Cytokine Profile in Striated Muscle Laminopathies: New Promising Biomarkers for Disease Prediction
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Healthy Controls
2.2. Serum Sample Collection
2.3. Cytokine, Chemokine and Growth Factor Quantification
2.4. Statistical Analysis
3. Results
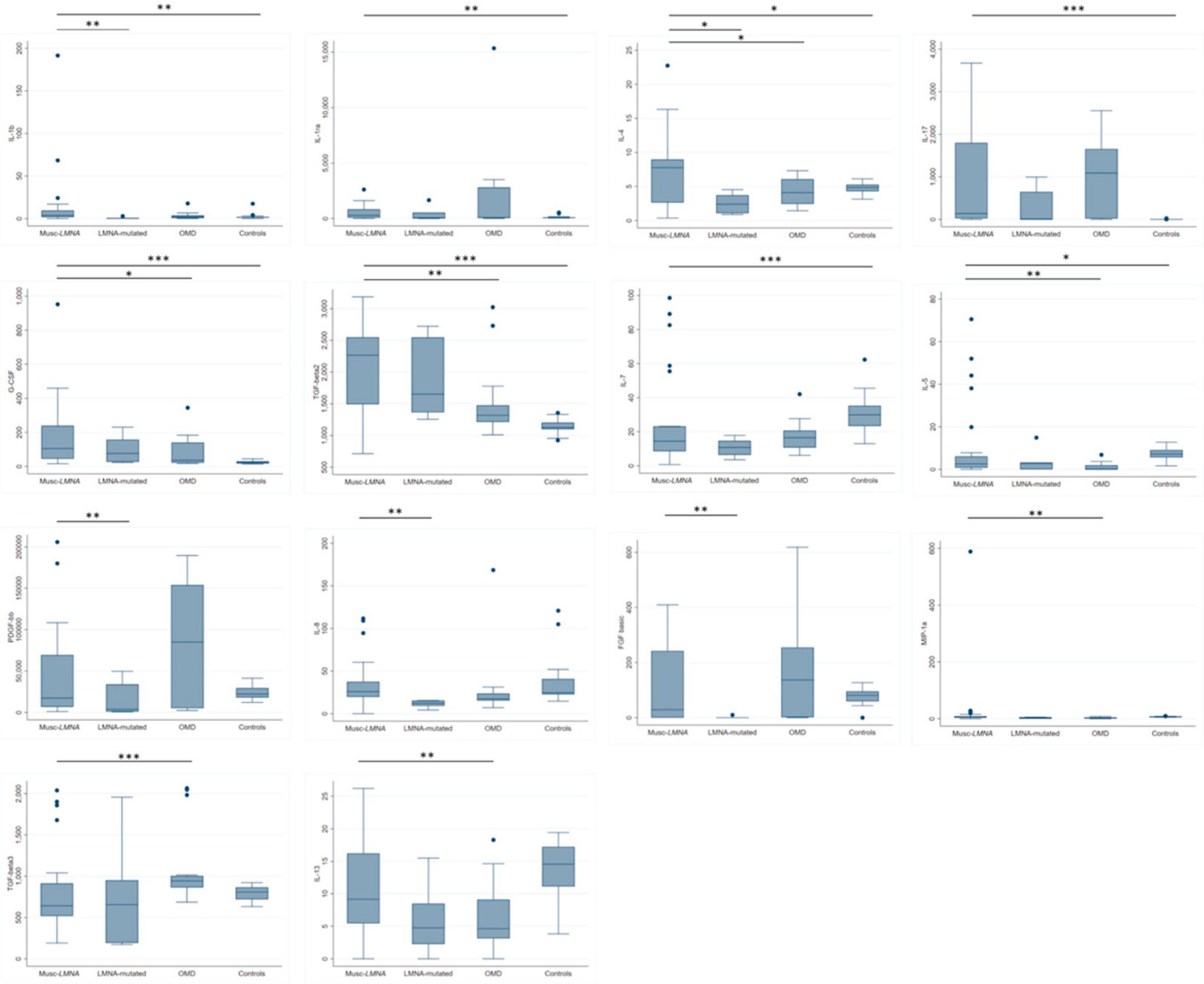
3.1. Evaluation of Cytokine Levels in the Sera of the Musc-LMNA Patients (n = 40) and Comparison with Those of Healthy Controls
3.2. IL-1β, IL-4 and IL-8 Levels in Musc-LMNA Compared to LMNA-Mutated Patients
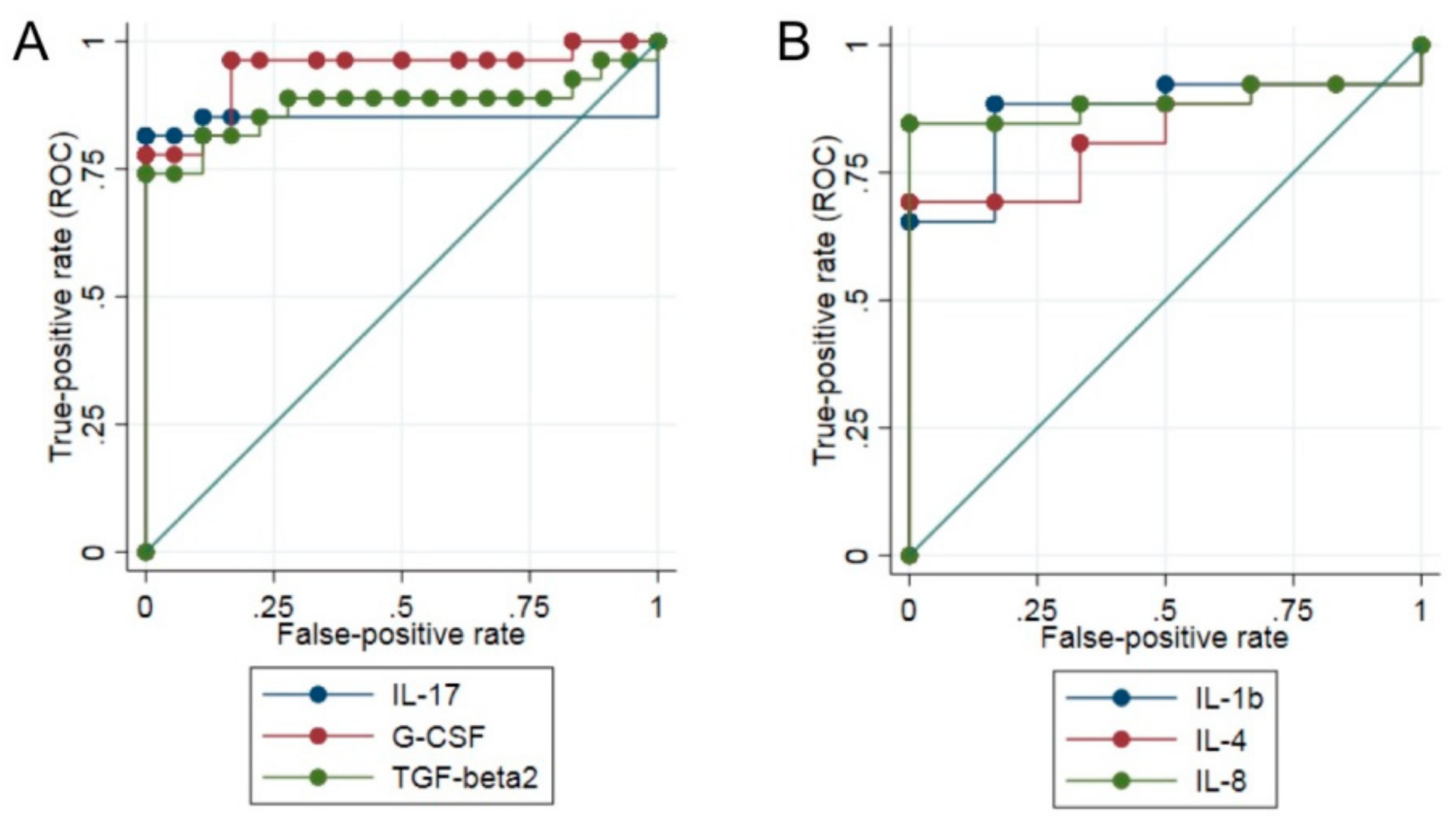
3.3. Differential Expression of Cytokines between Patients Affected by Musc-LMNA and Other Muscular Disorders
3.4. IL-17 Is Significantly Down-Regulated in Musc-LMNA Patients with Also Cardiac Involvement
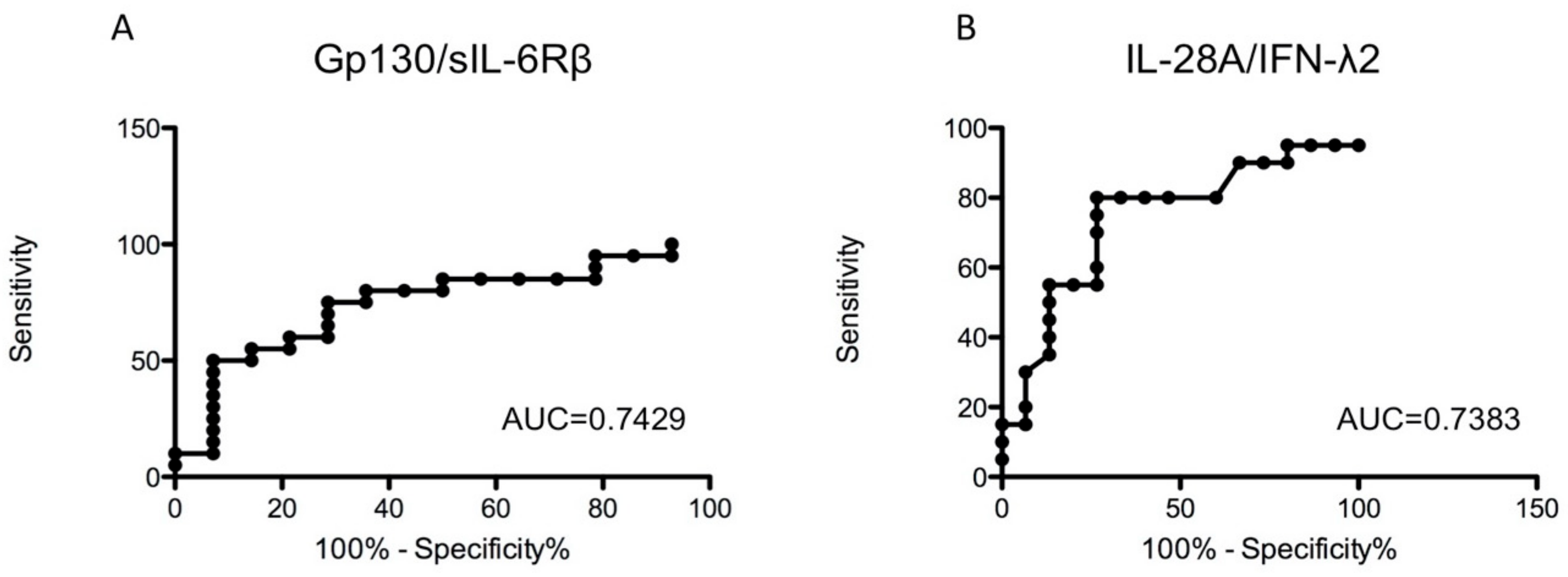
3.5. Analysis of TNF-α-Related Cytokines in Sera of Musc-LMNA Patients and Healthy Controls
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Camozzi, D.; Capanni, C.; Cenni, V.; Mattioli, E.; Columbaro, M.; Squarzoni, S.; Lattanzi, G. Diverse lamin-dependent mechanisms interact to control chromatin dynamics. Focus on laminopathies. Nucleus 2014, 5, 427–440. [Google Scholar] [CrossRef] [PubMed]
- Bonne, G.; Yaou, R.B.; Béroud, C.; Boriani, G. 108th ENMC International Workshop, 3rd Workshop of the MYO-CLUSTER project: EUROMEN, 7th International Emery-Dreifuss Muscular Dystrophy (EDMD) Workshop, 13–15 September 2002, Naarden, The Netherlands. Neuromuscul. Disord. 2003, 13, 508–515. [Google Scholar] [CrossRef]
- Turgay, Y.; Medalia, O. The structure of lamin filaments in somatic cells as revealed by cryo-electron tomography. Nucleus 2017, 8, 475–481. [Google Scholar] [CrossRef]
- Kang, S.M.; Yoon, M.H.; Park, B.J. Laminopathies; Mutations on single gene and various human genetic diseases. BMB Rep. 2018, 51, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Menezes, M.P.; Waddell, L.B.; Evesson, F.J.; Cooper, S.; Webster, R.; Jones, K.; Mowat, D.M.; Kiernan, C.; Johnston, H.M.; Corbett, A.; et al. Importance and challenge of making an early diagnosis in LMNA-related muscular dystrophy. Neurology 2012, 78, 1258–1263. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, I.; Rosa, I.; Bravo, S.B.; Guitián, E.; Pérez-Serra, A.; Campuzano, O.; Brugada, R.; Mangas, A.; García, Á.; Toro, R. Proteomic identification of putative biomarkers for early detection of sudden cardiac death in a family with a LMNA gene mutation causing dilated cardiomyopathy. J. Proteom. 2016, 148, 75–84. [Google Scholar] [CrossRef]
- Cappelletti, C.; Salerno, F.; Canioni, E.; Mora, M.; Mantegazza, R.; Bernasconi, P.; Maggi, L. Up-regulation of toll-like receptors 7 and 9 and its potential implications in the pathogenic mechanisms of LMNA-related myopathies. Nucleus 2018, 9, 398–409. [Google Scholar] [CrossRef]
- Cavaillon, J.M. Cytokines and Macrophages. Biomed. Pharmacother. 1994, 48, 445–453. [Google Scholar] [CrossRef]
- Arango Duque, G.; Descoteaux, A. Macrophage cytokines: Involvement in immunity and infectious diseases. Front. Immunol. 2014, 5, 491. [Google Scholar] [CrossRef]
- Monastero, R.N.; Pentyala, S. Cytokines as biomarkers and their respective clinical cutoff levels. Int. J. Inflam. 2017, 2017, 4309485. [Google Scholar] [CrossRef]
- Badrising, U.A.; Tsonaka, R.; Hiller, M.; Niks, E.H.; Evangelista, T.; Lochmüller, H.; Verschuuren, J.J.G.M.; Aartsma-Rus, A.; Spitali, P. Cytokine profiling of serum allows monitoring of disease progression in inclusion body myositis. J. Neuromuscul. Dis. 2017, 4, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Riaz, M.; Raz, Y.; van der Slujis, B.; Dickson, G.; van Engelen, B.; Vissing, J.; Raz, V. Cytokine genes as potential biomarkers for muscle weakness in OPMD. Hum. Mol. Genet. 2016, 25, 4282–4287. [Google Scholar] [CrossRef] [PubMed]
- Abdel Galil, S.M.; Ezzeldin, N.; El-Boshy, M.E. The role of serum IL-17 and IL-6 as biomarkers of disease activity and predictors of remission in patients with lupus nephritis. Cytokine 2015, 76, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Ball, D. Metabolic and endocrine response to exercise: Sympathoadrenal integration with skeletal muscle. J. Endocrinol. 2015, 224, R79–R95. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Febbraio, M.A. Muscle as an endocrine organ: Focus on muscle-derived interleukin-6. Physiol. Rev. 2008, 88, 1379–1406. [Google Scholar] [CrossRef] [PubMed]
- Ostrowski, K.; Rohde, T.; Zacho, M.; Asp, S.; Pedersen, B.K. Evidence that interleukin-6 is produced in human skeletal muscle during prolonged running. J. Physiol. 1998, 508, 949–953. [Google Scholar] [CrossRef] [PubMed]
- Bernasconi, P.; Carboni, N.; Ricci, G.; Siciliano, G.; Politano, L.; Maggi, L.; Mongini, T.; Vercelli, L.; Rodolico, C.; Biagini, E.; et al. Elevated TGF β2 serum levels in Emery-Dreifuss muscular dystrophy: Implications for myocyte and tenocyte differentiation and fibrogenic processess. Nucleus 2018, 9, 292–304. [Google Scholar] [CrossRef]
- Gong, D.; Shi, W.; Yi, S.J.; Chen, H.; Groffen, J.; Heisterkamp, N. TGFβ Signaling Plays a Critical Role in Promoting Alternative Macrophage Activation. BMC Immunol. 2012, 13, 31. [Google Scholar] [CrossRef]
- Pons, V.; Laflamme, N.; Préfontaine, P.; Rivest, S. Role of macrophage colony-stimulating factor receptor on the proliferation and survival of microglia following systemic nerve and cuprizone-induced injuries. Front. Immunol. 2020, 11, 47. [Google Scholar] [CrossRef]
- Preuße, C.; von Moers, A.; Kölbel, H.; Pehl, D.; Goebel, H.H.; Schara, U.; Stenzel, W. Inflammation-induced fibrosis in skeletal muscle of female carriers of Duchenne muscular dystrophy. Neuromuscul. Disord. 2019, 29, 487–496. [Google Scholar] [CrossRef]
- Speeckaert, R.; Lambert, J.; Grine, L.; Van Gele, M.; De Schepper, S.; van Geel, N. The many faces of interleukin-17 in inflammatory skin diseases. Br. J. Dermatol. 2016, 175, 892–901. [Google Scholar] [CrossRef]
- Erbel, C.; Akhavanpoor, M.; Okuyucu, D.; Wangler, S.; Dietz, A.; Zhao, L.; Stellos, K.; Little, K.M.; Lasitschka, F.; Doesch, A.; et al. IL-17A influences essential functions of the monocyte/macrophage lineage and is involved in advanced murine and human atherosclerosis. J. Immunol. 2014, 193, 4344–4355. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Atsuta, I.; Liu, S.; Chen, C.; Shi, S.; Shi, S.; Le, A.D. IL-17-mediated M1/M2 macrophage alteration contributes to pathogenesis of bisphosphonate-related osteonecrosis of the jaws. Clin. Cancer Res. 2013, 19, 3176–3188. [Google Scholar] [CrossRef] [PubMed]
- Hollmén, M.; Karaman, S.; Schwager, S.; Lisibach, A.; Christiansen, A.J.; Maksimow, M.; Varga, Z.; Jalkanen, S.; Detmar, M. G-CSF regulates macrophage phenotype and associates with poor overall survival in human triple-negative breast cancer. Oncoimmunology 2016, 5, e1115177. [Google Scholar] [CrossRef] [PubMed]
- ElKassar, N.; Gress, R.E. An overview of IL-7 biology and its use in immunotherapy. J. Immunotoxicol. 2010, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Keane, M.P.; Zhu, L.X.; Sharma, S.; Rozengurt, E.; Strieter, R.M.; Dubinett, S.M.; Huang, M. Interleukin-7 and transforming growth Factor-β play counter-regulatory roles in protein kinase c-δ-dependent control of fibroblast collagen synthesis in pulmonary fibrosis. J. Biol. Chem. 2004, 279, 28315–28319. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Sharma, S.; Zhu, L.X.; Keane, M.P.; Luo, J.; Zhang, L.; Burdick, M.D.; Lin, Y.Q.; Dohadwala, M.; Gardner, B.; et al. IL-7 inhibits fibroblast TGF-beta production and signaling in pulmonary fibrosis. J. Clin. Invest. 2002, 109, 931–937. [Google Scholar] [CrossRef]
- Bao, C.; Wang, B.; Yang, F.; Chen, L. Blockade of Interleukin-7 receptor shapes macrophage alternative activation and promotes functional recovery after spinal cord injury. Neuroscience 2018, 371, 518–527. [Google Scholar] [CrossRef]
- Fischer, P.; Hilfiker-Kleiner, D. Role of gp130-mediated signalling pathways in the heart and its impact on potential therapeutic aspects. Br. J. Pharmacol. 2008, 153, S414–S427. [Google Scholar] [CrossRef]
- Ayaub, E.A.; Dubey, A.; Imani, J.; Botelho, F.; Kolb, M.R.J.; Richards, C.D.; Ask, K. Overexpression of OSM and IL-6 impacts the polarization of pro-fibrotic macrophages and the development of bleomycin-induced lung fibrosis. Sci. Rep. 2017, 7, 13281. [Google Scholar] [CrossRef]
- Rückerl, D.; Seoane, P.I. The M2 triangle: gp130 binding cytokines drive macrophages to promote tumor growth. Immunol. Cell Biol. 2018, 96, 243–245. [Google Scholar] [CrossRef]
- Fischer, P.; Hilfiker-Kleiner, D. Survival pathways in hypertrophy and heart failure: The gp130-STAT axis. Basic Res. Cardiol. 2007, 102, 393–411. [Google Scholar] [CrossRef] [PubMed]
- Aukrust, P.; Ueland, T.; Lien, E.; Bendtzen, K.; Müller, F.; Andreassen, A.K.; Nordøy, I.; Aass, H.; Espevik, T.; Simonsen, S.; et al. Cytokine network in congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am. J. Cardiol. 1999, 83, 376–382. [Google Scholar] [CrossRef]
- Peretto, G.; Di Resta, C.; Perversi, J.; Forleo, C.; Maggi, L.; Politano, L.; Barison, A.; Previtali, S.C.; Carboni, N.; Brun, F.; et al. Italian network for laminopathies (NIL). Cardiac and neuromuscular features of patients with LMNA-related cardiomyopathy. Ann. Intern. Med. 2019, 171, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Maggi, L.; D’Amico, A.; Pini, A.; Sivo, S.; Pane, M.; Ricci, G.; Vercelli, L.; D’Ambrosio, P.; Travaglini, L.; Sala, S.; et al. LMNA-associated Myopathies: The Italian experience in a large cohort of patients. Neurology 2014, 83, 1634–1644. [Google Scholar] [CrossRef] [PubMed]
| Musc-LMNA 1st Group (n = 40) | Musc-LMNA 2nd Group (n = 13) | LMNA-Mutated (n = 10) | Other Muscular Diseases OMD (n = 22) | Healthy Controls 1st Group (n = 22) | Healthy Controls 2nd Group (n = 15) | |
|---|---|---|---|---|---|---|
| Age at time of blood collection (mean ± SD years) | 43.76 ± 16.80 | 49.18 ± 12.86 | 36.17 ± 15.41 | 32.5 ± 14.24 | 30.73 ± 7.99 | 41.25 ± 7.94 |
| Gender (F/M) | 15/25 | 6/7 | 5/5 | 3/19 | 19/3 | 9/6 |
| Skeletal muscle Disease duration (mean ± SD years) | 25 ± 14.2 | 25.19 ± 15.22 | 7.7 ± 1.5 | 14.6 ± 13 | - | - |
| Cardiac involvement (age of onset) | 31.15 ± 12.26 | 24.65 ± 16.58 | 28.67 ± 10.97 | 18.63 ±14.60 | - | - |
| Musc-LMNA (n = 40) | LMNA-Mutated (n = 10) | Other Muscular Disorders (n = 22) | Healthy Controls (n = 22) | |
|---|---|---|---|---|
| PDGF-BB | 17,315.96 (1161.56–205,948.70) | 3697.91 (686.09–49,690.25) | 84,812.43 (2505.20–189,606.70) | 22,222.5 (12,030.51–41,331.11) |
| IL-1b | 3.73 (0–191.50) | 0.15 (0–2.85) | 2.145 (0–17.73) | 1.69 (0–17.41) |
| IL-1ra | 294.61 (0–2616.60) | 70.43 (0–1659.02) | 98.27 (4.49–15,348.96) | 75.39 (40.99–543.56) |
| IL-2 | 9.67 (0–82.13) | 0 (0–0.79) | 11.87 (0–32.81) | 1.70 (0–24.23) |
| IL-4 | 7.77 (0.37–22.73) | 2.40 (0.89–4.54) | 4.10 (1.44–7.33) | 4.86 (3.13–6.13) |
| IL-5 | 2.62 (0–70.53) | 2.84 (0–14.91) | 0 (0–6.85) | 7.23 (1.64–12.77) |
| IL-6 | 10.14 (0–153.35) | 2.81 (0.31–11.75) | 6.77 (2.96–52.31) | 9.65 (4.90–64.69) |
| IL-7 | 14.43 (0.74–98.53) | 10.76 (3.57–17.86) | 16.48 (6.13–42.03) | 29.87 (13.00–62.31) |
| IL-8 | 25.79 (0–111.64) | 12.10 (4.31–15.56) | 17.47 (7.14–168.52) | 24.53 (14.64–120.83) |
| IL-9 | 32.62 (0–143.24) | 0 (0–53.83) | 33.27 (0–286.82) | 23.22 (10.99–60.36) |
| IL-10 | 12.28 (1.01–175.74) | 9.20 (1.12–188.78) | 9.72 (3.72–57.47) | 8.53 (3.95–62.91) |
| IL-12(p70) | 42.40 (0–139.44) | 5.70 (1.39–99.16) | 16.78 (5.06–79.27) | 34.23 (13.46–153.09) |
| IL-13 | 9.16 (0–26.20) | 4.77 (0–15.50) | 4.64 (0–18.29) | 14.57 (3.83–19.42) |
| IL-15 | 0 (0–37.61) | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| IL-17 | 137.84 (0–3670.05) | 0.17 (0–993.46) | 1088.45 (0–2552.44) | 0 (0–26.37) |
| Eotaxin | 201.02 (0–1470.63) | 134.36 (0–447.23) | 666.84 (2.25–3051.93) | 248.46 (109.92–1141.27) |
| FGF basic | 29.21 (0–409.01) | 0 (0–9.98) | 136.77 (0–618.20) | 81.19 (0–127.15) |
| G-CSF | 105.17 (16.07–951.87) | 76.42 (23.21–230.76) | 36.53 (17.14–344.58) | 25.07 (14.09–44.62) |
| GM-CSF | 0 (0–2603.30) | 0 (0–621.94) | 0 (0–2971.71) | 0 (0–112.41) |
| IFN-g | 284.82 (0–2380.49) | 138.48 (0–462.36) | 229.58 (0–600.53) | 177.33 (84.78–437.35) |
| IP-10 | 1353.57 (456.85–11,728.81) | 1979.34 (410.53–103,596.80) | 5276.79 (477.36–106,593.55) | 1703.98 (1030.39–3787.98) |
| MCP-1 (MCAF) | 152.44 (0–2333.73) | 43.82 (14.97–258,031.90) | 163.23 (0–2972.09) | 74.98 (28.88–327.95) |
| MIP-1a | 6.34 (0–588.43) | 3.33 (0–5.77) | 3.08 (0–8.04) | 6.61 (5.32–9.72) |
| MIP-1b | 139.07 (76.69–505.49) | 78.12 (51.70–167.59) | 224.45 (57.65–1002.74) | 155.91 (83.62–302.21) |
| TNF-a | 42.81 (5.48–423.51) | 38.92 (15.17–215.62) | 25.84 (9.51–61.89) | 35.45 (21.01–63.35) |
| VEGF | 114.81 (0–522.14) | 2.12 (0.03–360.84) | 48.05 (0–468.59) | 234.62 (45.76–488.52) |
| TGF-b1 | 92,399.89 (6544.34–205,045.20) | 64,905.52 (11,824.81–171,893.50) | 104,118.90 (38,641.36–133,449.20) | 72,071.24 (42,398.29–105,300.30) |
| TGF-b2 | 2265.20 (712.94–3186.47) | 1651.26 (1255.47–2722.26) | 1317.43 (1008.99–3022.97) | 1126.59 (922.55–1356.60) |
| TGF-b3 | 642.18 (190.97–2039.26) | 656.13 (173.47–1956.91) | 943.61 (686.71–2065.09) | 809.13 (632.75–921.18) |
| Group | ||||
|---|---|---|---|---|
| Myopathy | Cardiomyopathy | Myopathy + Cardiomyopathy | ||
| (n = 13) | (n = 11) | (n = 16) | p-Value * | |
| Median (Range) | Median (Range) | Median (Range) | ||
| PDGF-BB | 30,581.53 (5061.38–180,068.80) | 20,489.26 (4599.65–205,948.70) | 9564.63 (1161.56–68,897.70) | 0.348 |
| IL-1β | 4.53 (0–191.50) | 3.65 (0.82–24.07) | 3.62 (0.04–16.90) | 0.197 |
| IL-1ra | 710.35 (0–2616.60) | 294.61 (74.82–998.68) | 137.68 (7.30–1062.62) | 0.191 |
| IL-2 | 0.01 (0–82.13) | 9.94 (0–52.66) | 9.74 (0–40.63) | 0.776 |
| IL-4 | 7.24 (0.37–13.56) | 7.19 (2.10–22.73) | 7.97 (1.18–12.99) | 0.948 |
| IL-5 | 2.30 (0–51.97) | 1.39 (0–70.53) | 3.90 (0–38.08) | 0.462 |
| IL-6 | 7.91 (0–72.42) | 10.42 (4.25–153.35) | 10.69 (3.94–43.94) | 0.348 |
| IL-7 | 18.01 (0.74–98.53) | 14.43 (6.88–89.10) | 12.08 (5.34–55.48) | 0.191 |
| IL-8 | 23.12 (0–58.94) | 28.91 (16.6–111.64) | 25.93 (14.05–94.50) | 0.670 |
| IL-9 | 1.46 (0–111.50) | 59.11 (0–143.24) | 42.59 (0–109.43) | 0.443 |
| IL-10 | 12.10 (1.01–175.74) | 13.47 (5.39–76.50) | 11.66 (2.07–33.29) | 0.776 |
| IL-12(p70) | 35.44 (1.18–114.07) | 46.56 (11.63–134.04) | 46.92 (0–139.44) | 1.000 |
| IL-13 | 7.73 (0–23.04) | 9.42 (4.17–26.20) | 11.76 (4.66–18.27) | 0.104 |
| IL-15 | 0 (0–12.33) | 0 (0–37.61) | 0 (0–0) | 0.203 |
| IL-17 | 831.73 (0–3670.05) | 353.31 (3.98–2580.59) | 69.96 (0–1103.82) | 0.010 |
| Eotaxin | 302.70 (5.50–1470.63) | 429.69 (15.94–1462.28) | 81.01 (0–595.95) | 0.072 |
| FGF-b | 85.05 (0–372.76) | 117.38 (0–409.01) | 25.79 (0–271.26) | 0.776 |
| G-CSF | 200.57 (44.89–459.80) | 239.16 (31.51–951.87) | 44.64 (16.07–235.61) | 0.070 |
| GM-CSF | 0 (0–2603.30) | 0 (0–66.49) | 0 (0–0) | 0.085 |
| IFNγ | 356.67 (0–1462.33) | 350.35 (0–2380.49) | 252.11 (0–1171.02) | 0.776 |
| IP-10 | 2031.15 (492.37–11,728.81) | 1278.70 (705.29–3133.58) | 1187.12 (456.85–4185.00) | 0.104 |
| MCP-1(MCAF) | 288.82 (34.20–1358.13) | 132.90 (34.11–1567.18) | 111.38 (0–2333.73) | 0.438 |
| MIP-1α | 7.23 (2.36–588.43) | 4.01 (0.85–27.97) | 4.39 (0–18.84) | 0.462 |
| MIP-1β | 159.00 (90.58–505.49) | 158.68 (88.43–391.97) | 95.06 (76.69–207.82) | 0.124 |
| TNF-α | 52.56 (5.48–360.57) | 42.81 (27.39–423.51) | 37.19 (12.44–188.31) | 0.635 |
| VEGF | 52.54 (0.63–521.33) | 193.47 (22.18–522.14) | 106.01 (0–390.42) | 0.776 |
| TGF-β1 | 92,399.89 (6544.34–205,045.20) | 109,007.20 (37,750.73–168,194.40) | 74470.68 (27,310.81–178,799.60) | 0.435 |
| TGF-β2 | 2335.85 (712.94–3094.08) | 2252.80 (1303.16–2786.12) | 2342.04 (1222.87–3186.47) | 0.179 |
| TGF-β3 | 560.83 (190.97–989.91) | 541.31 (357.53–2039.26) | 697.98 (306.96–1901.91) | 0.472 |
| Cytokine | Musc-LMNA (n = 20) | Controls (n = 15) | p-Value |
|---|---|---|---|
| APRIL/TNFSF13 | 25,801.60 ± 22,051.82 | 21,467.51 ± 12,355.35 | 0.8705 |
| BAFF/TNFSF13B | 15.32 ± 3.65 | 14.08 ± 3.64 | 0.3941 |
| sCD30/TNFRSF8 | 610.56 ± 232.31 | 618.21 ± 285.94 | 0.8705 |
| sCD163 | 189,251.81 ± 84,913.40 | 178,724.79 ± 114,129.78 | 0.376 |
| Chitinase 3-like 1 | 16,470.27 ± 4435.62 | 16,516.77 ± 3408.92 | 0.4211 |
| gp130/sIL-6Rβ | 39,283.66 ± 5520.68 | 43,765.82 ± 7997.48 | 0.0149 |
| IFN-β | 22.31 ± 5.69 | 21.94 ± 4.64 | 0.8577 |
| IL-11 | 1.51 ± 0.52 | 1.41 ± 0.71 | 0.2927 |
| IL-19 | 76.52 ± 13.22 | 74.75 ± 13.96 | 0.5344 |
| IL-20 | 40.35 ± 13.87 | 36.24 ± 8.56 | 0.3163 |
| IL-26 | 13.72 ± 4.97 | 15.01 ± 5.78 | 0.5776 |
| IL-27 (p28) | 0.0 ± 0.0 | 0.0 ± 0.0 | - |
| IL-28A/IFN-λ2 | 21.10 ± 3.32 | 18.25 ± 2.68 | 0.0147 |
| IL-29/IFN-λ1 | 12.49 ± 3.63 | 13.53 ± 4.28 | 0.4815 |
| IL-32 1 | 5.90 ± 5.61 | 10.43 ± 4.22 | 0.1516 |
| IL-34 2 | 74.33 ± 53.86 | 60.47 ± 23.51 | 0.5812 |
| IL-35 | 112.27 ± 47.50 | 111.80 ± 57.47 | 0.8962 |
| LIGHT/TNFSF14 3 | 47.65 ± 41.68 | 49.12 ± 37.57 | 0.9776 |
| Osteocalcin | 4550.45 ± 1715.57 | 5580.24 ± 1975.88 | 0.1107 |
| Pentraxin-3 | 1170.04 ± 375.80 | 1191.73 ± 571.97 | 0.7443 |
| sTNF-R1 | 6198.05 ± 4290.42 | 5440.41 ± 1320.81 | 0.7443 |
| sTNF-R2 | 10,766.25 ± 8250.28 | 10,217.52 ± 6429.89 | 0.7691 |
| TSLP | 55.30 ± 22.31 | 51.67 ± 26.24 | 0.4029 |
| TWEAK/TNFSF12 | 766.58 ± 187.24 | 796.98 ± 178.46 | 0.7691 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cappelletti, C.; Tramacere, I.; Cavalcante, P.; Schena, E.; Politano, L.; Carboni, N.; Gambineri, A.; D’Amico, A.; Ruggiero, L.; Ricci, G.; et al. Cytokine Profile in Striated Muscle Laminopathies: New Promising Biomarkers for Disease Prediction. Cells 2020, 9, 1532. https://doi.org/10.3390/cells9061532
Cappelletti C, Tramacere I, Cavalcante P, Schena E, Politano L, Carboni N, Gambineri A, D’Amico A, Ruggiero L, Ricci G, et al. Cytokine Profile in Striated Muscle Laminopathies: New Promising Biomarkers for Disease Prediction. Cells. 2020; 9(6):1532. https://doi.org/10.3390/cells9061532
Chicago/Turabian StyleCappelletti, Cristina, Irene Tramacere, Paola Cavalcante, Elisa Schena, Luisa Politano, Nicola Carboni, Alessandra Gambineri, Adele D’Amico, Lucia Ruggiero, Giulia Ricci, and et al. 2020. "Cytokine Profile in Striated Muscle Laminopathies: New Promising Biomarkers for Disease Prediction" Cells 9, no. 6: 1532. https://doi.org/10.3390/cells9061532
APA StyleCappelletti, C., Tramacere, I., Cavalcante, P., Schena, E., Politano, L., Carboni, N., Gambineri, A., D’Amico, A., Ruggiero, L., Ricci, G., Siciliano, G., Boriani, G., Mongini, T. E., Vercelli, L., Biagini, E., Ziacchi, M., D’Apice, M. R., Lattanzi, G., Mantegazza, R., ... Bernasconi, P. (2020). Cytokine Profile in Striated Muscle Laminopathies: New Promising Biomarkers for Disease Prediction. Cells, 9(6), 1532. https://doi.org/10.3390/cells9061532










