Actomyosin Contractility in the Generation and Plasticity of Axons and Dendritic Spines
Abstract
:1. Introduction
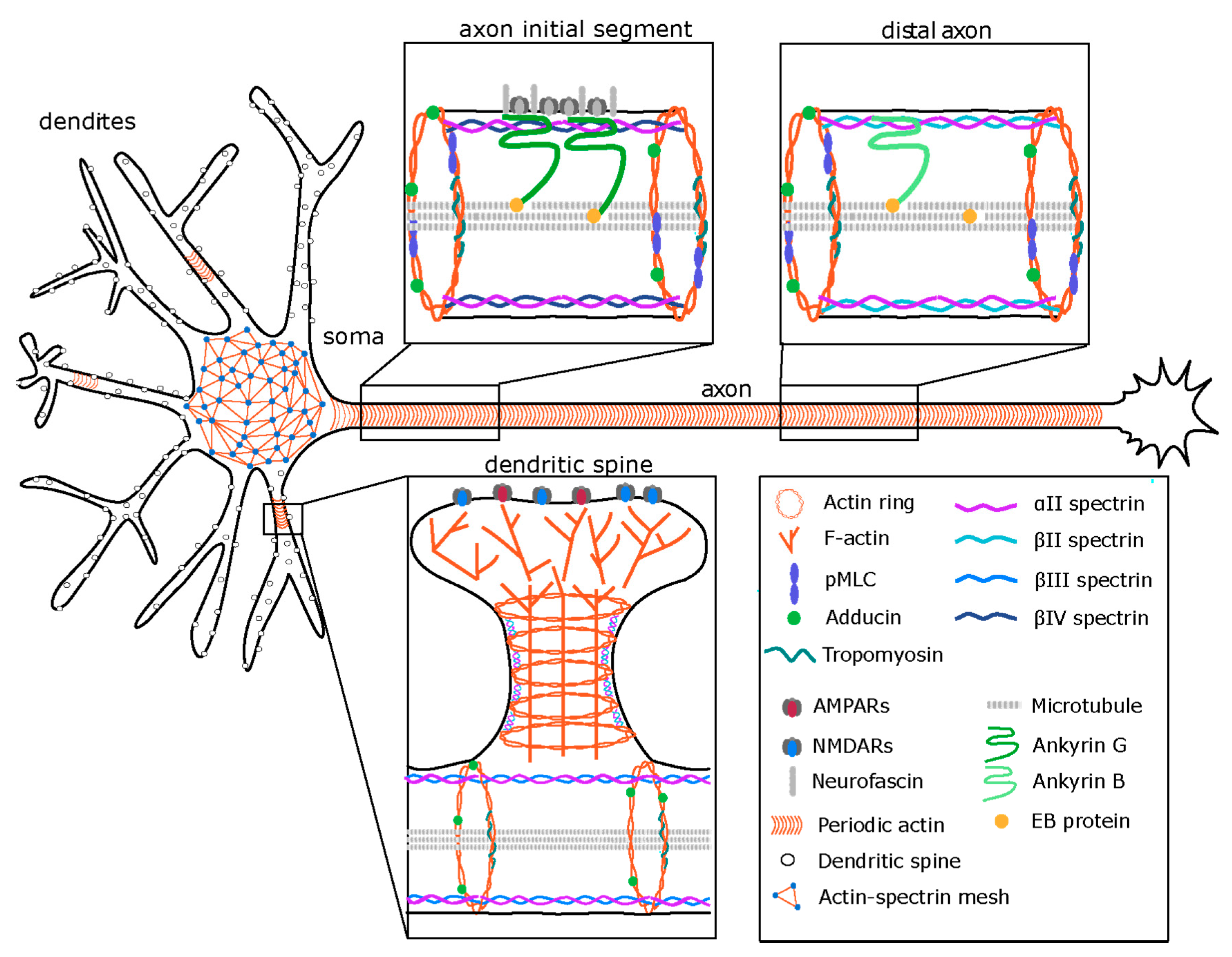
2. Neuronal Morphology and Compartmentalization
3. The Membrane-Associated Periodic Skeleton (MPS) in Neurons
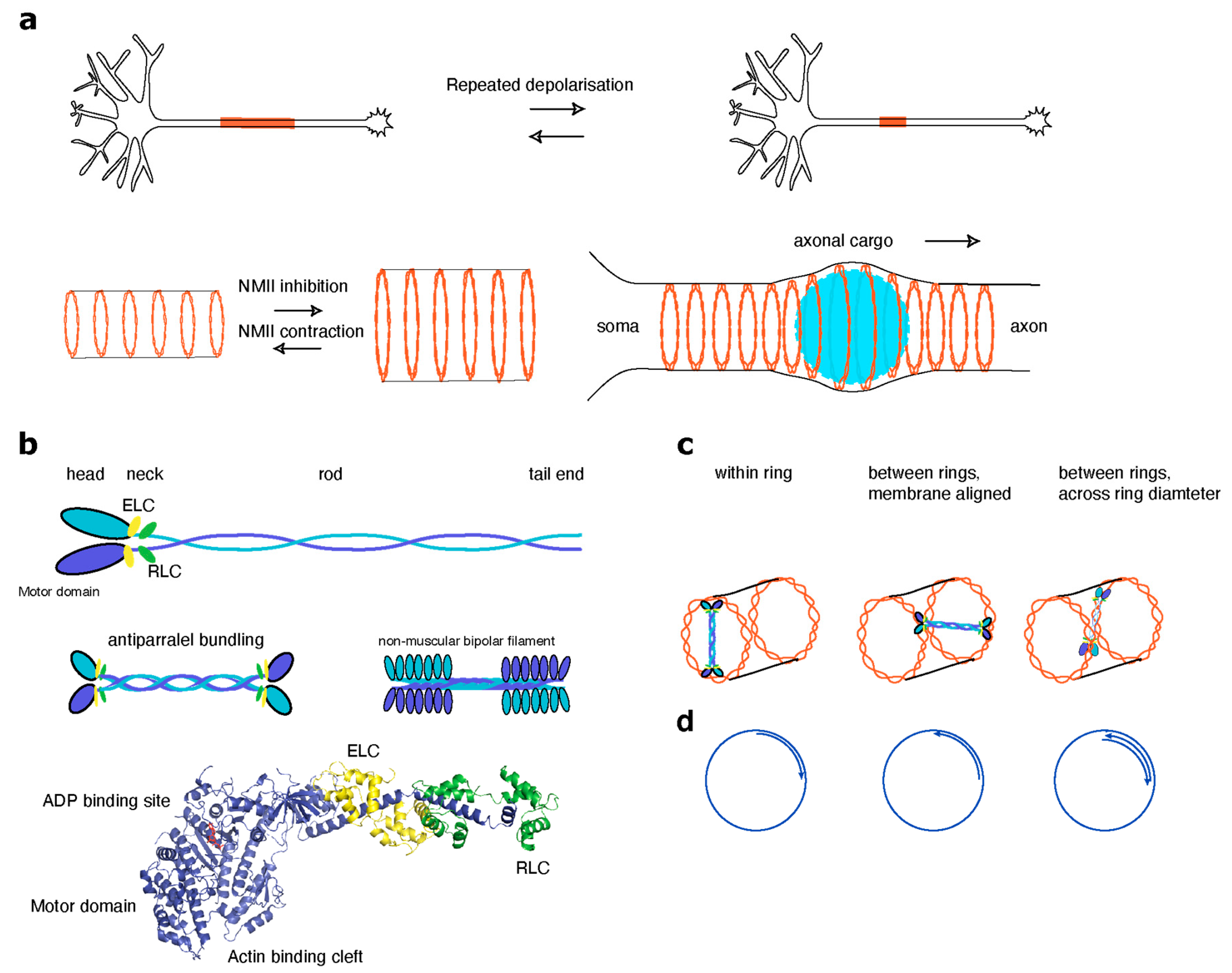
4. Regulation of the MPS
4.1. Regulation of the MPS by Calcium
4.2. Non-Muscle Myosins in Regulation of the MPS
5. Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Burnette, D.T.; Ji, L.; Schaefer, A.W.; Medeiros, N.A.; Danuser, G.; Forscher, P. Myosin II Activity Facilitates Microtubule Bundling in the Neuronal Growth Cone Neck. Dev. Cell 2008, 15, 163–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kollins, K.M.; Hy, J.; Bridgman, P.C.; Hyang, Y.Q.; Gallo, G. Myosin-II negatiwely regulates minor process extension and the temporal development of neuronal polarity. Dev. Neurobiol. 2009, 69, 279–298. [Google Scholar] [CrossRef] [Green Version]
- Yang, Q.; Zhang, X.F.; Pollard, T.D.; Forscher, P. Arp2/3 complex-dependent actin networks constrain myosin II function in driving retrograde actin flow. J. Cell Biol. 2012, 197, 939–956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medeiros, N.A.; Burnette, D.T.; Forscher, P. Myosin II functions in actin-bundle turnover in neuronal growth cones. Nat. Cell Biol. 2006, 8, 215–226. [Google Scholar] [CrossRef]
- Costa, A.C.R.; Sousa, S.C.; Pinto-Costa, R.; Mateus, J.C.; Lopes, C.D.F.; Costa, A.C.R.; Rosa, D.; Machado, D.; Pajuelo, L.; Wang, X.; et al. The membrane periodic skeleton is an actomyosin network that regulates axonal diameter and conduction. eLife 2020, 9, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Berger, S.L.; Leo-Macias, A.; Yuen, S.; Khatri, L.; Pfennig, S.; Zhang, Y.; Agullo-Pascual, E.; Caillol, G.; Zhu, M.S.; Rothenberg, E.; et al. Localized Myosin II Activity Regulates Assembly and Plasticity of the Axon Initial Segment. Neuron 2018, 97, 555–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morales, M.; Fifková, E. In situ localization of myosin and actin in dendritic spines with the immunogold technique. J. Comp. Neurol. 1989, 279, 666–674. [Google Scholar] [CrossRef]
- Ryu, J.; Liu, L.; Wong, T.P.; Wu, D.C.; Burette, A.; Weinberg, R.; Wang, Y.T.; Sheng, M. A critical role for myosin IIB in dendritic spine morphology and synaptic function. Neuron 2006, 49, 175–182. [Google Scholar] [CrossRef] [Green Version]
- Lin, W.H.; Hurley, J.T.; Raines, A.N.; Cheney, R.E.; Webb, D.J. Myosin X and its motorless isoform differentially modulate dendritic spine development by regulating trafficking and retention of vasodilator-stimulated phosphoprotein. J. Cell Sci. 2013, 126, 4756–4768. [Google Scholar] [CrossRef] [Green Version]
- Rubio, M.D.; Johnson, R.; Miller, C.A.; Huganir, R.L.; Rumbaugh, G. Regulation of synapse structure and function by distinct myosin II motors. J. Neurosci. 2011, 31, 1448–1460. [Google Scholar] [CrossRef]
- Müller, M.; Diensthuber, R.P.; Chizhov, I.; Claus, P.; Heissler, S.M.; Preller, M.; Taft, M.H.; Manstein, D.J. Distinct Functional Interactions between Actin Isoforms and Nonsarcomeric Myosins. PLoS ONE 2013, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Fox, P.; Lakonishok, M.; Davidson, M.W.; Gelfand, V.I. Initial neurite outgrowth in drosophila neurons is driven by kinesin-powered microtubule sliding. Curr. Biol. 2013, 23, 1018–1023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garvalov, B.K.; Flynn, K.C.; Neukirchen, D.; Meyn, L.; Teusch, N.; Wu, X.; Brakebusch, C.; Bamburg, J.R.; Bradke, F. Cdc42 regulates cofilin during the establishment of neuronal polarity. J. Neurosci. 2007, 27, 13117–13129. [Google Scholar] [CrossRef] [Green Version]
- Kapitein, L.C.; Hoogenraad, C.C. Building the Neuronal Microtubule Cytoskeleton. Neuron 2015, 87, 492–506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, A.H.; Chen, H.; Li, T.P.; Metzbower, S.R.; MacGillavry, H.D.; Blanpied, T.A. A trans-synaptic nanocolumn aligns neurotransmitter release to receptors. Nature 2016, 536, 210–214. [Google Scholar] [CrossRef]
- Biederer, T.; Kaeser, P.S.; Blanpied, T.A. Transcellular Nanoalignment of Synaptic Function. Neuron 2017, 96, 680–696. [Google Scholar] [CrossRef]
- Yuan, A.; Rao, M.V.; Nixon, R.A. Neurofilaments and neurofilament proteins in health and disease. Cold Spring Harb. Perspect. Biol. 2017, 9, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Suter, D.M.; Forscher, P. Substrate-cytoskeletal coupling as a mechanism for the regulation of growth cone motility and guidance. J. Neurobiol. 2000, 44, 97–113. [Google Scholar] [CrossRef]
- Geraldo, S.; Gordon-Weeks, P.R. Cytoskeletal dynamics in growth-cone steering. J. Cell Sci. 2009, 122, 3595–3604. [Google Scholar] [CrossRef] [Green Version]
- Xu, K.; Zhong, G.; Zhuang, X. Actin, Spectrin, and Associated Proteins Form a Periodic Cytoskeletal Structure in Axons. Science 2013, 339, 452–456. [Google Scholar] [CrossRef] [Green Version]
- Gao, M.; Maraspini, R.; Beutel, O.; Zehtabian, A.; Eickholt, B.; Honigmann, A.; Ewers, H. Expansion Stimulated Emission Depletion Microscopy (ExSTED). ACS Nano 2018, 12, 4178–4185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leterrier, C.; Potier, J.; Caillol, G.; Debarnot, C.; Rueda Boroni, F.; Dargent, B. Nanoscale Architecture of the Axon Initial Segment Reveals an Organized and Robust Scaffold. Cell Rep. 2015, 13, 2781–2793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Este, E.; Kamin, D.; Göttfert, F.; El-Hady, A.; Hell, S.W. STED Nanoscopy Reveals the Ubiquity of Subcortical Cytoskeleton Periodicity in Living Neurons. Cell Rep. 2015, 10, 1246–1251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bär, J.; Kobler, O.; Van Bommel, B.; Mikhaylova, M. Periodic F-actin structures shape the neck of dendritic spines. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef]
- Winterflood, C.M.; Platonova, E.; Albrecht, D.; Ewers, H. Dual-Color 3D Superresolution Microscopy by Combined Spectral-Demixing and Biplane Imaging. Biophys. J. 2015, 109, 3–6. [Google Scholar] [CrossRef] [Green Version]
- Vassilopoulos, S.; Gibaud, S.; Jimenez, A.; Caillol, G.; Leterrier, C. Ultrastructure of the axonal periodic scaffold reveals a braid-like organization of actin rings. Nat. Commun. 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Zhou, R.; Wu, Z.; Carrasco, M.A.; Kurshan, P.T.; Farley, J.E.; Simon, D.J.; Wang, G.; Han, B.; Hao, J.; et al. Prevalent presence of periodic actin-spectrin-based membrane skeleton in a broad range of neuronal cell types and animal species. Proc. Natl. Acad. Sci. USA 2016, 113, 6029–6034. [Google Scholar] [CrossRef] [Green Version]
- D’Este, E.; Kamin, D.; Velte, C.; Göttfert, F.; Simons, M.; Hell, S.W. Subcortical cytoskeleton periodicity throughout the nervous system. Sci. Rep. 2016, 6, 22741. [Google Scholar] [CrossRef] [Green Version]
- Hauser, M.; Yan, R.; Li, W.; Repina, N.A.; Schaffer, D.V.; Xu, K. The Spectrin-Actin-Based Periodic Cytoskeleton as a Conserved Nanoscale Scaffold and Ruler of the Neural Stem Cell Lineage. Cell Rep. 2018, 24, 1512–1522. [Google Scholar] [CrossRef] [Green Version]
- Han, B.; Zhou, R.; Xia, C.; Zhuang, X. Structural organization of the actin-spectrin–based membrane skeleton in dendrites and soma of neurons. Proc. Natl. Acad. Sci. USA 2017, 114, E6678–E6685. [Google Scholar] [CrossRef] [Green Version]
- Sidenstein, S.C.; D’Este, E.; Böhm, M.J.; Danzl, J.G.; Belov, V.N.; Hell, S.W. Multicolour multilevel STED nanoscopy of actin/spectrin organization at synapses. Sci. Rep. 2016, 6, 26725. [Google Scholar] [CrossRef] [Green Version]
- Hammarlund, M.; Jorgensen, E.M.; Bastiani, M.J. Axons break in animals lacking β-spectrin. J. Cell Biol. 2007, 176, 269–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubey, S.; Bhembre, N.; Bodas, S.; Veer, S.; Ghose, A.; Callan-Jones, A.; Pullarkat, P. The axonal actin-spectrin lattice acts as a tension buffering shock absorber. eLife 2020, 9, 1–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Efimova, N.; Korobova, F.; Stankewich, M.C.; Moberly, A.H.; Stolz, D.B.; Wang, J.; Kashina, A.; Ma, M. Βiii Spectrin Is Necessary for Formation of the Constricted Neck of Dendritic Spines and Regulation of Synaptic Activity in Neurons. J. Neurosci. 2017, 37, 6442–6459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshimura, T.; Rasband, M.N. Axon initial segments: Diverse and dynamic neuronal compartments. Curr. Opin. Neurobiol. 2014, 27, 96–102. [Google Scholar] [CrossRef] [Green Version]
- Rasband, M.N. The axon initial segment and the maintenance of neuronal polarity. Nat. Rev. Neurosci. 2010, 11, 552–562. [Google Scholar] [CrossRef]
- Hedstrom, K.L.; Ogawa, Y.; Rasband, M.N. AnkyrinG is required for maintenance of the axon initial segment and neuronal polarity. J. Cell Biol. 2008, 183, 635–640. [Google Scholar] [CrossRef] [Green Version]
- Sobotzik, J.M.; Sie, J.M.; Politi, C.; Del Turco, D.; Bennett, V.; Deller, T.; Schultz, C. AnkyrinG is required to maintain axo-dendritic polarity in vivo. Proc. Natl. Acad. Sci. USA 2009, 106, 17564–17569. [Google Scholar] [CrossRef] [Green Version]
- Schafer, D.P.; Jha, S.; Liu, F.; Akella, T.; McCullough, L.D.; Rasband, M.N. Disruption of the axon initial segment cytoskeleton is a new mechanism for neuronal injury. J. Neurosci. 2009, 29, 13242–13254. [Google Scholar] [CrossRef] [Green Version]
- Evans, M.D.; Sammons, R.P.; Lebron, S.; Dumitrescu, A.S.; Watkins, T.B.K.; Uebele, V.N.; Renger, J.J.; Grubb, M.S. Calcineurin signaling mediates activity-dependent relocation of the Axon Initial segment. J. Neurosci. 2013, 33, 6950–6963. [Google Scholar] [CrossRef] [Green Version]
- Kuba, H.; Oichi, Y.; Ohmori, H. Presynaptic activity regulates Na+ channel distribution at the axon initial segment. Nature 2010, 465, 1075–1078. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.D.; Tufo, C.; Dumitrescu, A.S.; Grubb, M.S. Myosin II activity is required for structural plasticity at the axon initial segment. Eur. J. Neurosci. 2017, 46, 1751–1757. [Google Scholar] [CrossRef] [Green Version]
- Zhong, G.; He, J.; Zhou, R.; Lorenzo, D.; Babcock, H.P.; Bennett, V.; Zhuang, X. Developmental mechanism of the periodic membrane skeleton in axons. eLife 2014, 3, 1–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Abiraman, K.; Li, H.; Pierce, D.M.; Tzingounis, A.V.; Lykotrafitis, G. Modeling of the axon membrane skeleton structure and implications for its mechanical properties. PLoS Comput. Biol. 2017, 13, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Li, W.; Martin, S.; Papadopulos, A.; Joensuu, M.; Liu, C.; Jiang, A.; Shamsollahi, G.; Amor, R.; Lanoue, V.; et al. Radial contractility of actomyosin rings facilitates axonal trafficking and structural stability. J. Cell Biol. 2020, 219. [Google Scholar] [CrossRef]
- Zhou, R.; Han, B.; Xia, C.; Zhuang, X. Membrane-associated periodic skeleton is a signaling platform for RTK transactivation in neurons. Science 2019, 365, 929–934. [Google Scholar] [CrossRef]
- Amini, M.; Ma, C.L.; Farazifard, R.; Zhu, G.; Zhang, Y.; Vanderluit, J.; Zoltewicz, J.S.; Hage, F.; Savitt, J.M.; Lagace, D.C.; et al. Conditional disruption of calpain in the CNS alters dendrite morphology, impairs LTP, and promotes neuronal survival following injury. J. Neurosci. 2013, 33, 5773–5784. [Google Scholar] [CrossRef] [Green Version]
- Kindler, S.; Dieterich, D.C.; Schütt, J.; Sahin, J.; Karpova, A.; Mikhaylova, M.; Schob, C.; Gundelfinger, E.D.; Kreienkamp, H.J.; Kreutz, M.R. Dendritic mRNA targeting of Jacob and N-methyl-D-aspartate-induced nuclear translocation after calpain-mediated proteolysis. J. Biol. Chem. 2009, 284, 25431–25440. [Google Scholar] [CrossRef] [Green Version]
- Perlson, E.; Hanz, S.; Ben-Yaakov, K.; Segal-Ruder, Y.; Seger, R.; Fainzilber, M. Vimentin-dependent spatial translocation of an activated MAP kinase in injured nerve. Neuron 2005, 45, 715–726. [Google Scholar] [CrossRef] [Green Version]
- Karpova, A.; Mikhaylova, M.; Bera, S.; Bär, J.; Reddy, P.P.; Behnisch, T.; Rankovic, V.; Spilker, C.; Bethge, P.; Sahin, J.; et al. Encoding and transducing the synaptic or extrasynaptic origin of NMDA receptor signals to the nucleus. Cell 2013, 152, 1119–1133. [Google Scholar] [CrossRef] [Green Version]
- Albrecht, D.; Winterflood, C.M.; Sadeghi, M.; Tschager, T.; Noé, F.; Ewers, H. Nanoscopic compartmentalization of membrane protein motion at the axon initial segment. J. Cell Biol. 2016, 215, 37–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karpenko, M.N.; Tikhomirova, M.S. The Role of Calpains in Regulating Synaptic Transmission. Neurosci. Behav. Physiol. 2015, 45, 952–956. [Google Scholar] [CrossRef]
- Lavoie-Cardinal, F.; Bilodeau, A.; Lemieux, M.; Gardner, M.-A.; Wiesner, T.; Laramée, G.; Gagné, C.; Koninck, P. De Neuronal activity remodels the F-actin based submembrane lattice in dendrites but not axons of hippocampal neurons. bioRxiv 2020. [Google Scholar] [CrossRef]
- Zadran, S.; Bi, X.; Baudry, M. Regulation of calpain-2 in neurons: Implications for synaptic plasticity. Mol. Neurobiol. 2010, 42, 143–150. [Google Scholar] [CrossRef]
- Berridge, M.J.; Lipp, P.; Bootman, M.D. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 2000, 1, 11–21. [Google Scholar] [CrossRef]
- Sabatini, B.L.; Oertner, T.G.; Svoboda, K. The life cycle of Ca2+ ions in dendritic spines. Neuron 2002, 33, 439–452. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Briz, V.; Chishti, A.; Bi, X.; Baudry, M. Distinct roles for μ-calpain and m-calpain in synaptic NMDAR-mediated neuroprotection and extrasynaptic NMDAR-mediated neurodegeneration. J. Neurosci. 2013, 33, 18880–18892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saez, M.E.; Ramirez-Lorca, R.; Moron, F.J.; Ruiz, A. The therapeutic potential of the calpain family: New aspects. Drug Discov. Today 2006, 11, 917–923. [Google Scholar] [CrossRef]
- Newell-Litwa, K.A.; Horwitz, R.; Lamers, M.L. Non-Muscle myosin II in disease: Mechanisms and therapeutic opportunities. DMM Dis. Model. Mech. 2015, 8, 1495–1515. [Google Scholar] [CrossRef] [Green Version]
- Heissler, S.M.; Sellers, J.R. Four things to know about myosin light chains as reporters for non-muscle myosin-2 dynamics in live cells. Cytoskeleton 2015, 72, 65–70. [Google Scholar] [CrossRef] [Green Version]
- Henson, J.H.; Ditzler, C.E.; Germain, A.; Irwin, P.M.; Vogt, E.T.; Yang, S.; Wu, X.; Shuster, C.B. The ultrastructural organization of actin and myosin II filaments in the contractile ring: New support for an old model of cytokinesis. Mol. Biol. Cell 2017, 28, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Dasbiswas, K.; Hu, S.; Schnorrer, F.; Safran, S.A.; Bershadsky, A.D. Ordering of myosin ii filaments driven by mechanical forces: Experiments and theory. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grubb, M.S.; Burrone, J. Activity-dependent relocation of the axon initial segment fine-tunes neuronal excitability. Nature 2010, 465, 1070–1074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heissler, S.M.; Sellers, J.R. Myosin light chains: Teaching old dogs new tricks. Bioarchitecture 2014, 4, 169–188. [Google Scholar] [CrossRef] [Green Version]
- Liewald, D.; Miller, R.; Logothetis, N.; Wagner, H.J.; Schüz, A. Distribution of axon diameters in cortical white matter: An electron-microscopic study on three human brains and a macaque. Biol. Cybern. 2014, 108, 541–557. [Google Scholar] [CrossRef] [Green Version]
- Korobova, F.; Svitkina, T.M. Molecular architecture of synaptic actin cytoskeleton in hippocampal neurons reveals a mechanism of dendritic spine morphogenesis. Mol. Biol. Cell 2010, 21, 165–176. [Google Scholar] [CrossRef] [Green Version]
- Hodges, J.L.; Newell-Litwa, K.; Asmussen, H.; Vicente-Manzanares, M.; Horwitz, A.R. Myosin IIB activity and phosphorylation status determines dendritic spine and post-synaptic density morphology. PLoS ONE 2011, 6. [Google Scholar] [CrossRef] [Green Version]
- Rex, C.S.; Gavin, C.F.; Rubio, M.D.; Kramar, E.A.; Chen, L.Y.; Jia, Y.; Huganir, R.L.; Muzyczka, N.; Gall, C.M.; Miller, C.A.; et al. Myosin IIb Regulates actin dynamics during synaptic plasticity and memory formation. Neuron 2010, 67, 603–617. [Google Scholar] [CrossRef] [Green Version]
- Kneussel, M.; Wagner, W. Myosin motors at neuronal synapses: Drivers of membrane transport and actin dynamics. Nat. Rev. Neurosci. 2013, 14, 233–247. [Google Scholar] [CrossRef]
- Krieg, M.; Stühmer, J.; Cueva, J.G.; Fetter, R.; Spilker, K.; Cremers, D.; Shen, K.; Dunn, A.R.; Goodman, M.B. Genetic defects in β-spectrin and tau sensitize C. Elegans axons to movement-induced damage via torque-tension coupling. eLife 2017, 6, 1–35. [Google Scholar] [CrossRef]
- Mitra, A.; Meißner, L.; Diez, S.; Gandhimathi, R.; Renger, R.; Ruhnow, F. Kinesin-14 motors drive a right-handed helical motion of antiparallel microtubules around each other. Nat. Commun. 2020, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bertling, E.; Englund, J.; Minkeviciene, R.; Koskinen, M.; Segerstråle, M.; Castrén, E.; Taira, T.; Hotulainen, P. Actin tyrosine-53-phosphorylation in neuronal maturation and synaptic plasticity. J. Neurosci. 2016, 36, 5299–5313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikhaylova, M.; Rentsch, J.; Ewers, H. Actomyosin Contractility in the Generation and Plasticity of Axons and Dendritic Spines. Cells 2020, 9, 2006. https://doi.org/10.3390/cells9092006
Mikhaylova M, Rentsch J, Ewers H. Actomyosin Contractility in the Generation and Plasticity of Axons and Dendritic Spines. Cells. 2020; 9(9):2006. https://doi.org/10.3390/cells9092006
Chicago/Turabian StyleMikhaylova, Marina, Jakob Rentsch, and Helge Ewers. 2020. "Actomyosin Contractility in the Generation and Plasticity of Axons and Dendritic Spines" Cells 9, no. 9: 2006. https://doi.org/10.3390/cells9092006
APA StyleMikhaylova, M., Rentsch, J., & Ewers, H. (2020). Actomyosin Contractility in the Generation and Plasticity of Axons and Dendritic Spines. Cells, 9(9), 2006. https://doi.org/10.3390/cells9092006




