Circulating miRNAs Associated with Dysregulated Vascular and Trophoblast Function as Target-Based Diagnostic Biomarkers for Preeclampsia
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Sample Collection
2.3. Cell Culture and Treatment
2.4. miRNA Isolation and Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
2.5. Measurement of sFlt-1, sEng, PlGF, Nitrite and Nitrate, and cGMP
2.6. Western Blot Analysis
2.7. Statistical Analysis
3. Results
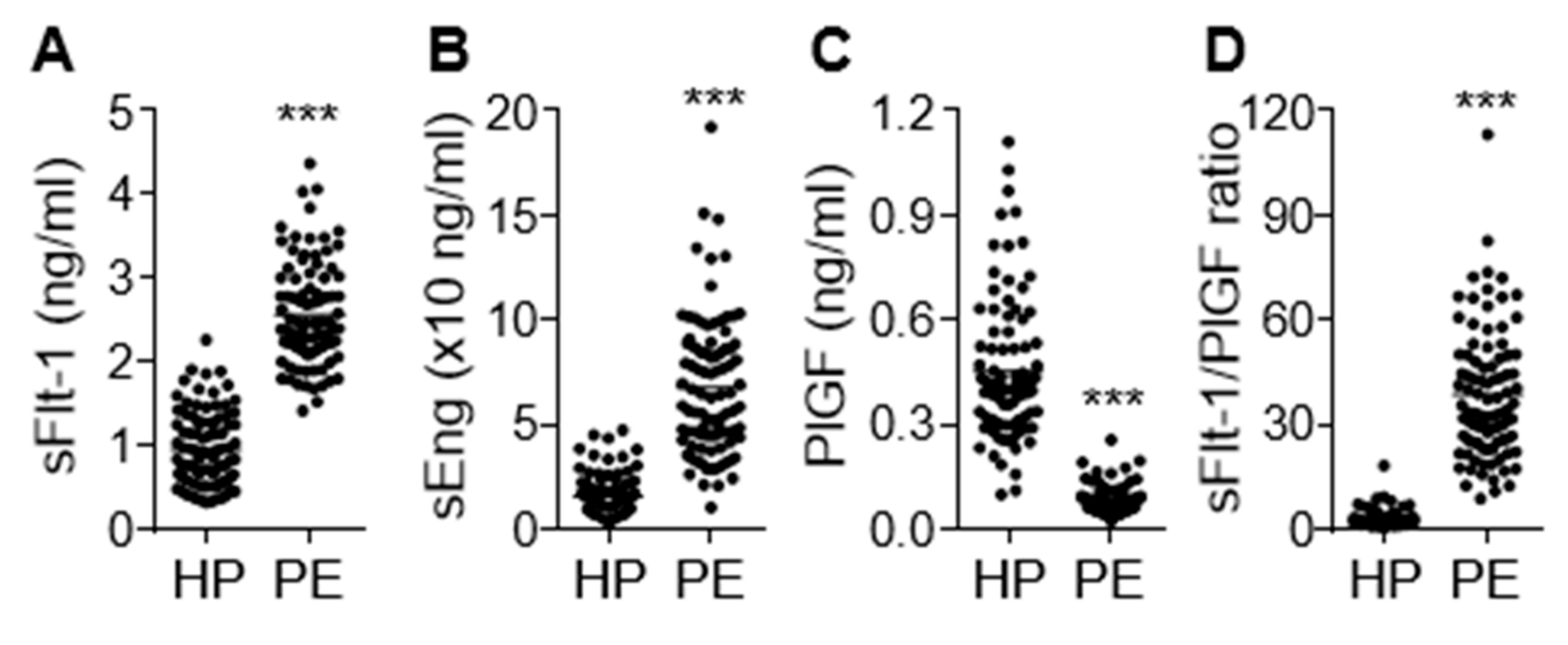
3.1. Levels of sFlt-1, sEng, and PlGF in Sera from Patients with PE
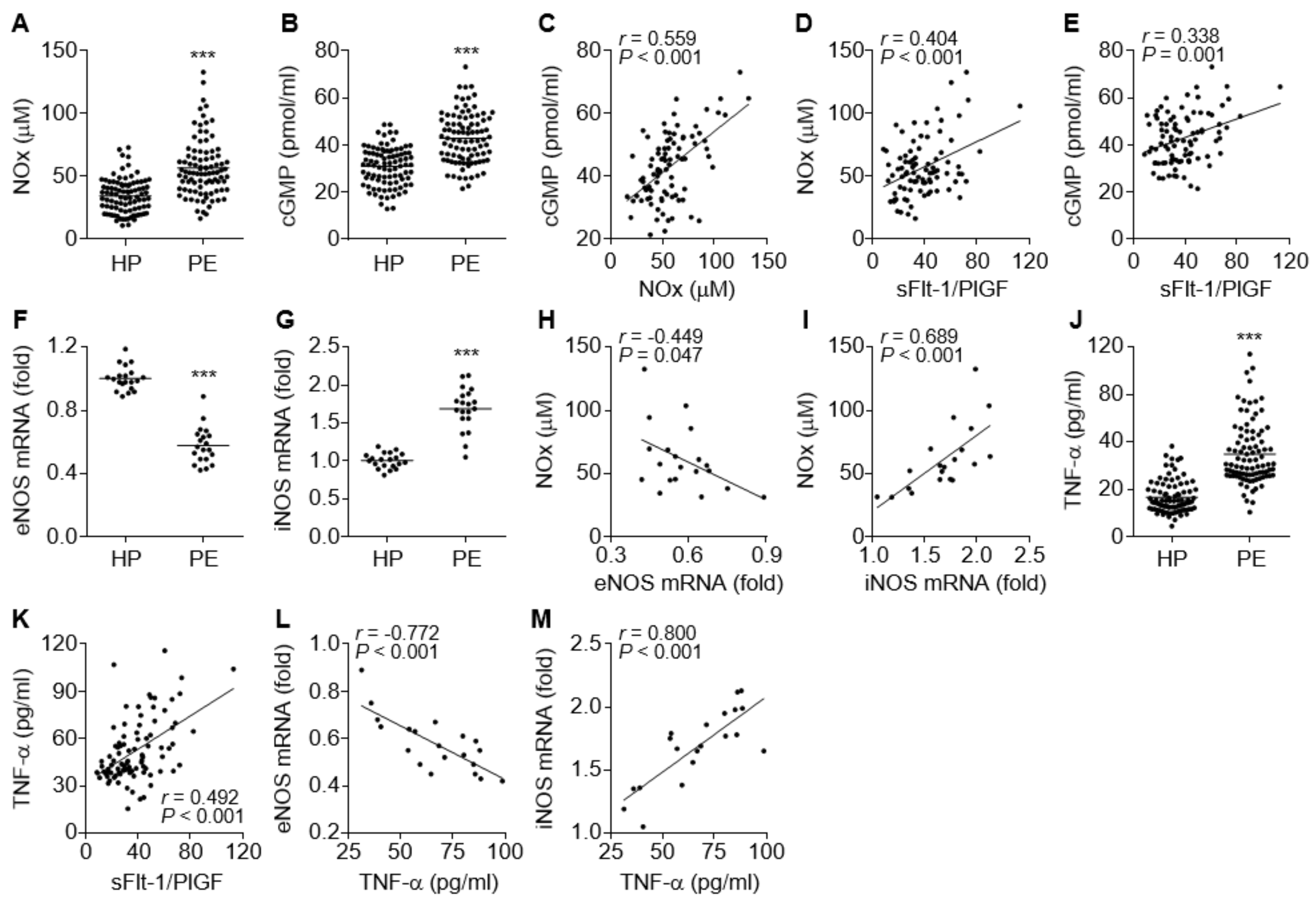
3.2. Comparative Levels of eNOS, iNOS, NO, cGMP, and TNF-α in PE Patients
3.3. Identification of miRNAs that are Positively and Negatively Regulated in PE Patients
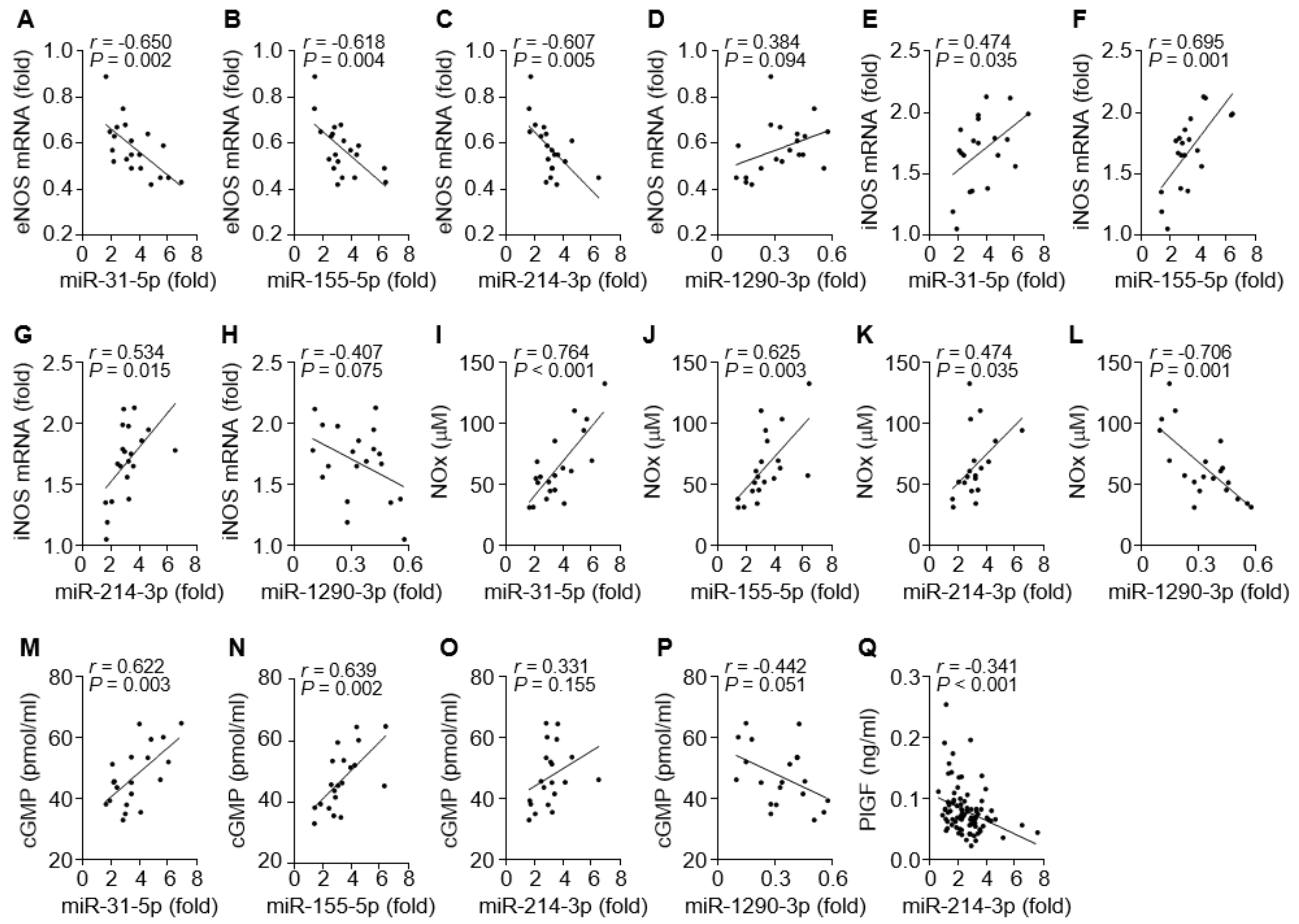
3.4. Correlation between the miRNAs and the NOS/NO/cGMP Axis or PlGF Level in PE Patients
3.5. The miRNAs Differentially Regulate iNOS, eNOS, and PlGF Expression
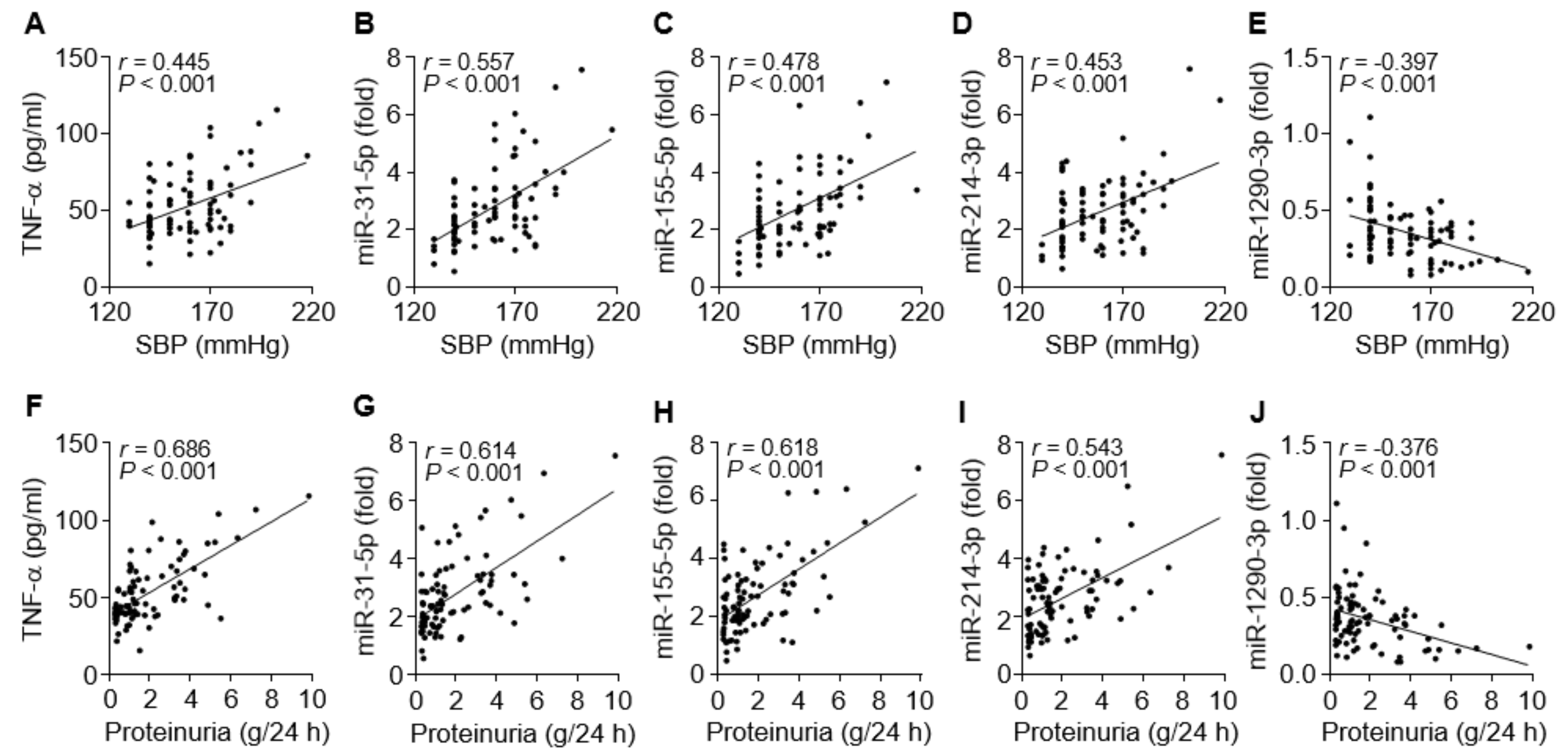
3.6. TNF-α and the miRNAs are Associated with Clinical Symptoms of PE
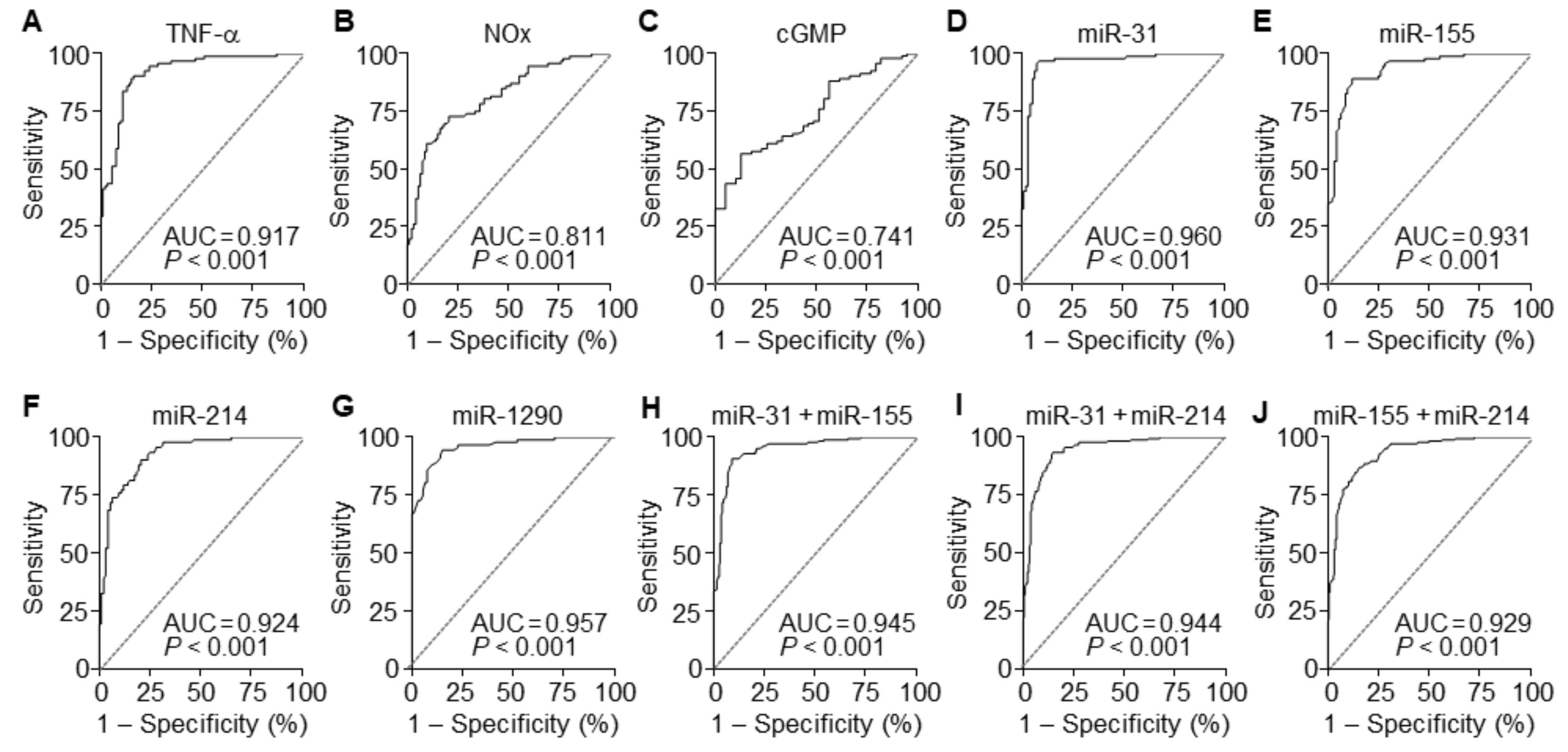
3.7. The miRNAs are Useful Serum Factors as Diagnostic Biomarkers in PE
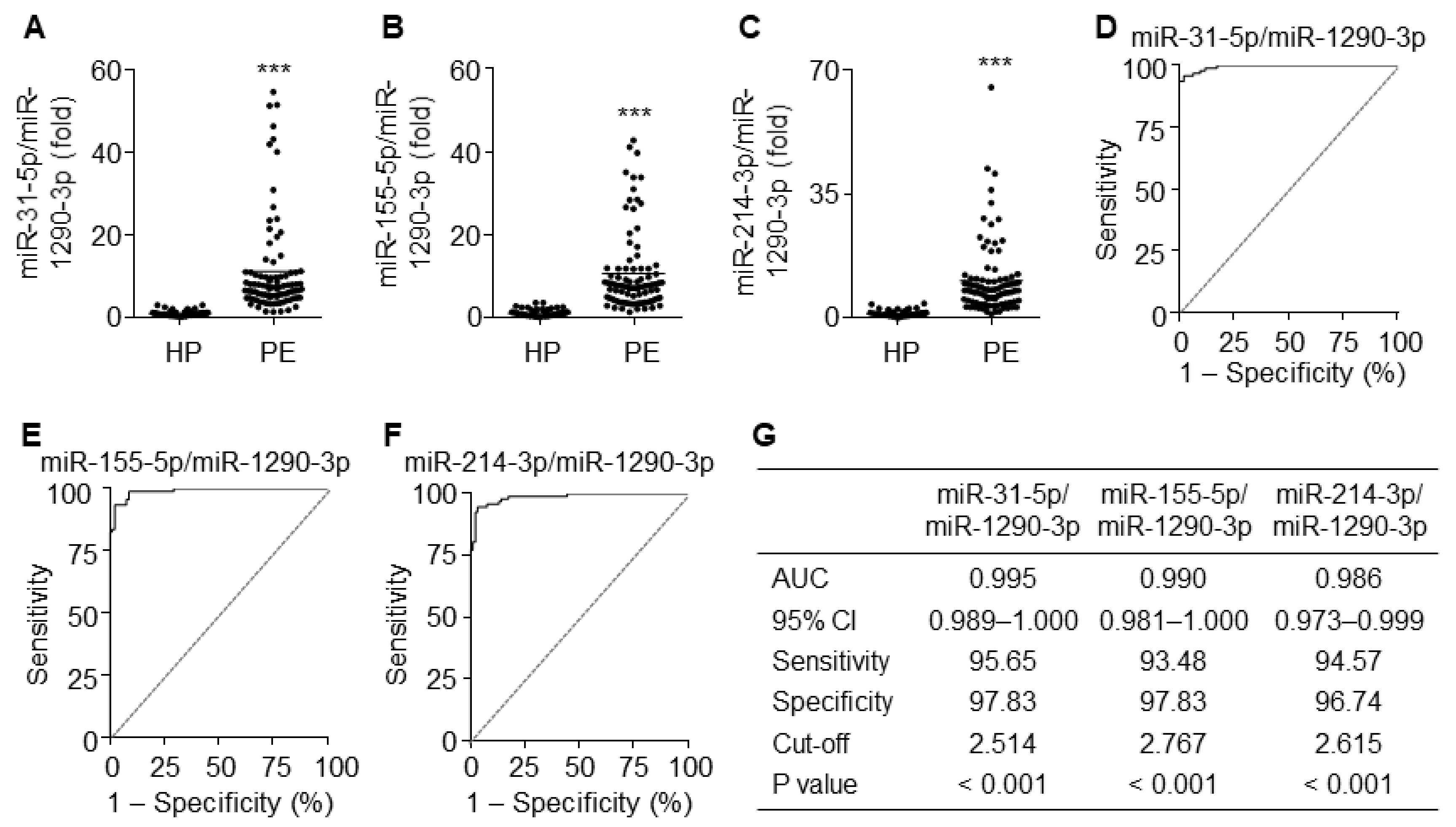
3.8. The Ratios of miR-31-5p, miR-155-5p, and miR-214-3p to miR-1290-3p Improve Diagnostic Accuracy
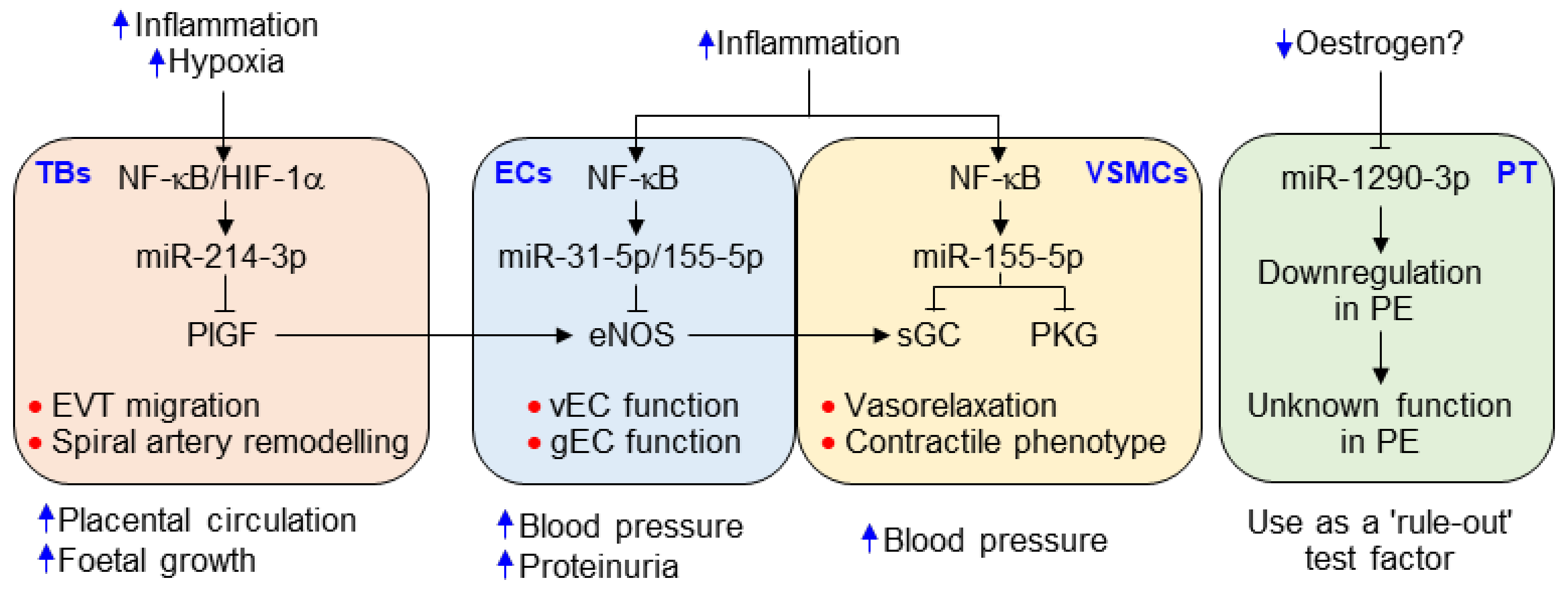
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Steegers, E.A.; von Dadelszen, P.; Duvekot, J.J.; Pijnenborg, R. Preeclampsia. Lancet 2010, 376, 631–644. [Google Scholar] [CrossRef]
- Duley, L. The global impact of pre-eclampsia and eclampsia. Semin Perinatol. 2009, 33, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Berg, C.J.; MacKay, A.P.; Qin, C.; Callaghan, W.M. Overview of maternal morbidity during hospitalization for labor and delivery in the united states: 1993–1997 and 2001–2005. Obstet. Gynecol. 2009, 113, 1075–1081. [Google Scholar] [CrossRef]
- Alice, W.; Sarosh, R.S.; Ananth, K. Preeclampsia: The role of angiogenic factors in its pathogenesis. Physiology 2009, 24, 147–158. [Google Scholar]
- Thangaratinam, S.; Coomarasamy, A.; O’Mahony, F.; Sharp, S.; Zamora, J.; Khan, K.S.; Ismail, K.M.K. Estimation of proteinuria as a predictor of complications of pre-eclampsia: A systematic review. BMC Med. 2009, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Zeisler, H.; Llurba, E.; Chantraine, F.J.; Vatish, M.; Staff, A.C.; Sennström, M.; Olovsson, M.; Brennecke, S.P.; Stepan, H.; Allegranza, D.; et al. Soluble fms-like tyrosine kinase-1 to placental growth factor ratio: Ruling out pre-eclampsia for up to 4 weeks and value of retesting. Ultrasound Obstet. Gynecol. 2019, 53, 367–375. [Google Scholar] [CrossRef]
- Chaiworapongsa, T.; Romero, R.; Korzeniewski, S.J.; Cortez, J.M.; Pappas, A.; Tarca, A.L.; Chaemsaithong, P.; Dong, Z.; Yeo, L.; Hassan, S.S. Plasma concentrations of angiogenic/anti-angiogenic factors have prognostic value in women presenting with suspected preeclampsia to the obstetrical triage area: A prospective study. J. Matern. Fetal. Neonatal. Med. 2014, 27, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.J.; Maynard, S.E.; Qian, C.; Lim, K.H.; England, L.J.; Yu, K.F.; Schisterman, E.F.; Thadhani, R.; Sachs, B.P.; Epstein, F.H.; et al. Circulating angiogenic factors and the risk of preeclampsia. N. Engl. J. Med. 2004, 350, 672–683. [Google Scholar] [CrossRef]
- Verlohren, S.; Galindo, A.; Schlembach, D.; Zeisler, H.; Herraiz, I.; Moertl, M.G.; Pape, J.; Dudenhausen, J.W.; Denk, B.; Stepan, H. An automated method for the determination of the sFlt-1/PIGF ratio in the assessment of preeclampsia. Am. J. Obstet. Gynecol. 2010, 202, 161.e1-161.e11. [Google Scholar] [CrossRef]
- Sunderji, S.; Gaziano, E.; Wothe, D.; Rogers, L.C.; Sibai, B.; Karumanchi, S.A.; Hodges-Savola, C. Automated assays for sVEGF R1 and PlGF as an aid in the diagnosis of preterm preeclampsia: A prospective clinical study. Am. J. Obstet. Gynecol. 2010, 202, 40.e1-40.e7. [Google Scholar] [CrossRef]
- Herraiz, I.; Llurba, E.; Verlohren, S.; Galindo, A. Spanish Group for the Study of Angiogenic Markers in Preeclampsia. Update on the diagnosis and prognosis of preeclampsia with the aid of the sFlt-1/PlGF ratio in singleton pregnancies. Fetal Diagn. Ther. 2018, 43, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Pant, V.; Yadav, B.K.; Sharma, J. A cross sectional study to assess the sFlt-1:PlGF ratio in pregnant women with and without preeclampsia. BMC Pregnancy Childbirth 2019, 19, 266. [Google Scholar]
- Munaut, C; Tebache, L; Blacher, S; Noël, A; Nisolle, M; Chantraine, F. Dysregulated circulating miRNAs in preeclampsia. Biomed. Rep. 2016, 5, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Hornakova, A; Kolkova, Z; Holubekova, V; Loderer, D; Lasabova, Z; Biringer, K; Halasova, E. Diagnostic Potential of MicroRNAs as Biomarkers in the Detection of Preeclampsia. Genet. Test. Mol. Biomarkers 2020, 24, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, K.S.; Kim, J.H.; Lee, D.K.; Park, M.; Choi, S.; Park, W.; Kim, S.; Choi, Y.K.; Hwang, J.Y.; et al. Aspirin prevents TNF-α-induced endothelial cell dysfunction by regulating the NF-κB-dependent miR-155/eNOS pathway: Role of a miR-155/eNOS axis in preeclampsia. Free Radic. Biol. Med. 2017, 104, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, K.S.; Choi, S.; Kim, J.; Lee, D.K.; Park, M.; Park, W.; Kim, T.H.; Hwang, J.Y.; Won, M.H.; et al. NF-κB-responsive miRNA-31-5p elicits endothelial dysfunction associated with preeclampsia via down-regulation of endothelial nitric-oxide synthase. J. Biol. Chem. 2018, 293, 18989–19000. [Google Scholar] [CrossRef]
- Lee, K.S.; Kim, J.; Kwak, S.N.; Lee, K.S.; Lee, D.K.; Ha, K.S.; Won, M.H.; Jeoung, D.; Lee, H.; Kwon, Y.G.; et al. Functional role of NF-κB in expression of human endothelial nitric oxide synthase. Biochem. Biophys. Res. Commun. 2014, 448, 101–107. [Google Scholar] [CrossRef]
- Park, M.; Choi, S.; Kim, S.; Kim, J.; Lee, D.K.; Park, W.; Kim, T.; Jung, J.; Hwang, J.Y.; Won, M.H.; et al. NF-κB-responsive miR-155 induces functional impairment of vascular smooth muscle cells by downregulating soluble guanylyl cyclase. Exp. Mol. Med. 2019, 51, 1–12. [Google Scholar] [CrossRef]
- Choi, S.; Park, M.; Kim, J.; Park, W.; Kim, S.; Lee, D.K.; Hwang, J.Y.; Choe, J.; Won, M.H.; Ryoo, S.; et al. TNF-α elicits phenotypic and functional alterations of vascular smooth muscle cells by miR-155-5p-dependent down-regulation of cGMP-dependent kinase 1. J. Biol. Chem. 2018, 293, 14812–14822. [Google Scholar] [CrossRef]
- Brown, M.A.; Magee, L.A.; Kenny, L.C.; Karumanchi, S.A.; McCarthy, F.P.; Saito, S.; Hall, D.R.; Warren, C.E.; Adoyi, G.; Ishaku, S. Hypertensive disorders of pregnancy: ISSHP Classification, diagnosis, and management recommendations for international practice. Hypertension 2018, 72, 24–43. [Google Scholar] [CrossRef]
- Salzman, A.; Denenberg, A.G.; Ueta, I.; O’Connor, M.; Linn, S.C.; Szabó, C. Induction and activity of nitric oxide synthase in cultured human intestinal epithelial monolayers. Am. J. Physiol. 1996, 270, G565–G573. [Google Scholar] [CrossRef] [PubMed]
- Breslau, N.; Paneth, N.S.; Lucia, V.C. The lingering academic deficits of low birth weight children. Pediatrics 2004, 114, 1035–1040. [Google Scholar] [CrossRef] [PubMed]
- Poon, L.C.; Tan, M.Y.; Yerlikaya, G.; Syngelaki, A.; Nicolaides, K.H. Birth weight in live births and stillbirths. Ultrasound Obstet. Gynecol. 2016, 48, 602–606. [Google Scholar] [CrossRef] [PubMed]
- Verlohren, S.; Stepan, H.; Dechend, R. Angiogenic growth factors in the diagnosis and prediction of pre-eclampsia. Clin. Sci. (Lond) 2012, 122, 43–52. [Google Scholar] [CrossRef]
- Li, F.; Hagaman, J.R.; Kim, H.S.; Maeda, N.; Jennette, J.C.; Faber, J.E.; Karumanchi, S.A.; Smithies, O.; Takahashi, N. eNOS deficiency acts through endothelin to aggravate sFlt-1-induced pre-eclampsia-like phenotype. J. Am. Soc. Nephrol. 2012, 23, 652–660. [Google Scholar] [CrossRef]
- Billiar, T.R.; Curran, R.D.; Harbrecht, B.G.; Stadler, J.; Williams, D.L.; Ochoa, J.B.; Di Silvio, M.; Simmons, R.L.; Murray, S.A. Association between synthesis and release of cGMP and nitric oxide biosynthesis by hepatocytes. Am. J. Physiol. 1992, 262, C1077–C1082. [Google Scholar] [CrossRef]
- Cantonwine, D.E.; McElrath, T.F.; Trabert, B.; Xu, X.; Sampson, J.; Roberts, J.M.; Hoover, R.N.; Troisi, R. Estrogen metabolism pathways in preeclampsia and normal pregnancy. Steroids 2019, 144, 8–14. [Google Scholar] [CrossRef]
- Chau, K.; Bobek, G.; Xu, B.; Stait-Gardner, T.; Price, W.; Hennessy, A.; Makris, A. Effect of placental growth factor in models of experimental pre-eclampsia and trophoblast invasion. Clin. Exp. Pharmacol. Physiol. 2020, 47, 49–59. [Google Scholar] [CrossRef]
- Gonsalves, C.S.; Li, C.; Mpollo, M.S.E.M.; Pullarkat, V.; Malik, P.; Tahara, S.M.; Kalra, V.K. Erythropoietin-mediated expression of placenta growth factor is regulated via activation of hypoxia-inducible factor-1α and post-transcriptionally by miR-214 in sickle cell disease. Biochem. J. 2015, 468, 409–423. [Google Scholar] [CrossRef]
- Taylor, B.D.; Ness, R.B.; Klebanoff, M.A.; Zoh, R.; Bass, D.; Hougaard, D.M.; Skogstrand, K.; Haggerty, C.L. First and second trimester immune biomarkers in preeclamptic and normotensive women. Pregnancy Hypertens. 2016, 6, 388–393. [Google Scholar] [CrossRef]
- Catarino, C.; Santos-Silva, A.; Belo, L.; Rocha-Pereira, P.; Rocha, S.; Patrício, B.; Quintanilha, A.; Rebelo, I. Inflammatory disturbances in preeclampsia: Relationship between maternal and umbilical cord blood. J. Pregnancy 2012, 2012, 684384. [Google Scholar] [CrossRef] [PubMed]
- Harmon, A.C.; Cornelius, D.C.; Amaral, L.M.; Faulkner, J.L.; Cunningham, M.W., Jr.; Wallace, K.; LaMarca, B. The role of inflammation in the pathology of preeclampsia. Clin. Sci. (Lond) 2016, 130, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Alexander, B.T.; Cockrell, K.L.; Massey, M.B.; Bennett, W.A.; Granger, J.P. Tumor necrosis factor-α-induced hypertension in pregnant rats results in decreased renal neuronal nitric oxide synthase expression. Am. J. Hypertens. 2002, 15, 170–175. [Google Scholar] [CrossRef]
- Sunderland, N.S.; Thomson, S.E.; Heffernan, S.J.; Lim, S.; Thompson, J.; Ogle, R.; McKenzie, P.; Kirwan, P.J.; Makris, A.; Hennessy, A. Tumor necrosis factor α induces a model of preeclampsia in pregnant baboons (Papio hamadryas). Cytokine 2011, 56, 192–199. [Google Scholar] [CrossRef]
- Baylis, C.; Mitruka, B.; Deng, A. Chronic blockade of nitric oxide synthesis in the rat produces systemic hypertension and glomerular damage, J. Clin. Invest. 1992, 90, 278–281. [Google Scholar] [CrossRef]
- Huang, P.L.; Huang, Z.; Mashimo, H.; Bloch, K.D.; Moskowitz, M.A.; Bevan, J.A.; Fishman, M.C. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature 1995, 377, 239–242. [Google Scholar] [CrossRef]
- López-Jaramillo, P.; Arenas, W.D.; García, R.G.; Rincon, M.Y.; López, M. The role of the L-arginine-nitric oxide pathway in preeclampsia. Ther. Adv. Cardiovasc. Dis. 2008, 2, 261–275. [Google Scholar] [CrossRef]
- Vaughan, J.E.; Walsh, S.W. Activation of NF-κB in placentas of women with preeclampsia. Hypertens. Pregnancy 2012, 31, 243–251. [Google Scholar] [CrossRef]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef]
- Watanabe, T.; Sato, T.; Amano, T.; Kawamura, Y.; Kawamura, N.; Kawaguchi, H.; Yamashita, N.; Kurihara, H.; Nakaoka, T. Dnm3os, a non-coding RNA, is required for normal growth and skeletal development in mice. Dev. Dyn. 2008, 237, 3738–3748. [Google Scholar] [CrossRef]
- Xu, P.; Zhao, Y.; Liu, M.; Wang, Y.; Wang, H.; Li, Y.X.; Zhu, X.; Yao, Y.; Wang, H.; Qiao, J.; et al. Variations of microRNAs in human placentas and plasma from preeclamptic pregnancy. Hypertension 2014, 63, 1276–1284. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.C.; Chin, T.M.; Yang, H.; Nga, M.E.; Lunny, D.P.; Lim, E.K.H.; Sun, L.L.; Pang, Y.H.; Leow, Y.N.; Malusay, S.R.Y.; et al. Tumour-initiating cell-specific miR-1246 and miR-1290 expression converge to promote non-small cell lung cancer progression. Nat. Commun. 2016, 7, 11702. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.; Meays, B.M.; Madduri, L.S.V.; Shahjin, F.; Chand, S.; Niu, M.; Albahrani, A.; Guda, C.; Pendyala, G.; Fox, S.H.; et al. Downregulation of an evolutionary young miR-1290 in an iPSC-derived neural stem cell model of autism spectrum disorder. Stem Cells Int. 2019, 2019, 8710180. [Google Scholar] [CrossRef] [PubMed]
- Ponsuksili, S.; Tesfaye, D.; Schellander, K.; Hoelker, M.; Hadlich, F.; Schwerin, M.; Wimmers, K. Differential expression of miRNAs and their target mRNAs in endometria prior to maternal recognition of pregnancy associates with endometrial receptivity for in vivo- and in vitro-produced bovine embryos. Biol. Reprod. 2014, 91, 135. [Google Scholar] [CrossRef] [PubMed]
- Endo, Y.; Yamashita, H.; Takahashi, S.; Sato, S.; Yoshimoto, N.; Asano, T.; Hato, Y.; Dong, Y.; Fujii, Y.; Toyama, T. Immunohistochemical determination of the miR-1290 target arylamine N-acetyltransferase 1 (NAT1) as a prognostic biomarker in breast cancer. BMC Cancer 2014, 4, 990. [Google Scholar] [CrossRef]
- Troisi, R.; Potischman, N.; Roberts, J.M.; Ness, R.; Crombleholme, W.; Lykins, D.; Siiteri, P.; Hoover, R.N. Maternal serum oestrogen and androgen concentrations in preeclamptic and uncomplicated pregnancies. Int. J. Epidemiol. 2003, 32, 455–460. [Google Scholar] [CrossRef]
- Atamer, Y.; Erden, A.C.; Demir, B.; Koçyigit, Y.; Atamer, A. The relationship between plasma levels of leptin and androgen in healthy and preeclamptic pregnant women. Acta. Obstet. Gynecol. Scand. 2004, 83, 425–430. [Google Scholar] [CrossRef]
- Tenório, M.B.; Ferreira, R.C.; Moura, F.A.; Bueno, N.B.; de Oliveira, A.C.M.; Goulart, M.O.F. Cross-Talk between Oxidative Stress and Inflammation in Preeclampsia. Oxid. Med. Cell Longev. 2019, 2019, 8238727. [Google Scholar] [CrossRef]
- Hu, S.; Zhu, W.; Zhang, L.F.; Pei, M.; Liu, M.F. MicroRNA-155 broadly orchestrates inflammation-induced changes of microRNA expression in breast cancer. Cell Res. 2014, 24, 254–257. [Google Scholar] [CrossRef]
- Gaudet, A.D.; Fonken, L.K.; Gushchina, L.V.; Aubrecht, T.G.; Maurya, S.K.; Periasamy, M.; Nelson, R.J.; Popovich, P.G. miR-155 deletion in female mice prevents diet-induced obesity. Sci. Rep. 2016, 6, 22862. [Google Scholar] [CrossRef]
- Yang, S.W.; Vosch, T. Rapid detection of microRNA by a silver nanocluster DNA probe. Anal. Chem. 2011, 83, 6935–6939. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | Healthy pregnancy (n = 92) | Preeclampsia (n = 92) | p-Value |
|---|---|---|---|
| Age at pregnancy (year) | 31.49 ± 0.50 | 32.73 ± 0.54 | 0.0946 |
| Gestational age at delivery (week) | 37.52 ± 0.38 | 35.72 ± 0.28 | 0.0002 |
| Systolic blood pressure (mmHg) | 115.20 ± 0.84 | 157.80 ± 1.90 | <0.0001 |
| Diastolic blood pressure (mmHg) | 74.89 ± 0.78 | 99.85 ± 1.20 | <0.0001 |
| Urinary protein (g/24 h) | N/A | 1.89 ± 0.19 | N/A |
| Birth weight (kg) | 3.08 ± 0.06 | 2.39 ± 0.07 | <0.0001 |
| Serum Factors | AUC | 95% CI | Sensitivity | Specificity | Cut-off | p-Value |
|---|---|---|---|---|---|---|
| TNF-α | 0.917 | 0.876–0.958 | 90.22 | 83.70 | 34.89 | <0.001 |
| NOx | 0.811 | 0.750–0.873 | 72.83 | 79.35 | 44.59 | <0.001 |
| cGMP | 0.741 | 0.655–0.826 | 88.04 | 43.59 | 31.77 | <0.001 |
| miR-31-5p | 0.960 | 0.931–0.990 | 95.65 | 92.39 | 1.275 | <0.001 |
| miR-155-5p | 0.931 | 0.895–0.967 | 89.13 | 88.04 | 1.365 | <0.001 |
| miR-214-3p | 0.924 | 0.887–0.962 | 90.22 | 79.35 | 1.250 | <0.001 |
| miR-1290-3p | 0.957 | 0.931–0.984 | 94.57 | 84.78 | 0.595 | <0.001 |
| miR-31-5p+miR-155-5p | 0.945 | 0.922–0.968 | 90.76 | 90.76 | 1.365 | <0.001 |
| miR-31-5p+miR-214-3p | 0.944 | 0.921–0.967 | 93.48 | 85.33 | 1.265 | <0.001 |
| miR-214-3p+miR-155-5p | 0.929 | 0.903–0.955 | 86.96 | 84.24 | 1.355 | <0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Park, M.; Kim, J.-Y.; Kim, T.; Hwang, J.Y.; Ha, K.-S.; Won, M.-H.; Ryoo, S.; Kwon, Y.-G.; Kim, Y.-M. Circulating miRNAs Associated with Dysregulated Vascular and Trophoblast Function as Target-Based Diagnostic Biomarkers for Preeclampsia. Cells 2020, 9, 2003. https://doi.org/10.3390/cells9092003
Kim S, Park M, Kim J-Y, Kim T, Hwang JY, Ha K-S, Won M-H, Ryoo S, Kwon Y-G, Kim Y-M. Circulating miRNAs Associated with Dysregulated Vascular and Trophoblast Function as Target-Based Diagnostic Biomarkers for Preeclampsia. Cells. 2020; 9(9):2003. https://doi.org/10.3390/cells9092003
Chicago/Turabian StyleKim, Suji, Minsik Park, Ji-Yoon Kim, Taesam Kim, Jong Yun Hwang, Kwon-Soo Ha, Moo-Ho Won, Sungwoo Ryoo, Young-Guen Kwon, and Young-Myeong Kim. 2020. "Circulating miRNAs Associated with Dysregulated Vascular and Trophoblast Function as Target-Based Diagnostic Biomarkers for Preeclampsia" Cells 9, no. 9: 2003. https://doi.org/10.3390/cells9092003
APA StyleKim, S., Park, M., Kim, J.-Y., Kim, T., Hwang, J. Y., Ha, K.-S., Won, M.-H., Ryoo, S., Kwon, Y.-G., & Kim, Y.-M. (2020). Circulating miRNAs Associated with Dysregulated Vascular and Trophoblast Function as Target-Based Diagnostic Biomarkers for Preeclampsia. Cells, 9(9), 2003. https://doi.org/10.3390/cells9092003






