Mechanisms behind the Immunoregulatory Dialogue between Mesenchymal Stem Cells and Th17 Cells
Abstract
1. Introduction
2. Immunoregulatory Properties of MSC
2.1. Innate Cells
2.2. Adaptive Cells
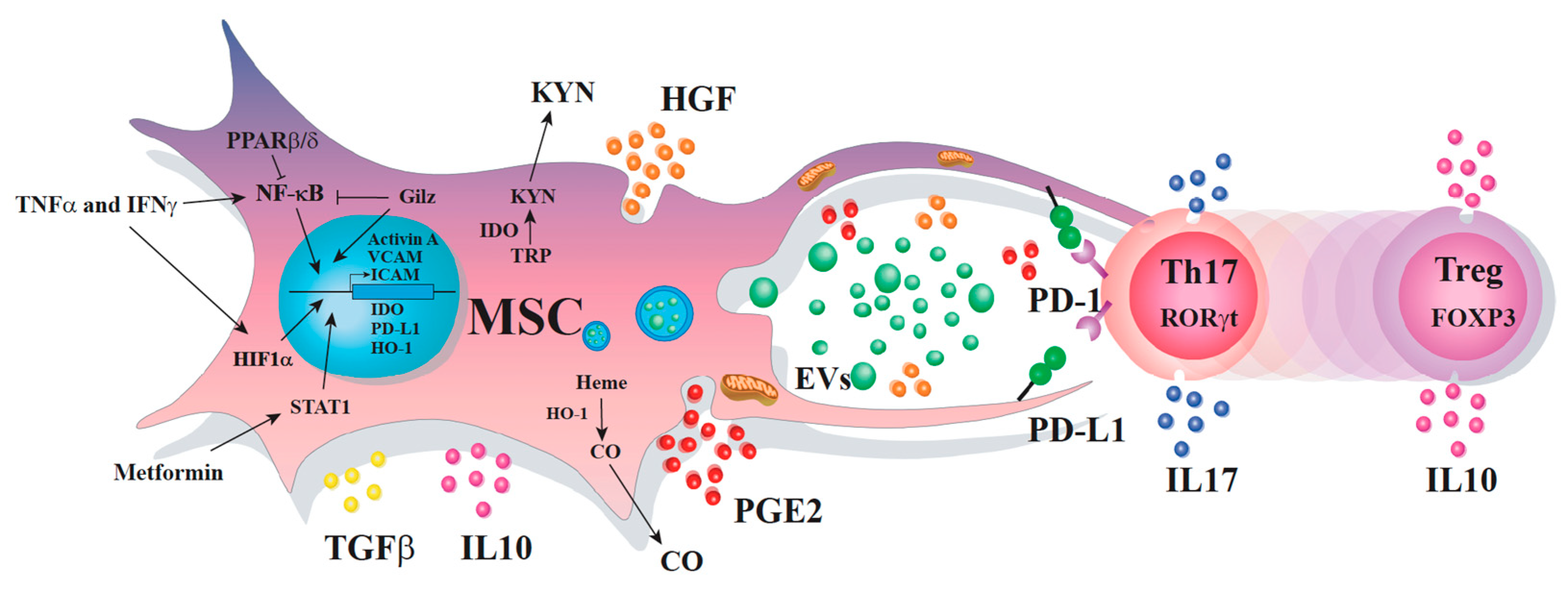
3. Mechanisms behind the MSC Immunosuppressive Effect on Th17 Cells
3.1. Soluble Factors
3.2. Cell-to-Cell Contact
3.3. Extracellular Vesicles
3.4. Transfer of Mitochondria
4. MSC Enhancement to Improve Their Therapeutic Potential
5. Conclusions
Funding
Conflicts of Interest
References
- Friedenstein, A.J.; Petrakova, K.V.; Kurolesova, A.I.; Frolova, G.P. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation 1968, 6, 230–247. [Google Scholar] [CrossRef]
- Owen, M.; Friedenstein, A.J. Stromal stem cells: Marrow-derived osteogenic precursors. Ciba Found. Symp. 1988, 136, 42–60. [Google Scholar] [CrossRef]
- Friedenstein, A.J.; Chailakhyan, R.K.; Gerasimov, U.V. Bone marrow osteogenic stem cells: In vitro cultivation and transplantation in diffusion chambers. Cell Tissue Kinet. 1987, 20, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Piersma, A.H.; Brockbank, K.G.; Ploemacher, R.E.; van Vliet, E.; Brakel-van Peer, K.M.; Visser, P.J. Characterization of fibroblastic stromal cells from murine bone marrow. Exp. Hematol. 1985, 13, 237–243. [Google Scholar]
- Caplan, A.I. Mesenchymal stem cells. J. Orthop. Res. 1991, 9, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Romanov, Y.A.; Darevskaya, A.N.; Merzlikina, N.V.; Buravkova, L.B. Mesenchymal stem cells from human bone marrow and adipose tissue: Isolation, characterization, and differentiation potentialities. Bull. Exp. Biol. Med. 2005, 140, 138–143. [Google Scholar] [CrossRef]
- Kassis, I.; Zangi, L.; Rivkin, R.; Levdansky, L.; Samuel, S.; Marx, G.; Gorodetsky, R. Isolation of mesenchymal stem cells from G-CSF-mobilized human peripheral blood using fibrin microbeads. Bone Marrow Transplant. 2006, 37, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Alcayaga-Miranda, F.; Cuenca, J.; Luz-Crawford, P.; Aguila-Diaz, C.; Fernandez, A.; Figueroa, F.E.; Khoury, M. Characterization of menstrual stem cells: Angiogenic effect, migration and hematopoietic stem cell support in comparison with bone marrow mesenchymal stem cells. Stem Cell Res. Ther. 2015, 6, 32. [Google Scholar] [CrossRef] [PubMed]
- Djouad, F.; Bony, C.; Häupl, T.; Uzé, G.; Lahlou, N.; Louis-Plence, P.; Apparailly, F.; Canovas, F.; Rème, T.; Sany, J.; et al. Transcriptional Profiles Discriminate Bone Marrow-Derived and Synovium-Derived Mesenchymal Stem Cells. Arthritis Res. Ther. 2005, 7, R1304. [Google Scholar] [CrossRef] [PubMed]
- De Bari, C.; Dell’Accio, F.; Tylzanowski, P.; Luyten, F.P. Multipotent Mesenchymal Stem Cells from Adult Human Synovial Membrane. Arthritis Rheum. 2001, 44, 1928–1942. [Google Scholar] [CrossRef]
- Huang, G.T.; Yamaza, T.; Shea, L.D.; Djouad, F.; Kuhn, N.Z.; Tuan, R.S.; Shi, S. Stem/Progenitor Cell-Mediated De Novo Regeneration of Dental Pulp with Newly Deposited Continuous Layer of Dentin in an In Vivo Model. Tissue Eng. Part A 2010, 16, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Erices, A.A.; Allers, C.I.; Conget, P.A.; Rojas, C.V.; Minguell, J.J. Human Cord Blood-Derived Mesenchymal Stem Cells Home and Survive in the Marrow of Immunodeficient Mice after Systemic Infusion. Cell Transplant. 2003, 12, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Miao, Z.; Jin, J.; Chen, L.; Zhu, J.; Huang, W.; Zhao, J.; Qian, H.; Zhang, X. Isolation of mesenchymal stem cells from human placenta: Comparison with human bone marrow mesenchymal stem cells. Cell Biol. Int. 2006, 30, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Weiss, M.L.; Anderson, C.; Medicetty, S.; Seshareddy, K.B.; Weiss, R.J.; VanderWerff, I.; Troyer, D.; McIntosh, K.R. Immune Properties of Human Umbilical Cord Wharton’s Jelly-Derived Cells. Stem Cells 2008, 26, 2865–2874. [Google Scholar] [CrossRef] [PubMed]
- Marongiu, F.; Gramignoli, R.; Sun, Q.; Tahan, V.; Miki, T.; Dorko, K.; Ellis, E.; Strom, S.C. Isolation of amniotic mesenchymal stem cells. Curr. Protoc. Stem Cell Biol. 2010, 12, 1E.5.1–1E.5.11. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Barhanpurkar-Naik, A.; Mhaske, S.T.; Pote, S.T.; Singh, K.; Wani, M.R. Interleukin-3 enhances the migration of human mesenchymal stem cells by regulating expression of CXCR4. Stem Cell Res. Ther. 2017, 8, 168. [Google Scholar] [CrossRef]
- Nishikawa, G.; Kawada, K.; Nakagawa, J.; Toda, K.; Ogawa, R.; Inamoto, S.; Mizuno, R.; Itatani, Y.; Sakai, Y. Bone marrow-derived mesenchymal stem cells promote colorectal cancer progression via CCR5. Cell Death Dis. 2019, 10, 264. [Google Scholar] [CrossRef]
- Croitoru-Lamoury, J.; Lamoury, F.M.; Zaunders, J.J.; Veas, L.A.; Brew, B.J. Human mesenchymal stem cells constitutively express chemokines and chemokine receptors that can be upregulated by cytokines, IFN-beta, and Copaxone. J. Interferon Cytokine Res. 2007, 27, 53–64. [Google Scholar] [CrossRef]
- Sordi, V.; Malosio, M.L.; Marchesi, F.; Mercalli, A.; Melzi, R.; Giordano, T.; Belmonte, N.; Ferrari, G.; Leone, B.E.; Bertuzzi, F.; et al. Bone marrow mesenchymal stem cells express a restricted set of functionally active chemokine receptors capable of promoting migration to pancreatic islets. Blood 2005, 106, 419–427. [Google Scholar] [CrossRef]
- Li, B.; Zhang, H.; Zeng, M.; He, W.; Li, M.; Huang, X.; Deng, D.Y.; Wu, J. Bone Marrow Mesenchymal Stem Cells Protect Alveolar Macrophages from Lipopolysaccharide-Induced Apoptosis Partially by Inhibiting the Wnt/β-Catenin Pathway. Cell Biol. Int. 2015, 39, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Guo, Y.H.; Zhu, X.J.; Gao, W.; Mao, J.M. Anti-apoptotic Effects of Mesenchymal Stem Cells on Cardiac Myocytes: In Vitro Study with Rats. Zhonghua Yi Xue Za Zhi 2007, 87, 271–274. [Google Scholar] [PubMed]
- Huang, W.; Lv, B.; Zeng, H.; Shi, D.; Liu, Y.; Chen, F.; Li, F.; Liu, X.; Zhu, R.; Yu, L.; et al. Paracrine Factors Secreted by MSCs Promote Astrocyte Survival Associated with GFAP Downregulation after Ischemic Stroke via p38 MAPK and JNK. J. Cell. Physiol. 2015, 230, 2461–2475. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.L.; Zhao, Q.; Qin, X.; Shen, L.; Cheng, L.; Ge, J.; Phillips, M.I. Paracrine Action Enhances the Effects of Autologous Mesenchymal Stem Cell Transplantation on Vascular Regeneration in Rat Model of Myocardial Infarction. Ann. Thorac. Surg. 2005, 80, 229–237. [Google Scholar] [CrossRef]
- Ucuzian, A.A.; Gassman, A.A.; East, A.T.; Greisler, H.P. Molecular Mediators of Angiogenesis. J. Burn Care Res. 2010, 31, 158–175. [Google Scholar] [CrossRef] [PubMed]
- Melero-Martin, J.M.; De Obaldia, M.E.; Kang, S.Y.; Khan, Z.A.; Yuan, L.; Oettgen, P.; Bischoff, J. Engineering robust and functional vascular networks in vivo with human adult and cord blood-derived progenitor cells. Circ. Res. 2008, 103, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.Z.; Moreno-Luna, R.; Zhou, B.; Pu, W.T.; Melero-Martin, J.M. Equal modulation of endothelial cell function by four distinct tissue-specific mesenchymal stem cells. Angiogenesis 2012, 15, 443–455. [Google Scholar] [CrossRef]
- Traktuev, D.O.; Prater, D.N.; Merfeld-Clauss, S.; Sanjeevaiah, A.R.; Saadatzadeh, M.R.; Murphy, M.; Johnstone, B.H.; Ingram, D.A.; March, K.L. Robust functional vascular network formation in vivo by cooperation of adipose progenitor and endothelial cells. Circ. Res. 2009, 104, 1410–1420. [Google Scholar] [CrossRef]
- Kachgal, S.; Putnam, A.J. Mesenchymal stem cells from adipose and bone marrow promote angiogenesis via distinct cytokine and protease expression mechanisms. Angiogenesis 2011, 14, 47–59. [Google Scholar] [CrossRef]
- Roubelakis, M.G.; Tsaknakis, G.; Pappa, K.I.; Anagnou, N.P.; Watt, S.M. Spindle Shaped Human Mesenchymal Stem/Stromal Cells from Amniotic Fluid Promote Neovascularization. PLoS ONE 2013, 8, e54747. [Google Scholar] [CrossRef]
- Merfeld-Clauss, S.; Gollahalli, N.; March, K.L.; Traktuev, D.O. Adipose tissue progenitor cells directly interact with endothelial cells to induce vascular network formation. Tissue Eng. Part A 2010, 16, 2953–2966. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.M.; Hur, S.M.; Park, K.Y.; Kim, C.K.; Kim, Y.M.; Kim, H.S.; Shin, H.C.; Won, M.H.; Ha, K.S.; Kwon, Y.G.; et al. Multiple paracrine factors secreted by mesenchymal stem cells contribute to angiogenesis. Vasc. Pharm. 2014, 63, 19–28. [Google Scholar] [CrossRef]
- Razban, V.; Lotfi, A.S.; Soleimani, M.; Ahmadi, H.; Massumi, M.; Khajeh, S.; Ghaedi, M.; Arjmand, S.; Najavand, S.; Khoshdel, A. HIF-1α Overexpression Induces Angiogenesis in Mesenchymal Stem Cells. BioRes. Open Access 2012, 1, 174–183. [Google Scholar] [CrossRef]
- Ranganath, S.H.; Levy, O.; Inamdar, M.S.; Karp, J.M. Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Cell Stem Cell 2012, 10, 244–258. [Google Scholar] [CrossRef]
- Liu, L.; Gao, J.; Yuan, Y.; Chang, Q.; Liao, Y.; Lu, F. Hypoxia preconditioned human adipose derived mesenchymal stem cells enhance angiogenic potential via secretion of increased VEGF and bFGF. Cell Biol. Int. 2013, 37, 551–560. [Google Scholar] [CrossRef]
- El Agha, E.; Kramann, R.; Schneider, R.K.; Li, X.; Seeger, W.; Humphreys, B.D.; Bellusci, S. Mesenchymal Stem Cells in Fibrotic Disease. Cell Stem Cell 2017, 21, 166–177. [Google Scholar] [CrossRef]
- Rockel, J.S.; Rabani, R.; Viswanathan, S. Anti-Fibrotic Mechanisms of Exogenously-Expanded Mesenchymal Stromal Cells for Fibrotic Diseases. Semin. Cell Dev. Biol. 2020, 101, 87–103. [Google Scholar] [CrossRef]
- Firestein, G.S. Evolving Concepts of Rheumatoid Arthritis. Nature 2003, 423, 356–361. [Google Scholar] [CrossRef]
- Müller-Ladner, U.; Ospelt, C.; Gay, S.; Distler, O.; Pap, T. Cells of the Synovium in Rheumatoid Arthritis. Synovial Fibroblasts. Arthritis Res. Ther. 2007, 9, 223. [Google Scholar] [CrossRef]
- Yang, Z.; Shen, Y.; Oishi, H.; Matteson, E.L.; Tian, L.; Goronzy, J.J.; Weyand, C.M. Restoring Oxidant Signaling Suppresses Proarthritogenic T Cell Effector Functions in Rheumatoid Arthritis. Sci. Transl. Med. 2016, 8. [Google Scholar] [CrossRef]
- Luque-Campos, N.; Contreras-Lopez, R.A.; Jose Paredes-Martinez, M.; Torres, M.J.; Bahraoui, S.; Wei, M.; Espinoza, F.; Djouad, F.; Elizondo-Vega, R.J.; Luz-Crawford, P. Mesenchymal Stem Cells Improve Rheumatoid Arthritis Progression by Controlling Memory T Cell Response. Front. Immunol. 2019, 10, 798. [Google Scholar] [CrossRef]
- Ehrenstein, M.R.; Evans, J.G.; Singh, A.; Moore, S.; Warnes, G.; Isenberg, D.A.; Mauri, C. Compromised Function of Regulatory T Cells in Rheumatoid Arthritis and Reversal by Anti-TNFalpha Therapy. J. Exp. Med. 2004, 200, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.; Isaacs, J. Novel Immunotherapies for Rheumatoid Arthritis. Clin. Med. 2013, 13, 391–394. [Google Scholar] [CrossRef]
- Luz-Crawford, P.; Noël, D.; Fernandez, X.; Khoury, M.; Figueroa, F.; Carrión, F.; Jorgensen, C.; Djouad, F. Mesenchymal Stem Cells Repress Th17 Molecular Program through the PD-1 Pathway. PLoS ONE 2012, 7, e45272. [Google Scholar] [CrossRef]
- Luz-Crawford, P.; Tejedor, G.; Mausset-Bonnefont, A.L.; Beaulieu, E.; Morand, E.F.; Jorgensen, C.; Noel, D.; Djouad, F. Glucocorticoid-induced leucine zipper governs the therapeutic potential of mesenchymal stem cells by inducing a switch from pathogenic to regulatory Th17 cells in a mouse model of collagen-induced arthritis. Arthritis Rheumatol. 2015, 67, 1514–1524. [Google Scholar] [CrossRef]
- Luz-Crawford, P.; Jorgensen, C.; Djouad, F. Mesenchymal Stem Cells Direct the Immunological Fate of Macrophages. In Macrophages; Part of the Results and Problems in Cell Differentiation Book Series; Springer: Cham, Germany, 2017; Volume 62, pp. 61–72. [Google Scholar] [CrossRef]
- Luz-Crawford, P.; Hernandez, J.; Djouad, F.; Luque-Campos, N.; Caicedo, A.; Carrère-Kremer, S.; Brondello, J.M.; Vignais, M.L.; Pène, J.; Jorgensen, C. Mesenchymal Stem Cell Repression of Th17 Cells Is Triggered by Mitochondrial Transfer. Stem Cell Res. Ther. 2019, 10, 232. [Google Scholar] [CrossRef]
- Rozenberg, A.; Rezk, A.; Boivin, M.N.; Darlington, P.J.; Nyirenda, M.; Li, R.; Jalili, F.; Winer, R.; Artsy, E.A.; Uccelli, A.; et al. Human Mesenchymal Stem Cells Impact Th17 and Th1 Responses Through a Prostaglandin E2 and Myeloid-Dependent Mechanism. Stem Cells Transl. Med. 2016, 5, 1506–1514. [Google Scholar] [CrossRef]
- Leyendecker, A.; Pinheiro, C.C.G.; Amano, M.T.; Bueno, D.F. The Use of Human Mesenchymal Stem Cells as Therapeutic Agents for the In Vivo Treatment of Immune-Related Diseases: A Systematic Review. Front. Immunol. 2018, 9, 2056. [Google Scholar] [CrossRef]
- Li, X.; Dong, Y.; Yin, H.; Qi, Z.; Wang, D.; Ren, S. Mesenchymal Stem Cells Induced Regulatory Dendritic Cells from Hemopoietic Progenitor Cells through Notch Pathway and TGF-β Synergistically. Immunol. Lett. 2020, 222, 49–57. [Google Scholar] [CrossRef]
- Chen, P.; Huang, Y.; Womer, K.L. Effects of Mesenchymal Stromal Cells on Human Myeloid Dendritic Cell Differentiation and Maturation in a Humanized Mouse Model. J. Immunol. Methods 2015, 427, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Peng, J.; Xie, Q.; Xiao, N.; Su, X.; Mei, H.; Lu, Y.; Zhou, J.; Dai, Y.; Wang, S.; et al. Mesenchymal Stem Cells Alleviate Moderate-to-Severe Psoriasis by Reducing the Production of Type I Interferon (IFN-I) by Plasmacytoid Dendritic Cells (pDCs). Stem Cells Int. 2019, 2019, 6961052. [Google Scholar] [CrossRef]
- Lu, Z.; Chang, W.; Meng, S.; Xu, X.; Xie, J.; Guo, F.; Yang, Y.; Qiu, H.; Liu, L. Mesenchymal Stem Cells Induce Dendritic Cell Immune Tolerance via Paracrine Hepatocyte Growth Factor to Alleviate Acute Lung Injury. Stem Cell Res. Ther. 2019, 10, 372. [Google Scholar] [CrossRef] [PubMed]
- Favaro, E.; Carpanetto, A.; Caorsi, C.; Giovarelli, M.; Angelini, C.; Cavallo-Perin, P.; Tetta, C.; Camussi, G.; Zanone, M.M. Human Mesenchymal Stem Cells and Derived Extracellular Vesicles Induce Regulatory Dendritic Cells in Type 1 Diabetic Patients. Diabetologia 2016, 59, 325–333. [Google Scholar] [CrossRef]
- He, X.; Dong, Z.; Cao, Y.; Wang, H.; Liu, S.; Liao, L.; Jin, Y.; Yuan, L.; Li, B. MSC-Derived Exosome Promotes M2 Polarization and Enhances Cutaneous Wound Healing. Stem Cells Int. 2019, 2019, 7132708. [Google Scholar] [CrossRef]
- Liu, F.; Qiu, H.; Xue, M.; Zhang, S.; Zhang, X.; Xu, J.; Chen, J.; Yang, Y.; Xie, J. MSC-secreted TGF-β Regulates Lipopolysaccharide-Stimulated Macrophage M2-like Polarization via the Akt/FoxO1 Pathway. Stem Cell Res. Ther. 2019, 10, 34. [Google Scholar] [CrossRef]
- Morrison, T.J.; Jackson, M.V.; Cunningham, E.K.; Kissenpfennig, A.; McAuley, D.F.; O’Kane, C.M.; Krasnodembskaya, A.D. Mesenchymal Stromal Cells Modulate Macrophages in Clinically Relevant Lung Injury Models by Extracellular Vesicle Mitochondrial Transfer. Am. J. Respir. Crit. Care Med. 2017, 196, 1275–1286. [Google Scholar] [CrossRef]
- Akiyama, K.; Chen, C.; Wang, D.; Xu, X.; Qu, C.; Yamaza, T.; Cai, T.; Chen, W.; Sun, L.; Shi, S. Mesenchymal-stem-cell-induced immunoregulation involves FAS-ligand-/FAS-mediated T cell apoptosis. Cell Stem Cell 2012, 10, 544–555. [Google Scholar] [CrossRef] [PubMed]
- Murphy, N.; Treacy, O.; Lynch, K.; Morcos, M.; Lohan, P.; Howard, L.; Fahy, G.; Griffin, M.D.; Ryan, A.E.; Ritter, T. TNF-α/IL-1β-licensed Mesenchymal Stromal Cells Promote Corneal Allograft Survival via Myeloid Cell-Mediated Induction of Foxp3 + Regulatory T Cells in the Lung. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2019, 33, 9404–9421. [Google Scholar] [CrossRef] [PubMed]
- Henao Agudelo, J.S.; Braga, T.T.; Amano, M.T.; Cenedeze, M.A.; Cavinato, R.A.; Peixoto-Santos, A.R.; Muscará, M.N.; Teixeira, S.A.; Cruz, M.C.; Castoldi, A.; et al. Mesenchymal Stromal Cell-Derived Microvesicles Regulate an Internal Pro-Inflammatory Program in Activated Macrophages. Front. Immunol. 2017, 8, 881. [Google Scholar] [CrossRef]
- Zhu, Y.G.; Feng, X.M.; Abbott, J.; Fang, X.H.; Hao, Q.; Monsel, A.; Qu, J.M.; Matthay, M.A.; Lee, J.W. Human Mesenchymal Stem Cell Microvesicles for Treatment of Escherichia Coli Endotoxin-Induced Acute Lung Injury in Mice. Stem Cells 2014, 32, 116–125. [Google Scholar] [CrossRef]
- Jackson, M.V.; Morrison, T.J.; Doherty, D.F.; McAuley, D.F.; Matthay, M.A.; Kissenpfennig, A.; O’Kane, C.M.; Krasnodembskaya, A.D. Mitochondrial Transfer via Tunneling Nanotubes Is an Important Mechanism by Which Mesenchymal Stem Cells Enhance Macrophage Phagocytosis in the In Vitro and In Vivo Models of ARDS. Stem Cells 2016, 34, 2210–2223. [Google Scholar] [CrossRef]
- Hu, C.D.; Kosaka, Y.; Marcus, P.; Rashedi, I.; Keating, A. Differential Immunomodulatory Effects of Human Bone Marrow-Derived Mesenchymal Stromal Cells on Natural Killer Cells. Stem Cells Dev. 2019, 28, 933–943. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, J.; Liu, Y.; Qin, Y.; Luo, Q.; Wang, Q.; Duan, H. TLR4 Plays a Crucial Role in MSC-induced Inhibition of NK Cell Function. Biochem. Biophys. Res. Commun. 2015, 464, 541–547. [Google Scholar] [CrossRef]
- Blanco, B.; Herrero-Sánchez, M.D.; Rodríguez-Serrano, C.; García-Martínez, M.L.; Blanco, J.F.; Muntión, S.; García-Arranz, M.; Sánchez-Guijo, F.; Del Cañizo, C. Immunomodulatory Effects of Bone Marrow versus Adipose Tissue-Derived Mesenchymal Stromal Cells on NK Cells: Implications in the Transplantation Setting. Eur. J. Haematol. 2016, 97, 528–537. [Google Scholar] [CrossRef]
- Di Trapani, M.; Bassi, G.; Midolo, M.; Gatti, A.; Kamga, P.T.; Cassaro, A.; Carusone, R.; Adamo, A.; Krampera, M. Differential and Transferable Modulatory Effects of Mesenchymal Stromal Cell-Derived Extracellular Vesicles on T, B and NK Cell Functions. Sci. Rep. 2016, 6, 24120. [Google Scholar] [CrossRef]
- Alves, V.B.F.; de Sousa, B.C.; Fonseca, M.T.C.; Ogata, H.; Caliári-Oliveira, C.; Yaochite, J.N.U.; Rodrigues Júnior, V.; Chica, J.E.L.; da Silva, J.S.; Malmegrim, K.C.R.; et al. A Single Administration of Human Adipose Tissue-Derived Mesenchymal Stromal Cells (MSC) Induces Durable and Sustained Long-Term Regulation of Inflammatory Response in Experimental Colitis. Clin. Exp. Immunol. 2019, 196. [Google Scholar] [CrossRef] [PubMed]
- Van Hoeven, V.; Munneke, J.M.; Cornelissen, A.S.; Omar, S.Z.; Spruit, M.J.; Kleijer, M.; Bernink, J.H.; Blom, B.; Voermans, C.; Hazenberg, M.D. Mesenchymal Stromal Cells Stimulate the Proliferation and IL-22 Production of Group 3 Innate Lymphoid Cells. J. Immunol. 2018, 201, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Ma, T.; Zhou, X.; Jiang, Y.; Han, Y.; Li, H. B Lymphocytes Are the Target of Mesenchymal Stem Cells Immunoregulatory Effect in a Murine Graft-versus-Host Disease Model. Cell Transplant. 2019, 28, 1279–1288. [Google Scholar] [CrossRef]
- Luk, F.; Carreras-Planella, L.; Korevaar, S.S.; de Witte, S.F.H.; Borràs, F.E.; Betjes, M.G.H.; Baan, C.C.; Hoogduijn, M.J.; Franquesa, M. Inflammatory Conditions Dictate the Effect of Mesenchymal Stem or Stromal Cells on B Cell Function. Front. Immunol. 2017, 8, 1042. [Google Scholar] [CrossRef]
- Wang, H.; Qi, F.; Dai, X.; Tian, W.; Liu, T.; Han, H.; Zhang, B.; Li, H.; Zhang, Z.; Du, C. Requirement of B7-H1 in Mesenchymal Stem Cells for Immune Tolerance to Cardiac Allografts in Combination Therapy with Rapamycin. Transpl. Immunol. 2014, 31, 65–74. [Google Scholar] [CrossRef]
- Cho, K.A.; Lee, J.K.; Kim, Y.H.; Park, M.; Woo, S.Y.; Ryu, K.H. Mesenchymal stem cells ameliorate B-cell-mediated immune responses and increase IL-10-expressing regulatory B cells in an EBI3-dependent manner. Cell. Mol. Immunol. 2017, 14, 895–908. [Google Scholar] [CrossRef] [PubMed]
- Gupte, K.S.; Vanikar, A.V.; Trivedi, H.L.; Patel, C.N.; Patel, J.V. In-Vitro Generation of Interleukin-10 Secreting B-Regulatory Cells From Donor Adipose Tissue Derived Mesenchymal Stem Cells and Recipient Peripheral Blood Mononuclear Cells for Potential Cell Therapy. Biomed. J. 2017, 40, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Franquesa, M.; Mensah, F.K.; Huizinga, R.; Strini, T.; Boon, L.; Lombardo, E.; DelaRosa, O.; Laman, J.D.; Grinyó, J.M.; Weimar, W.; et al. Human Adipose Tissue-Derived Mesenchymal Stem Cells Abrogate Plasmablast Formation and Induce Regulatory B Cells Independently of T Helper Cells. Stem Cells 2015, 33, 880–891. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.G.; Lee, J.; Hong, S.M.; Kwok, S.K.; Cho, M.L.; Park, S.H. Metformin Enhances the Immunomodulatory Potential of Adipose-Derived Mesenchymal Stem Cells through STAT1 in an Animal Model of Lupus. Rheumatology 2020, 59, 1426–1438. [Google Scholar] [CrossRef]
- Kiernan, C.H.; Asmawidjaja, P.S.; Fahy, N.; Witte-Bouma, J.; Wolvius, E.B.; Brama, P.A.J.; Lubberts, E.; Farrell, E.; Physiol, M. Allogeneic Chondrogenic Mesenchymal Stromal Cells alter Helper T Cell Subsets in CD4+ Memory T Cells. Tissue Eng. Part A 2020, 26, 490–502. [Google Scholar] [CrossRef]
- Lee, K.; Park, N.; Jung, H.; Rim, Y.A.; Nam, Y.; Lee, J.; Park, S.H.; Ju, J.H. Mesenchymal Stem Cells Ameliorate Experimental Arthritis via Expression of interleukin-1 Receptor Antagonist. PLoS ONE 2018, 13, e0193086. [Google Scholar] [CrossRef]
- Pianta, S.; Bonassi Signoroni, P.; Muradore, I.; Rodrigues, M.F.; Rossi, D.; Silini, A.; Parolini, O. Amniotic Membrane Mesenchymal Cells-Derived Factors Skew T Cell Polarization Toward Treg and Downregulate Th1 and Th17 Cells Subsets. Stem Cell Rev. Rep. 2015, 11, 394–407. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, J.; Huang, F.; Wang, J.; Su, W.; Zhou, J.; Qi, Q.; Cao, F.; Sun, B.; Liu, Z.; et al. Human Gingiva Tissue-Derived MSC Ameliorates Immune-Mediated Bone Marrow Failure of Aplastic Anemia via Suppression of Th1 and Th17 Cells and Enhancement of CD4+Foxp3+ Regulatory T Cells Differentiation. Am. J. Transl. Res. 2019, 11, 7627–7643. [Google Scholar]
- Ng, J.; Hynes, K.; White, G.; Sivanathan, K.N.; Vandyke, K.; Bartold, P.M.; Gronthos, S. Immunomodulatory Properties of Induced Pluripotent Stem Cell-Derived Mesenchymal Cells. J. Cell. Biochem. 2016, 117, 2844–2853. [Google Scholar] [CrossRef]
- Cosenza, S.; Toupet, K.; Maumus, M.; Luz-Crawford, P.; Blanc-Brude, O.; Jorgensen, C.; Noël, D. Mesenchymal Stem Cells-Derived Exosomes Are More Immunosuppressive Than Microparticles in Inflammatory Arthritis. Theranostics 2018, 8, 1399–1410. [Google Scholar] [CrossRef]
- Del Fattore, A.; Luciano, R.; Pascucci, L.; Goffredo, B.M.; Giorda, E.; Scapaticci, M.; Fierabracci, A.; Muraca, M. Immunoregulatory Effects of Mesenchymal Stem Cell-Derived Extracellular Vesicles on T Lymphocytes. Cell Transplant. 2015, 24, 2615–2627. [Google Scholar] [CrossRef] [PubMed]
- Laso-García, F.; Ramos-Cejudo, J.; Carrillo-Salinas, F.; Otero-Ortega, L.; Feliú, A.; Gómez-de Frutos, M.; Mecha, M.; Díez-Tejedor, E.; Guaza, C.; Gutiérrez-Fernández, M. Therapeutic Potential of Extracellular Vesicles Derived from Human Mesenchymal Stem Cells in a Model of Progressive Multiple Sclerosis. PLoS ONE 2018, 13, e0202590. [Google Scholar] [CrossRef] [PubMed]
- Shigemoto-Kuroda, T.; Oh, J.Y.; Kim, D.; Jeong, H.J.; Park, S.Y.; Lee, H.J.; Park, J.W.; Kim, T.W.; An, S.Y.; Prockop, D.J.; et al. MSC-Derived Extracellular Vesicles Attenuate Immune Responses in Two Autoimmune Murine Models: Type 1 Diabetes and Uveoretinitis. Stem Cell Rep. 2017, 8, 1214–1225. [Google Scholar] [CrossRef]
- Court, A.C.; Le-Gatt, A.; Luz-Crawford, P.; Parra, E.; Aliaga-Tobar, V.; Bátiz, L.F.; Contreras, R.A.; Ortúzar, M.I.; Kurte, M.; Elizondo-Vega, R.; et al. Mitochondrial Transfer from MSCs to T Cells Induces Treg Differentiation and Restricts Inflammatory Response. EMBO Rep. 2020, 21, e48052. [Google Scholar] [CrossRef]
- Bai, L.; Lennon, D.P.; Eaton, V.; Maier, K.; Caplan, A.I.; Miller, S.D.; Miller, R.H. Human Bone Marrow-Derived Mesenchymal Stem Cells Induce Th2-polarized Immune Response and Promote Endogenous Repair in Animal Models of Multiple Sclerosis. Glia 2009, 57, 1192–1203. [Google Scholar] [CrossRef]
- Hur, J.; Kang, J.Y.; Kim, Y.K.; Lee, S.Y.; Jeon, S.; Kim, Y.; Jung, C.K.; Rhee, C.K. Evaluation of Human MSCs Treatment Frequency on Airway Inflammation in a Mouse Model of Acute Asthma. J. Korean Med. Sci. 2020, 35, e188. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.B.; Zhang, H.Y.; Meng, X.C.; Wang, C.; He, B.X.; Peng, Y.Q.; Xu, Z.B.; Fan, X.L.; Wu, Z.J.; Wu, Z.C.; et al. Small Extracellular Vesicles Derived from Human MSCs Prevent Allergic Airway Inflammation via Immunomodulation on Pulmonary Macrophages. Cell Death Dis. 2020, 11, 409. [Google Scholar] [CrossRef]
- Chan, C.K.; Lin, T.C.; Huang, Y.A.; Chen, Y.S.; Wu, C.L.; Lo, H.Y.; Kuo, M.L.; Wu, K.H.; Huang, J.L. The Modulation of Th2 Immune Pathway in the Immunosuppressive Effect of Human Umbilical Cord Mesenchymal Stem Cells in a Murine Asthmatic Model. Inflamm. Res. Off. J. Eur. Histamine Res. Soc. 2016, 65, 795–801. [Google Scholar] [CrossRef]
- Patel, S.A.; Dave, M.A.; Bliss, S.A.; Giec-Ujda, A.B.; Bryan, M.; Pliner, L.F.; Rameshwar, P. Treg/Th17 Polarization by Distinct Subsets of Breast Cancer Cells Is Dictated by the Interaction with Mesenchymal Stem Cells. J. Cancer Stem Cell Res. 2014, 2014, e1003. [Google Scholar] [CrossRef]
- Geng, L.; Tang, X.; Wang, S.; Sun, Y.; Wang, D.; Tsao, B.P.; Feng, X.; Sun, L. Reduced Let-7f in Bone Marrow-Derived Mesenchymal Stem Cells Triggers Treg/Th17 Imbalance in Patients with Systemic Lupus Erythematosus. Front. Immunol. 2020, 11, 233. [Google Scholar] [CrossRef]
- Cao, Y.; Jin, X.; Sun, Y.; Wen, W. Therapeutic Effect of Mesenchymal Stem Cell on Hashimoto’s Thyroiditis in a Rat Model by Modulating Th17/Treg Cell Balance. Autoimmunity 2020, 53, 35–45. [Google Scholar] [CrossRef]
- Wang, M.; Chen, B.; Sun, X.X.; Zhao, X.D.; Zhao, Y.Y.; Sun, L.; Xu, C.G.; Shen, B.; Su, Z.L.; Xu, W.R.; et al. Gastric Cancer Tissue-Derived Mesenchymal Stem Cells Impact Peripheral Blood Mononuclear Cells via Disruption of Treg/Th17 Balance to Promote Gastric Cancer Progression. Exp. Cell Res. 2017, 361, 19–29. [Google Scholar] [CrossRef]
- Chen, W.; Huang, Y.; Han, J.; Yu, L.; Li, Y.; Lu, Z.; Li, H.; Liu, Z.; Shi, C.; Duan, F.; et al. Immunomodulatory Effects of Mesenchymal Stromal Cells-Derived Exosome. Immunol. Res. 2016, 64, 831–840. [Google Scholar] [CrossRef]
- Monguió-Tortajada, M.; Roura, S.; Gálvez-Montón, C.; Pujal, J.M.; Aran, G.; Sanjurjo, L.; Franquesa, M.; Sarrias, M.R.; Bayes-Genis, A.; Borràs, F.E. Nanosized UCMSC-Derived Extracellular Vesicles but Not Conditioned Medium Exclusively Inhibit the Inflammatory Response of Stimulated T Cells: Implications for Nanomedicine. Theranostics 2017, 7, 270–284. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Huang, S.; Yuan, X.; Liang, J.; Xu, R.; Yao, G.; Feng, X.; Sun, L. The Regulation of the Treg/Th17 Balance by Mesenchymal Stem Cells in Human Systemic Lupus Erythematosus. Cell. Mol. Immunol. 2017, 14, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Wang, J.; Fang, Q.; Liu, P.; Chen, S.; Zhe, N.; Lin, X.; Zhang, Y.; Zhao, J.; Zhou, Z. High expression of heme oxygenase-1 in target organs may attenuate acute graft-versus-host disease through regulation of immune balance of TH17/Treg. Transpl. Immunol. 2016, 37, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Yu, T.; Liu, D.; Shi, S.; Zhou, Y. Hydrogen sulfide promotes immunomodulation of gingiva-derived mesenchymal stem cells via the Fas/FasL coupling pathway. Stem Cell Res. Ther. 2018, 9, 62. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Yan, B.; Li, J.; Zhang, T.; Yang, R.; Wang, X.; Liu, Y.; Liu, D. Acetylsalicylic acid rescues the immunomodulation of inflamed gingiva-derived mesenchymal stem cells via upregulating FasL in mice. Stem Cell Res. Ther. 2019, 10, 368. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, R.I.; Minskaia, E.; Fernandes-Platzgummer, A.; Vieira, A.I.S.; da Silva, C.L.; Cabral, J.M.S.; Lacerda, J.F. Mesenchymal Stromal Cells Induce Regulatory T Cells via Epigenetic Conversion of Human Conventional CD4 T Cells In Vitro. Stem Cells 2020. [Google Scholar] [CrossRef]
- Djouad, F.; Charbonnier, L.M.; Bouffi, C.; Louis-Plence, P.; Bony, C.; Apparailly, F.; Cantos, C.; Jorgensen, C.; Noel, D. Mesenchymal stem cells inhibit the differentiation of dendritic cells through an interleukin-6-dependent mechanism. Stem Cells 2007, 25, 2025–2032. [Google Scholar] [CrossRef]
- Gao, W.X.; Sun, Y.Q.; Shi, J.; Li, C.L.; Fang, S.B.; Wang, D.; Deng, X.Q.; Wen, W.; Fu, Q.L. Effects of Mesenchymal Stem Cells from Human Induced Pluripotent Stem Cells on Differentiation, Maturation, and Function of Dendritic Cells. Stem Cell Res. Ther. 2017, 8, 48. [Google Scholar] [CrossRef]
- Abumaree, M.H.; Al Jumah, M.A.; Kalionis, B.; Jawdat, D.; Al Khaldi, A.; Abomaray, F.M.; Fatani, A.S.; Chamley, L.W.; Knawy, B.A. Human Placental Mesenchymal Stem Cells (pMSCs) Play a Role as Immune Suppressive Cells by Shifting Macrophage Differentiation from Inflammatory M1 to Anti-Inflammatory M2 Macrophages. Stem Cell Rev. Rep. 2013, 9, 620–641. [Google Scholar] [CrossRef]
- Fan, L.; Hu, C.; Chen, J.; Cen, P.; Wang, J.; Li, L. Interaction between Mesenchymal Stem Cells and B-Cells. Int. J. Mol. Sci. 2016, 17, 650. [Google Scholar] [CrossRef] [PubMed]
- Miossec, P.; Korn, T.; Kuchroo, V.K. Interleukin-17 and Type 17 Helper T Cells. N. Engl. J. Med. 2009, 361, 888–898. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, K.; Takeuchi, Y.; Hirota, K. The pathogenicity of Th17 cells in autoimmune diseases. Semin. Immunopathol. 2019, 41, 283–297. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.R. The Balance of Th17 versus Treg Cells in Autoimmunity. Int. J. Mol. Sci. 2018, 19, 730. [Google Scholar] [CrossRef] [PubMed]
- Ueno, A.; Jeffery, L.; Kobayashi, T.; Hibi, T.; Ghosh, S.; Jijon, H. Th17 Plasticity and Its Relevance to Inflammatory Bowel Disease. J. Autoimmun. 2018, 87, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Li, B. The Functional Stability of FOXP3 and RORγt in Treg and Th17 and Their Therapeutic Applications. Adv. Protein Chem. Struct. Biol. 2017, 107, 155–189. [Google Scholar] [CrossRef]
- Harris, S.G.; Padilla, J.; Koumas, L.; Ray, D.; Phipps, R.P. Prostaglandins as modulators of immunity. Trends Immunol. 2002, 23, 144–150. [Google Scholar] [CrossRef]
- Yao, C.; Sakata, D.; Esaki, Y.; Li, Y.; Matsuoka, T.; Kuroiwa, K.; Sugimoto, Y.; Narumiya, S. Prostaglandin E2-EP4 signaling promotes immune inflammation through Th1 cell differentiation and Th17 cell expansion. Nat. Med. 2009, 15, 633–640. [Google Scholar] [CrossRef]
- Boniface, K.; Bak-Jensen, K.S.; Li, Y.; Blumenschein, W.M.; McGeachy, M.J.; McClanahan, T.K.; McKenzie, B.S.; Kastelein, R.A.; Cua, D.J.; de Waal Malefyt, R. Prostaglandin E2 regulates Th17 cell differentiation and function through cyclic AMP and EP2/EP4 receptor signaling. J. Exp. Med. 2009, 206, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.M.; Pindjakova, J.; Hanley, S.A.; McCarthy, C.; Weidhofer, G.A.; Sweeney, E.M.; English, K.; Shaw, G.; Murphy, J.M.; Barry, F.P.; et al. Mesenchymal stem cell inhibition of T-helper 17 cell-differentiation is triggered by cell-cell contact and mediated by prostaglandin E2 via the EP4 receptor. Eur. J. Immunol. 2011, 41, 2840–2851. [Google Scholar] [CrossRef] [PubMed]
- Kalinski, P. Regulation of immune responses by prostaglandin E2. J. Immunol. 2012, 188, 21–28. [Google Scholar] [CrossRef]
- Vasilev, G.; Ivanova, M.; Ivanova-Todorova, E.; Tumangelova-Yuzeir, K.; Krasimirova, E.; Stoilov, R.; Kyurkchiev, D. Secretory Factors Produced by Adipose Mesenchymal Stem Cells Downregulate Th17 and Increase Treg Cells in Peripheral Blood Mononuclear Cells from Rheumatoid Arthritis Patients. Rheumatol. Int. 2019, 39, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Liu, X.; Cheng, K.; Yang, R.; Zhao, R.C. Mesenchymal Stem Cells Inhibit Th17 Cell Differentiation by IL-10 Secretion. Exp. Hematol. 2012, 40, 761–770. [Google Scholar] [CrossRef]
- Najar, M.; Lombard, C.A.; Fayyad-Kazan, H.; Faour, W.H.; Merimi, M.; Sokal, E.M.; Lagneaux, L.; Fahmi, H. Th17 Immune Response to Adipose Tissue-Derived Mesenchymal Stromal Cells. J. Cell. Physiol. 2019, 234, 21145–21152. [Google Scholar] [CrossRef]
- Svobodova, E.; Krulova, M.; Zajicova, A.; Pokorna, K.; Prochazkova, J.; Trosan, P.; Holan, V. The Role of Mouse Mesenchymal Stem Cells in Differentiation of Naive T-cells into Anti-Inflammatory Regulatory T-cell or Proinflammatory Helper T-cell 17 Population. Stem Cells Dev. 2012, 21, 901–910. [Google Scholar] [CrossRef]
- Li, C.L.; Leng, Y.; Zhao, B.; Gao, C.; Du, F.F.; Jin, N.; Lian, Q.Z.; Xu, S.Y.; Yan, G.L.; Xia, J.J.; et al. Human iPSC-MSC-Derived Xenografts Modulate Immune Responses by Inhibiting the Cleavage of Caspases. Stem Cells 2017, 35, 1719–1732. [Google Scholar] [CrossRef]
- Bouffi, C.; Bony, C.; Courties, G.; Jorgensen, C.; Noël, D. IL-6-Dependent PGE2 Secretion by Mesenchymal Stem Cells Inhibits Local Inflammation in Experimental Arthritis. PLoS ONE 2010, 5, e14247. [Google Scholar] [CrossRef]
- Harrell, C.R.; Markovic, B.S.; Fellabaum, C.; Arsenijevic, N.; Djonov, V.; Volarevic, V. The Role of Interleukin 1 Receptor Antagonist in Mesenchymal Stem Cell-Based Tissue Repair and Regeneration. BioFactors 2020, 46, 263–275. [Google Scholar] [CrossRef]
- Ren, G.; Su, J.; Zhang, L.; Zhao, X.; Ling, W.; L’Huillie, A.; Zhang, J.; Lu, Y.; Roberts, A.I.; Ji, W.; et al. Species variation in the mechanisms of mesenchymal stem cell-mediated immunosuppression. Stem Cells 2009, 27, 1954–1962. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Chen, X.; Huang, Y.; Li, W.; Li, J.; Cao, K.; Cao, G.; Zhang, L.; Li, F.; Roberts, A.I.; et al. Phylogenetic distinction of iNOS and IDO function in mesenchymal stem cell-mediated immunosuppression in mammalian species. Cell Death Differ. 2014, 21, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Volarevic, V.; Markovic, B.S.; Jankovic, M.G.; Djokovic, B.; Jovicic, N.; Harrell, C.R.; Fellabaum, C.; Djonov, V.; Arsenijevic, N.; Lukic, M.L. Galectin 3 Protects from Cisplatin-Induced Acute Kidney Injury by Promoting TLR-2-Dependent Activation of IDO1/Kynurenine Pathway in Renal DCs. Theranostics 2019, 9, 5976–6001. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, K.; Chen, Y.; Gong, Y.; Liang, Y. Indoleamine 2,3-Dioxygenase (IDO) Regulates Th17/Treg Immunity in Experimental IgA Nephropathy. Folia Biol. 2019, 65, 101–108. [Google Scholar]
- Routy, J.P.; Routy, B.; Graziani, G.M.; Mehraj, V. The Kynurenine Pathway Is a Double-Edged Sword in Immune-Privileged Sites and in Cancer: Implications for Immunotherapy. Int. J. Tryptophan Res. 2016, 9, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Milosavljevic, N.; Gazdic, M.; Simovic Markovic, B.; Arsenijevic, A.; Nurkovic, J.; Dolicanin, Z.; Jovicic, N.; Jeftic, I.; Djonov, V.; Arsenijevic, N.; et al. Mesenchymal stem cells attenuate liver fibrosis by suppressing Th17 cells–An experimental study. Transpl. Int. 2018, 31, 102–115. [Google Scholar] [CrossRef]
- Grochot-Przeczek, A.; Dulak, J.; Jozkowicz, A. Haem oxygenase-1: Non-canonical roles in physiology and pathology. Clin. Sci. 2012, 122, 93–103. [Google Scholar] [CrossRef]
- Shen, Z.Y.; Wu, B.; Liu, T.; Yang, Y.; Yin, M.L.; Zheng, W.P.; Zhang, B.Y.; Song, H.L. Immunomodulatory Effects of Bone Marrow Mesenchymal Stem Cells Overexpressing Heme Oxygenase-1: Protective Effects on Acute Rejection Following Reduced-Size Liver Transplantation in a Rat Model. Cell. Immunol. 2017, 313, 10–24. [Google Scholar] [CrossRef]
- Langrzyk, A.; Nowak, W.N.; Stępniewski, J.; Jaźwa, A.; Florczyk-Soluch, U.; Józkowicz, A.; Dulak, J. Critical View on Mesenchymal Stromal Cells in Regenerative Medicine. Antioxid. Redox Signal. 2018, 29, 169–190. [Google Scholar] [CrossRef]
- Ren, G.; Zhang, L.; Zhao, X.; Xu, G.; Zhang, Y.; Roberts, A.I.; Zhao, R.C.; Shi, Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell 2008, 2, 141–150. [Google Scholar] [CrossRef]
- Niedbala, W.; Cai, B.; Liew, F.Y. Role of Nitric Oxide in the Regulation of T Cell Functions. Ann. Rheum. Dis. 2006, 65 (Suppl. 3), iii37–iii40. [Google Scholar] [CrossRef]
- Sato, K.; Ozaki, K.; Oh, I.; Meguro, A.; Hatanaka, K.; Nagai, T.; Muroi, K.; Ozawa, K. Nitric Oxide Plays a Critical Role in Suppression of T-cell Proliferation by Mesenchymal Stem Cells. Blood 2007, 109, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Zhang, Y.; Tang, Q.; Gao, J.; Yang, H.; Gao, Z.; Zhao, R.C. Mechanisms of the Immunomodulation Effects of Bone Marrow-Derived Mesenchymal Stem Cells on Facial Nerve Injury in Sprague-Dawley Rats. Stem Cells Dev. 2019, 28, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.H.; Wu, F.; Liu, L.; Chen, H.B.; Zheng, R.Q.; Wang, H.L.; Yu, L.N. Mesenchymal Stem Cells Regulate the Th17/Treg Cell Balance Partly through Hepatocyte Growth Factor In Vitro. Stem Cell Res. Ther. 2020, 11, 91. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.P.; Han, D.M.; Zhao, L.; Guo, Z.K.; Xiao, F.J.; Zhang, Y.K.; Zhang, X.Y.; Wang, L.S.; Wang, H.X.; Wang, H. Hepatocyte Growth Factor Enhances the Inflammation-Alleviating Effect of Umbilical Cord-Derived Mesenchymal Stromal Cells in a Bronchiolitis Obliterans Model. Cytotherapy 2016, 18, 402–412. [Google Scholar] [CrossRef] [PubMed]
- Hübel, J.; Hieronymus, T. HGF/Met-Signaling Contributes to Immune Regulation by Modulating Tolerogenic and Motogenic Properties of Dendritic Cells. Biomedicines 2015, 3, 138–148. [Google Scholar] [CrossRef]
- Keir, M.E.; Butte, M.J.; Freeman, G.J.; Sharpe, A.H. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 2008, 26, 677–704. [Google Scholar] [CrossRef]
- Kim, J.Y.; Park, M.; Kim, Y.H.; Ryu, K.H.; Lee, K.H.; Cho, K.A.; Woo, S.Y. Tonsil-derived mesenchymal stem cells (T-MSCs) prevent Th17-mediated autoimmune response via regulation of the programmed death-1/programmed death ligand-1 (PD-1/PD-L1) pathway. J. Tissue Eng. Regen. Med. 2018, 12, e1022–e1033. [Google Scholar] [CrossRef]
- Luz-Crawford, P.; Djouad, F.; Toupet, K.; Bony, C.; Franquesa, M.; Hoogduijn, M.J.; Jorgensen, C.; Noël, D. Mesenchymal Stem Cell-Derived Interleukin 1 Receptor Antagonist Promotes Macrophage Polarization and Inhibits B Cell Differentiation. Stem Cells 2016, 34, 483–492. [Google Scholar] [CrossRef]
- Ren, G.; Zhao, X.; Zhang, L.; Zhang, J.; L’Huillier, A.; Ling, W.; Roberts, A.I.; Le, A.D.; Shi, S.; Shao, C.; et al. Inflammatory Cytokine-Induced Intercellular Adhesion Molecule-1 and Vascular Cell Adhesion Molecule-1 in Mesenchymal Stem Cells Are Critical for Immunosuppression. J. Immunol. 2010, 184, 2321–2328. [Google Scholar] [CrossRef]
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, Secretion, and Intercellular Interactions of Exosomes and Other Extracellular Vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef] [PubMed]
- L Ramos, T.; Sánchez-Abarca, L.I.; Muntión, S.; Preciado, S.; Puig, N.; López-Ruano, G.; Hernández-Hernández, Á.; Redondo, A.; Ortega, R.; Rodríguez, C.; et al. MSC Surface Markers (CD44, CD73, and CD90) Can Identify Human MSC-derived Extracellular Vesicles by Conventional Flow Cytometry. Cell Commun. Signal. 2016, 14, 2. [Google Scholar] [CrossRef]
- Quesenberry, P.J.; Goldberg, L.R.; Aliotta, J.M.; Dooner, M.S.; Pereira, M.G.; Wen, S.; Camussi, G. Cellular Phenotype and Extracellular Vesicles: Basic and Clinical Considerations. Stem Cells Dev. 2014, 23, 1429–1436. [Google Scholar] [CrossRef] [PubMed]
- Montecalvo, A.; Larregina, A.T.; Shufesky, W.J.; Stolz, D.B.; Sullivan, M.L.; Karlsson, J.M.; Baty, C.J.; Gibson, G.A.; Erdos, G.; Wang, Z.; et al. Mechanism of Transfer of Functional microRNAs between Mouse Dendritic Cells via Exosomes. Blood 2012, 119, 756–766. [Google Scholar] [CrossRef]
- Hidalgo-Garcia, L.; Galvez, J.; Rodriguez-Cabezas, M.E.; Anderson, P.O. Can a Conversation between Mesenchymal Stromal Cells and Macrophages Solve the Crisis in the Inflamed Intestine? Front. Pharmacol. 2018, 9, 179. [Google Scholar] [CrossRef] [PubMed]
- Rad, F.; Ghorbani, M.; Mohammadi Roushandeh, A.; Habibi Roudkenar, M. Mesenchymal Stem Cell-Based Therapy for Autoimmune Diseases: Emerging Roles of Extracellular Vesicles. Mol. Biol. Rep. 2019, 46, 1533–1549. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Dong, C.; Yang, J.; Jin, Y.; Zheng, W.; Zhou, Q.; Liang, Y.; Bao, L.; Feng, G.; Ji, J.; et al. Exosomal microRNA-155-5p from PDLSCs Regulated Th17/Treg Balance by Targeting Sirtuin-1 in Chronic Periodontitis. J. Cell. Physiol. 2019, 234, 20662–20674. [Google Scholar] [CrossRef]
- Song, N.; Zhang, T.; Xu, X.; Lu, Z.; Yu, X.; Fang, Y.; Hu, J.; Jia, P.; Teng, J.; Ding, X. miR-21 Protects against Ischemia/Reperfusion-Induced Acute Kidney Injury by Preventing Epithelial Cell Apoptosis and Inhibiting Dendritic Cell Maturation. Front. Physiol. 2018, 9, 790. [Google Scholar] [CrossRef]
- Gu, D.; Zou, X.; Ju, G.; Zhang, G.; Bao, E.; Zhu, Y. Mesenchymal Stromal Cells Derived Extracellular Vesicles Ameliorate Acute Renal Ischemia Reperfusion Injury by Inhibition of Mitochondrial Fission through miR-30. Stem Cells Int. 2016, 2016, 2093940. [Google Scholar] [CrossRef]
- Harrell, C.R.; Fellabaum, C.; Jovicic, N.; Djonov, V.; Arsenijevic, N.; Volarevic, V. Molecular Mechanisms Responsible for Therapeutic Potential of Mesenchymal Stem Cell-Derived Secretome. Cells 2019, 8, 467. [Google Scholar] [CrossRef]
- Kapnick, S.M.; Pacheco, S.E.; McGuire, P.J. The Emerging Role of Immune Dysfunction in Mitochondrial Diseases as a Paradigm for Understanding Immunometabolism. Metab. Clin. Exp. 2018, 81, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Torralba, D.; Baixauli, F.; Sánchez-Madrid, F. Mitochondria Know No Boundaries: Mechanisms and Functions of Intercellular Mitochondrial Transfer. Front. Cell Dev. Biol. 2016, 4, 107. [Google Scholar] [CrossRef]
- Berridge, M.V.; McConnell, M.J.; Grasso, C.; Bajzikova, M.; Kovarova, J.; Neuzil, J. Horizontal Transfer of Mitochondria between Mammalian Cells: Beyond Co-Culture Approaches. Curr. Opin. Genet. Dev. 2016, 38, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.L.; Zhang, Y.; Li, X.; Fu, Q.L. Mechanisms Underlying the Protective Effects of Mesenchymal Stem Cell-Based Therapy. Cell. Mol. Life Sci. 2020, 77, 2771–2794. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Qiu, Y.; Shi, Y.; Cai, J.; Wang, B.; Wei, X.; Ke, Q.; Sui, X.; Wang, Y.; et al. Cell Adhesion-Mediated Mitochondria Transfer Contributes to Mesenchymal Stem Cell-Induced Chemoresistance on T Cell Acute Lymphoblastic Leukemia Cells. J. Hematol. Oncol. 2018, 11, 11. [Google Scholar] [CrossRef]
- Ahmad, T.; Mukherjee, S.; Pattnaik, B.; Kumar, M.; Singh, S.; Kumar, M.; Rehman, R.; Tiwari, B.K.; Jha, K.A.; Barhanpurkar, A.P.; et al. Miro1 Regulates Intercellular Mitochondrial Transport & Enhances Mesenchymal Stem Cell Rescue Efficacy. EMBO J. 2014, 33, 994–1010. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Hwang, J.W.; Yun, C.K.; Lee, Y.; Choi, Y.S. Delivery of Exogenous Mitochondria via Centrifugation Enhances Cellular Metabolic Function. Sci. Rep. 2018, 8, 3330. [Google Scholar] [CrossRef] [PubMed]
- Konari, N.; Nagaishi, K.; Kikuchi, S.; Fujimiya, M. Mitochondria Transfer from Mesenchymal Stem Cells Structurally and Functionally Repairs Renal Proximal Tubular Epithelial Cells in Diabetic Nephropathy In Vivo. Sci. Rep. 2019, 9, 5184. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.N.; Das, S.R.; Emin, M.T.; Wei, M.; Sun, L.; Westphalen, K.; Rowlands, D.J.; Quadri, S.K.; Bhattacharya, S.; Bhattacharya, J. Mitochondrial Transfer from Bone-Marrow-Derived Stromal Cells to Pulmonary Alveoli Protects against Acute Lung Injury. Nat. Med. 2012, 18, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Gao, F.; Zhang, Y.; Wong, D.S.; Li, Q.; Tse, H.F.; Xu, G.; Yu, Z.; Lian, Q. Mitochondrial Transfer of Mesenchymal Stem Cells Effectively Protects Corneal Epithelial Cells From Mitochondrial Damage. Cell Death Dis. 2016, 7, e2467. [Google Scholar] [CrossRef]
- Luz-Crawford, P.; Torres, M.J.; Noël, D.; Fernandez, A.; Toupet, K.; Alcayaga-Miranda, F.; Tejedor, G.; Jorgensen, C.; Illanes, S.E.; Figueroa, F.E.; et al. The Immunosuppressive Signature of Menstrual Blood Mesenchymal Stem Cells Entails Opposite Effects on Experimental Arthritis and Graft versus Host Diseases. Stem Cells 2016, 34, 456–469. [Google Scholar] [CrossRef] [PubMed]
- Noronha, N.C.; Mizukami, A.; Caliári-Oliveira, C.; Cominal, J.G.; Rocha, J.L.M.; Covas, D.T.; Swiech, K.; Malmegrim, K.C.R. Priming Approaches to Improve the Efficacy of Mesenchymal Stromal Cell-Based Therapies. Stem Cell Res. Ther. 2019, 10, 131. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yang, Q.; Wang, Z.; Tong, H.; Ma, L.; Zhang, Y.; Shan, F.; Meng, Y.; Yuan, Z. Comparative Analysis of Human Mesenchymal Stem Cells from Fetal-Bone Marrow, Adipose Tissue, and Warton’s Jelly as Sources of Cell Immunomodulatory Therapy. Hum. Vaccines Immunother. 2016, 12, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Carrero, R.; Cerrada, I.; Lledó, E.; Dopazo, J.; García-García, F.; Rubio, M.P.; Trigueros, C.; Dorronsoro, A.; Ruiz-Sauri, A.; Montero, J.A.; et al. IL1β Induces Mesenchymal Stem Cells Migration and Leucocyte Chemotaxis Through NF-κB. Stem Cell Rev. Rep. 2012, 8, 905–916. [Google Scholar] [CrossRef]
- Fan, H.; Zhao, G.; Liu, L.; Liu, F.; Gong, W.; Liu, X.; Yang, L.; Wang, J.; Hou, Y. Pre-treatment with IL-1β Enhances the Efficacy of MSC Transplantation in DSS-induced Colitis. Cell. Mol. Immunol. 2012, 9, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Yang, Q.; Lin, L.; Xu, C.; Zheng, C.; Chen, X.; Han, Y.; Li, M.; Cao, W.; Cao, K.; et al. Interleukin-17 Enhances Immunosuppression by Mesenchymal Stem Cells. Cell Death Differ. 2014, 21, 1758–1768. [Google Scholar] [CrossRef]
- Kurte, M.; Luz-Crawford, P.; Vega-Letter, A.; Contreras, R.; Tejedor, G.; Elizondo-Vega, R.; Martinez-Viola, L.; Fernández-O’Ryan, C.; Figueroa, F.; Jorgensen, C.; et al. IL17/IL17RA As a Novel Signaling Axis Driving Mesenchymal Stem Cell Therapeutic Function in Experimental Autoimmune Encephalomyelitis. Front. Immunol. 2018, 9, 802. [Google Scholar] [CrossRef]
- Sivanathan, K.N.; Rojas-Canales, D.M.; Hope, C.M.; Krishnan, R.; Carroll, R.P.; Gronthos, S.; Grey, S.T.; Coates, P.T. Interleukin-17A-Induced Human Mesenchymal Stem Cells Are Superior Modulators of Immunological Function. Stem Cells 2015, 33, 2850–2863. [Google Scholar] [CrossRef]
- Zimmermann, J.A.; McDevitt, T.C. Pre-Conditioning Mesenchymal Stromal Cell Spheroids for Immunomodulatory Paracrine Factor Secretion. Cytotherapy 2014, 16, 331–345. [Google Scholar] [CrossRef]
- Spencer, J.A.; Ferraro, F.; Roussakis, E.; Klein, A.; Wu, J.; Runnels, J.M.; Zaher, W.; Mortensen, L.J.; Alt, C.; Turcotte, R.; et al. Direct Measurement of Local Oxygen Concentration in the Bone Marrow of Live Animals. Nature 2014, 508, 269–273. [Google Scholar] [CrossRef]
- Saparov, A.; Ogay, V.; Nurgozhin, T.; Jumabay, M.; Chen, W.C. Preconditioning of Human Mesenchymal Stem Cells to Enhance Their Regulation of the Immune Response. Stem Cells Int. 2016, 2016, 3924858. [Google Scholar] [CrossRef] [PubMed]
- Haque, N.; Rahman, M.T.; Abu Kasim, N.H.; Alabsi, A.M. Hypoxic Culture Conditions As a Solution for Mesenchymal Stem Cell Based Regenerative Therapy. Sci. World J. 2013, 2013, 632972. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Lopez, R.; Elizondo-Vega, R.; Paredes, M.J.; Luque-Campos, N.; Torres, M.J.; Tejedor, G.; Vega-Letter, A.M.; Figueroa-Valdés, A.; Pradenas, C.; Oyarce, K.; et al. HIF1α-Dependent Metabolic Reprogramming Governs Mesenchymal Stem/Stromal Cell Immunoregulatory Functions. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2020, 34, 8250–8264. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, P.A. Metabolic and Nonmetabolic Regulatory Functions of Peroxisome Proliferator-Activated Receptor Beta. Curr. Opin. Lipidol. 2010, 21, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Palomer, X.; Barroso, E.; Pizarro-Delgado, J.; Peña, L.; Botteri, G.; Zarei, M.; Aguilar, D.; Montori-Grau, M.; Vázquez-Carrera, M. PPARβ/δ: A Key Therapeutic Target in Metabolic Disorders. Int. J. Mol. Sci. 2018, 19, 913. [Google Scholar] [CrossRef]
- Luz-Crawford, P.; Ipseiz, N.; Espinosa-Carrasco, G.; Caicedo, A.; Tejedor, G.; Toupet, K.; Loriau, J.; Scholtysek, C.; Stoll, C.; Khoury, M.; et al. PPARβ/δ Directs the Therapeutic Potential of Mesenchymal Stem Cells in Arthritis. Ann. Rheum. Dis. 2016, 75, 2166–2174. [Google Scholar] [CrossRef] [PubMed]
- Luz-Crawford, P.; Espinosa-Carrasco, G.; Ipseiz, N.; Contreras, R.; Tejedor, G.; Medina, D.A.; Vega-Letter, A.M.; Ngo, D.; Morand, E.F.; Pène, J.; et al. Gilz-Activin A As a Novel Signaling Axis Orchestrating Mesenchymal Stem Cell and Th17 Cell Interplay. Theranostics 2018, 8, 846–859. [Google Scholar] [CrossRef]
- Palkar, P.S.; Borland, M.G.; Naruhn, S.; Ferry, C.H.; Lee, C.; Sk, U.H.; Sharma, A.K.; Amin, S.; Murray, I.A.; Anderson, C.R.; et al. Cellular and Pharmacological Selectivity of the Peroxisome Proliferator-Activated Receptor-β/δ Antagonist GSK3787. Mol. Pharmacol. 2010, 78, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Hong, F.; Xu, P.; Zhai, Y. The Opportunities and Challenges of Peroxisome Proliferator-Activated Receptors Ligands in Clinical Drug Discovery and Development. Int. J. Mol. Sci. 2018, 19, 2189. [Google Scholar] [CrossRef] [PubMed]
| Target Cell | Involved Mechanism | Observed Effect | References |
|---|---|---|---|
| Innate Cells | |||
| DCs | TGF-β, HGF, EVs | ↓MHC-II, CD86, CD40; ↑phagocytic function; ↓IL-6, IL-12, TNF-α, IFN-α; ↑IL-10, TGF-β | [50,51,52,53,54] |
| Macrophages | TGF-β, iNOS, mitochondrial transfer, EVs | ↓CD86; ↑phagocytic function, ↓IL-6, IL-8, TNF-α, IL-1β; ↑IL-10, TGF-β; ↑M2 type polarization | [55,56,57,58,59,60,61,62] |
| NK cells | IDO, PGE2, EVs | ↓IFN-γ; ↓cytotoxic activity, proliferation | [63,64,65,66] |
| Neutrophils | TGF-β, EVs | ↓CRAMP and MPO messenger RNA (mRNA), ↓IL-17 | [52,57,61,67] |
| ILC | PGE2, IL-7 | ↑IL-22; ↑proliferation | [68] |
| Adaptive Cells | |||
| B cells | PD-1, IDO, TGF-β, EVs | ↓IgG production; ↓CD69, CD83, CD86; ↓IL-4 mRNA; ↓proliferation, plasmablast differentiation; ↑IL-10 | [66,69,70,71,72,73,74,75] |
| Th1 cells | PD-1, IDO, Fas, IL-6, TGF-β, IL-1Ra, EVs, mitochondrial transfer | ↓IFN-γ, IL-1β, TNF-α; ↑apoptosis; ↓proliferation; differentiation | [52,58,67,76,77,78,79,80,81,82,83,84,85] |
| Th2 cells | IL-6, EVs | ↑differentiation; ↑IL-4, IL5; ↓IL-4, IL-5, IL-13 | [67,76,86,87,88,89] |
| Th17 cells | PGE2, Fas, IDO, IL-6, TGF-β, IL-1Ra, EVs, mitochondrial transfer | ↓IL-17, IL-22; ↑apoptosis; ↓proliferation; differentiation; ↑interconversion to Treg cells. | [54,58,67,75,76,77,78,79,81,82,83,84,90,91,92,93,94,95,96,97,98,99] |
| Regulatory T (Treg) cells | PGE2, Fas, IDO, IL-6, iNOS, TGF-β, IL-1Ra, EVs, mitochondrial transfer | ↑PD-1, ↑IL-10, TGF-β; ↑proliferation; differentiation; ↑conversion from Th17 cells. | [54,58,59,66,67,75,76,77,78,79,80,81,82,85,90,91,92,93,94,96,97,98,99,100] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terraza-Aguirre, C.; Campos-Mora, M.; Elizondo-Vega, R.; Contreras-López, R.A.; Luz-Crawford, P.; Jorgensen, C.; Djouad, F. Mechanisms behind the Immunoregulatory Dialogue between Mesenchymal Stem Cells and Th17 Cells. Cells 2020, 9, 1660. https://doi.org/10.3390/cells9071660
Terraza-Aguirre C, Campos-Mora M, Elizondo-Vega R, Contreras-López RA, Luz-Crawford P, Jorgensen C, Djouad F. Mechanisms behind the Immunoregulatory Dialogue between Mesenchymal Stem Cells and Th17 Cells. Cells. 2020; 9(7):1660. https://doi.org/10.3390/cells9071660
Chicago/Turabian StyleTerraza-Aguirre, Claudia, Mauricio Campos-Mora, Roberto Elizondo-Vega, Rafael A. Contreras-López, Patricia Luz-Crawford, Christian Jorgensen, and Farida Djouad. 2020. "Mechanisms behind the Immunoregulatory Dialogue between Mesenchymal Stem Cells and Th17 Cells" Cells 9, no. 7: 1660. https://doi.org/10.3390/cells9071660
APA StyleTerraza-Aguirre, C., Campos-Mora, M., Elizondo-Vega, R., Contreras-López, R. A., Luz-Crawford, P., Jorgensen, C., & Djouad, F. (2020). Mechanisms behind the Immunoregulatory Dialogue between Mesenchymal Stem Cells and Th17 Cells. Cells, 9(7), 1660. https://doi.org/10.3390/cells9071660







