Loganin Attenuates High Glucose-Induced Schwann Cells Pyroptosis by Inhibiting ROS Generation and NLRP3 Inflammasome Activation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and Drug Treatment
2.2. Cell Viability Assays
2.3. Measurement of Intracellular Reactive Oxygen Species
2.4. ELISA Measurement of IL-1β Release
2.5. Western Blot Analysis
2.6. Cell Death Assay
2.7. Immunocytochemistry
2.8. RNA Extraction, cDNA Synthesis and Quantitative Real-Time PCR (qRT-PCR)
2.9. Statistical Analysis
3. Results
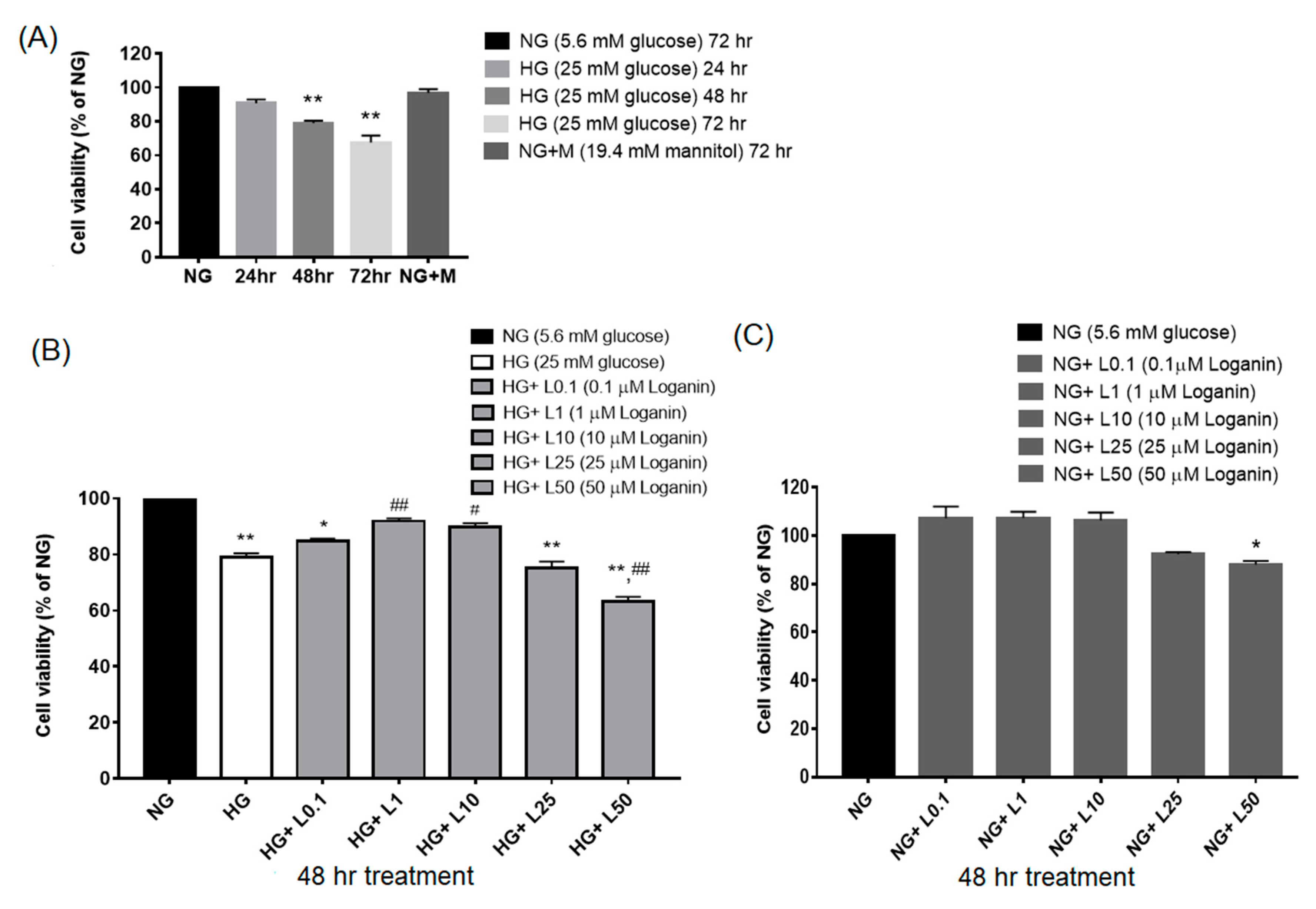
3.1. Loganin Effects on Cell Viability in High-Glucose-Treated RSC96 Schwann Cells
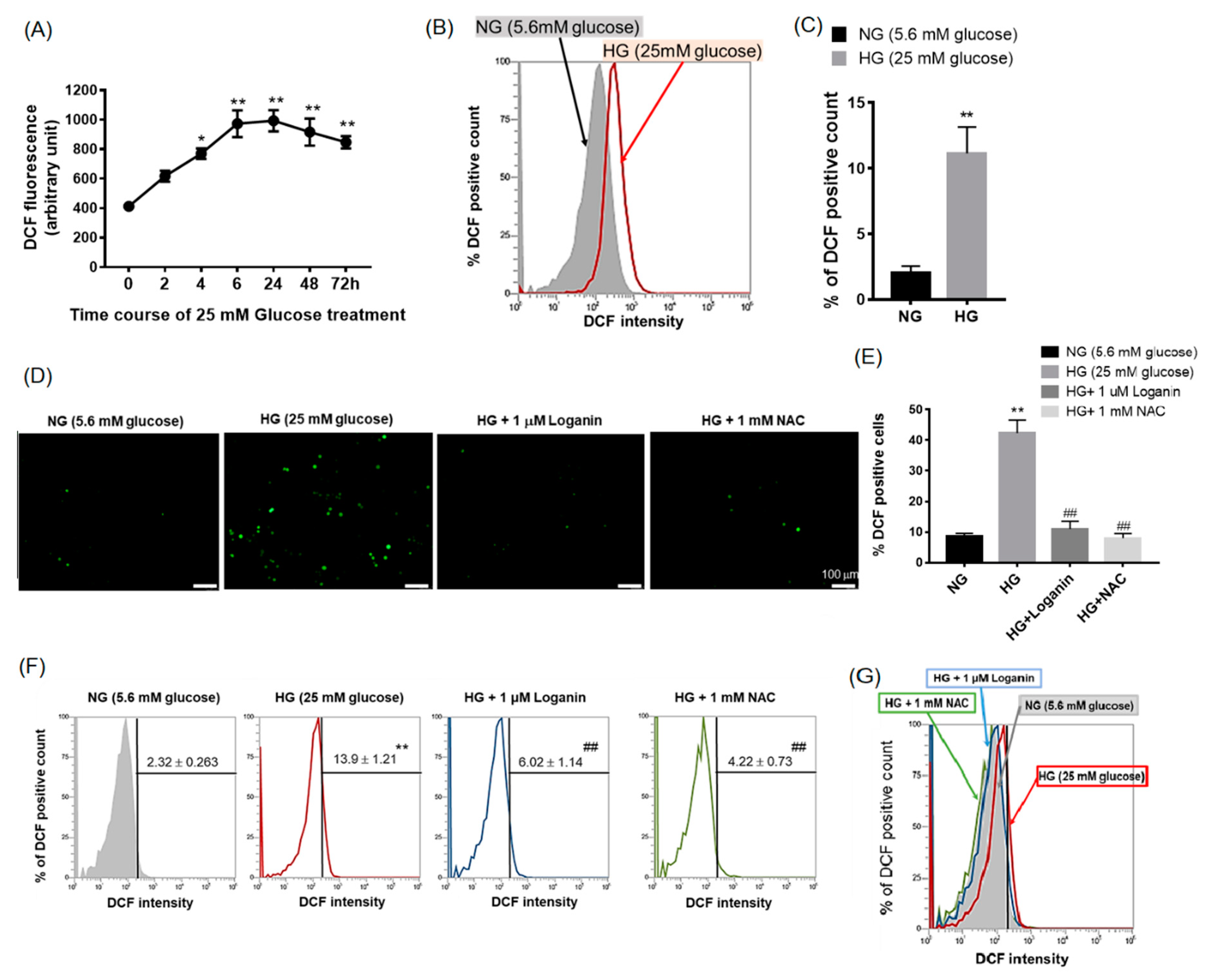
3.2. Loganin Diminished Intracellular ROS Generation in High-Glucose-Treated RSC96 Schwann Cells
3.3. Loganin Attenuated Cell Death in High-Glucose-Treated RSC96 Schwann Cells
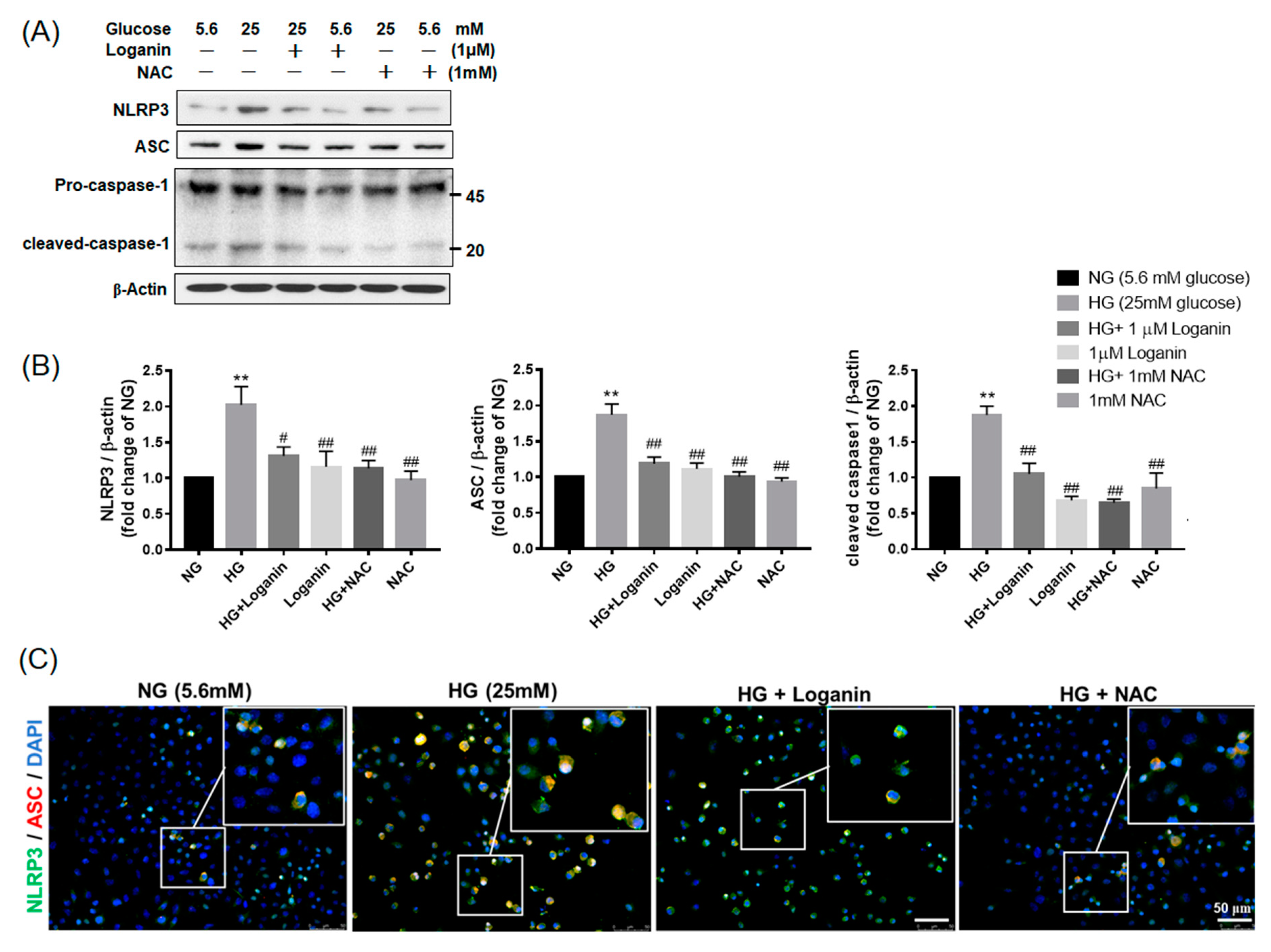
3.4. Loganin Decreased NLRP3 Inflammasome Assembly in High-Glucose-Treated RSC96 Schwann Cells
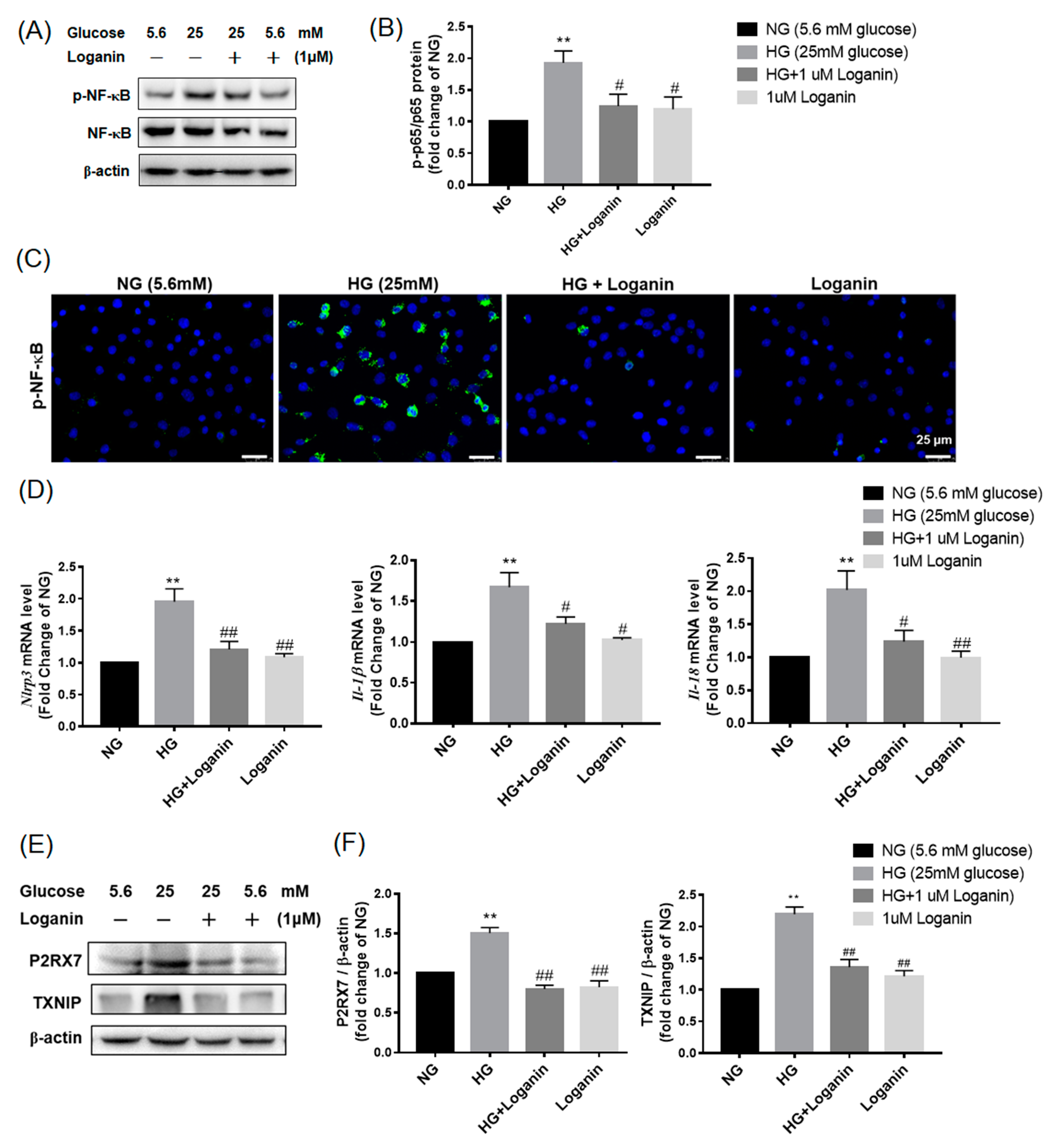
3.5. Loganin Inhibited NLRP3 Inflammasome Activation in High-Glucose-Treated RSC96 Schwann Cells
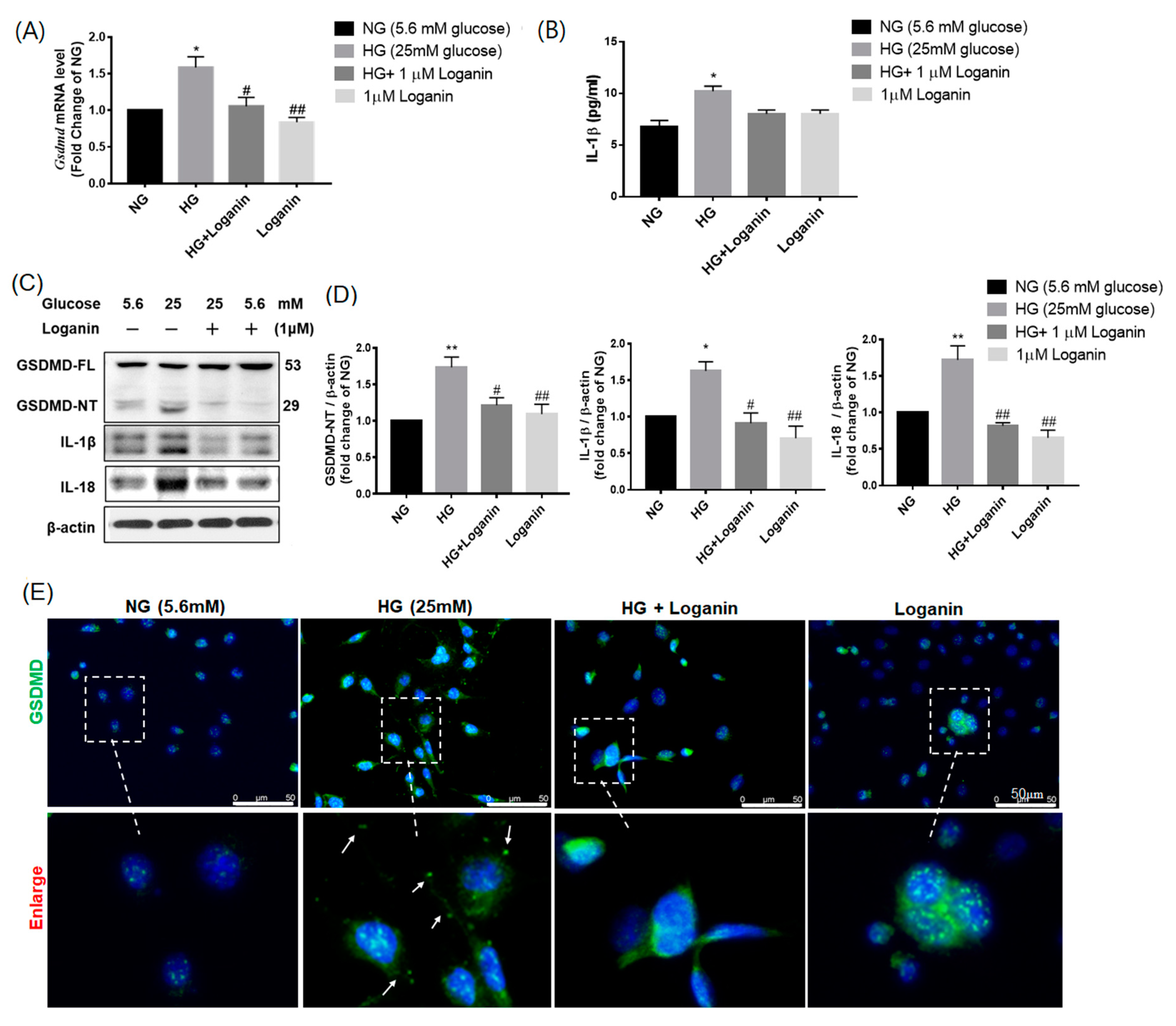
3.6. Loganin Reduced GSDMD-Mediated Pyroptosis in High-Glucose-Treated RSC96 Schwann Cells
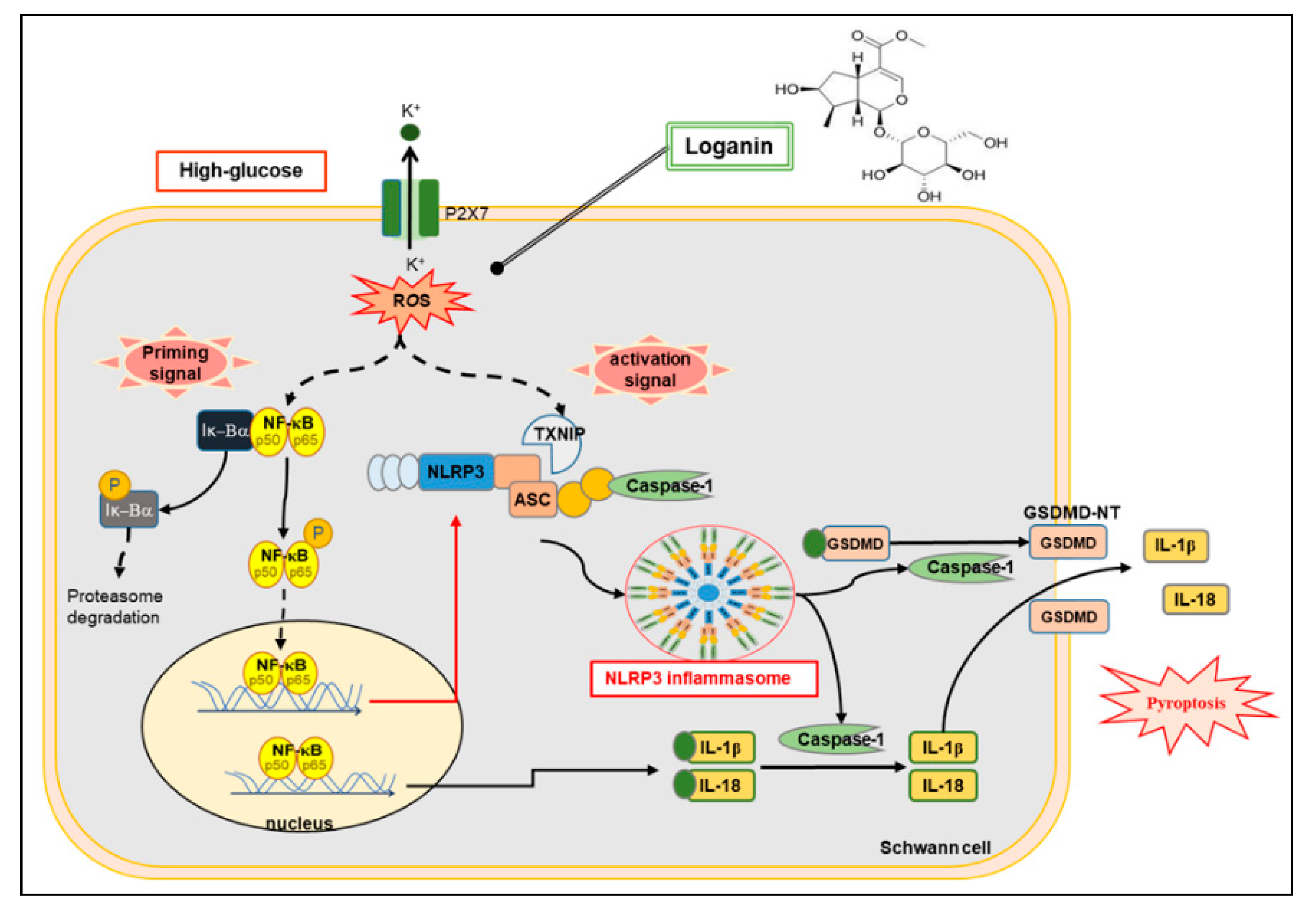
4. Discussion
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ASC | apoptosis-associated speck-like protein containing a caspase recruitment domain |
| DCF | dichlorofluorescein |
| DPN | diabetic peripheral neuropathy |
| GSDMD | gasdermin D |
| GSDMD–NT | gasdermin D-N-terminal |
| HG | high glucose |
| IL-1β | interleukin-1β |
| IL-18 | interleukin-18 |
| NAC | N-acetyl-L-cysteine |
| NF-κB | nuclear factor κB |
| NG | normal glucose |
| NLRP3 | NOD-like receptor protein 3 |
| P2RX7 | purinergic receptor P2 × 7 |
| PI | propidium iodide |
| ROS | reactive oxygen species |
| TXNIP | thioredoxin-interacting protein |
References
- Dixit, S.; Maiya, A. Diabetic peripheral neuropathy and its evaluation in a clinical scenario: A review. J. Postgrad. Med. 2014, 60, 33–40. [Google Scholar] [CrossRef]
- Sendi, R.A.; Mahrus, A.M.; Saeed, R.M.; Mohammed, M.A.; Al-Dubai, S.A.R. Diabetic peripheral neuropathy among Saudi diabetic patients: A multicenter cross-sectional study at primary health care setting. J. Fam. Med. Prim. Care 2020, 9, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Impellizzeri, D.; Peritore, A.F.; Cordaro, M.; Gugliandolo, E.; Siracusa, R.; Crupi, R.; D’Amico, R.; Fusco, R.; Evangelista, M.; Cuzzocrea, S.; et al. The neuroprotective effects of micronized PEA (PEA-m) formulation on diabetic peripheral neuropathy in mice. FASEB J. 2019, 33, 11364–11380. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Sloan, G.; Ye, Y.; Wang, S.; Duan, B.; Tesfaye, S.; Gao, L. New Perspective in Diabetic Neuropathy: From the Periphery to the Brain, a Call for Early Detection, and Precision Medicine. Front. Endocrinol. 2019, 10, 929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kosacka, J.; Woidt, K.; Toyka, K.V.; Paeschke, S.; Kloting, N.; Bechmann, I.; Bluher, M.; Thiery, J.; Ossmann, S.; Baum, P.; et al. The role of dietary non-heme iron load and peripheral nerve inflammation in the development of peripheral neuropathy (PN) in obese non-diabetic leptin-deficient ob/ob mice. Neurol. Res. 2019, 41, 341–353. [Google Scholar] [CrossRef]
- Mrakic-Sposta, S.; Vezzoli, A.; Maderna, L.; Gregorini, F.; Montorsi, M.; Moretti, S.; Greco, F.; Cova, E.; Gussoni, M. R(+)-Thioctic Acid Effects on Oxidative Stress and Peripheral Neuropathy in Type II Diabetic Patients: Preliminary Results by Electron Paramagnetic Resonance and Electroneurography. Oxid. Med. Cell. Longev. 2018, 2018, 1767265. [Google Scholar] [CrossRef]
- Goncalves, N.P.; Vaegter, C.B.; Andersen, H.; Ostergaard, L.; Calcutt, N.A.; Jensen, T.S. Schwann cell interactions with axons and microvessels in diabetic neuropathy. Nat. Rev. Neurol. 2017, 13, 135–147. [Google Scholar] [CrossRef]
- Naruse, K. Schwann Cells as Crucial Players in Diabetic Neuropathy. Adv. Exp. Med. Biol. 2019, 1190, 345–356. [Google Scholar]
- Goncalves, N.P.; Vaegter, C.B.; Pallesen, L.T. Peripheral Glial Cells in the Development of Diabetic Neuropathy. Front. Neurol. 2018, 9, 268. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.J.; Liu, X.; Xia, S.; Zhang, Z.; Zhang, Y.; Zhao, J.; Ruan, J.; Luo, X.; Lou, X.; Bai, Y.; et al. FDA-approved disulfiram inhibits pyroptosis by blocking gasdermin D pore formation. Nat. Immunol. 2020, 21, 736–745. [Google Scholar] [CrossRef]
- Li, K.; Shi, X.; Luo, M.; Inam, U.L.; Wu, P.; Zhang, M.; Zhang, C.; Li, Q.; Wang, Y.; Piao, F. Taurine protects against myelin damage of sciatic nerve in diabetic peripheral neuropathy rats by controlling apoptosis of schwann cells via NGF/Akt/GSK3beta pathway. Exp. Cell Res. 2019, 383, 111557. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Hao, J.; Cheng, M.; Zhang, C.; Huo, C.; Liu, Y.; Du, W.; Zhang, X. Hyperglycemia-induced Bcl-2/Bax-mediated apoptosis of Schwann cells via mTORC1/S6K1 inhibition in diabetic peripheral neuropathy. Exp. Cell Res. 2018, 367, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Ma, J.; Wu, Y.; Nangle, M.; Zou, S.; Li, Y.; Yin, J.; Zhao, Y.; Xu, H.; Zhang, H.; et al. Dual Delivery of NGF and bFGF Coacervater Ameliorates Diabetic Peripheral Neuropathy via Inhibiting Schwann Cells Apoptosis. Int. J. Biol. Sci. 2017, 13, 640–651. [Google Scholar] [CrossRef] [PubMed]
- Volpe, C.M.; Anjos, P.M.; Nogueira-Machado, J.A. Inflammasome as a New Therapeutic Target for Diabetic Complications. Recent Pat. Endocr. Metab. Immune Drug Discov. 2016, 10, 56–62. [Google Scholar] [CrossRef]
- Fusco, R.; Siracusa, R.; Genovese, T.; Cuzzocrea, S.; Di Paola, R. Focus on the Role of NLRP3 Inflammasome in Diseases. Int. J. Mol. Sci. 2020, 21, 4223. [Google Scholar] [CrossRef] [PubMed]
- Fusco, R.; D’Amico, R.; Cordaro, M.; Gugliandolo, E.; Siracusa, R.; Peritore, A.F.; Crupi, R.; Impellizzeri, D.; Cuzzocrea, S.; Di Paola, R. Absence of formyl peptide receptor 1 causes endometriotic lesion regression in a mouse model of surgically-induced endometriosis. Oncotarget 2018, 9, 31355–31366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Amico, R.; Fusco, R.; Cordaro, M.; Siracusa, R.; Peritore, A.F.; Gugliandolo, E.; Crupi, R.; Scuto, M.; Cuzzocrea, S.; Di Paola, R.; et al. Modulation of NLRP3 Inflammasome through Formyl Peptide Receptor 1 (Fpr-1) Pathway as a New Therapeutic Target in Bronchiolitis Obliterans Syndrome. Int. J. Mol. Sci. 2020, 21, 2144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mascarenhas, D.P.; Zamboni, D.S. Inflammasome biology taught by Legionella pneumophila. J. Leukoc. Biol. 2017, 101, 841–849. [Google Scholar] [CrossRef] [Green Version]
- Fusco, R.; Gugliandolo, E.; Biundo, F.; Campolo, M.; Di Paola, R.; Cuzzocrea, S. Inhibition of inflammasome activation improves lung acute injury induced by carrageenan in a mouse model of pleurisy. FASEB J. 2017, 31, 3497–3511. [Google Scholar] [CrossRef] [Green Version]
- Gan, J.; Huang, M.; Lan, G.; Liu, L.; Xu, F. High Glucose Induces the Loss of Retinal Pericytes Partly via NLRP3-Caspase-1-GSDMD-Mediated Pyroptosis. Biomed. Res. Int. 2020, 2020, 4510628. [Google Scholar] [CrossRef]
- Karmakar, M.; Minns, M.; Greenberg, E.N.; Diaz-Aponte, J.; Pestonjamasp, K.; Johnson, J.L.; Rathkey, J.K.; Abbott, D.W.; Wang, K.; Shao, F.; et al. N-GSDMD trafficking to neutrophil organelles facilitates IL-1beta release independently of plasma membrane pores and pyroptosis. Nat. Commun. 2020, 11, 2212. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.W.; Zhang, J.; Li, X.; Wang, Y.; Fu, Y.H.; Gao, X.Y. A new research hot spot: The role of NLRP3 inflammasome activation, a key step in pyroptosis, in diabetes and diabetic complications. Life Sci. 2020, 240, 117138. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Wang, K.J.; Cheng, C.S.; Yan, G.Q.; Lu, W.L.; Ge, J.F.; Cheng, Y.X.; Li, N. Bioactive compounds from Cornus officinalis fruits and their effects on diabetic nephropathy. J. Ethnopharmacol. 2014, 153, 840–845. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Jiao, N.; Jiang, M.; Liu, L.; Zhu, Y.; Wu, H.; Chen, J.; Fu, Y.; Du, Q.; Xu, H.; et al. Loganin alleviates testicular damage and germ cell apoptosis induced by AGEs upon diabetes mellitus by suppressing the RAGE/p38MAPK/NF-kappaB pathway. J. Cell. Mol. Med. 2020, 24, 6083–6095. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Chen, J.; Jiang, M.; Fu, Y.; Zhu, Y.; Jiao, N.; Liu, L.; Du, Q.; Wu, H.; Xu, H.; et al. Loganin and catalpol exert cooperative ameliorating effects on podocyte apoptosis upon diabetic nephropathy by targeting AGEs-RAGE signaling. Life Sci. 2020, 252, 117653. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Xu, H.; Lv, G.; Liu, B.; Lee, M.K.; Lu, C.; Lv, X.; Wu, Y. Loganin attenuates diabetic nephropathy in C57BL/6J mice with diabetes induced by streptozotocin and fed with diets containing high level of advanced glycation end products. Life Sci. 2015, 123, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Yamabe, N.; Noh, J.S.; Park, C.H.; Kang, K.S.; Shibahara, N.; Tanaka, T.; Yokozawa, T. Evaluation of loganin, iridoid glycoside from Corni Fructus, on hepatic and renal glucolipotoxicity and inflammation in type 2 diabetic db/db mice. Eur. J. Pharmacol. 2010, 648, 179–187. [Google Scholar] [CrossRef]
- Park, C.H.; Tanaka, T.; Kim, J.H.; Cho, E.J.; Park, J.C.; Shibahara, N.; Yokozawa, T. Hepato-protective effects of loganin, iridoid glycoside from Corni Fructus, against hyperglycemia-activated signaling pathway in liver of type 2 diabetic db/db mice. Toxicology 2011, 290, 14–21. [Google Scholar] [CrossRef]
- Lee, C.M.; Jung, H.A.; Oh, S.H.; Park, C.H.; Tanaka, T.; Yokozawa, T.; Choi, J.S. Kinetic and molecular docking studies of loganin and 7-O-galloyl-D-sedoheptulose from Corni Fructus as therapeutic agents for diabetic complications through inhibition of aldose reductase. Arch. Pharm. Res. 2015, 38, 1090–1098. [Google Scholar] [CrossRef]
- Kim, M.J.; Bae, G.S.; Jo, I.J.; Choi, S.B.; Kim, D.G.; Shin, J.Y.; Lee, S.K.; Kim, M.J.; Shin, S.; Song, H.J.; et al. Loganin protects against pancreatitis by inhibiting NF-kappaB activation. Eur. J. Pharmacol. 2015, 765, 541–550. [Google Scholar] [CrossRef]
- Rajabi, M.; Mohaddes, G.; Farajdokht, F.; Nayebi Rad, S.; Mesgari, M.; Babri, S. Impact of loganin on pro-inflammatory cytokines and depression- and anxiety-like behaviors in male diabetic rats. Physiol. Int. 2018, 105, 199–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tseng, Y.T.; Lin, W.J.; Chang, W.H.; Lo, Y.C. The novel protective effects of loganin against 1-methyl-4-phenylpyridinium-induced neurotoxicity: Enhancement of neurotrophic signaling, activation of IGF-1R/GLP-1R, and inhibition of RhoA/ROCK pathway. Phytother. Res. 2019, 33, 690–701. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.W.; Cheng, K.I.; Chen, J.Y.; Cheng, Y.C.; Chang, Y.C.; Yeh, J.L.; Hsu, J.H.; Dai, Z.K.; Wu, B.N. Loganin prevents chronic constriction injury-provoked neuropathic pain by reducing TNF-alpha/IL-1beta-mediated NF-kappaB activation and Schwann cell demyelination. Phytomedicine 2020, 67, 153166. [Google Scholar] [CrossRef] [PubMed]
- Clement, S.; Braithwaite, S.S.; Magee, M.F.; Ahmann, A.; Smith, E.P.; Schafer, R.G.; Hirsch, I.B.; American Diabetes Association Diabetes in Hospitals Writing C. Management of diabetes and hyperglycemia in hospitals. Diabetes Care 2004, 27, 553–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madonna, R.; Geng, Y.J.; Shelat, H.; Ferdinandy, P.; De Caterina, R. High glucose-induced hyperosmolarity impacts proliferation, cytoskeleton remodeling and migration of human induced pluripotent stem cells via aquaporin-1. Biochim. Biophys. Acta 2014, 1842, 2266–2275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rharass, T.; Lucas, S. High Glucose Level Impairs Human Mature Bone Marrow Adipocyte Function Through Increased ROS Production. Front. Endocrinol. 2019, 10, 607. [Google Scholar] [CrossRef]
- Grosick, R.; Alvarado-Vazquez, P.A.; Messersmith, A.R.; Romero-Sandoval, E.A. High glucose induces a priming effect in macrophages and exacerbates the production of pro-inflammatory cytokines after a challenge. J. Pain Res. 2018, 11, 1769–1778. [Google Scholar] [CrossRef] [Green Version]
- Moustafa, P.E.; Abdelkader, N.F.; El Awdan, S.A.; El-Shabrawy, O.A.; Zaki, H.F. Liraglutide ameliorated peripheral neuropathy in diabetic rats: Involvement of oxidative stress, inflammation and extracellular matrix remodeling. J. Neurochem. 2018, 146, 173–185. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Shen, J.; Liu, H.; Shi, Y.; Li, L.; Wei, M. Morroniside and loganin extracted from Cornus officinalis have protective effects on rat mesangial cell proliferation exposed to advanced glycation end products by preventing oxidative stress. Can. J. Physiol. Pharmacol. 2006, 84, 1267–1273. [Google Scholar] [CrossRef]
- Babri, S.; Hasani Azami, S.; Mohaddes, G. Effect of Acute Administration of loganin on Spatial Memory in Diabetic Male Rats. Adv. Pharm. Bull. 2013, 3, 91–95. [Google Scholar]
- Li, C.; Xu, X.; Wang, Z.; Wang, Y.; Luo, L.; Cheng, J.; Chen, S.F.; Liu, H.; Wan, Q.; Wang, Q. Exercise ameliorates post-stroke depression by inhibiting PTEN elevation-mediated upregulation of TLR4/NF-kappaB/NLRP3 signaling in mice. Brain Res. 2020, 1736, 146777. [Google Scholar] [CrossRef] [PubMed]
- Chao, G.; Tian, X.; Zhang, W.; Ou, X.; Cong, F.; Song, T. Blocking Smad2 signalling with loganin attenuates SW10 cell cycle arrest induced by TNF-alpha. PLoS ONE 2017, 12, e0176965. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Ma, Y.; Zhou, L.; Jiang, H.; Wang, M.; Zhan, X. Protective effect of cornel iridoid glycoside in D-galactosamine/tumor necrosis factor-alpha-injured L02 hepatocytes and its mechanism. J. Intercult. Ethnopharmacol. 2014, 3, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, H.; Li, Y.; Zhao, Y.; Xiong, F.; Liu, Y.; Xue, H.; Yang, Z.; Ni, S.; Sahil, A.; et al. Coriolus versicolor alleviates diabetic cardiomyopathy by inhibiting cardiac fibrosis and NLRP3 inflammasome activation. Phytother. Res. 2019, 33, 2737–2748. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Cheng, W.; Zhang, G.; Ma, Q.; Li, X.; Zhang, B.; Hu, T.; Song, G. Protective effects of iridoid glycosides on acute colitis via inhibition of the inflammatory response mediated by the STAT3/NF-small ka, CyrillicB pathway. Int. Immunopharmacol. 2020, 81, 106240. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Liu, Q.; Hu, N.; Zheng, F.; Zhang, X.; Ni, Y.; Liu, J. Downregulation of hsa_circ_0068087 ameliorates TLR4/NF-kappaB/NLRP3 inflammasome-mediated inflammation and endothelial cell dysfunction in high glucose conditioned by sponging miR-197. Gene 2019, 709, 1–7. [Google Scholar] [CrossRef]
- Ding, S.; Xu, S.; Ma, Y.; Liu, G.; Jang, H.; Fang, J. Modulatory Mechanisms of the NLRP3 Inflammasomes in Diabetes. Biomolecules 2019, 9, 850. [Google Scholar] [CrossRef] [Green Version]
- Rovira-Llopis, S.; Apostolova, N.; Banuls, C.; Muntane, J.; Rocha, M.; Victor, V.M. Mitochondria, the NLRP3 Inflammasome, and Sirtuins in Type 2 Diabetes: New Therapeutic Targets. Antioxid. Redox Signal. 2018, 29, 749–791. [Google Scholar] [CrossRef] [Green Version]
- Jia, M.; Wu, C.; Gao, F.; Xiang, H.; Sun, N.; Peng, P.; Li, J.; Yuan, X.; Li, H.; Meng, X.; et al. Activation of NLRP3 inflammasome in peripheral nerve contributes to paclitaxel-induced neuropathic pain. Mol. Pain 2017, 13, 1744806917719804. [Google Scholar] [CrossRef] [Green Version]
- Jo, E.K.; Kim, J.K.; Shin, D.M.; Sasakawa, C. Molecular mechanisms regulating NLRP3 inflammasome activation. Cell. Mol. Immunol. 2016, 13, 148–159. [Google Scholar] [CrossRef] [Green Version]
- Sagulenko, V.; Thygesen, S.J.; Sester, D.P.; Idris, A.; Cridland, J.A.; Vajjhala, P.R.; Roberts, T.L.; Schroder, K.; Vince, J.E.; Hill, J.M.; et al. AIM2 and NLRP3 inflammasomes activate both apoptotic and pyroptotic death pathways via ASC. Cell Death Differ. 2013, 20, 1149–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, Y.; Zhang, H.; Wang, C.; Jiao, F.; Xu, H.; Wang, X.; Luan, W.; Ma, F.; Ni, L.; Tang, X.; et al. Activation of ROS/MAPKs/NF-kappaB/NLRP3 and inhibition of efferocytosis in osteoclast-mediated diabetic osteoporosis. FASEB J. 2019, 33, 12515–12527. [Google Scholar] [CrossRef] [Green Version]
- Zhong, Z.; Umemura, A.; Sanchez-Lopez, E.; Liang, S.; Shalapour, S.; Wong, J.; He, F.; Boassa, D.; Perkins, G.; Ali, S.R.; et al. NF-kappaB Restricts Inflammasome Activation via Elimination of Damaged Mitochondria. Cell 2016, 164, 896–910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-kappaB signaling in inflammation. Signal Transduct. Target Ther. 2017, 2, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, C.; Liu, S.; Wang, H.; Dou, H. Role of the thioredoxin interacting protein in diabetic nephropathy and the mechanism of regulating NODlike receptor protein 3 inflammatory corpuscle. Int. J. Mol. Med. 2019, 43, 2440–2450. [Google Scholar]
- Zhou, R.; Tardivel, A.; Thorens, B.; Choi, I.; Tschopp, J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol. 2010, 11, 136–140. [Google Scholar] [CrossRef]
- Jacobson, K.A.; Muller, C.E. Medicinal chemistry of adenosine, P2Y and P2X receptors. Neuropharmacology 2016, 104, 31–49. [Google Scholar] [CrossRef] [Green Version]
- Savio, L.E.B.; de Andrade Mello, P.; da Silva, C.G.; Coutinho-Silva, R. The P2X7 Receptor in Inflammatory Diseases: Angel or Demon? Front. Pharmacol. 2018, 9, 52. [Google Scholar] [CrossRef] [Green Version]
- Karmakar, M.; Katsnelson, M.A.; Dubyak, G.R.; Pearlman, E. Neutrophil P2X7 receptors mediate NLRP3 inflammasome-dependent IL-1beta secretion in response to ATP. Nat. Commun. 2016, 7, 10555. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Wang, H.; Gao, H.; Zhang, H.; Zhang, H.; Wang, Q.; Sun, Z. P2X7 receptor mediates NLRP3 inflammasome activation in depression and diabetes. Cell Biosci. 2020, 10, 28. [Google Scholar] [CrossRef] [Green Version]
- Fink, S.L.; Cookson, B.T. Apoptosis, pyroptosis, and necrosis: Mechanistic description of dead and dying eukaryotic cells. Infect. Immun. 2005, 73, 1907–1916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, D.; Kang, R.; Berghe, T.V.; Vandenabeele, P.; Kroemer, G. The molecular machinery of regulated cell death. Cell Res. 2019, 29, 347–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.Y.; Liu, X.L.; Zhao, R. Induction of Pyroptosis and Its Implications in Cancer Management. Front. Oncol. 2019, 9, 971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roshanravan, N.; Alamdari, N.M.; Jafarabadi, M.A.; Mohammadi, A.; Shabestari, B.R.; Nasirzadeh, N.; Asghari, S.; Mansoori, B.; Akbarzadeh, M.; Ghavami, A.; et al. Effects of oral butyrate and inulin supplementation on inflammation-induced pyroptosis pathway in type 2 diabetes: A randomized, double-blind, placebo-controlled trial. Cytokine 2020, 131, 155101. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Z.; Ruan, J.; Pan, Y.; Magupalli, V.G.; Wu, H.; Lieberman, J. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 2016, 535, 153–158. [Google Scholar] [CrossRef] [Green Version]
- He, W.T.; Wan, H.; Hu, L.; Chen, P.; Wang, X.; Huang, Z.; Yang, Z.H.; Zhong, C.Q.; Han, J. Gasdermin D is an executor of pyroptosis and required for interleukin-1beta secretion. Cell Res. 2015, 25, 1285–1298. [Google Scholar] [CrossRef]
- De Vasconcelos, N.M.; Lamkanfi, M. Recent Insights on Inflammasomes, Gasdermin Pores, and Pyroptosis. Cold Spring Harb. Perspect. Biol. 2020, 12, a036392. [Google Scholar] [CrossRef]

| Gene | Accession No. | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|---|
| Nlrp3 | NM_001191642.1 | CGGTGACCTTGTGTGTGCTT | TCATGTCCTGAGCCATGGAAG |
| Gsdmd | NM_001130553.1 | AAGATCGTGGATCATGCCGT | CTCAGGAGGCAGTAGGGCTT |
| Il-1 β | NM_031512.2 | AAATGCCTCGTGCTGTCTGA | AGGCCACAGGGATTTTGTCG |
| Il-18 | NM_019165.1 | ACCACTTTGGCAGACTTCACT | ACACAGGCGGGTTTCTTTTG |
| β-actin | NM_031144.3 | GACCCAGATCATGTTTGAGACC | AGGCATACAGGGACAACACA |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Y.-C.; Chu, L.-W.; Chen, J.-Y.; Hsieh, S.-L.; Chang, Y.-C.; Dai, Z.-K.; Wu, B.-N. Loganin Attenuates High Glucose-Induced Schwann Cells Pyroptosis by Inhibiting ROS Generation and NLRP3 Inflammasome Activation. Cells 2020, 9, 1948. https://doi.org/10.3390/cells9091948
Cheng Y-C, Chu L-W, Chen J-Y, Hsieh S-L, Chang Y-C, Dai Z-K, Wu B-N. Loganin Attenuates High Glucose-Induced Schwann Cells Pyroptosis by Inhibiting ROS Generation and NLRP3 Inflammasome Activation. Cells. 2020; 9(9):1948. https://doi.org/10.3390/cells9091948
Chicago/Turabian StyleCheng, Yu-Chi, Li-Wen Chu, Jun-Yih Chen, Su-Ling Hsieh, Yu-Chin Chang, Zen-Kong Dai, and Bin-Nan Wu. 2020. "Loganin Attenuates High Glucose-Induced Schwann Cells Pyroptosis by Inhibiting ROS Generation and NLRP3 Inflammasome Activation" Cells 9, no. 9: 1948. https://doi.org/10.3390/cells9091948
APA StyleCheng, Y.-C., Chu, L.-W., Chen, J.-Y., Hsieh, S.-L., Chang, Y.-C., Dai, Z.-K., & Wu, B.-N. (2020). Loganin Attenuates High Glucose-Induced Schwann Cells Pyroptosis by Inhibiting ROS Generation and NLRP3 Inflammasome Activation. Cells, 9(9), 1948. https://doi.org/10.3390/cells9091948






