Evidence Supporting an Antimicrobial Origin of Targeting Peptides to Endosymbiotic Organelles
Abstract
1. Introduction
2. Methods and Materials
2.1. Sequence Data Set
2.2. Peptide Description and Auto-Cross Covariance (ACC) Terms
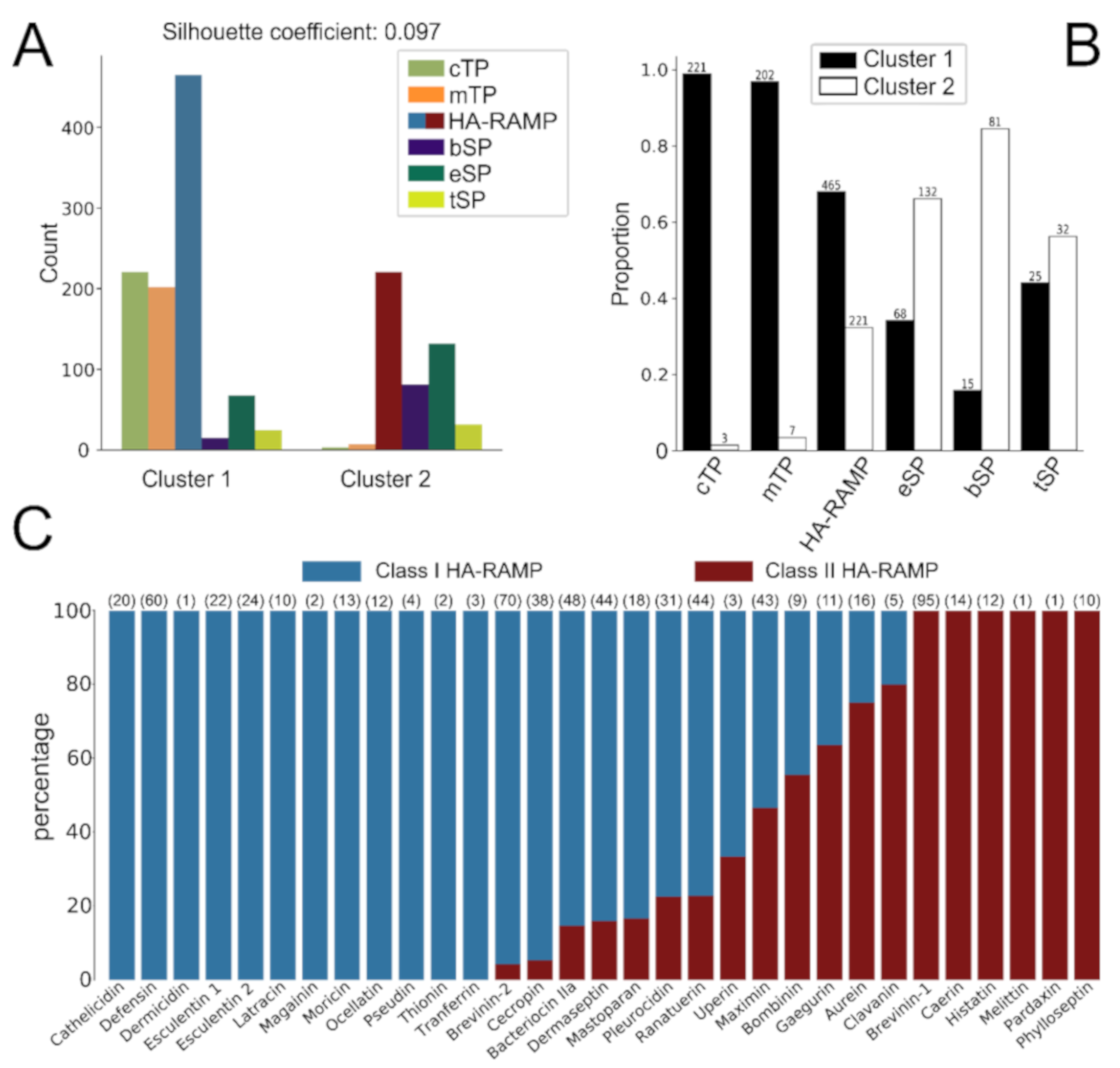
2.3. K-Means Clustering
2.4. Distance Trees
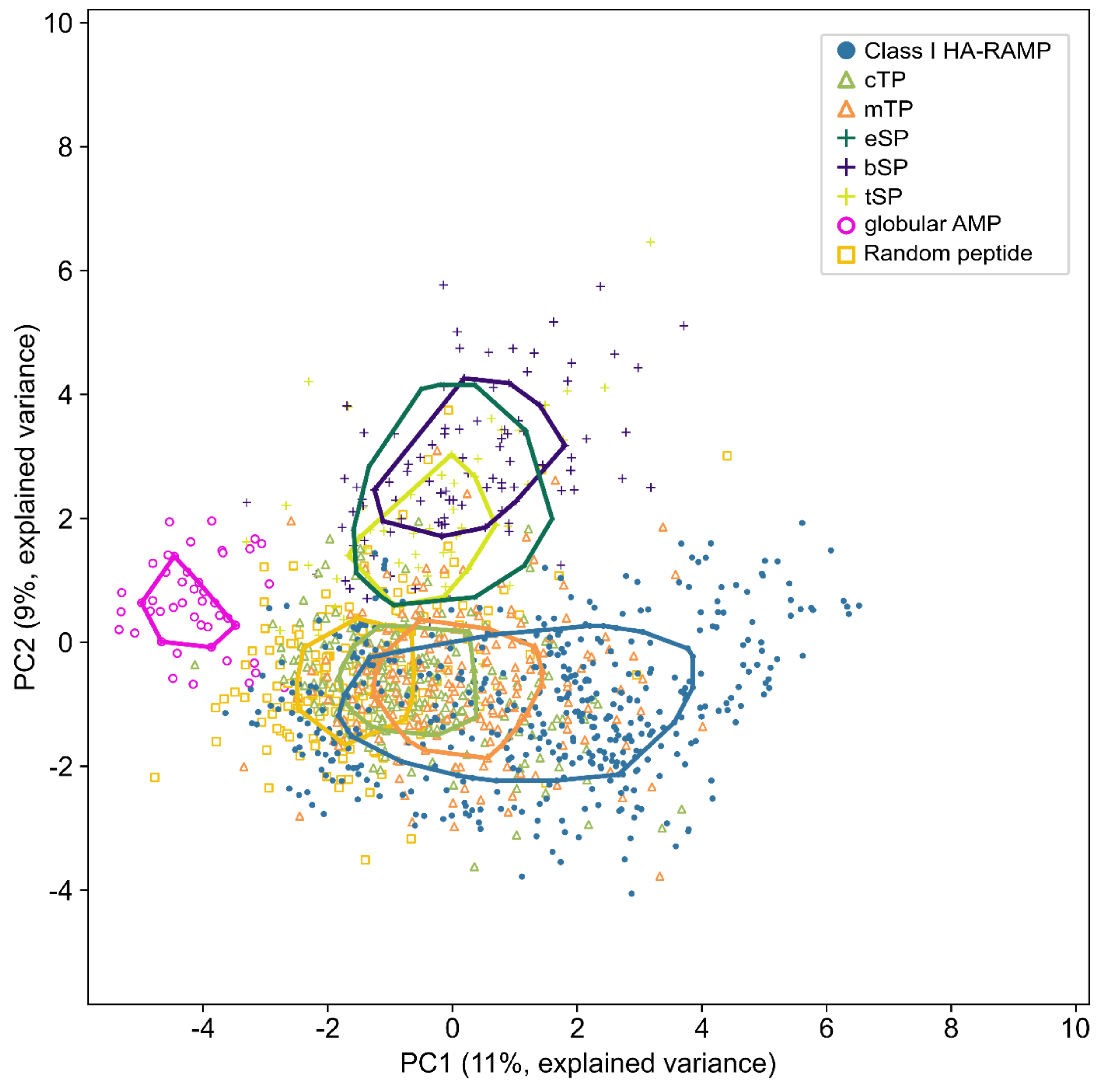
2.5. Vizualisation of Peptide Properties
2.6. Detection of cTP Motifs in HA-RAMPs and TP Predictions
2.7. Strains and Culture Conditions
2.8. Generation of Constructs
2.9. Microscopy
2.10. Biochemistry
2.11. Antimicrobial Activity Assays
2.12. Statistical Analysis
2.13. Code Availability
3. Results
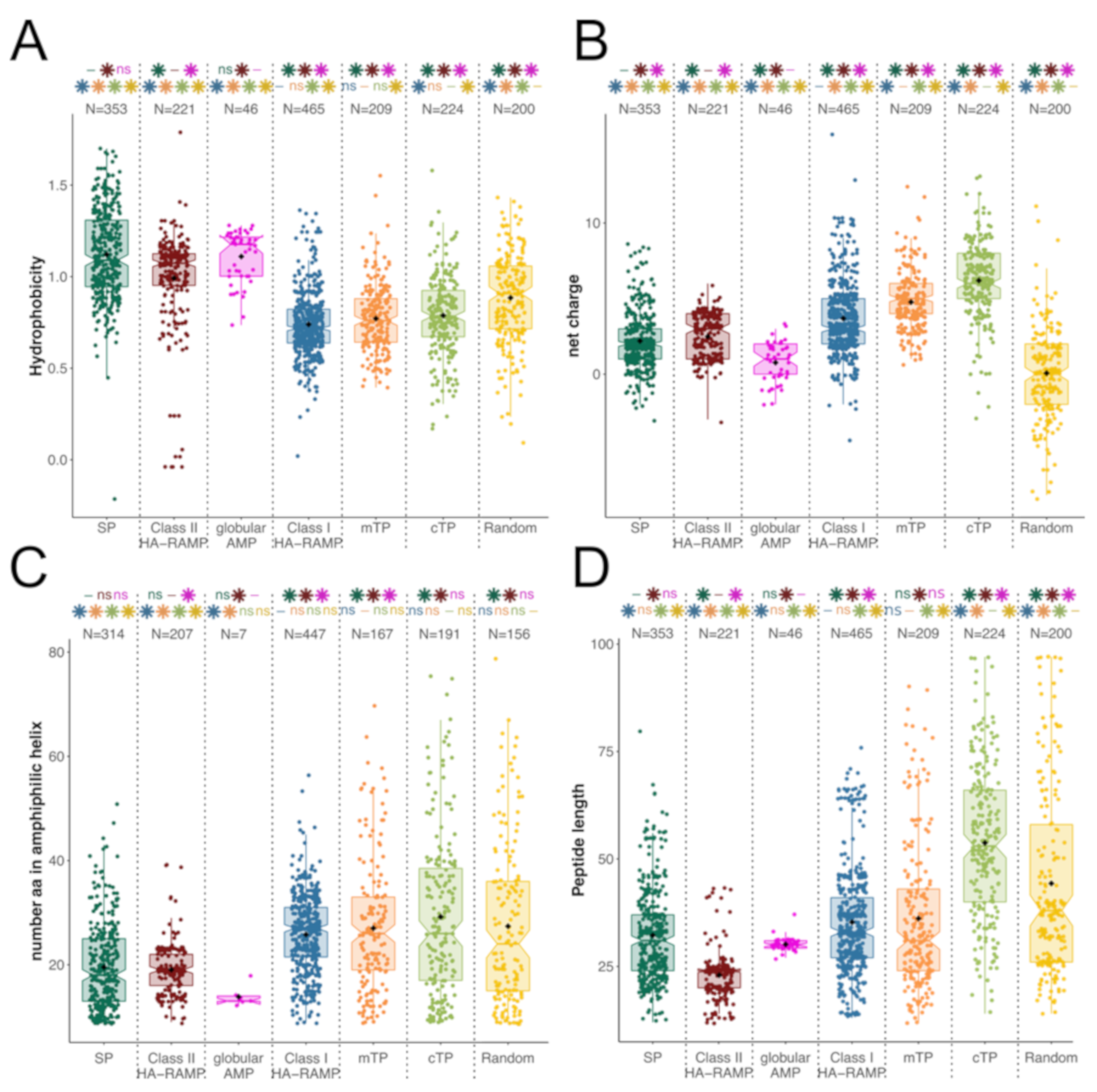
3.1. TPs and HA-RAMPs Share a Common Set of Physico-Chemical Properties
Peptide Families and Their Descriptors
3.2. HA-RAMPs Can Be Divided into Two Distinct Classes
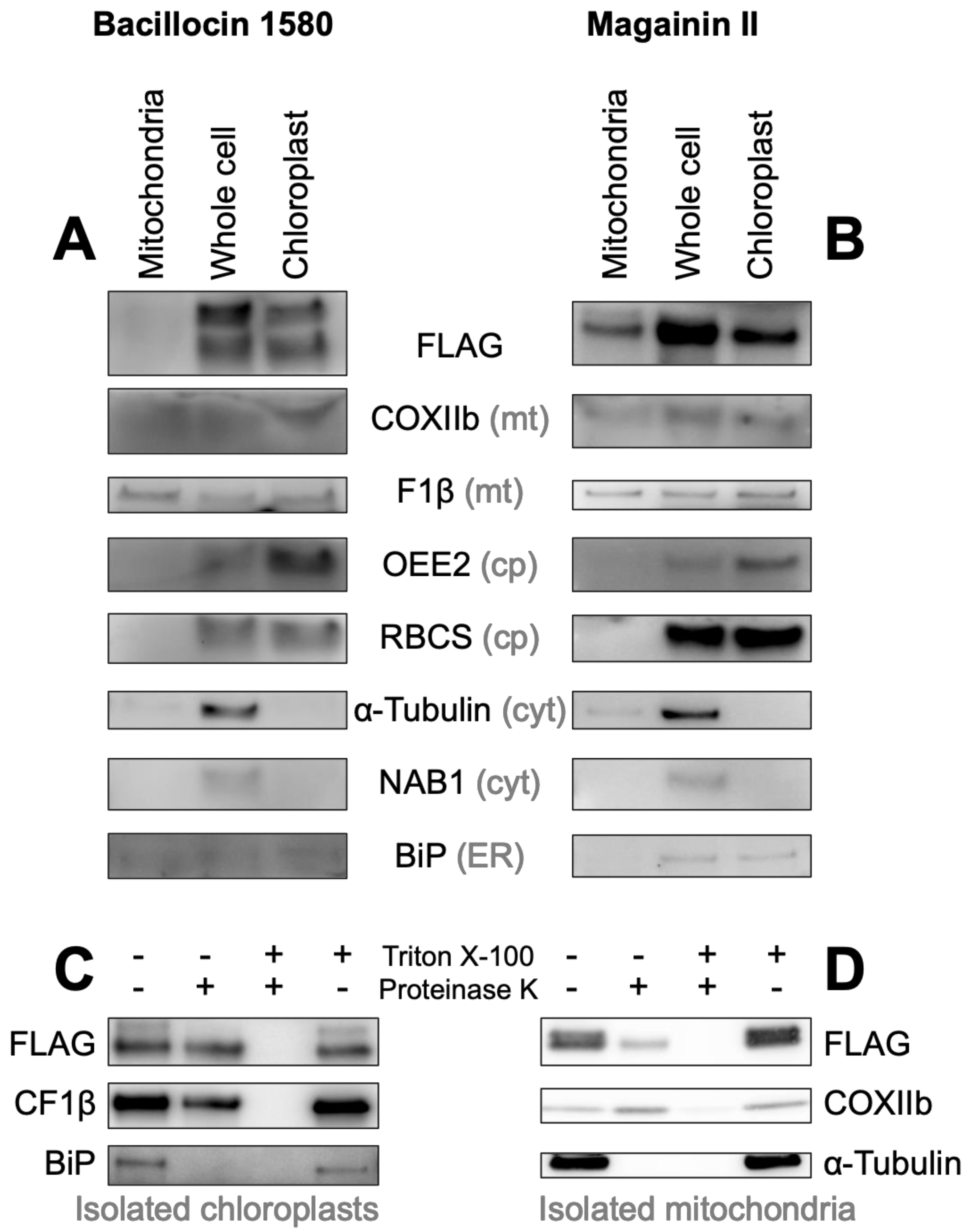
3.3. HA-RAMPs and TPs Show Dual Targeting and Antimicrobial Activities
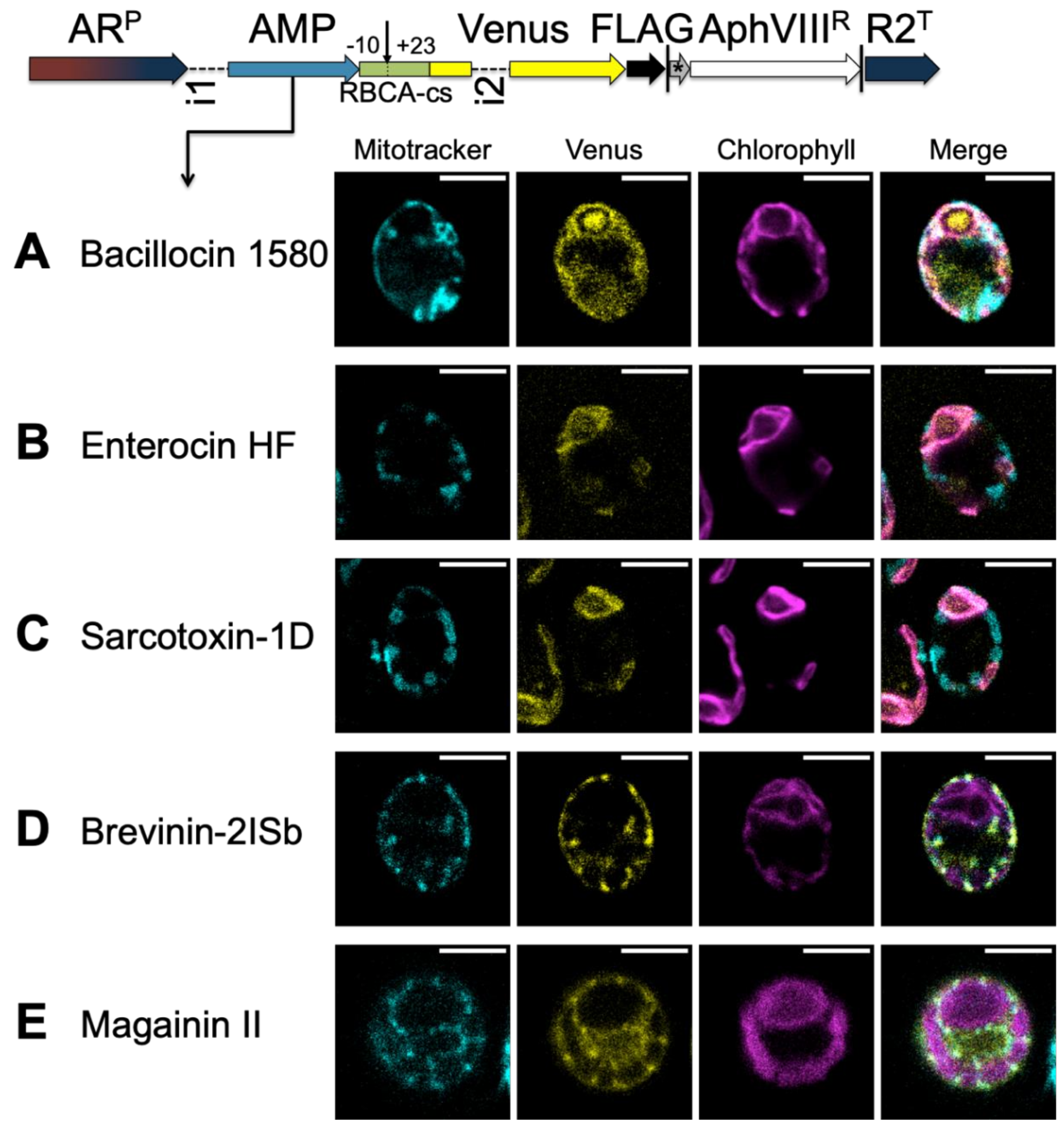
3.3.1. A TP Cleavage-Site Fragment Is Required for Import of the Venus Reporter
3.3.2. HA-RAMPs Target Venus to Endosymbiotic Organelles
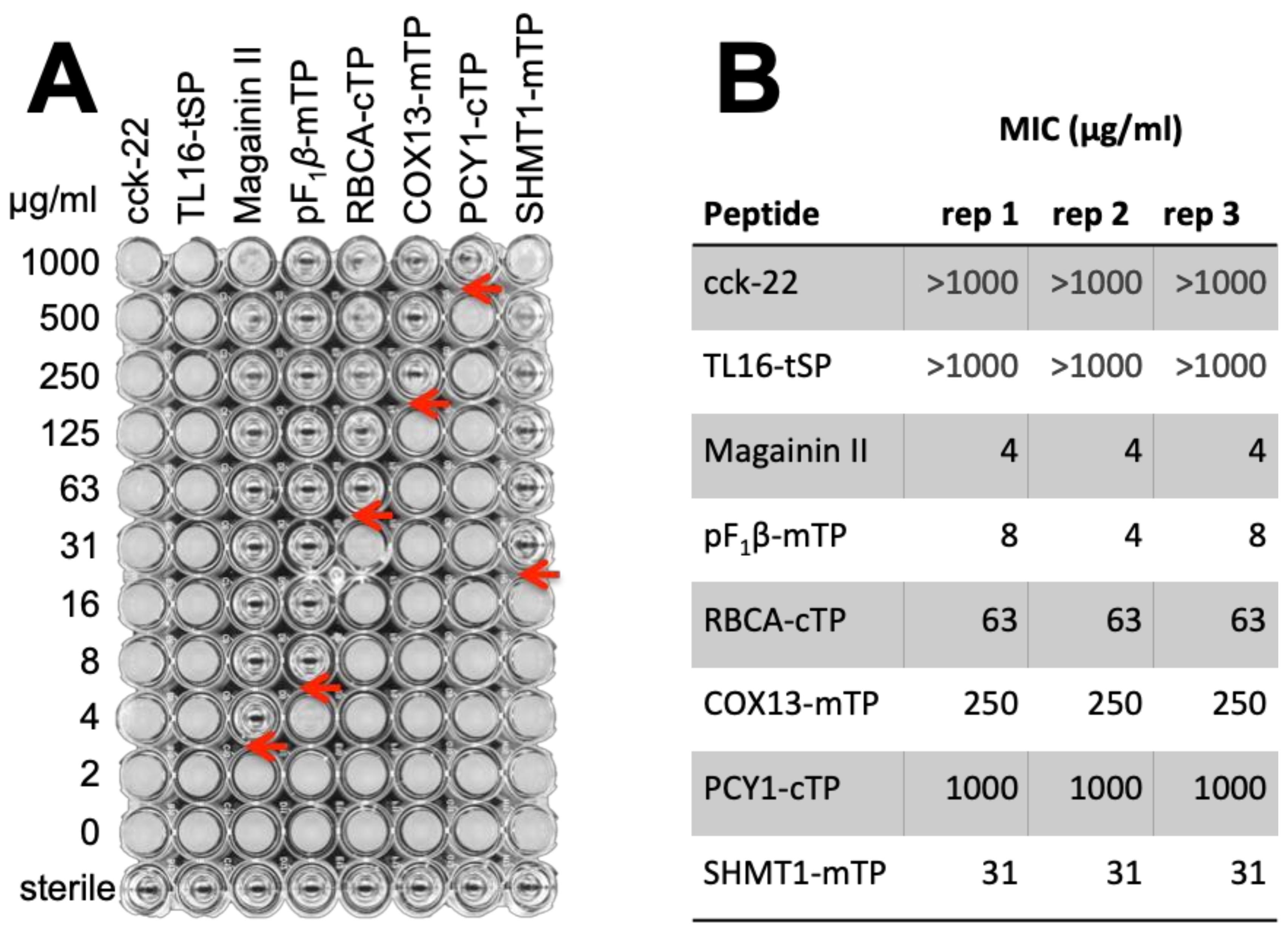
3.3.3. TPs Show Antimicrobial Activity
4. Discussion
4.1. Diversity of HA-RAMPs
4.2. Evidence for a Common Origin of TPs with a Class of HA-RAMPs
4.3. Efficient targeting to Extant Organelles Requires Specific Sequences Besides the Amphiphilic Helix of TPs
4.4. Targeting Peptides and the Translocation Machinery
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Martin, W.F.; Garg, S.; Zimorski, V. Endosymbiotic theories for eukaryote origin. Philos. Trans. R. Soc. Lond B Biol. Sci. 2015, 370. [Google Scholar] [CrossRef] [PubMed]
- Archibald, J.M. Endosymbiosis and Eukaryotic Cell Evolution. Curr. Biol. 2015, 25, R911–R921. [Google Scholar] [CrossRef] [PubMed]
- Timmis, J.N.; Ayliffe, M.A.; Huang, C.Y.; Martin, W. Endosymbiotic gene transfer: Organelle genomes forge eukaryotic chromosomes. Nat. Rev. Genet. 2004, 5, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Neupert, W.; Herrmann, J.M. Translocation of proteins into mitochondria. Annu. Rev. Biochem. 2007, 76, 723–749. [Google Scholar] [CrossRef] [PubMed]
- Chotewutmontri, P.; Holbrook, K.; Bruce, B.D. Chapter Six-Plastid Protein Targeting: Preprotein Recognition and Translocation. In International Review of Cell and Molecular Biology; Galluzzi, L., Ed.; Academic Press, 2017; Volume 330, pp. 227–294. [Google Scholar]
- von Heijne, G.; Steppuhn, J.; Herrmann, R.G. Domain structure of mitochondrial and chloroplast targeting peptides. Eur. J. Biochem. 1989, 180, 535–545. [Google Scholar] [CrossRef]
- Wiedemann, N.; Pfanner, N. Mitochondrial Machineries for Protein Import and Assembly. Annu. Rev. Biochem. 2017, 86, 685–714. [Google Scholar] [CrossRef]
- Nakai, M. New Perspectives on Chloroplast Protein Import. Plant Cell Physiol. 2018, 59, 1111–1119. [Google Scholar] [CrossRef]
- Fukasawa, Y.; Oda, T.; Tomii, K.; Imai, K. Origin and Evolutionary Alteration of the Mitochondrial Import System in Eukaryotic Lineages. Mol. Biol. Evol. 2017, 34, 1574–1586. [Google Scholar] [CrossRef]
- Reumann, S.; Inoue, K.; Keegstra, K. Evolution of the general protein import pathway of plastids (review). Mol. Membr. Biol. 2005, 22, 73–86. [Google Scholar] [CrossRef]
- Wollman, F.-A. An antimicrobial origin of transit peptides accounts for early endosymbiotic events. Traffic 2016, 17, 1322–1328. [Google Scholar] [CrossRef]
- Maróti, G.; Kereszt, A.; Kondorosi, É.; Mergaert, P.; Maro, G. Natural roles of antimicrobial peptides in microbes, plants and animals. Res. Microbiol. 2011, 162, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Besse, A.; Peduzzi, J.; Rebuffat, S.; Carré-Mlouka, A. Antimicrobial peptides and proteins in the face of extremes: Lessons from archaeocins. Biochimie 2015, 118, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Mergaert, P.; Kikuchi, Y.; Shigenobu, S.; Nowack, E.C.M. Metabolic Integration of Bacterial Endosymbionts through Antimicrobial Peptides. Trends Microbiol. 2017, 25, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Mergaert, P. Role of antimicrobial peptides in controlling symbiotic bacterial populations. Nat. Prod. Rep. 2018, 35, 336–356. [Google Scholar] [CrossRef] [PubMed]
- Ball, S.G.; Bhattacharya, D.; Weber, A.P.M. Pathogen to powerhouse. Science 2016, 351, 659–660. [Google Scholar] [CrossRef] [PubMed]
- Zachar, I.; Szathmáry, E. Breath-giving cooperation: Critical review of origin of mitochondria hypotheses. Biol. Direct 2017, 12, 19. [Google Scholar] [CrossRef] [PubMed]
- Andersson, S.G.E.; Zomorodipour, A.; Andersson, J.O.; Sicheritz-Pontén, T.; Alsmark, U.C.M.; Podowski, R.M.; Näslund, A.K.; Eriksson, A.-S.; Winkler, H.H.; Kurland, C.G. The genome sequence of Rickettsia prowazekii and the origin of mitochondria. Nature 1998, 396, 133–140. [Google Scholar] [CrossRef]
- Andrä, J.; Herbst, R.; Leippe, M. Amoebapores, archaic effector peptides of protozoan origin, are discharged into phagosomes and kill bacteria by permeabilizing their membranes. Dev. Comp. Immunol. 2003, 27, 291–304. [Google Scholar] [CrossRef]
- Mason, K.M.; Bruggeman, M.E.; Munson, R.S.; Bakaletz, L.O. The non-typeable Haemophilus influenzae Sap transporter provides a mechanism of antimicrobial peptide resistance and SapD-dependent potassium acquisition. Mol. Microbiol. 2006, 62, 1357–1372. [Google Scholar] [CrossRef]
- Hiron, A.; Falord, M.; Valle, J.; Débarbouillé, M.; Msadek, T. Bacitracin and nisin resistance in Staphylococcus aureus: A novel pathway involving the BraS/BraR two-component system (SA2417/SA2418) and both the BraD/BraE and VraD/VraE ABC transporters. Mol. Microbiol. 2011, 81, 602–622. [Google Scholar] [CrossRef]
- Shelton, C.L.; Raffel, F.K.; Beatty, W.L.; Johnson, S.M.; Mason, K.M. Sap transporter mediated import and subsequent degradation of antimicrobial peptides in Haemophilus. PLoS Pathog. 2011, 7, e1002360. [Google Scholar] [CrossRef] [PubMed]
- Rinker, S.D.; Gu, X.; Fortney, K.R.; Zwickl, B.W.; Katz, B.P.; Janowicz, D.M.; Spinola, S.M.; Bauer, M.E. Permeases of the Sap Transporter Are Required for Cathelicidin Resistance and Virulence of Haemophilus ducreyi in Humans. J. Infect. Dis. 2012, 206, 1407–1414. [Google Scholar] [CrossRef] [PubMed]
- Runti, G.; Lopez Ruiz, M.D.C.; Stoilova, T.; Hussain, R.; Jennions, M.; Choudhury, H.G.; Benincasa, M.; Gennaro, R.; Beis, K.; Scocchi, M. Functional characterization of SbmA, a bacterial inner membrane transporter required for importing the antimicrobial peptide Bac7(1-35). J. Bacteriol. 2013, 195, 5343–5351. [Google Scholar] [CrossRef] [PubMed]
- Guefrachi, I.; Pierre, O.; Timchenko, T.; Alunni, B.; Barrière, Q.; Czernic, P.; Villaécija-Aguilar, J.-A.; Verly, C.; Bourge, M.; Fardoux, J.; et al. Bradyrhizobium BclA Is a Peptide Transporter Required for Bacterial Differentiation in Symbiosis with Aeschynomene Legumes. MPMI 2015, 28, 1155–1166. [Google Scholar] [CrossRef]
- Wang, Z.; Bie, P.; Cheng, J.; Lu, L.; Cui, B.; Wu, Q. The ABC transporter YejABEF is required for resistance to antimicrobial peptides and the virulence of Brucella melitensis. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef]
- Ford Doolittle, W. You are what you eat: A gene transfer ratchet could account for bacterial genes in eukaryotic nuclear genomes. Trends Genet. 1998, 14, 307–311. [Google Scholar] [CrossRef]
- Clarke, M.; Lohan, A.J.; Liu, B.; Lagkouvardos, I.; Roy, S.; Zafar, N.; Bertelli, C.; Schilde, C.; Kianianmomeni, A.; Bürglin, T.R.; et al. Genome of Acanthamoeba castellanii highlights extensive lateral gene transfer and early evolution of tyrosine kinase signaling. Genome Biol. 2013, 14, R11. [Google Scholar] [CrossRef]
- Bechinger, B.; Zasloff, M.; Opella, S.J. Structure and orientation of the antibiotic peptide magainin in membranes by solid-state nuclear magnetic resonance spectroscopy. Protein Sci. 1993, 2, 2077–2084. [Google Scholar] [CrossRef]
- Bechinger, B. The structure, dynamics and orientation of antimicrobial peptides in membranes by multidimensional solid-state NMR spectroscopy. Biochim. Biophys. Acta 1999, 1462, 157–183. [Google Scholar] [CrossRef]
- Roise, D.; Horvath, S.J.; Tomich, J.M.; Richards, J.H.; Schatz, G. A chemically synthesized pre-sequence of an imported mitochondrial protein can form an amphiphilic helix and perturb natural and artificial phospholipid bilayers. EMBO J. 1986, 5, 1327–1334. [Google Scholar] [CrossRef]
- Roise, D.; Theiler, F.; Horvath, S.J.; Tomich, J.M.; Richards, J.H.; Allison, D.S.; Schatz, G. Amphiphilicity is essential for mitochondrial presequence function. EMBO J. 1988, 7, 649–653. [Google Scholar] [CrossRef] [PubMed]
- Lemire, B.D.; Fankhauser, C.; Baker, A.; Schatz, G. The mitochondrial targeting function of randomly generated peptide sequences correlates with predicted helical amphiphilicity. J. Biol. Chem. 1989, 264, 20206–20215. [Google Scholar] [PubMed]
- Bhushan, S.; Kuhn, C.; Berglund, A.K.; Roth, C.; Glaser, E. The role of the N-terminal domain of chloroplast targeting peptides in organellar protein import and miss-sorting. FEBS Lett. 2006, 580, 3966–3972. [Google Scholar] [CrossRef] [PubMed]
- Chotewutmontri, P.; Bruce, B.D. Non-native, N-terminal Hsp70 molecular motor recognition elements in transit peptides support plastid protein translocation. J. Biol. Chem. 2015, 290, 7602–7621. [Google Scholar] [CrossRef]
- Lee, D.W.; Lee, S.; Lee, J.; Woo, S.; Razzak, M.A.; Vitale, A.; Hwang, I. Molecular mechanism of protein import specificity to chloroplasts and mitochondria in plant cells. Mol. Plant 2019, 12, 951–966. [Google Scholar] [CrossRef] [PubMed]
- von Heijne, G.; Nishikawa, K. Chloroplast transit peptides. The perfect random coil? FEBS Lett. 1991, 278, 1–3. [Google Scholar] [CrossRef]
- Lancelin, J.M.; Bally, I.; Arlaud, G.J.; Blackledge, M.; Gans, P.; Stein, M.; Jacquot, J.P. NMR structures of ferredoxin chloroplastic transit peptide from Chlamydomonas reinhardtii promoted by trifluoroethanol in aqueous solution. FEBS Lett. 1994, 343, 261–266. [Google Scholar] [CrossRef]
- Krimm, I.; Gans, P.; Hernandez, J.F.; Arlaud, G.J.; Lancelin, J.M. A coil-helix instead of a helix-coil motif can be induced in a chloroplast transit peptide from Chlamydomonas reinhardtii. Eur. J. Biochem. 1999, 265, 171–180. [Google Scholar] [CrossRef]
- Wienk, H.L.; Wechselberger, R.W.; Czisch, M.; de Kruijff, B. Structure, dynamics, and insertion of a chloroplast targeting peptide in mixed micelles. Biochemistry 2000, 39, 8219–8227. [Google Scholar] [CrossRef]
- Bruce, B.D. The paradox of plastid transit peptides: Conservation of function despite divergence in primary structure. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2001, 1541, 2–21. [Google Scholar] [CrossRef]
- Waghu, F.H.; Barai, R.S.; Gurung, P.; Idicula-Thomas, S. CAMPR3: A database on sequences, structures and signatures of antimicrobial peptides. Nucleic Acids Res. 2016, 44, D1094–D1097. [Google Scholar] [CrossRef] [PubMed]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Rea, M.; Ross, R.; Cotter, D.; Hill, C. Part II Classification of Prokaryotic Antimicrobial Peptides, Chapter 3 Classification of Bacteriocins from Gram-Positive Bacteria. In Prokaryotic Antimicrobial Peptides: From Genes to Applications; Drider, D., Rebuffat, S., Eds.; Springer: New York, NY, USA, 2011. [Google Scholar]
- Hammami, R.; Zouhir, A.; Le Lay, C.; Ben Hamida, J.; Fliss, I. BACTIBASE second release: A database and tool platform for bacteriocin characterization. BMC Microbiol. 2010, 10, 22. [Google Scholar] [CrossRef] [PubMed]
- Mookherjee, N.; Brown, K.L.; Hancock, R.E.W. Chapter 15-Cathelicidins. In Handbook of Biologically Active Peptides, 2nd ed.; Kastin, A.J., Ed.; Academic Press: Boston, MA, USA, 2013; pp. 77–84. [Google Scholar]
- Hellberg, S.; Sjoestroem, M.; Skagerberg, B.; Wold, S. Peptide quantitative structure-activity relationships, a multivariate approach. J. Med. Chem. 1987, 30, 1126–1135. [Google Scholar] [CrossRef]
- Wold, S.; Jonsson, J.; Sjörström, M.; Sandberg, M.; Rännar, S. DNA and peptide sequences and chemical processes multivariately modelled by principal component analysis and partial least-squares projections to latent structures. Anal. Chim. Acta 1993, 277, 239–253. [Google Scholar] [CrossRef]
- Fauchère, J.-L.; Pliska, V. Hydrophobic parameters πof amino-acid side chains from the partitioning of N-acetyl-amino-acid amides. Eur. J. Med. Chem. 1983, 18, 369–375. [Google Scholar]
- Gautier, R.; Douguet, D.; Antonny, B.; Drin, G. HELIQUEST: A web server to screen sequences with specific alpha-helical properties. Bioinformatics 2008, 24, 2101–2102. [Google Scholar] [CrossRef]
- Rousseeuw, P.J. Silhouettes: A graphical aid to the interpretation and validation of cluster analysis. J. Comput. Appl. Math. 1987, 20, 53–65. [Google Scholar] [CrossRef]
- Salichos, L.; Stamatakis, A.; Rokas, A. Novel Information Theory-Based Measures for Quantifying Incongruence among Phylogenetic Trees. Mol. Biol. Evol. 2014, msu061. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML Version 8: A tool for Phylogenetic Analysis and Post-Analysis of Large Phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree of Life (iTOL) v4: Recent updates and new developments. Nucleic Acids Res. 2019, 47, W256–W259. [Google Scholar] [CrossRef] [PubMed]
- Ivey, R.A.; Subramanian, C.; Bruce, B.D. Identification of a Hsp70 recognition domain within the rubisco small subunit transit peptide. Plant Physiol. 2000, 122, 1289–1299. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chotewutmontri, P.; Reddick, L.E.; McWilliams, D.R.; Campbell, I.M.; Bruce, B.D. Differential transit peptide recognition during preprotein binding and translocation into flowering plant plastids. Plant Cell 2012, 24, 3040–3059. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Harris, E. The Chlamydomonas Sourcebook, 1st ed.; Academic Press: San Diego, CA, USA, 1989. [Google Scholar]
- Onishi, M.; Pringle, J.R. Robust Transgene Expression from Bicistronic mRNA in the Green Alga Chlamydomonas reinhardtii. G3 Genes Genomes Genet. 2016, 6, 4115–4125. [Google Scholar] [CrossRef]
- Dunn, K.W.; Kamocka, M.M.; McDonald, J.H. A practical guide to evaluating colocalization in biological microscopy. Am. J. Physiol. Cell Physiol. 2011, 300, C723–C742. [Google Scholar] [CrossRef]
- Willmund, F.; Schroda, M. HEAT SHOCK PROTEIN 90C is a bona fide Hsp90 that interacts with plastidic HSP70B in Chlamydomonas reinhardtii. Plant Physiol. 2005, 138, 2310–2322. [Google Scholar] [CrossRef]
- Craige, B.; Brown, J.M.; Witman, G.B. Isolation of chlamydomonas flagella. Curr. Protoc. Cell Biol. 2013, 1–9. [Google Scholar] [CrossRef]
- Chua, N.H.; Blomberg, F. Immunochemical studies of thylakoid membrane polypeptides from spinach and Chlamydomonas reinhardtii. A modified procedure for crossed immunoelectrophoresis of dodecyl sulfate.protein complexes. J. Biol. Chem. 1979, 254, 215–223. [Google Scholar]
- de Vitry, C.; Olive, J.; Drapier, D.; Recouvreur, M.; Wollman, F.A. Posttranslational events leading to the assembly of photosystem II protein complex: A study using photosynthesis mutants from Chlamydomonas reinhardtii. J. Cell Biol. 1989, 109, 991–1006. [Google Scholar] [CrossRef]
- Whitney, S.M.; John Andrews, T. The gene for the ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) small subunit relocated to the plastid genome of tobacco directs the synthesis of small subunits that assemble into Rubisco. Plant Cell 2001, 13, 193–205. [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef] [PubMed]
- von Heijne, G. The signal peptide. J. Membr. Biol. 1990, 115, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Frain, K.M.; Gangl, D.; Jones, A.; Zedler, J.A.Z.; Robinson, C. Protein translocation and thylakoid biogenesis in cyanobacteria. Biochim. Biophys. Acta (Bba) Bioenerg. 2016, 1857, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Schatz, G.; Dobberstein, B. Common Principles of Protein Translocation Across Membranes. Science 1996, 271, 1519–1526. [Google Scholar] [CrossRef] [PubMed]
- van Westen, G.J.; Swier, R.F.; Wegner, J.K.; IJzerman, A.P.; van Vlijmen, H.W.; Bender, A. Benchmarking of protein descriptor sets in proteochemometric modeling (part 1): Comparative study of 13 amino acid descriptor sets. J. Cheminform. 2013, 5, 41. [Google Scholar] [CrossRef]
- van Westen, G.J.; Swier, R.F.; Cortes-Ciriano, I.; Wegner, J.K.; Overington, J.P.; IJzerman, A.P.; van Vlijmen, H.W.; Bender, A. Benchmarking of protein descriptor sets in proteochemometric modeling (part 2): Modeling performance of 13 amino acid descriptor sets. J. Cheminform. 2013, 5, 42. [Google Scholar] [CrossRef]
- Franzen, L.G.; Rochaix, J.D.; von Heijne, G. Chloroplast transit peptides from the green alga Chlamydomonas reinhardtii share features with both mitochondrial and higher plant chloroplast presequences. FEBS Lett. 1990, 260, 165–168. [Google Scholar] [CrossRef]
- Nagai, T.; Ibata, K.; Park, E.S.; Kubota, M.; Mikoshiba, K.; Miyawaki, A. A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications. Nat. Biotechnol. 2002, 20, 87–90. [Google Scholar] [CrossRef]
- Bionda, T.; Tillmann, B.; Simm, S.; Beilstein, K.; Ruprecht, M.; Schleiff, E. Chloroplast import signals: The length requirement for translocation in vitro and in vivo. J. Mol. Biol. 2010, 402, 510–523. [Google Scholar] [CrossRef]
- Razzak, A.; Lee, D.W.; Yoo, Y.; Hwang, I. Evolution of rubisco complex small subunit transit peptides from algae to plants. Sci. Rep. 2017, 7, 9279. [Google Scholar] [CrossRef]
- Mackinder, L.C.M.; Chen, C.; Leib, R.D.; Patena, W.; Blum, S.R.; Rodman, M.; Ramundo, S.; Adams, C.M.; Jonikas, M.C. A Spatial Interactome Reveals the Protein Organization of the Algal CO2-Concentrating Mechanism. Cell 2017, 171, 133–147. [Google Scholar] [CrossRef] [PubMed]
- Hugosson, M.; Andreu, D.; Boman, H.G.; Glaser, E. Antibacterial peptides and mitochonrial presequences affect mitochonrial coupling, respiration and protein import. Eur. J. Biochem. 1994, 223, 1027–1033. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.; Schatz, G. Sequences from a prokaryotic genome or the mouse dihydrofolate reductase gene can restore the import of a truncated precursor protein into yeast mitochondria. Proc. Natl. Acad. Sci. USA 1987, 84, 3117–3121. [Google Scholar] [CrossRef] [PubMed]
- Dunn, C.D.; Paavilainen, V.O. Wherever I may roam: Organellar protein targeting and evolvability. Curr. Opin. Genet. Dev. 2019, 58–59, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, C.A.; Preuss, D.; Grisafi, P.; Botstein, D. Many random sequences functionally replace the secretion signal sequence of yeast invertase. Science 1987, 235, 312–317. [Google Scholar] [CrossRef]
- Miras, S.; Salvi, D.; Piette, L.; Seigneurin-Berny, D.; Grunwald, D.; Reinbothe, C.; Joyard, J.; Reinbothe, S.; Rolland, N. Toc159- and Toc75-independent import of a transit sequence-less precursor into the inner envelope of chloroplasts. J. Biol. Chem. 2007, 282, 29482–29492. [Google Scholar] [CrossRef]
- Moyet, L.; Salvi, D.; Bouchnak, I.; Miras, S.; Perrot, L.; Seigneurin-Berny, D.; Kuntz, M.; Rolland, N. Calmodulin is involved in the dual subcellular location of two chloroplast proteins. J. Biol. Chem. 2019, 294, 17543–17554. [Google Scholar] [CrossRef]
- Baslam, M.; Oikawa, K.; Kitajima-Koga, A.; Kaneko, K.; Mitsui, T. Golgi-to-plastid trafficking of proteins through secretory pathway: Insights into vesicle-mediated import toward the plastids. Plant Signal. Behav. 2016, 11, e1221558. [Google Scholar] [CrossRef]
- Villarejo, A.; Buren, S.; Larsson, S.; Dejardin, A.; Monne, M.; Rudhe, C.; Karlsson, J.; Jansson, S.; Lerouge, P.; Rolland, N.; et al. Evidence for a protein transported through the secretory pathway en route to the higher plant chloroplast. Nat. Cell Biol. 2005, 7, 1224–1231. [Google Scholar] [CrossRef] [PubMed]
- Hurt, E.C.; Soltanifar, N.; Goldschmidt-Clermont, M.; Rochaix, J.D.; Schatz, G. The cleavable pre-sequence of an imported chloroplast protein directs attached polypeptides into yeast mitochondria. EMBO J. 1986, 5, 1343–1350. [Google Scholar] [CrossRef]
- Cleary, S.P.; Tan, F.C.; Nakrieko, K.A.; Thompson, S.J.; Mullineaux, P.M.; Creissen, G.P.; von Stedingk, E.; Glaser, E.; Smith, A.G.; Robinson, C. Isolated plant mitochondria import chloroplast precursor proteins in vitro with the same efficiency as chloroplasts. J. Biol. Chem. 2002, 277, 5562–5569. [Google Scholar] [CrossRef]
- Huang, J.; Hack, E.; Thornburg, R.W.; Myers, A.M. A yeast mitochondrial leader peptide functions in vivo as a dual targeting signal for both chloroplasts and mitochondria. Plant Cell 1990, 2, 1249–1260. [Google Scholar] [CrossRef] [PubMed]
- Rödiger, A.; Baudisch, B.; Langner, U.; Klösgen, R.B. Dual targeting of a mitochondrial protein: The case study of cytochrome c1. Mol. Plant 2011, 4, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Ge, C.; Spanning, E.; Glaser, E.; Wieslander, A. Import determinants of organelle-specific and dual targeting peptides of mitochondria and chloroplasts in Arabidopsis thaliana. Mol. Plant 2014, 7, 121–136. [Google Scholar] [CrossRef] [PubMed]
- McKinnon, L.; Theg, S.M. Determinants of the Specificity of Protein Targeting to Chloroplasts or Mitochondria. Mol. Plant 2019, 12, 893–895. [Google Scholar] [CrossRef] [PubMed]
- Robert, V.; Volokhina, E.B.; Senf, F.; Bos, M.P.; Van Gelder, P.; Tommassen, J. Assembly factor Omp85 recognizes its outer membrane protein substrates by a species-specific C-terminal motif. PLoS Biol. 2006, 4, e377. [Google Scholar] [CrossRef] [PubMed]
- Sommer, M.S.; Daum, B.; Gross, L.E.; Weis, B.L.M.; Mirus, O.; Abram, L.; Maier, U.-G.; Kühlbrandt, W.; Schleiff, E. Chloroplast Omp85 proteins change orientation during evolution. Proc. Natl. Acad. Sci. USA 2011, 108, 13841–13846. [Google Scholar] [CrossRef]
- Wunder, T.; Martin, R.; Löffelhardt, W.; Schleiff, E.; Steiner, J.M. The invariant phenylalanine of precursor proteins discloses the importance of Omp85 for protein translocation into cyanelles. BMC Evol. Biol. 2007, 7, 236. [Google Scholar] [CrossRef]
- Knopp, M.; Garg, S.G.; Handrich, M.; Gould, S.B. Major Changes in Plastid Protein Import and the Origin of the Chloroplastida. iScience 2020, 23, 100896. [Google Scholar] [CrossRef]
- Sato, H.; Feix, J.B. Peptide–membrane interactions and mechanisms of membrane destruction by amphipathic α-helical antimicrobial peptides. Biochim. Biophys. Acta (BBA) Biomembr. 2006, 1758, 1245–1256. [Google Scholar] [CrossRef]
- Piers, K.L.; Brown, M.H.; Hancock, R.E. Improvement of outer membrane-permeabilizing and lipopolysaccharide-binding activities of an antimicrobial cationic peptide by C-terminal modification. Antimicrob. Agents Chemother. 1994, 38, 2311–2316. [Google Scholar] [CrossRef]
- Vimala, A.; Ramakrishnan, C.; Gromiha, M.M. Identifying a potential receptor for the antibacterial peptide of sponge Axinella donnani endosymbiont. Gene 2015, 566, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Tripp, J.; Hahn, A.; Koenig, P.; Flinner, N.; Bublak, D.; Brouwer, E.M.; Ertel, F.; Mirus, O.; Sinning, I.; Tews, I.; et al. Structure and conservation of the periplasmic targeting factor Tic22 protein from plants and cyanobacteria. J. Biol. Chem. 2012, 287, 24164–24173. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Theg, S. The chloroplast protein import system: From algae to trees. Biochim. Biophys. Acta 2013, 1833, 314–331. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, E.M.; Ngo, G.; Yadav, S.; Ladig, R.; Schleiff, E. Tic22 from Anabaena sp. PCC 7120 with holdase function involved in outer membrane protein biogenesis shuttles between plasma membrane and Omp85. Mol. Microbiol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Delaye, L.; Valadez-Cano, C.; Pérez-Zamorano, B. How Really Ancient Is Paulinella Chromatophora? PLoS Curr. 2016, 8. [Google Scholar] [CrossRef]
- Singer, A.; Poschmann, G.; Mühlich, C.; Valadez-Cano, C.; Hänsch, S.; Hüren, V.; Rensing, S.A.; Stühler, K.; Nowack, E.C.M. Massive Protein Import into the Early-Evolutionary-Stage Photosynthetic Organelle of the Amoeba Paulinella chromatophora. Curr. Biol. 2017, 27, 2763–2773. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garrido, C.; Caspari, O.D.; Choquet, Y.; Wollman, F.-A.; Lafontaine, I. Evidence Supporting an Antimicrobial Origin of Targeting Peptides to Endosymbiotic Organelles. Cells 2020, 9, 1795. https://doi.org/10.3390/cells9081795
Garrido C, Caspari OD, Choquet Y, Wollman F-A, Lafontaine I. Evidence Supporting an Antimicrobial Origin of Targeting Peptides to Endosymbiotic Organelles. Cells. 2020; 9(8):1795. https://doi.org/10.3390/cells9081795
Chicago/Turabian StyleGarrido, Clotilde, Oliver D. Caspari, Yves Choquet, Francis-André Wollman, and Ingrid Lafontaine. 2020. "Evidence Supporting an Antimicrobial Origin of Targeting Peptides to Endosymbiotic Organelles" Cells 9, no. 8: 1795. https://doi.org/10.3390/cells9081795
APA StyleGarrido, C., Caspari, O. D., Choquet, Y., Wollman, F.-A., & Lafontaine, I. (2020). Evidence Supporting an Antimicrobial Origin of Targeting Peptides to Endosymbiotic Organelles. Cells, 9(8), 1795. https://doi.org/10.3390/cells9081795




