
- Dhanabal, M.; Ramchandran, R.; Waterman, M.J.F.; Lu, H.; Knebelmann, B.; Segal, M.; Sukhatme, V.P. Endostatin induces endothelial cell apoptosis. J. Biol. Chem. 1999, 274, 11721–11726. [Google Scholar] [CrossRef] [Green Version]
- Dixelius, J.; Larsson, H.; Sasaki, T.; Holmqvist, K.; Lu, L.; Engström, K.; Timpl, R.; Welsh, M.; Claesson-Welsh, L. Endostatin-induced tyrosine kinase signaling through the Shb adaptor protein regulates endothelial cell apoptosis. Blood 2000, 95, 3403–3411. [Google Scholar] [CrossRef]
- Wickström, S.A.; Veikkola, T.; Rehn, M.; Pihlajaniemi, T.; Alitalo, K.; Keski-Oja, J. Endostatin-induced modulation of plasminogen activation with concomitant loss of focal adhesions and actin stress fibers in cultured human endothelial cells. Cancer Res. 2001, 61, 6511–6516. [Google Scholar]
- Wickström, S.A.; Alitalo, K.; Keski-Oja, J. Endostatin associates with lipid rafts and induces reorganization of the actin cytoskeleton via down-regulation of RhoA activity. J. Biol. Chem. 2003, 278, 37895–37901. [Google Scholar] [CrossRef] [Green Version]
- Bix, G.; Fu, J.; Gonzalez, E.M.; Macro, L.; Barker, A.; Campbell, S.; Zutter, M.M.; Santoro, S.A.; Kim, J.K.; Höök, M.; et al. Endorepellin causes endothelial cell disassembly of actin cytoskeleton and focal adhesions through α2β1 integrin. J. Cell Biol. 2004, 166, 97–109. [Google Scholar] [CrossRef] [Green Version]
- Gunda, V.; Verma, R.K.; Sudhakar, Y.A. Inhibition of elastin peptide-mediated angiogenic signaling mechanism(s) in choroidal endothelial cells by the α6(IV)NC1 collagen fragment. Investig. Ophthalmol. Vis. Sci. 2013, 54, 7828–7835. [Google Scholar] [CrossRef]
- Chelladurai, P.; Seeger, W.; Pullamsetti, S.S. Matrix metalloproteinases and their inhibitors in pulmonary hypertension. Eur. Respir. J. 2012, 40, 766–782. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, J.; Wilhelm, J.; Marsh, L.M.; Ghanim, B.; Klepetko, W.; Kovacs, G.; Olschewski, H.; Olschewski, A.; Kwapiszewska, G. Distinct differences in gene expression patterns in pulmonary arteries of patients with chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis with pulmonary hypertension. Am. J. Respir. Crit. Care Med. 2014, 190, 98–111. [Google Scholar] [CrossRef]
- Bertero, T.; Cottrill, K.A.; Lu, Y.; Haeger, C.M.; Dieffenbach, P.; Annis, S.; Hale, A.; Bhat, B.; Kaimal, V.; Zhang, Y.-Y.; et al. Matrix Remodeling Promotes Pulmonary Hypertension through Feedback Mechanoactivation of the YAP/TAZ-miR-130/301 Circuit. Cell Rep. 2015, 13, 1016–1032. [Google Scholar] [CrossRef] [Green Version]
- Meyrick, B.; Clarke, S.W.; Symons, C.; Woodgate, D.J.; Reid, L. Primary pulmonary hypertension a case report including electronmicroscopic study. Br. J. Dis. Chest 1974, 68, 11–20. [Google Scholar] [CrossRef]
- Lipke, D.W.; Arcot, S.S.; Gillespie, M.N.; Olson, J.W. Temporal alterations in specific basement membrane components in lungs from monocrotaline-treated rats. Am. J. Respir. Cell Mol. Biol. 1993, 9, 418–428. [Google Scholar] [CrossRef]
- Vyas-Somani, A.C.; Aziz, S.M.; Arcot, S.A.; Gillespie, M.N.; Olson, J.W.; Lipke, D.W. Temporal Alterations in Basement Membrane Components in the Pulmonary Vasculature of the Chronically Hypoxic Rat: Impact of Hypoxia and Recovery. Am. J. Med. Sci. 1996, 312, 54–67. [Google Scholar] [CrossRef]
- Rhodes, C.J.; Im, H.; Cao, A.; Hennigs, J.K.; Wang, L.; Sa, S.; Chen, P.I.; Nickel, N.P.; Miyagawa, K.; Hopper, R.K.; et al. RNA sequencing analysis detection of a novel pathway of endothelial dysfunction in pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2015, 192, 356–366. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.T.; Chan, C.K.; Eriksson, I.; Johnson, P.Y.; Cao, X.; Westöö, C.; Norvik, C.; Andersson-Sjöland, A.; Westergren-Thorsson, G.; Johansson, S.; et al. Versican accumulates in vascular lesions in pulmonary arterial hypertension. Pulm. Circ. 2016, 6, 347–359. [Google Scholar] [CrossRef] [Green Version]
- Abdul-Salam, V.B.; Wharton, J.; Cupitt, J.; Berryman, M.; Edwards, R.J.; Wilkins, M.R. Proteomic Analysis of Lung Tissues From Patients With Pulmonary Arterial Hypertension. Circulation 2010, 122, 2058–2067. [Google Scholar] [CrossRef] [Green Version]
- De Jesus Perez, V.A.; Alastalo, T.P.; Wu, J.C.; Axelrod, J.D.; Cooke, J.P.; Amieva, M.; Rabinovitch, M. Bone morphogenetic protein 2 induces pulmonary angiogenesis via Wnt -β-catenin and Wnt—RhoA—Rac1 pathways. J. Cell Biol. 2009, 184, 83–99. [Google Scholar] [CrossRef] [Green Version]
- Goyanes, A.M.; Moldobaeva, A.; Marimoutou, M.; Varela, L.C.; Wang, L.; Johnston, L.F.; Aladdin, M.M.; Peloquin, G.L.; Kim, B.S.; Damarla, M.; et al. Functional impact of human genetic variants of COL18A1/endostatin on pulmonary endothelium. Am. J. Respir. Cell Mol. Biol. 2020, 62, 524–534. [Google Scholar] [CrossRef]
- Liu, F.; Haeger, C.M.; Dieffenbach, P.B.; Sicard, D.; Chrobak, I.; Coronata, A.M.F.; Velandia, M.M.S.; Vitali, S.; Colas, R.A.; Norris, P.C.; et al. Distal vessel stiffening is an early and pivotal mechanobiological regulator of vascular remodeling and pulmonary hypertension. JCI Insight 2016, 1, e86987. [Google Scholar] [CrossRef]
- Kudryashova, T.V.; Goncharov, D.A.; Pena, A.; Kelly, N.; Vanderpool, R.; Baust, J.; Kobir, A.; Shufesky, W.; Mora, A.L.; Morelli, A.E.; et al. HIPPO-integrin-linked kinase cross-talk controls self-sustaining proliferation and survival in pulmonary hypertension. Am. J. Respir. Crit. Care Med. 2016, 194, 866–877. [Google Scholar] [CrossRef] [Green Version]
- Spiekerkoetter, E.; Goncharova, E.A.; Guignabert, C.; Stenmark, K.; Kwapiszewska, G.; Rabinovitch, M.; Voelkel, N.; Bogaard, H.J.; Graham, B.; Pullamsetti, S.S.; et al. Hot topics in the mechanisms of pulmonary arterial hypertension disease: Cancer-like pathobiology, the role of the adventitia, systemic involvement, and right ventricular failure. Pulm. Circ. 2019, 9, 2045894019889775. [Google Scholar] [CrossRef] [Green Version]
- Soubrier, F.; Chung, W.K.; Machado, R.; Grünig, E.; Aldred, M.; Geraci, M.; Loyd, J.E.; Elliott, C.G.; Trembath, R.C.; Newman, J.H.; et al. Genetics and genomics of pulmonary arterial hypertension. J. Am. Coll. Cardiol. 2013, 62, D13–D21. [Google Scholar] [CrossRef] [Green Version]
- West, J.; Austin, E.; Fessel, J.P.; Loyd, J.; Hamid, R. Rescuing the BMPR2 signaling axis in pulmonary arterial hypertension. Drug Discov. Today 2014, 19, 1241–1245. [Google Scholar] [CrossRef] [Green Version]
- Hiepen, C.; Jatzlau, J.; Hildebrandt, S.; Kampfrath, B.; Goktas, M.; Murgai, A.; Cuellar Camacho, J.L.; Haag, R.; Ruppert, C.; Sengle, G.; et al. BMPR2 acts as a gatekeeper to protect endothelial cells from increased TGFβ responses and altered cell mechanics. PLoS Biol. 2019, 17, e3000557. [Google Scholar] [CrossRef] [Green Version]
- De Jesus Perez, V.A.; Yuan, K.; Lyuksyutova, M.A.; Dewey, F.; Orcholski, M.E.; Shuffle, E.M.; Mathur, M.; Yancy, L.; Rojas, V.; Li, C.G.; et al. Whole-exome sequencing reveals TopBP1 as a novel gene in idiopathic pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2014, 189, 1260–1272. [Google Scholar] [CrossRef] [Green Version]
- Dixelius, J.; Cross, M.; Matsumoto, T.; Sasaki, T.; Timpl, R.; Claesson-Welsh, L. Endostatin regulates endothelial cell adhesion and cytoskeletal organization. Cancer Res. 2002, 62, 1944–1947. [Google Scholar]
- Veith, C.; Marsh, L.M.; Wygrecka, M.; Rutschmann, K.; Seeger, W.; Weissmann, N.; Kwapiszewska, G. Paxillin regulates pulmonary arterial smooth muscle cell function in pulmonary hypertension. Am. J. Pathol. 2012, 181, 1621–1633. [Google Scholar] [CrossRef]
- Veith, C.; Zakrzewicz, D.; Dahal, B.K.; Bálint, Z.; Murmann, K.; Wygrecka, M.; Seeger, W.; Schermuly, R.T.; Weissmann, N.; Kwapiszewska, G. Hypoxia- or PDGF-BB-dependent paxillin tyrosine phosphorylation in pulmonary hypertension is reversed by HIF-1α depletion or imatinib treatment. Thromb. Haemost. 2014, 112, 1288–1303. [Google Scholar] [CrossRef]
- Paulin, R.; Meloche, J.; Courboulin, A.; Lambert, C.; Haromy, A.; Courchesne, A.; Bonnet, P.; Provencher, S.; Michelakis, E.D.; Bonnet, S. Targeting cell motility in pulmonary arterial hypertension. Eur. Respir. J. 2014, 43, 531–544. [Google Scholar] [CrossRef] [Green Version]
- Jandl, K.; Thekkekara Puthenparampil, H.; Marsh, L.M.; Hoffmann, J.; Wilhelm, J.; Veith, C.; Sinn, K.; Klepetko, W.; Olschewski, H.; Olschewski, A.; et al. Long non-coding RNAs influence the transcriptome in pulmonary arterial hypertension: The role of PAXIP1-AS1. J. Pathol. 2019, 247, 357–370. [Google Scholar] [CrossRef] [Green Version]
- Chettimada, S. Caveolae, caveolin-1 and cavin-1: Emerging roles in pulmonary hypertension. World J. Respirol. 2015, 5, 126. [Google Scholar] [CrossRef]
- Filippini, A.; Sica, G.; D’Alessio, A. The caveolar membrane system in endothelium: From cell signaling to vascular pathology. J. Cell. Biochem. 2018, 119, 5060–5071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuo, A.; Lee, M.Y.; Yang, K.; Gross, R.W.; Sessa, W.C. Caveolin-1 regulates lipid droplet metabolism in endothelial cells via autocrine prostacyclin–stimulated, cAMP-mediated lipolysis. J. Biol. Chem. 2018, 293, 973–983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saliez, J.; Bouzin, C.; Rath, G.; Ghisdal, P.; Desjardins, F.; Rezzani, R.; Rodella, L.F.; Vriens, J.; Nilius, B.; Feron, O.; et al. Role of caveolar compartmentation in endothelium-derived hyperpolarizing factor-mediated relaxation-ca2+ signals and gap junction function are regulated by caveolin in endothelial cells. Circulation 2008, 117, 1065–1074. [Google Scholar] [CrossRef] [Green Version]
- Achcar, R.O.D.; Demura, Y.; Rai, P.R.; Taraseviciene-Stewart, L.; Kasper, M.; Voelkel, N.F.; Cool, C.D. Loss of caveolin and heme oxygenase expression in severe pulmonary hypertension. Chest 2006, 129, 696–705. [Google Scholar] [CrossRef]
- Geraci, M.W.; Moore, M.; Gesell, T.; Yeager, M.E.; Alger, L.; Golpon, H.; Gao, B.; Loyd, J.E.; Tuder, R.M.; Voelkel, N.F. Gene expression patterns in the lungs of patients with primary pulmonary hypertension: A gene microarray analysis. Circ. Res. 2001, 88, 555–562. [Google Scholar] [CrossRef] [Green Version]
- Austin, E.D.; Ma, L.; LeDuc, C.; Rosenzweig, E.B.; Borczuk, A.; Phillips, J.A.; Palomero, T.; Sumazin, P.; Kim, H.R.; Talati, M.H.; et al. Whole exome sequencing to identify a novel gene (Caveolin-1) associated with human pulmonary arterial hypertension. Circ. Cardiovasc. Genet. 2012, 5, 336–343. [Google Scholar] [CrossRef] [Green Version]
- Ju, H.; Zou, R.; Venema, V.J.; Venema, R.C. Direct interaction of endothelial nitric-oxide synthase and caveolin-1 inhibits synthase activity. J. Biol. Chem. 1997, 272, 18522–18525. [Google Scholar] [CrossRef] [Green Version]
- Wenzel, D.; Schmidt, A.; Reimann, K.; Hescheler, J.; Pfitzer, G.; Bloch, W.; Fleischmann, B.K. Endostatin, the proteolytic fragment of collagen XVIII, induces vasorelaxation. Circ. Res. 2006, 98, 1203–1211. [Google Scholar] [CrossRef] [Green Version]
- Sunshine, S.B.; Dallabrida, S.M.; Durand, E.; Ismail, N.S.; Bazinet, L.; Birsner, A.E.; Sohn, R.; Ikeda, S.; Pu, W.T.; Kulke, M.H.; et al. Endostatin lowers blood pressure via nitric oxide and prevents hypertension associated with VEGF inhibition. Proc. Natl. Acad. Sci. USA 2012, 109, 11306–11311. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Harris, M.B.; Venema, V.J.; Venema, R.C. Endostatin induces acute endothelial nitric oxide and prostacyclin release. Biochem. Biophys. Res. Commun. 2005, 329, 873–878. [Google Scholar] [CrossRef]
- Galbiati, F.; Volonte, D.; Brown, A.M.C.; Weinstein, D.E.; Ben-Ze’ev, A.; Pestell, R.G.; Lisantia, M.P. Caveolin-1 expression inhibits Wnt/β-catenin/Lef-1 signaling by recruting β-catenin to caveolae membrane domains. J. Biol. Chem. 2000, 275, 23368–23377. [Google Scholar] [CrossRef] [Green Version]
- Hanai, J.I.; Gloy, J.; Ananth Karumanchi, S.; Kale, S.; Tang, J.; Hu, G.; Chan, B.; Ramchandran, R.; Jha, V.; Sukhatme, V.P.; et al. Endostatin is a potential inhibitor of Wnt signaling. J. Cell Biol. 2002, 158, 529–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhan, T.; Rindtorff, N.; Boutros, M. Wnt signaling in cancer. Oncogene 2017, 36, 1461–1473. [Google Scholar] [CrossRef] [PubMed]
- Laumanns, I.P.; Fink, L.; Wilhelm, J.; Wolff, J.-C.; Mitnacht-Kraus, R.; Graef-Hoechst, S.; Stein, M.M.; Bohle, R.M.; Klepetko, W.; Hoda, M.A.R.; et al. The Noncanonical WNT Pathway Is Operative in Idiopathic Pulmonary Arterial Hypertension. Am. J. Respir. Cell Mol. Biol. 2009, 40, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Talbot, C.C.; Liu, Q.; Jing, Z.C.; Damico, R.L.; Tuder, R.; Barnes, K.C.; Hassoun, P.M.; Gao, L. Identifying microRNAs targeting Wnt/β-catenin pathway in end-stage idiopathic pulmonary arterial hypertension. J. Mol. Med. 2016, 94, 875–885. [Google Scholar] [CrossRef] [PubMed]
- Murdoch, A.D.; Liu, B.; Schwarting, R.; Tuan, R.S.; Iozzo, R.V. Widespread expression of perlecan proteoglycan in basement membranes and extracellular matrices of human tissues as detected by a novel monoclonal antibody against Domain III and by in situ hybridization. J. Histochem. Cytochem. 1994, 42, 239–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zoeller, J.J.; Whitelock, J.M.; Iozzo, R.V. Perlecan regulates developmental angiogenesis by modulating the VEGF-VEGFR2 axis. Matrix Biol. 2009, 28, 284–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gotha, L.; Lim, S.Y.; Osherov, A.B.; Wolff, R.; Qiang, B.; Erlich, I.; Nili, N.; Pillarisetti, S.; Chang, Y.T.; Tran, P.K.; et al. Heparan sulfate side chains have a critical role in the inhibitory effects of perlecan on vascular smooth muscle cell response to arterial injury. Am. J. Physiol. Hear. Circ. Physiol. 2014, 307, H337–H345. [Google Scholar] [CrossRef] [Green Version]
- Tran-Lundmark, K.; Tannenberg, P.; Rauch, B.H.; Ekstrand, J.; Tran, P.K.; Hedin, U.; Kinsella, M.G. Perlecan heparan sulfate is required for the inhibition of smooth muscle cell proliferation by all-trans-retinoic acid. J. Cell. Physiol. 2015, 230, 482–487. [Google Scholar] [CrossRef]
- Lindblom, P.; Gerhardt, H.; Liebner, S.; Abramsson, A.; Enge, M.; Hellström, M.; Bäckström, G.; Fredriksson, S.; Landegren, U.; Nyström, H.C.; et al. Endothelial PDGF-B retention is required for proper investment of pericytes in the microvessel wall. Genes Dev. 2003, 17, 1835–1840. [Google Scholar] [CrossRef] [Green Version]
- Andrae, J.; Gallini, R.; Betsholtz, C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008, 22, 1276–1312. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.T.; Tseng, C.N.; Tannenberg, P.; Eriksson, L.; Yuan, K.; De Jesus Perez, V.A.; Lundberg, J.; Lengquist, M.; Botusan, I.R.; Catrina, S.B.; et al. Perlecan heparan sulfate deficiency impairs pulmonary vascular development and attenuates hypoxic pulmonary hypertension. Cardiovasc. Res. 2015, 107, 20–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nonaka, R.; Iesaki, T.; de Vega, S.; Daida, H.; Okada, T.; Sasaki, T.; Arikawa-Hirasawa, E. Perlecan deficiency causes endothelial dysfunction by reducing the expression of endothelial nitric oxide synthase. Physiol. Rep. 2015, 3, e12272. [Google Scholar] [CrossRef]
- Peng, Z. A10450 Cathepsin L Contributes to the development of pulmonary arterial hypertension via degradation of Bone Morphogenetic Protein Type II Receptor. J. Hypertens. 2018, 36, e59. [Google Scholar] [CrossRef]
- Chakraborty, S.; Njah, K.; Pobbati, A.V.; Lim, Y.B.; Raju, A.; Lakshmanan, M.; Tergaonkar, V.; Lim, C.T.; Hong, W. Agrin as a Mechanotransduction Signal Regulating YAP through the Hippo Pathway. Cell Rep. 2017, 18, 2464–2479. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.J.; La Pierre, D.P.; Wu, J.; Yee, A.J.; Yang, B.B. The interaction of versican with its binding partners. Cell Res. 2005, 15, 483–494. [Google Scholar] [CrossRef]
- Stacher, E.; Graham, B.B.; Hunt, J.M.; Gandjeva, A.; Groshong, S.D.; McLaughlin, V.V.; Jessup, M.; Grizzle, W.E.; Aldred, M.A.; Cool, C.D.; et al. Modern age pathology of pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2012, 186, 261–272. [Google Scholar] [CrossRef] [Green Version]
- Savai, R.; Pullamsetti, S.S.; Kolbe, J.; Bieniek, E.; Voswinckel, R.; Fink, L.; Scheed, A.; Ritter, C.; Dahal, B.K.; Vater, A.; et al. Immune and inflammatory cell involvement in the pathology of idiopathic pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2012, 186, 897–908. [Google Scholar] [CrossRef]
- Marsh, L.M.; Jandl, K.; Grünig, G.; Foris, V.; Bashir, M.; Ghanim, B.; Klepetko, W.; Olschewski, H.; Olschewski, A.; Kwapiszewska, G. The inflammatory cell landscape in the lungs of patients with idiopathic pulmonary arterial hypertension. Eur. Respir. J. 2018, 51. [Google Scholar] [CrossRef] [Green Version]
- Goetzl, E.J.; Banda, M.J.; Leppert, D. Matrix metalloproteinases in immunity. J. Immunol. 1996, 156, 1–4. [Google Scholar]
- Matsui, K.; Takano, Y.; Yu, Z.X.; Hi, J.E.S.; Stetler-Stevenson, W.G.; Travis, W.D.; Ferrans, V.J. Immunohistochemical study of endothelin-1 and matrix metalloproteinases in plexogenic pulmonary arteriopathy. Pathol. Res. Pract. 2002, 198, 403–412. [Google Scholar] [CrossRef]
- Lepetit, H.; Eddahibi, S.; Fadel, E.; Frisdal, E.; Munaut, C.; Noel, A.; Humbert, M.; Adnot, S.; D’Ortho, M.P.; Lafuma, C. Smooth muscle cell matrix metalloproteinases in idiopathic pulmonary arterial hypertension. Eur. Respir. J. 2005, 25, 834–842. [Google Scholar] [CrossRef]
- Arvidsson, M.; Ahmed, A.; Bouzina, H.; Rådegran, G. Matrix metalloproteinase 7 in diagnosis and differentiation of pulmonary arterial hypertension. Pulm. Circ. 2019, 9, 2045894019895414. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.-J.; Hsu, H.-C.; Ho, W.-J.; Chang, G.-J.; Pang, J.-H.S.; Chen, W.-J.; Huang, C.-C.; Lai, Y.-J. Cathepsin S promotes the development of pulmonary arterial hypertension. Am. J. Physiol. Lung Cell Mol. Physiol. 2019, 317, 1–13. [Google Scholar] [CrossRef]
- Di Girolamo, N.; Indoh, I.; Jackson, N.; Wakefield, D.; McNeil, H.P.; Yan, W.; Geczy, C.; Arm, J.P.; Tedla, N. Human Mast Cell-Derived Gelatinase B (Matrix Metalloproteinase-9) Is Regulated by Inflammatory Cytokines: Role in Cell Migration. J. Immunol. 2006, 177, 2638–2650. [Google Scholar] [CrossRef] [Green Version]
- Clair, J.M.-S.; Shi, G.P.; Sutherland, R.E.; Chapman, H.A.; Caughey, G.H.; Wolters, P.J. Cathepsins L and S are not required for activation of dipeptidyl peptidase I (cathepsin C) in mice. Biol. Chem. 2006, 387, 1143–1146. [Google Scholar]
- Kwapiszewska, G.; Markart, P.; Dahal, B.K.; Kojonazarov, B.; Marsh, L.M.; Schermuly, R.T.; Taube, C.; Meinhardt, A.; Ghofrani, H.A.; Steinhoff, M.; et al. PAR-2 inhibition reverses experimental pulmonary hypertension. Circ. Res. 2012, 110, 1179–1191. [Google Scholar] [CrossRef]
- Farha, S.; Sharp, J.; Asosingh, K.; Park, M.; Comhair, S.A.A.; Wilson Tang, W.H.; Thomas, J.; Farver, C.; Hsieh, F.; Loyd, J.E.; et al. Mast cell number, phenotype, and function in human pulmonary arterial hypertension. Pulm. Circ. 2012, 2, 220–228. [Google Scholar] [CrossRef] [Green Version]
- Kosanovic, D.; Dahal, B.K.; Peters, D.M.; Seimetz, M.; Wygrecka, M.; Hoffmann, K.; Antel, J.; Reiss, I.; Ghofrani, H.A.; Weissmann, N.; et al. Histological characterization of mast cell chymase in patients with pulmonary hypertension and chronic obstructive pulmonary disease. Pulm. Circ. 2014, 4, 128–136. [Google Scholar] [CrossRef] [Green Version]
- Johnson, J.L.; Jackson, C.L.; Angelini, G.D.; George, S.J. Activation of Matrix-Degrading Metalloproteinases by Mast Cell Proteases in Atherosclerotic Plaques. Arterioscler. Thromb. Vasc. Biol. 1998, 18, 1707–1715. [Google Scholar] [CrossRef] [Green Version]
- Hosokawa, S.; Haraguchi, G.; Sasaki, A.; Arai, H.; Muto, S.; Itai, A.; Doi, S.; Mizutani, S.; Isobe, M. Pathophysiological roles of nuclear factor kappaB (NF-kB) in pulmonary arterial hypertension: Effects of synthetic selective NF-kB inhibitor IMD-0354. Cardiovasc. Res. 2013, 99, 35–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farkas, D.; Alhussaini, A.A.; Kraskauskas, D.; Kraskauskiene, V.; Cool, C.D.; Nicolls, M.R.; Natarajan, R.; Farkas, L. Nuclear factor κB inhibition reduces lung vascular lumen obliteration in severe pulmonary hypertension in rats. Am. J. Respir. Cell Mol. Biol. 2014, 51, 413–425. [Google Scholar] [CrossRef]
- Hayden, M.S.; Ghosh, S. Regulation of NF-κB by TNF family cytokines. Semin. Immunol. 2014, 26, 253–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soon, E.; Holmes, A.M.; Treacy, C.M.; Doughty, N.J.; Southgate, L.; MacHado, R.D.; Trembath, R.C.; Jennings, S.; Barker, L.; Nicklin, P.; et al. Elevated levels of inflammatory cytokines predict survival in idiopathic and familial pulmonary arterial hypertension. Circulation 2010, 122, 920–927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
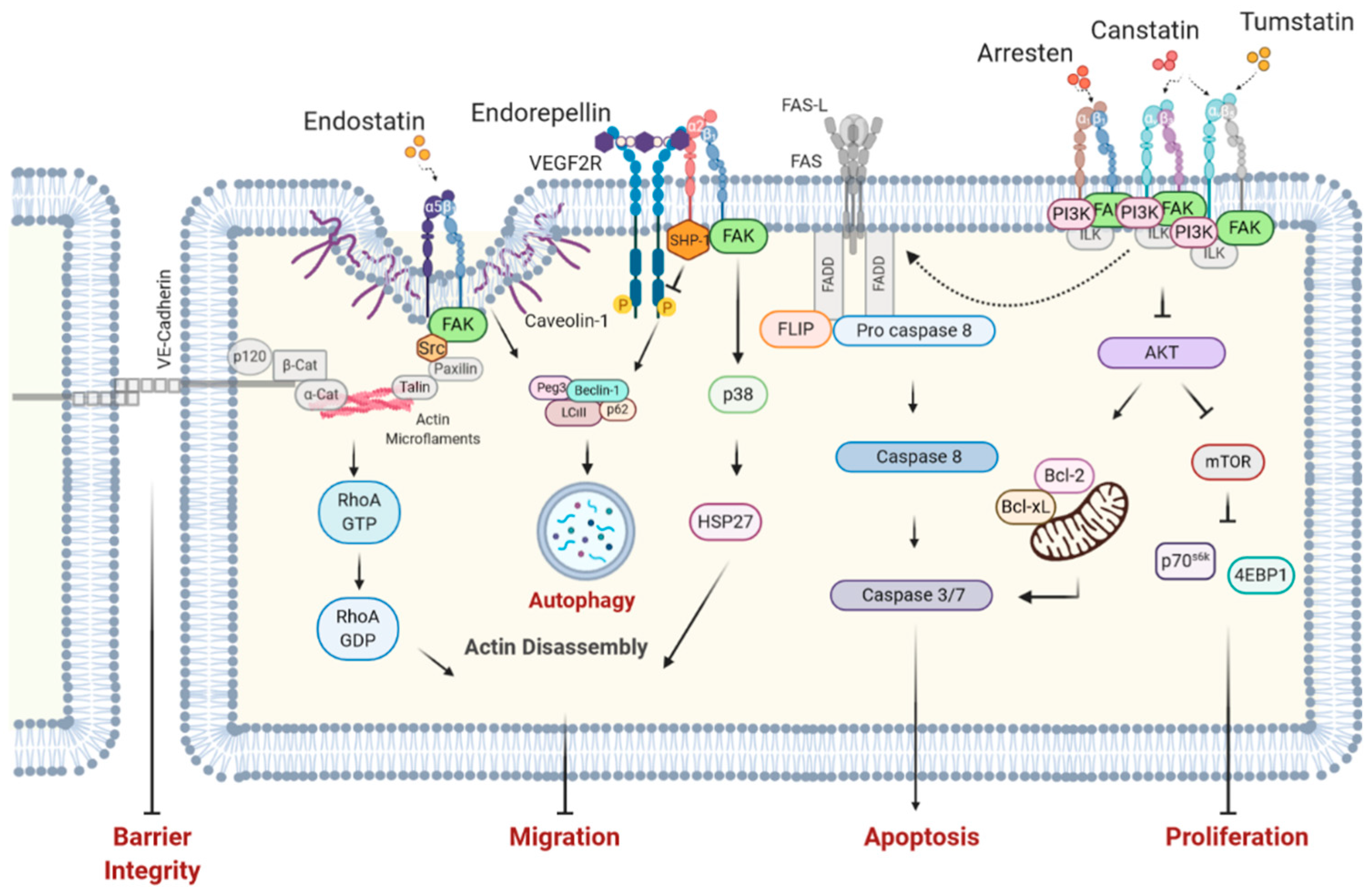
| BM Component | Function in BM |
|---|---|
| BM Glycoprotein | |
| Laminin | Assembly |
| Nidogen-1 | Assembly and Integrity |
| Nidogen-2 | Integrity |
| BM Type IV Collagens | |
| Type IV Collagen heterotrimers | Integrity and Maintenance |
| BM HSPGs | |
| Perlecan | Integrity and Stability |
| Type XVIII Collagen α1 | Integrity and Stability |
| Agrin | Integrity and Stability |
| Parent Protein | BM Matrikine | Proteolytic Enzyme | Molecular Weight (kDa) | Receptor on ECs |
|---|---|---|---|---|
| BM Glycoproteins | ||||
| Laminin α5 | AQARSAASKVKVSMKF [105] | n/a | n/a | n/a |
| Nidogen-1 | G3 Domain | MMP-19 [106] CathepsinS [107] Mephrin-α [108] | 90 | n/a |
| BM Type IV Collagens | ||||
| α1 chain | Arresten [109] | Cathepsin S [110] MMP-14 MMP-15 [111] | 26 | α1β1 integrin [102] |
| α2 chain | Canstatin [112] | Cathepsin S [110] MMP-14 MMP-15 [111] | 24 | αVβ1 integrin [113] αVβ3 αVβ5 integrins [113,114] |
| α3 chain | Tumstatin [115,116] | MMP-9 [116] | 28 | αVβ3 αVβ5 integrins [56,113] |
| α4 chain | Tetrastatin (α4(IV)NC1 domain) [117] | n/a | 28 | n/a |
| Tetrastatins [118] | n/a | ~2 | n/a | |
| α5 chain | Lamstatin (α5(IV)NC1) [119] | n/a | 25 | n/a |
| Pentastatin [118] | ~2 | β1 and β3 integrins [120] | ||
| α6 chain | α6(IV)NC 1 domain [103] | n/a | 25 | αVβ3 integrin [113] |
| Hexastatin [118] | n/a | ~2 | n/a | |
| BM HSPGs | ||||
| Perlecan | Endorepellin [121] | Cathepsin L [122] | 81 | α2β1 integrin [123,124] VEGFR2 [124] |
| Endorepellin LG3 Domain [125] | BMP1/TLD-like protease [125] Cathepsin L [122] t-PA [122] | 23 | n/a | |
| Type XVIII Collagen α1 | Endostatin (ES) [126] | Elastase [127] Cathepsin L Cathepsin B Cathepsin K Cathepsin S Cathepsin D [128] MMP-3 MMP-9 MMP-12 MMP-13 MMP-20 [126] | 20 | α5β1 integrins [129] Caveolin-1 [129] VEGFR2 [130] Glypican 1/2 [131] Nucleolin [132] |
| Neostatin 7 [133] | MMP-7 [133] | 28 | n/a | |
| Neostatin 14 [134] | MMP-14 [134] | 28 | n/a | |
| Agrin | C-Terminal Agrin Fragment [135] | Neurotrypsin [135] | 22 | n/a |
| BM CSPGs | ||||
| Versican | Versikine [136] | ADAMTS [136,137] | 49 | n/a |
| Matrikine | Anti-Angiogenic | Anti-Migratory | Anti-Proliferative | Pro-Apoptotic | Actin Disassembly |
|---|---|---|---|---|---|
| BM-Type IV Collagen | |||||
| Arresten | + | + | + | + | − |
| Canstatin | + | + | + | + | − |
| Tumstatin | + | + | + | + | − |
| α4 NC1 | − | + | + | n/a | − |
| α5 NC1 | − | + | + | n/a | − |
| α6 NC1 | + | + | + | n/a | − |
| Tetrastatin | − | + | + | n/a | − |
| Pentastatin | − | + | + | n/a | − |
| Hexastatin | − | + | + | n/a | − |
| BM HSPGs | |||||
| Endostatin | + | + | + | + | + |
| Endorepellin | + | + | + | + | + |
| Gene | Mutation Detection Method | Variant | Amino Acid [Codon] | Reference |
|---|---|---|---|---|
| COL18A1 | WGS | rs12483377 | D [GAC] > N [AAC] | [41] |
| VCAN | WES | NS | NS | [184] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mutgan, A.C.; Jandl, K.; Kwapiszewska, G. Endothelial Basement Membrane Components and Their Products, Matrikines: Active Drivers of Pulmonary Hypertension? Cells 2020, 9, 2029. https://doi.org/10.3390/cells9092029
Mutgan AC, Jandl K, Kwapiszewska G. Endothelial Basement Membrane Components and Their Products, Matrikines: Active Drivers of Pulmonary Hypertension? Cells. 2020; 9(9):2029. https://doi.org/10.3390/cells9092029
Chicago/Turabian StyleMutgan, Ayse Ceren, Katharina Jandl, and Grazyna Kwapiszewska. 2020. "Endothelial Basement Membrane Components and Their Products, Matrikines: Active Drivers of Pulmonary Hypertension?" Cells 9, no. 9: 2029. https://doi.org/10.3390/cells9092029
APA StyleMutgan, A. C., Jandl, K., & Kwapiszewska, G. (2020). Endothelial Basement Membrane Components and Their Products, Matrikines: Active Drivers of Pulmonary Hypertension? Cells, 9(9), 2029. https://doi.org/10.3390/cells9092029





