Decreased Equilibrative Nucleoside Transporter 1 (ENT1) Activity Contributes to the High Extracellular Adenosine Levels in Mesenchymal Glioblastoma Stem-Like Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Pharmacological Agents
2.2. Cell Culture
2.3. Glioblastoma Stem-Like Cell Culture
2.4. Adenosine Quantification
2.5. Nucleoside Transport Activity
2.6. Western Blots
2.7. RNA Extraction and qRT-PCR
2.8. Adenosine Accumulation
2.9. CD73 and PAP Activity
2.10. Statistics
3. Results
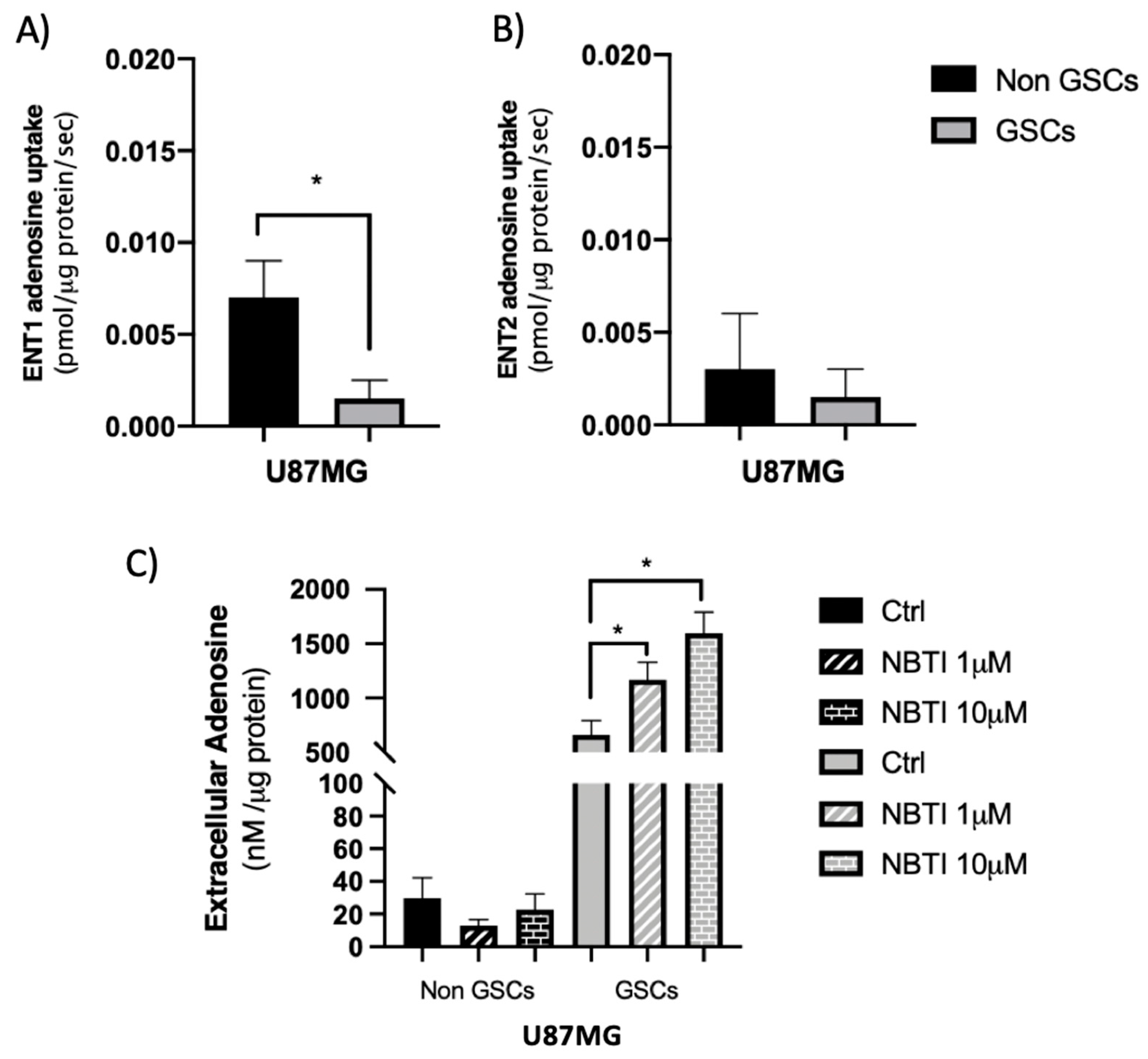
3.1. ENTs Contribute to the High Level of Extracellular Adenosine in U87MG GSCs
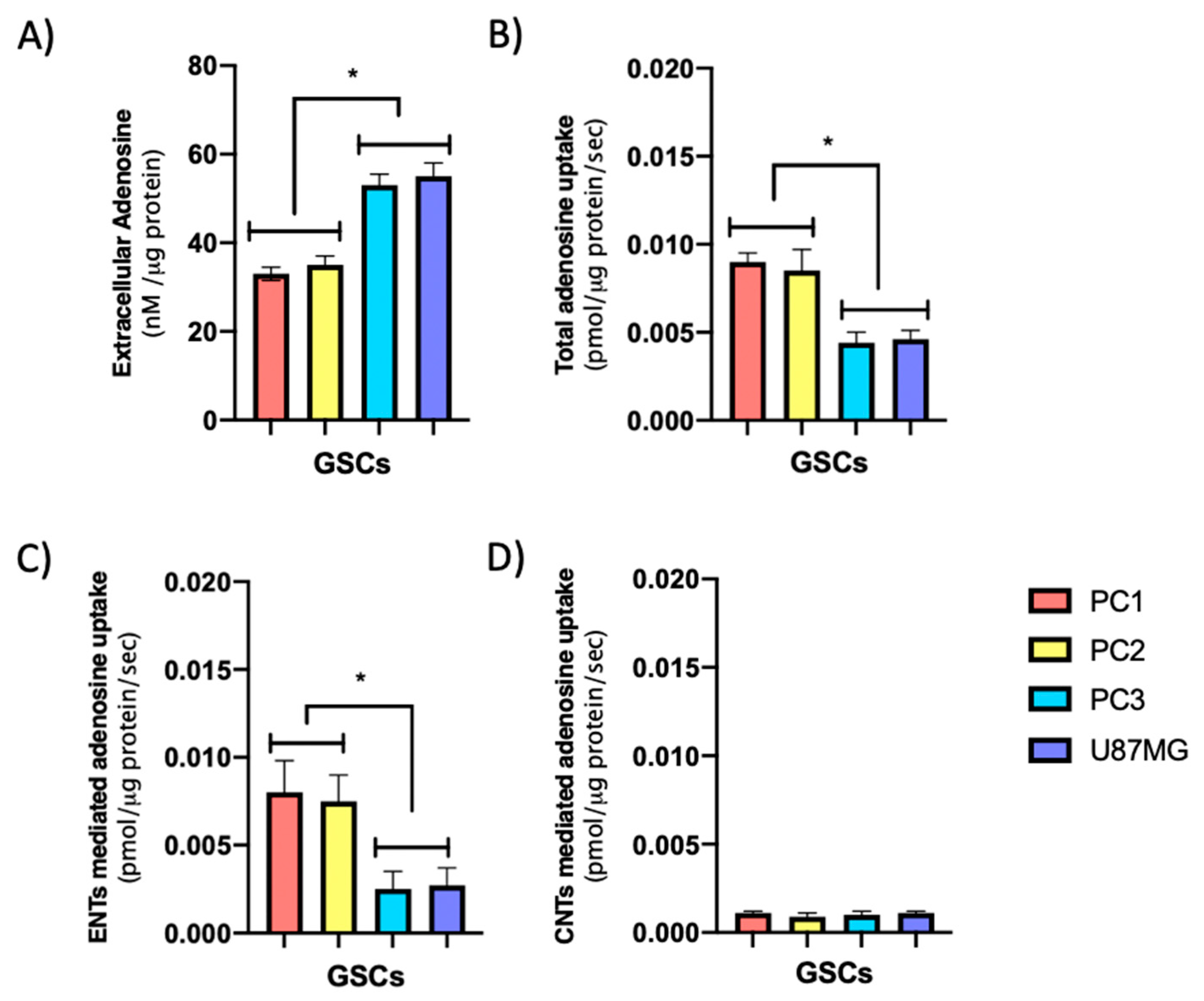
3.2. GSCs Subtypes Exhibit Differential Expression and Activity Levels of ENTs Which Correlate with Their Extracellular Adenosine Levels
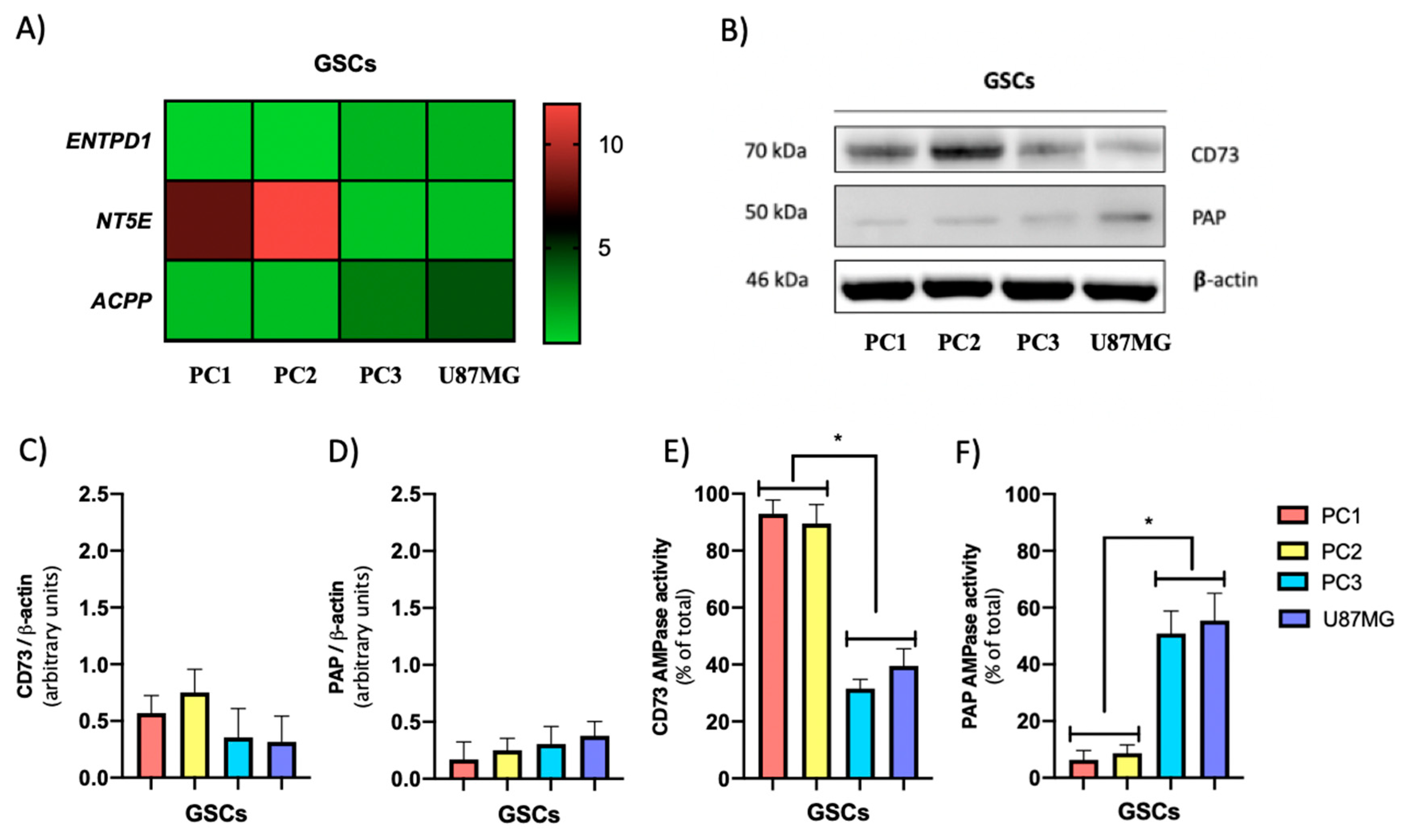
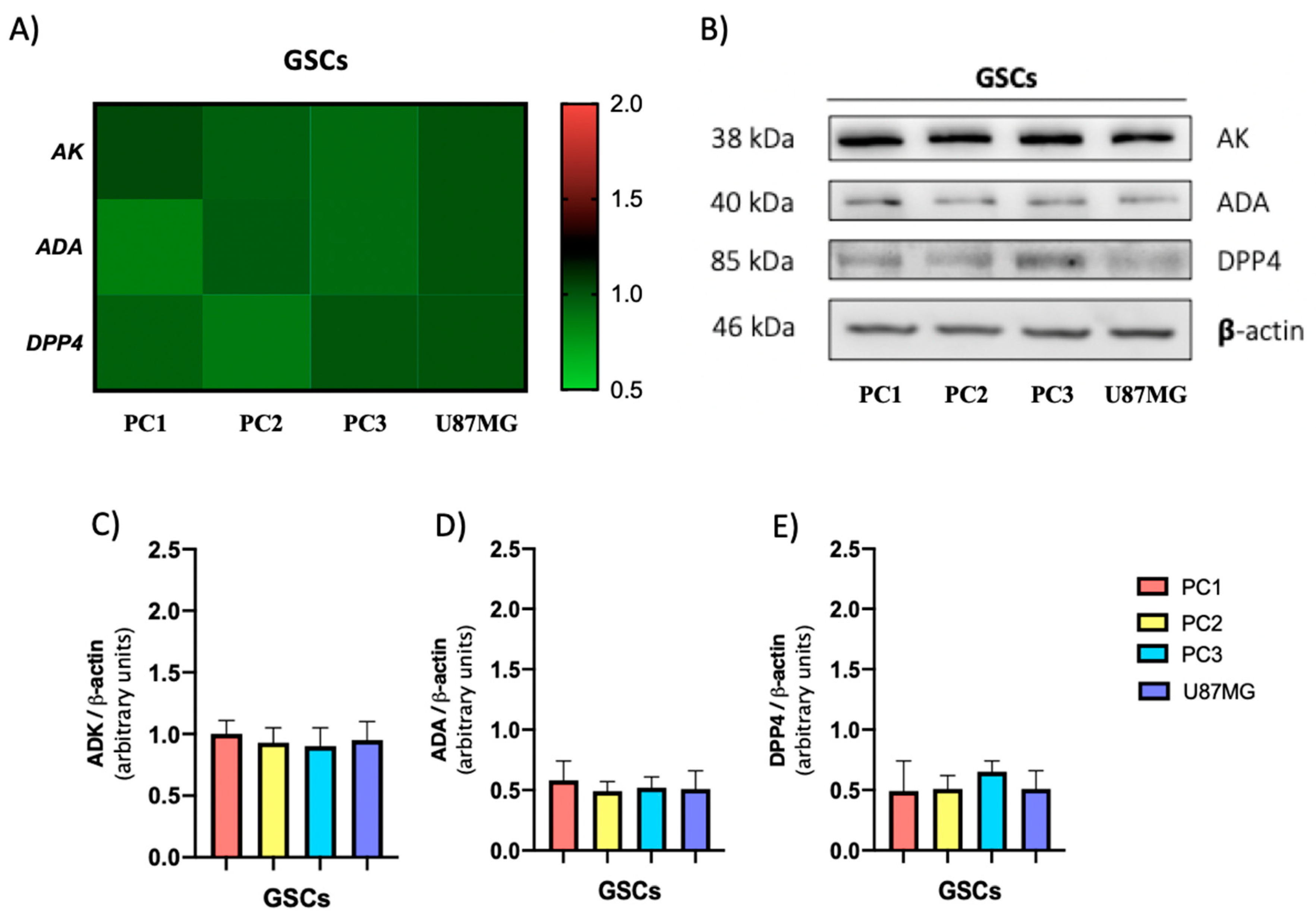
3.3. The GSC Subtypes Differentially Express Adenosine Producing Enzymes But Not Adenosine Metabolizing Enzymes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hanif, F.; Muzaffar, K.; Perveen, K.; Malhi, S.M.; Simjee, S.U. Glioblastoma multiforme: A review of its epidemiology and pathogenesis through clinical presentation and treatment. Asian Pac. J. Cancer Prev. 2017. [Google Scholar]
- Bahadur, S.; Kumar Sahu, A.; Baghel, P.; Saha, S. Current promising treatment strategy for glioblastoma multiform: A review. Oncol. Rev. 2019. [Google Scholar] [CrossRef]
- Lee, D.H.; Ryu, H.W.; Won, H.R.; Kwon, S.H. Advances in epigenetic glioblastoma therapy. Oncotarget 2017. [Google Scholar] [CrossRef]
- Wolf, K.J.; Chen, J.; Coombes, J.D.; Aghi, M.K.; Kumar, S. Dissecting and rebuilding the glioblastoma microenvironment with engineered materials. Nat. Rev. Mater. 2019. [Google Scholar] [CrossRef]
- Shergalis, A.; Bankhead, A.; Luesakul, U.; Muangsin, N.; Neamati, N. Current challenges and opportunities in treating glioblastomas. Pharmacol. Rev. 2018. [Google Scholar] [CrossRef]
- Torres, Á.; Erices, J.I.; Sanchez, F.; Ehrenfeld, P.; Turchi, L.; Virolle, T.; Uribe, D.; Niechi, I.; Spichiger, C.; Rocha, J.D.; et al. Extracellular adenosine promotes cell migration/invasion of Glioblastoma Stem-like Cells through A 3 Adenosine Receptor activation under hypoxia. Cancer Lett. 2019. [Google Scholar] [CrossRef]
- Kalra, B.; Kannan, S.; Gupta, T. Optimal adjuvant therapy in elderly glioblastoma: Results from a systematic review and network meta-analysis. J. Neurooncol. 2020. [Google Scholar] [CrossRef]
- Zhu, P.; Du, X.L.; Lu, G.; Zhu, J.J. Survival benefit of glioblastoma patients after FDA approval of temozolomide concomitant with radiation and bevacizumab: A population-based study. Oncotarget 2017. [Google Scholar] [CrossRef]
- Bradshaw, A.; Wickremsekera, A.; Tan, S.T.; Peng, L.; Davis, P.F.; Itinteang, T. Cancer Stem Cell Hierarchy in Glioblastoma Multiforme. Front. Surg. 2016. [Google Scholar] [CrossRef]
- Safa, A.R.; Saadatzadeh, M.R.; Cohen-Gadol, A.A.; Pollok, K.E.; Bijangi-Vishehsaraei, K. Glioblastoma stem cells (GSCs) epigenetic plasticity and interconversion between differentiated non-GSCs and GSCs. Genes Dis. 2015. [Google Scholar] [CrossRef] [PubMed]
- Krichevsky, A.M.; Uhlmann, E.J. Oligonucleotide Therapeutics as a New Class of Drugs for Malignant Brain Tumors: Targeting mRNAs, Regulatory RNAs, Mutations, Combinations, and Beyond. Neurotherapeutics 2019. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Alvarez, A.; Pangeni, R.; Horbinski, C.; Lu, S.; James, C.D.; Raizer, J.; Brenann, C.; Sulman, E.; Finocchiaro, G.; et al. CSIG-08. A regulatory circuit of miR-125b/miR-20b and Wnt signalling controls glioblastoma phenotypes through FZD6-modulated pathways. Neuro. Oncol. 2016. [Google Scholar] [CrossRef]
- Bhat, K.P.L.; Balasubramaniyan, V.; Vaillant, B.; Ezhilarasan, R.; Hummelink, K.; Hollingsworth, F.; Wani, K.; Heathcock, L.; James, J.D.; Goodman, L.D.; et al. Mesenchymal Differentiation Mediated by NF-κB Promotes Radiation Resistance in Glioblastoma. Cancer Cell 2013. [Google Scholar] [CrossRef] [PubMed]
- Mao, P.; Joshi, K.; Li, J.; Kim, S.H.; Li, P.; Santana-Santos, L.; Luthra, S.; Chandran, U.R.; Benos, P.V.; Smith, L.; et al. Mesenchymal glioma stem cells are maintained by activated glycolytic metabolism involving aldehyde dehydrogenase 1A3. Proc. Natl. Acad. Sci. USA 2013. [Google Scholar] [CrossRef]
- Minata, M.; Audia, A.; Shi, J.; Lu, S.; Bernstock, J.; Pavlyukov, M.S.; Das, A.; Kim, S.H.; Shin, Y.J.; Lee, Y.; et al. Phenotypic Plasticity of Invasive Edge Glioma Stem-like Cells in Response to Ionizing Radiation. Cell Rep. 2019. [Google Scholar] [CrossRef] [PubMed]
- Garnier, D.; Renoult, O.; Alves-Guerra, M.C.; Paris, F.; Pecqueur, C. Glioblastoma stem-like cells, Metabolic strategy to kill a challenging target. Front. Oncol. 2019. [Google Scholar] [CrossRef]
- Auffinger, B.; Spencer, D.; Pytel, P.; Ahmed, A.U.; Lesniak, M.S. The role of glioma stem cells in chemotherapy resistance and glioblastoma multiforme recurrence. Expert Rev. Neurother. 2015. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Hsieh, I.Y.; Huang, X.; Li, J.; Zhao, W. Glioblastoma stem-like cells: Characteristics, microenvironment, and therapy. Front. Pharmacol. 2016. [Google Scholar] [CrossRef]
- Torres, A.; Vargas, Y.; Uribe, D.; Jaramillo, C.; Gleisner, A.; Salazar-Onfray, F.; López, M.N.; Melo, R.; Oyarzún, C.; Martín, R.S.; et al. Adenosine A 3 receptor elicits chemoresistance mediated by multiple resistance-associated protein-1 in human glioblastoma stem-like cells. Oncotarget 2016. [Google Scholar] [CrossRef]
- Ceruti, S.; Abbracchio, M.P. Adenosine Signaling in Glioma Cells. In Advances in Experimental Medicine and Biology; Springer: Dordrecht, The Netherlands, 2020. [Google Scholar]
- Yan, A.; Joachims, M.L.; Thompson, L.F.; Miller, A.D.; Canoll, P.D.; Bynoe, M.S. CD73 promotes glioblastoma pathogenesis and enhances its chemoresistance via A2B adenosine receptor signaling. J. Neurosci. 2019. [Google Scholar] [CrossRef]
- Soliman, A.M.; Fathalla, A.M.; Moustafa, A.A. Adenosine role in brain functions: Pathophysiological influence on Parkinson’s disease and other brain disorders. Pharmacol. Rep. 2018. [Google Scholar] [CrossRef]
- Fried, N.T.; Elliott, M.B.; Oshinsky, M.L. The role of adenosine signaling in headache: A review. Brain Sci. 2017, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, K.A.; Gao, Z.G. Adenosine receptors as therapeutic targets. Nat. Rev. Drug Discov. 2006. [Google Scholar] [CrossRef] [PubMed]
- Niechi, I.; Uribe-Ojeda, A.; Erices, J.I.; Torres, Á.; Uribe, D.; Rocha, J.D.; Silva, P.; Richter, H.G.; San Martín, R.; Quezada, C. Adenosine Depletion as A New Strategy to Decrease Glioblastoma Stem-Like Cells Aggressiveness. Cells 2019, 8, 1353. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.Z.; Wang, X.; Bai, Y.F.; Liao, H.Z.; Qiu, S.C.; Yang, Y.Q.; Yan, X.H.; Chen, J.; Guo, H.B.; Zhang, S.Z. The HIF-2alpha dependent induction of PAP and adenosine synthesis regulates glioblastoma stem cell function through the A2B adenosine receptor. Int. J. Biochem. Cell Biol. 2014. [Google Scholar] [CrossRef] [PubMed]
- Quezada, C.; Garrido, W.; Oyarzún, C.; Fernández, K.; Segura, R.; Melo, R.; Casanello, P.; Sobrevia, L.; San Martín, R. 5′-ectonucleotidase mediates multiple-drug resistance in glioblastoma multiforme cells. J. Cell. Physiol. 2013. [Google Scholar] [CrossRef]
- Boswell-Casteel, R.C.; Hays, F.A. Equilibrative nucleoside transporters—A review. Nucleosides Nucleotides Nucleic Acids 2017. [Google Scholar] [CrossRef]
- Pastor-Anglada, M.; Pérez-Torras, S. Emerging roles of nucleoside transporters. Front. Pharmacol. 2018. [Google Scholar] [CrossRef]
- Pastor-Anglada, M.; Pérez-Torras, S. Who is who in Adenosine transport. Front. Pharmacol. 2018. [Google Scholar] [CrossRef]
- Köhler, D.; Streißenberger, A.; Morote-García, J.C.; Granja, T.F.; Schneider, M.; Straub, A.; Boison, D.; Rosenberger, P. Inhibition of Adenosine Kinase Attenuates Acute Lung Injury. Crit. Care Med. 2016. [Google Scholar] [CrossRef]
- Alarcón, S.; Garrido, W.; Cappelli, C.; Suárez, R.; Oyarzún, C.; Quezada, C.; San Martín, R. Deficient Insulin-mediated Upregulation of the Equilibrative Nucleoside Transporter 2 Contributes to Chronically Increased Adenosine in Diabetic Glomerulopathy. Sci. Rep. 2017. [Google Scholar] [CrossRef]
- Kretschmar, C.; Oyarzún, C.; Villablanca, C.; Jaramillo, C.; Alarcón, S.; Perez, G.; Díaz-Encarnación, M.M.; Pastor-Anglada, M.; Garrido, W.; Quezada, C.; et al. Reduced Adenosine Uptake and Its Contribution to Signaling that Mediates Profibrotic Activation in Renal Tubular Epithelial Cells: Implication in Diabetic Nephropathy. PLoS ONE 2016, 11, e0147430. [Google Scholar] [CrossRef] [PubMed]
- Rehan, S.; Shahid, S.; Salminen, T.A.; Jaakola, V.P.; Paavilainen, V.O. Current Progress on Equilibrative Nucleoside Transporter Function and Inhibitor Design. SLAS Discov. 2019. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zeng, X.; Shi, Y.; Liu, M. Functional characterization of human equilibrative nucleoside transporter 1. Protein Cell 2017. [Google Scholar] [CrossRef] [PubMed]
- García-Romero, N.; González-Tejedo, C.; Carrión-Navarro, J.; Esteban-Rubio, S.; Rackov, G.; Rodríguez-Fanjul, V.; Oliver-De La Cruz, J.; Prat-Acín, R.; Peris-Celda, M.; Blesa, D.; et al. Cancer stem cells from human glioblastoma resemble but do not mimic original tumors after in vitro passaging in serum-free media. Oncotarget 2016. [Google Scholar] [CrossRef] [PubMed]
- Oyarzún, C.; Salinas, C.; Gómez, D.; Jaramillo, K.; Pérez, G.; Alarcón, S.; Podestá, L.; Flores, C.; Quezada, C.; San Martín, R. Increased levels of adenosine and ecto 5′-nucleotidase (CD73) activity precede renal alterations in experimental diabetic rats. Biochem. Biophys. Res. Commun. 2015. [Google Scholar] [CrossRef]
- Guardia, G.D.A.; Correa, B.R.; Araujo, P.R.; Qiao, M.; Burns, S.; Penalva, L.O.F.; Galante, P.A.F. Proneural and mesenchymal glioma stem cells display major differences in splicing and lncRNA profiles. NPJ Genom. Med. 2020. [Google Scholar] [CrossRef]
- Rocha, R.; Torres, Á.; Ojeda, K.; Uribe, D.; Rocha, D.; Erices, J.; Niechi, I.; Ehrenfeld, P.; Martín, R.S.; Quezada, C. The adenosine A3 receptor regulates differentiation of glioblastoma stem-like cells to endothelial cells under hypoxia. Int. J. Mol. Sci. 2018, 19, 1228. [Google Scholar] [CrossRef]
- Cho, S.Y.; Polster, J.; Engles, J.M.; Hilton, J.; Abraham, E.H.; Wahl, R.L. In vitro evaluation of adenosine 5′-monophosphate as an imaging agent of tumor metabolism. J. Nucl. Med. 2006. [Google Scholar]
- Westermeier, F.; Salomón, C.; González, M.; Puebla, C.; Guzmán-Gutiérrez, E.; Cifuentes, F.; Leiva, A.; Casanello, P.; Sobrevia, L. Insulin restores gestational diabetes mellitus-reduced adenosine transport involving differential expression of insulin receptor isoforms in human umbilical vein endothelium. Diabetes 2011. [Google Scholar] [CrossRef]
- Aguayo, C.; Casado, J.; González, M.; Pearson, J.D.; San Martín, R.; Casanello, P.; Pastor-Anglada, M.; Sobrevia, L. Equilibrative nucleoside transporter 2 is expressed in human umbilical vein endothelium, but is not involved in the inhibition of adenosine transport induced by hyperglycaemia. Placenta 2005. [Google Scholar] [CrossRef]
- Parodi, J.; Flores, C.; Aguayo, C.; Rudolph, M.I.; Casanello, P.; Sobrevia, L. Inhibition of nitrobenzylthioinosine-sensitive adenosine transport by elevated elevated D-glucose involves activation of P2Y2 purinoceptors in human umbilical vein endothelial cells. Circ. Res. 2002. [Google Scholar] [CrossRef] [PubMed]
- Bone, D.B.J.; Robillard, K.R.; Stolk, M.; Hammond, J.R. Differential regulation of mouse equilibrative nucleoside transporter 1 (mENT1) splice variants by protein kinase CK2. Mol. Membr. Biol. 2007. [Google Scholar] [CrossRef] [PubMed]
- Grañe-Boladeras, N.; Williams, D.; Tarmakova, Z.; Stevanovic, K.; Villani, L.A.; Mehrabi, P.; Michael Siu, K.W.; Pastor-Anglada, M.; Coe, I.R. Oligomerization of equilibrative nucleoside transporters: A novel regulatory and functional mechanism involving PKC and PP1. FASEB J. 2019. [Google Scholar] [CrossRef]
- Eltzschig, H.K.; Abdulla, P.; Hoffman, E.; Hamilton, K.E.; Daniels, D.; Schönfeld, C.; Löffler, M.; Reyes, G.; Duszenko, M.; Karhausen, J.; et al. HIF-1-dependent repression of equilibrative nucleoside transporter (ENT) in hypoxia. J. Exp. Med. 2005. [Google Scholar] [CrossRef] [PubMed]
- Morote-Garcia, J.C.; Rosenberger, P.; Nivillac, N.M.I.; Coe, I.R.; Eltzschig, H.K. Hypoxia-Inducible Factor-Dependent Repression of Equilibrative Nucleoside Transporter 2 Attenuates Mucosal Inflammation During Intestinal Hypoxia. Gastroenterology 2009. [Google Scholar] [CrossRef]
- Jin, X.; Kim, L.J.Y.; Wu, Q.; Wallace, L.C.; Prager, B.C.; Sanvoranart, T.; Gimple, R.C.; Wang, X.; Mack, S.C.; Miller, T.E.; et al. Targeting glioma stem cells through combined BMI1 and EZH2 inhibition. Nat. Med. 2017. [Google Scholar] [CrossRef]
- Behnan, J.; Finocchiaro, G.; Hanna, G. The landscape of the mesenchymal signature in brain tumours. Brain 2019. [Google Scholar] [CrossRef]
- Ho, I.A.W.; Shim, W.S.N. Contribution of the microenvironmental niche to glioblastoma heterogeneity. Biomed Res. Int. 2017. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, C.; Shi, H.; Wu, D.; Ying, W. Extracellular degradation into adenosine and the activities of adenosine kinase and AMPK mediate extracellular NAD+-produced increases in the adenylate pool of BV2 microglia under basal conditions. Front. Cell. Neurosci. 2018. [Google Scholar] [CrossRef]
- Pardo, F.; Arroyo, P.; Salomón, C.; Westermeier, F.; Salsoso, R.; Sáez, T.; Guzmán-Gutiérrez, E.; Leiva, A.; Sobrevia, L. Role of equilibrative adenosine transporters and adenosine receptors as modulators of the human placental endothelium in gestational diabetes mellitus. Placenta 2013. [Google Scholar] [CrossRef]
- Escudero, C.; Casanello, P.; Sobrevia, L. Human Equilibrative Nucleoside Transporters 1 and 2 may be Differentially Modulated by A2B Adenosine Receptors in Placenta Microvascular Endothelial Cells from Pre-eclampsia. Placenta 2008. [Google Scholar] [CrossRef] [PubMed]
- Shan, D.; Haroutunian, V.; Meador-Woodruff, J.H.; McCullumsmith, R.E. Expression of equilibrative nucleoside transporter type 1 protein in elderly patients with schizophrenia. Neuroreport 2012. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cui, M.; Bai, X.; Li, T.; Chen, F.; Dong, Q.; Zhao, Y.; Liu, X. Decreased Extracellular Adenosine Levels Lead to Loss of Hypoxia-Induced Neuroprotection after Repeated Episodes of Exposure to Hypoxia. PLoS ONE 2013, 8, e57065. [Google Scholar] [CrossRef]
- Kao, Y.H.; Lin, M.S.; Chen, C.M.; Wu, Y.R.; Chen, H.M.; Lai, H.L.; Chern, Y.; Lin, C.J. Targeting ENT1 and adenosine tone for the treatment of Huntington’s disease. Hum. Mol. Genet. 2017. [Google Scholar] [CrossRef] [PubMed]
- Rose, J.B.; Naydenova, Z.; Bang, A.; Eguchi, M.; Sweeney, G.; Choi, D.S.; Hammond, J.R.; Coe, I.R. Equilibrative nucleoside transporter 1 plays an essential role in cardioprotection. Am. J. Physiol.-Hear. Circ. Physiol. 2010. [Google Scholar] [CrossRef]
- Rose, J.B.; Naydenova, Z.; Bang, A.; Ramadan, A.; Klawitter, J.; Schram, K.; Sweeney, G.; Grenz, A.; Eltzschig, H.; Hammond, J.; et al. Absence of equilibrative nucleoside transporter 1 in ENT1 knockout mice leads to altered nucleoside levels following hypoxic challenge. Life Sci. 2011. [Google Scholar] [CrossRef]
- You, G.; Morris, M.E. Drug Transporters: Molecular Characterization and Role in Drug Disposition; John Wiley and Sons: Hoboken, NJ, USA, 2006; ISBN 9780471784913. [Google Scholar]
- Cappellari, A.R.; Rockenbach, L.; Dietrich, F.; Clarimundo, V.; Glaser, T.; Braganhol, E.; Abujamra, A.L.; Roesler, R.; Ulrich, H.; Liveira Battastini, A.M. Characterization of Ectonucleotidases in Human Medulloblastoma Cell Lines: Ecto-5′NT/CD73 in Metastasis as Potential Prognostic Factor. PLoS ONE 2012, 7, e47468. [Google Scholar] [CrossRef]
- Dziembor-Gryszkiewicz, E.; Fikus, M.; Kazimierczuk, Z.; Ostrowski, W. Activity of human prostatic acid phosphatase toward purine 5′-phosphonucleosides. Bull. Acad. Pol. Sci. Ser. Sci. Biol. 1978. [Google Scholar]
- Lam, K.W.; Li, O.; Li, C.Y.; Yam, L.T. Biochemical properties of human prostatic acid phosphatase. Clin. Chem. 1973. [Google Scholar] [CrossRef]
- Raza, R.; Saeed, A.; Lecka, J.; Sevigny, J.; Iqbal, J. Identification of Small Molecule Sulfonic Acids as Ecto-5′-Nucleotidase Inhibitors. Med. Chem. (Los Angeles) 2012. [Google Scholar] [CrossRef]
- Schneider, E.; Rissiek, A.; Winzer, R.; Puig, B.; Rissiek, B.; Haag, F.; Mittrücker, H.W.; Magnus, T.; Tolosa, E. Generation and Function of Non-cell-bound CD73 in Inflammation. Front. Immunol. 2019. [Google Scholar] [CrossRef]
- Jiang, B. Aerobic glycolysis and high level of lactate in cancer metabolism and microenvironment. Genes Dis. 2017. [Google Scholar] [CrossRef]
- Hu, X.; Chao, M.; Wu, H. Central role of lactate and proton in cancer cell resistance to glucose deprivation and its clinical translation. Signal Transduct. Target. Ther. 2017. [Google Scholar] [CrossRef]
- Huber, V.; Camisaschi, C.; Berzi, A.; Ferro, S.; Lugini, L.; Triulzi, T.; Tuccitto, A.; Tagliabue, E.; Castelli, C.; Rivoltini, L. Cancer acidity: An ultimate frontier of tumor immune escape and a novel target of immunomodulation. Semin. Cancer Biol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Sowa, N.A.; Vadakkan, K.I.; Zylka, M.J. Recombinant mouse PAP has pH-dependent ectonucleotidase activity and acts through A1-adenosine receptors to mediate antinociception. PLoS ONE 2009, 4, e4248. [Google Scholar] [CrossRef] [PubMed]
- Mandapathil, M.; Szczepanski, M.J.; Harasymczuk, M.; Ren, J.; Cheng, D.; Jackson, E.K.; Gorelik, E.; Johnson, J.T.; Lang, S.; Whiteside, T.L. CD26 expression and adenosine deaminase activity in regulatory T cells (Treg) and cd4+ T effector cells in patients with head and neck squamous cell carcinoma. Oncoimmunology 2012. [Google Scholar] [CrossRef] [PubMed]
- Flinn, A.M.; Gennery, A.R. Adenosine deaminase deficiency: A review. Orphanet J. Rare Dis. 2018. [Google Scholar] [CrossRef]
- Whitmore, K.V.; Gaspar, H.B. Adenosine deaminase deficiency—more than just an immunodeficiency. Front. Immunol. 2016. [Google Scholar] [CrossRef]
- Silva-Vilches, C.; Ring, S.; Mahnke, K. ATP and its metabolite adenosine as regulators of dendritic cell activity. Front. Immunol. 2018. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alarcón, S.; Toro, M.d.l.Á.; Villarreal, C.; Melo, R.; Fernández, R.; Ayuso Sacido, A.; Uribe, D.; San Martín, R.; Quezada, C. Decreased Equilibrative Nucleoside Transporter 1 (ENT1) Activity Contributes to the High Extracellular Adenosine Levels in Mesenchymal Glioblastoma Stem-Like Cells. Cells 2020, 9, 1914. https://doi.org/10.3390/cells9081914
Alarcón S, Toro MdlÁ, Villarreal C, Melo R, Fernández R, Ayuso Sacido A, Uribe D, San Martín R, Quezada C. Decreased Equilibrative Nucleoside Transporter 1 (ENT1) Activity Contributes to the High Extracellular Adenosine Levels in Mesenchymal Glioblastoma Stem-Like Cells. Cells. 2020; 9(8):1914. https://doi.org/10.3390/cells9081914
Chicago/Turabian StyleAlarcón, Sebastián, María de los Ángeles Toro, Carolina Villarreal, Rómulo Melo, Rodrigo Fernández, Angel Ayuso Sacido, Daniel Uribe, Rody San Martín, and Claudia Quezada. 2020. "Decreased Equilibrative Nucleoside Transporter 1 (ENT1) Activity Contributes to the High Extracellular Adenosine Levels in Mesenchymal Glioblastoma Stem-Like Cells" Cells 9, no. 8: 1914. https://doi.org/10.3390/cells9081914
APA StyleAlarcón, S., Toro, M. d. l. Á., Villarreal, C., Melo, R., Fernández, R., Ayuso Sacido, A., Uribe, D., San Martín, R., & Quezada, C. (2020). Decreased Equilibrative Nucleoside Transporter 1 (ENT1) Activity Contributes to the High Extracellular Adenosine Levels in Mesenchymal Glioblastoma Stem-Like Cells. Cells, 9(8), 1914. https://doi.org/10.3390/cells9081914




