Actin and Myosin in Non-Neuronal Exocytosis
Abstract
1. Introduction
2. Vesicle Transport
3. Docking
4. Fusion Pore
5. Post Fusion
5.1. Vesicle Content Release
5.2. Endocytosis
6. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Burgoyne, R.D.; Morgan, A. Secretory granule exocytosis. Physiol. Rev. 2003, 83, 581–632. [Google Scholar] [CrossRef]
- Rudolf, R.; Kögel, T.; Kuznetsov, S.A.; Salm, T.; Schlicker, O.; Hellwig, A.; Hammer, J.A.; Gerdes, H.H. Myosin Va facilitates the distribution of secretory granules in the F-actin rich cortex of PC12 cells. J. Cell Sci. 2003, 116, 1339–1348. [Google Scholar] [CrossRef]
- Pulido, I.R.; Nightingale, T.D.; Darchen, F.; Seabra, M.C.; Cutler, D.F.; Gerke, V. Myosin Va acts in concert with Rab27a and MyRIP to regulate acute von-Willebrand factor release from endothelial cells. Traffic 2011, 12, 1371–1382. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Bademosi, A.T.; Luo, J.; Meunier, F.A. Actin Remodeling in Regulated Exocytosis: Toward a Mesoscopic View. Trends Cell Biol. 2018, 28, 685–697. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, L.M.; Villanueva, J. The role of F-actin in the transport and secretion of chromaffin granules: An historic perspective. Pflugers Arch. Eur. J. Physiol. 2018, 470, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Meunier, F.A.; Gutiérrez, L.M. Captivating New Roles of F-Actin Cortex in Exocytosis and Bulk Endocytosis in Neurosecretory Cells. Trends Neurosci. 2016, 39, 605–613. [Google Scholar] [CrossRef]
- Papadopulos, A. Membrane shaping by actin and myosin during regulated exocytosis. Mol. Cell. Neurosci. 2017, 84, 93–99. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Papadopulos, A.; Tomatis, V.M.; Kasula, R.; Meunier, F.A. The Cortical Acto-Myosin Network: From Diffusion Barrier to Functional Gateway in the Transport of Neurosecretory Vesicles to the Plasma Membrane. Front. Endocrinol. (Lausanne) 2013, 4, 20–24. [Google Scholar] [CrossRef]
- Porat-Shliom, N.; Milberg, O.; Masedunskas, A.; Weigert, R. Multiple roles for the actin cytoskeleton during regulated exocytosis. Cell. Mol. Life Sci. 2013, 70, 2099–2121. [Google Scholar] [CrossRef]
- Barlan, K.; Gelfand, V.I. Microtubule-based transport and the distribution, tethering, and organization of organelles. Cold Spring Harb. Perspect. Biol. 2017, 9, 1–12. [Google Scholar] [CrossRef]
- Noordstra, I.; Akhmanova, A. Linking cortical microtubule attachment and exocytosis [version 1; referees: 2 approved]. F1000Research 2017, 6, 1–12. [Google Scholar] [CrossRef]
- Conte, I.L.; Hellen, N.; Bierings, R.; Mashanov, G.I.; Manneville, J.B.; Kiskin, N.I.; Hannah, M.J.; Molloy, J.E.; Carter, T. Interaction between MyRIP and the actin cytoskeleton regulates Weibel-Palade body trafficking and exocytosis. J. Cell Sci. 2016, 129, 592–603. [Google Scholar] [CrossRef]
- Nightingale, T.; Cutler, D. The secretion of von Willebrand factor from endothelial cells; an increasingly complicated story. J. Thromb. Haemost. 2013, 11, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Geron, E.; Schejter, E.D.; Shilo, B.Z. Directing exocrine secretory vesicles to the apical membrane by actin cables generated by the formin mDia1. Proc. Natl. Acad. Sci. USA 2013, 110, 10652–10657. [Google Scholar] [CrossRef] [PubMed]
- Massarwa, R.; Schejter, E.D.; Shilo, B.Z. Apical Secretion in Epithelial Tubes of the Drosophila Embryo Is Directed by the Formin-Family Protein Diaphanous. Dev. Cell 2009, 16, 877–888. [Google Scholar] [CrossRef] [PubMed]
- Geron, E.; Schejter, E.D.; Shilo, B.Z. Targeting secretion to the apical surface by mDia1-built actin tracks. Commun. Integr. Biol. 2013, 6, e25660-1–e25660-2. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hammer, J.A.; Sellers, J.R. Walking to work: Roles for class v myosins as cargo transporters. Nat. Rev. Mol. Cell Biol. 2012, 13, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Reck-Peterson, S.; Provance, D.; Mooseker, M.; Mercer, J. Class V myosins. Biochim. Biophys. Acta 2000, 1496, 36–51. [Google Scholar] [CrossRef]
- Dolce, L.G.; Ohbayashi, N.; da Silva, D.F.C.; Ferrari, A.J.R.; Pirolla, R.A.S.; de A.P. Schwarzer, A.C.; Zanphorlin, L.M.; Cabral, L.; Fioramonte, M.; Ramos, C.H.I.; et al. Unveiling the interaction between the molecular motor Myosin Vc and the small GTPase Rab3A. J. Proteom. 2020, 212, 1–9. [Google Scholar] [CrossRef]
- Hsueh, P.Y.; Edman, M.C.; Sun, G.; Shi, P.; Xu, S.; Lin, Y.A.; Cui, H.; Hamm-Alvarez, S.F.; Mackay, J.A. Tear-mediated delivery of nanoparticles through transcytosis of the lacrimal gland. J. Control. Release 2015, 208, 2–13. [Google Scholar] [CrossRef]
- Brozzi, F.; Diraison, F.; Lajus, S.; Rajatileka, S.; Philips, T.; Regazzi, R.; Fukuda, M.; Verkade, P.; Molnár, E.; Váradi, A. Molecular mechanism of Myosin Va recruitment to dense core secretory granules. Traffic 2012, 13, 54–69. [Google Scholar] [CrossRef]
- Stenmark, H. Rab GTPases as coordinators of vesicle traffic. Nat. Rev. Mol. Cell Biol. 2009, 10, 513–525. [Google Scholar] [CrossRef]
- Welz, T.; Kerkhoff, E. Exploring the iceberg: Prospects of coordinated myosin V and actin assembly functions in transport processes. Small GTPases 2019, 10, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, M. Membrane traffic in the secretory pathway: Regulation of secretory vesicle traffic by Rab small GTPases. Cell. Mol. Life Sci. 2008, 65, 2801–2813. [Google Scholar] [CrossRef]
- Tolmachova, T.; Anders, R.; Stinchcombe, J.; Bossi, G.; Griffiths, G.; Huxley, C.; Seabra, M. A General Role for Rab27a in Secretory Cells. Mol. Biol. Cell 2004, 15, 332–344. [Google Scholar] [CrossRef] [PubMed]
- Nightingale, T.D.; Pattni, K.; Hume, A.N.; Seabra, M.C.; Cutler, D.F. Rab27a and MyRIP regulate the amount and multimeric state of VWF released from endothelial cells. Blood 2009, 113, 5010–5018. [Google Scholar] [CrossRef]
- Cheeseman, L.P.; Boulanger, J.; Bond, L.M.; Schuh, M. Two pathways regulate cortical granule translocation to prevent polyspermy in mouse oocytes. Nat. Commun. 2016, 7, 1–13. [Google Scholar] [CrossRef]
- Bustos, M.A.; Lucchesi, O.; Ruete, M.C.; Mayorga, L.S.; Tomes, C.N. Rab27 and Rab3 sequentially regulate human sperm dense-core granule exocytosis. Proc. Natl. Acad. Sci. USA 2012, 109, E2057–E2066. [Google Scholar] [CrossRef]
- Wu, X.; Rao, K.; Bowers, M.B.; Copeland, N.G.; Jenkins, N.A.; Hammer, J.A. Rab27a enables myosin Va-dependent melanosome capture by recruiting the myosin to the organelle. J. Cell Sci. 2001, 114, 1091–1100. [Google Scholar]
- Stinchcombe, J.C.; Barral, D.C.; Mules, E.H.; Booth, S.; Hume, A.N.; Machesky, L.M.; Seabra, M.C.; Griffiths, G.M. Rab27a is required for regulated secretion in cytotoxic T lymphocytes. J. Cell Biol. 2001, 152, 825–833. [Google Scholar] [CrossRef]
- Nagashima, K.; Torii, S.; Yi, Z.; Igarashi, M.; Okamoto, K.; Takeuchi, T.; Izumi, T. Melanophilin directly links Rab27a and myosin Va through its distinct coiled-coil regions. FEBS Lett. 2002, 517, 233–238. [Google Scholar] [CrossRef]
- Schuh, M. An actin-dependent mechanism for long-range vesicle transport. Nat. Cell Biol. 2011, 13, 1431–1436. [Google Scholar] [CrossRef]
- Chugh, P.; Paluch, E.K. The actin cortex at a glance. J. Cell Sci. 2018, 131, 1–9. [Google Scholar] [CrossRef]
- Kawaguchi, K.; Yoshida, S.; Hatano, R.; Asano, S. Pathophysiological roles of ezrin/radixin/moesin proteins. Biol. Pharm. Bull. 2017, 40, 381–390. [Google Scholar] [CrossRef]
- McIntosh, B.B.; Ostap, E.M. Myosin-I molecular motors at a glance. J. Cell Sci. 2016, 129, 2689–2695. [Google Scholar] [CrossRef]
- Chaigne, A.; Campillo, C.; Gov, N.S.; Voituriez, R.; Azoury, J.; Umaña-Diaz, C.; Almonacid, M.; Queguiner, I.; Nassoy, P.; Sykes, C.; et al. A soft cortex is essential for asymmetric spindle positioning in mouse oocytes. Nat. Cell Biol. 2013, 15, 958–966. [Google Scholar] [CrossRef]
- Courtemanche, N. Mechanisms of formin-mediated actin assembly and dynamics. Biophys. Rev. 2018, 10, 1553–1569. [Google Scholar] [CrossRef]
- Rottner, K.; Faix, J.; Bogdan, S.; Linder, S.; Kerkhoff, E. Actin assembly mechanisms at a glance. J. Cell Sci. 2017, 130, 3427–3435. [Google Scholar] [CrossRef]
- Eghiaian, F.; Rigato, A.; Scheuring, S. Structural, mechanical, and dynamical variability of the actin cortex in living cells. Biophys. J. 2015, 108, 1330–1340. [Google Scholar] [CrossRef]
- Clausen, M.P.; Colin-York, H.; Schneider, F.; Eggeling, C.; Fritzsche, M. Dissecting the actin cortex density and membrane-cortex distance in living cells by super-resolution microscopy. J. Phys. D Appl. Phys. 2017, 50, 1–11. [Google Scholar] [CrossRef]
- Eitzen, G. Actin remodeling to facilitate membrane fusion. Biochim. Biophys. Acta-Mol. Cell Res. 2003, 1641, 175–181. [Google Scholar] [CrossRef]
- Gutiérrez, L.M. New Insights into the Role of the Cortical Cytoskeleton in Exocytosis from Neuroendocrine Cells. Int. Rev. Cell Mol. Biol. 2012, 295, 109–137. [Google Scholar] [CrossRef]
- Nightingale, T.D.; White, I.J.; Doyle, E.L.; Turmaine, M.; Harrison-Lavoie, K.J.; Webb, K.F.; Cramer, L.P.; Cutler, D.F. Actomyosin II contractility expels von Willebrand factor from Weibel-Palade bodies during exocytosis. J. Cell Biol. 2011, 194, 613–629. [Google Scholar] [CrossRef]
- Singh, R.K.; Mizuno, K.; Wasmeier, C.; Wavre-Shapton, S.T.; Recchi, C.; Catz, S.D.; Futter, C.; Tolmachova, T.; Hume, A.N.; Seabra, M.C. Distinct and opposing roles for Rab27a/Mlph/MyoVa and Rab27b/Munc13-4 in mast cell secretion. FEBS J. 2013, 280, 892–903. [Google Scholar] [CrossRef]
- Klein, O.; Krier-Burris, R.A.; Lazki-Hagenbach, P.; Gorzalczany, Y.; Mei, Y.; Ji, P.; Bochner, B.S.; Sagi-Eisenberg, R. Mammalian diaphanous-related formin 1 (mDia1) coordinates mast cell migration and secretion through its actin-nucleating activity. J. Allergy Clin. Immunol. 2019, 144, 1074–1090. [Google Scholar] [CrossRef]
- Carisey, A.F.; Mace, E.M.; Saeed, M.B.; Davis, D.M.; Orange, J.S. Nanoscale Dynamism of Actin Enables Secretory Function in Cytolytic Cells. Curr. Biol. 2018, 28, 489–502.e9. [Google Scholar] [CrossRef]
- Jerdeva, G.V.; Wu, K.; Yarber, F.A.; Rhodes, C.J.; Kalman, D.; Schechter, J.E.; Hamm-Alvarez, S.F. Actin and non-muscle myosin II facilitate apical exocytosis of tear proteins in rabbit lacrimal acinar epithelial cells. J. Cell Sci. 2005, 118, 4797–4812. [Google Scholar] [CrossRef]
- Ma, W.; Chang, J.; Tong, J.; Ho, U.; Yau, B.; Kebede, M.A.; Thorn, P. Arp2/3 nucleates F-actin coating of fusing insulin granules in pancreatic β cells to control insulin secretion. J. Cell Sci. 2020, 133, 1–11. [Google Scholar] [CrossRef]
- Colin-York, H.; Li, D.; Korobchevskaya, K.; Chang, V.T.; Betzig, E.; Eggeling, C.; Fritzsche, M. Cytoskeletal actin patterns shape mast cell activation. Commun. Biol. 2019, 2, 1–12. [Google Scholar] [CrossRef]
- Iizuka, Y.; Cichocki, F.; Sieben, A.; Sforza, F.; Karim, R.; Coughlin, K.; Vogel, R.I.; Gavioli, R.; McCullar, V.; Lenvik, T.; et al. UNC-45A Is a Nonmuscle Myosin IIA Chaperone Required for NK Cell Cytotoxicity via Control of Lytic Granule Secretion. J. Immunol. 2015, 195, 4760–4770. [Google Scholar] [CrossRef]
- Steppan, X.D.; Pan, L.; Gross, K.W.; Kurtz, A. Analysis of the calcium paradox of renin secretion. Am. J. Physiol. Ren. Physiol. 2018, 315, F834–F843. [Google Scholar] [CrossRef]
- Bose, A.; Robida, S.; Furcinitti, P.S.; Chawla, A.; Fogarty, K.; Corvera, S.; Czech, M.P. Unconventional myosin Myo1c promotes membrane fusion in a regulated exocytic pathway. Mol. Cell. Biol. 2004, 24, 5447–5458. [Google Scholar] [CrossRef]
- Kittelberger, N.; Breunig, M.; Martin, R.; Knölker, H.J.; Miklavc, P.; Knölker, H.-J.; Miklavc, P. The role of myosin 1c and myosin 1b in surfactant exocytosis. J. Cell Sci. 2016, 129, 1685–1696. [Google Scholar] [CrossRef]
- Maravillas-Montero, J.L.; López-Ortega, O.; Patiño-López, G.; Santos-Argumedo, L. Myosin 1g regulates cytoskeleton plasticity, cell migration, exocytosis, and endocytosis in B lymphocytes. Eur. J. Immunol. 2014, 44, 877–886. [Google Scholar] [CrossRef]
- Saarikangas, J.; Zhao, H.; Lappalainen, P. Regulation of the actin cytoskeleton-plasma membrane interplay by phosphoinositides. Physiol. Rev. 2010, 90, 259–289. [Google Scholar] [CrossRef]
- Janmey, P.A.; Bucki, R.; Radhakrishnan, R. Regulation of actin assembly by PI(4,5)P2 and other inositol phospholipids: An update on possible mechanisms. Biochem. Biophys. Res. Commun. 2019, 506, 307–314. [Google Scholar] [CrossRef]
- Bader, M.F.; Doussau, F.; Chasserot-Golaz, S.; Vitale, N.; Gasman, S. Coupling actin and membrane dynamics during calcium-regulated exocytosis: A role for Rho and ARF GTPases. Biochim. Biophys. Acta Mol. Cell Res. 2004, 1742, 37–49. [Google Scholar] [CrossRef]
- Ory, S.; Gasman, S. Rho GTPases and exocytosis: What are the molecular links? Semin. Cell Dev. Biol. 2011, 22, 27–32. [Google Scholar] [CrossRef]
- Sit, S.T.; Manser, E. Rho GTPases and their role in organizing the actin cytoskeleton. J. Cell Sci. 2011, 124, 679–683. [Google Scholar] [CrossRef]
- Singer, M.; Martin, L.D.; Vargaftig, B.B.; Park, J.; Gruber, A.D.; Li, Y.; Adler, K.B. A MARCKS-related peptide blocks mucus hypersecretion in a mouse model of asthma. Nat. Med. 2004, 10, 193–196. [Google Scholar] [CrossRef]
- Agrawal, A.; Rengarajan, S.; Adler, K.B.; Ram, A.; Ghosh, B.; Fahim, M.; Dickey, B.F. Inhibition of mucin secretion with MARCKS-related peptide improves airway obstruction in a mouse model of asthma. J. Appl. Physiol. 2007, 102, 399–405. [Google Scholar] [CrossRef]
- Sheats, M.K.; Yin, Q.; Fang, S.; Park, J.; Crews, A.L.; Parikh, I.; Dickson, B.; Adler, K.B. Marcks and lung disease. Am. J. Respir. Cell Mol. Biol. 2019, 60, 16–27. [Google Scholar] [CrossRef]
- Malacombe, M.; Bader, M.F.; Gasman, S. Exocytosis in neuroendocrine cells: New tasks for actin. Biochim. Biophys. Acta-Mol. Cell Res. 2006, 1763, 1175–1183. [Google Scholar] [CrossRef]
- Tran, D.T.; Masedunskas, A.; Weigert, R.; Hagen, K.G.T. Arp2/3-mediated F-actin formation controls regulated exocytosis in vivo. Nat. Commun. 2015, 6, 1–10. [Google Scholar] [CrossRef]
- Verhage, M.; Sørensen, J.B. Vesicle docking in regulated exocytosis. Traffic 2008, 9, 1414–1424. [Google Scholar] [CrossRef]
- Varadi, A.; Tsuboi, T.; Rutter, G. Myosin Va Transports Dense Core Secretory Vesicles in Pancreatic MIN6 β-Cells. Mol. Biol. Cell 2005, 16, 2670–2680. [Google Scholar] [CrossRef]
- Südhof, T. The Presynaptic Active Zone. Neuron 2012, 75, 11–25. [Google Scholar] [CrossRef]
- Fan, F.; Matsunaga, K.; Wang, H.; Ishizaki, R.; Kobayashi, E.; Kiyonari, H.; Mukumoto, Y.; Okunishi, K.; Izumi, T. Exophilin-8 assembles secretory granules for exocytosis in the actin cortex via interaction with RIM-BP2 and myosin-VIIa. Elife 2017, 6, 1–23. [Google Scholar] [CrossRef]
- Encarnação, M.; Espada, L.; Escrevente, C.; Mateus, D.; Ramalho, J.; Michelet, X.; Santarino, I.; Hsu, V.W.; Brenner, M.B.; Barral, D.C.; et al. A Rab3a-dependent complex essential for lysosome positioning and plasma membrane repair. J. Cell Biol. 2016, 213, 631–640. [Google Scholar] [CrossRef]
- Machado, E.; White-Gilbertson, S.; Van De Vlekkert, D.; Janke, L.; Moshiach, S.; Campos, Y.; Finkelstein, D.; Gomero, E.; Mosca, R.; Qiu, X.; et al. Regulated lysosomal exocytosis mediates cancer progression. Sci. Adv. 2015, 1, 1–16. [Google Scholar] [CrossRef]
- Gerelsaikhan, T.; Chen, X.L.; Chander, A. Secretagogues of lung surfactant increase annexin A7 localization with ABCA3 in alveolar type II cells. Biochim. Biophys. Acta Mol. Cell Res. 2011, 1813, 2017–2025. [Google Scholar] [CrossRef] [PubMed]
- Knop, M.; Aareskjold, E.; Bode, G.; Gerke, V. Rab3D and annexin A2 play a role in regulated of vWF, but not tPA, from endothelial cells. EMBO J. 2004, 23, 2982–2992. [Google Scholar] [CrossRef] [PubMed]
- Chehab, T.; Santos, N.C.; Holthenrich, A.; Koerdt, S.N.; Disse, J.; Schuberth, C.; Nazmi, A.R.; Neeft, M.; Koch, H.; Man, K.N.M.; et al. A novel Munc13-4/S100A10/annexin A2 complex promotes Weibel-Palade body exocytosis in endothelial cells. Mol. Biol. Cell 2017, 28, 1688–1700. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.A.; Hoffman, L.M.; Beckerle, M.C. LIM proteins in actin cytoskeleton mechanoresponse. Trends Cell Biol. 2014, 24, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Li, P.; Yang, Z.; Huang, X.; Wei, G.; Sun, Y.; Kang, X.; Hu, X.; Deng, Q.; Chen, L.; et al. Zyxin regulates endothelial von Willebrand factor secretion by reorganizing actin filaments around exocytic granules. Nat. Commun. 2017, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- McLeish, K.R.; Merchant, M.L.; Creed, T.M.; Tandon, S.; Barati, M.T.; Uriarte, S.M.; Ward, R.A. Frontline Science: Tumor necrosis factor-α stimulation and priming of human neutrophil granule exocytosis. J. Leukoc. Biol. 2017, 102, 19–29. [Google Scholar] [CrossRef]
- Wilson, J.D.; Shelby, S.A.; Holowka, D.; Baird, B. Rab11 Regulates the Mast Cell Exocytic Response. Traffic 2016, 17, 1027–1041. [Google Scholar] [CrossRef]
- Südhof, T.C.; Rothman, J.E. Membrane fusion: Grappling with SNARE and SM proteins. Science 2009, 323, 474–477. [Google Scholar] [CrossRef]
- Jorgačevski, J.; Kreft, M.; Zorec, R. Exocytotic fusion pores as a target for therapy. Cell Calcium 2017, 66, 71–77. [Google Scholar] [CrossRef]
- Miklavc, P.; Hecht, E.; Hobi, N.; Wittekindt, O.H.; Dietl, P.; Kranz, C.; Frick, M. Actin coating and compression of fused secretory vesicles are essential for surfactant secretion: A role for Rho, formins and myosin II. J. Cell Sci. 2012, 125, 2765–2774. [Google Scholar] [CrossRef]
- Miklavc, P.; Mair, N.; Wittekindt, O.H.; Haller, T.; Dietl, P.; Felder, E.; Timmler, M.; Frick, M. Fusion-activated Ca 2+ entry via vesicular P2X 4 receptors promotes fusion pore opening and exocytotic content release in pneumocytes. Proc. Natl. Acad. Sci. USA 2011, 108, 14503–14508. [Google Scholar] [CrossRef] [PubMed]
- Larina, O.; Bhat, P.; Pickett, J.A.; Launikonis, B.S.; Shah, A.; Kruger, W.A.; Edwardson, J.M.; Thorn, P. Dynamic regulation of the large exocytotic fusion pore in pancreatic acinar cells. Mol. Biol. Cell 2007, 18, 3502–3511. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Peruchena, C.; Navas, S.; Montes, M.A.; Álvarez De Toledo, G. Fusion pore regulation of transmitter release. Brain Res. Rev. 2005, 49, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Shin, W.; Ge, L.; Arpino, G.; Villarreal, S.A.; Hamid, E.; Liu, H.; Zhao, W.D.; Wen, P.J.; Chiang, H.C.; Wu, L.G. Visualization of Membrane Pore in Live Cells Reveals a Dynamic-Pore Theory Governing Fusion and Endocytosis. Cell 2018, 173, 934–945. [Google Scholar] [CrossRef] [PubMed]
- Haller, T.; Dietl, P.; Pfaller, K.; Frick, M.; Mair, N.; Paulmichl, M.; Hess, M.W.; Fürst, J.; Maly, K. Fusion pore expansion is a slow, discontinuous, and Ca2+-dependent process regulating secretion from alveolar type II cells. J. Cell Biol. 2001, 155, 279–289. [Google Scholar] [CrossRef]
- Doreian, B.W.; Fulop, T.G.; Smith, C.B. Myosin II activation and actin reorganization regulate the mode of quantal exocytosis in mouse adrenal chromaffin cells. J. Neurosci. 2008, 28, 4470–4478. [Google Scholar] [CrossRef]
- Ñeco, P.; Fernández-Peruchena, C.; Navas, S.; Gutiérrez, L.M.; Álvarez De Toledo, G.; Alés, E. Myosin II contributes to fusion pore expansion during exocytosis. J. Biol. Chem. 2008, 283, 10949–10957. [Google Scholar] [CrossRef]
- Bhat, P.; Thorn, P. Myosin 2 maintains an open exocytic fusion pore in secretory epithelial cells. Mol. Biol. Cell 2009, 20, 1795–1803. [Google Scholar] [CrossRef]
- Anantharam, A.; Bittner, M.A.; Aikman, R.L.; Stuenkel, E.L.; Schmid, S.L.; Axelrod, D.; Holz, R.W. A new role for the dynamin GTPase in the regulation of fusion pore expansion. Mol. Biol. Cell 2011, 22, 1907–1918. [Google Scholar] [CrossRef]
- Jaiswal, J.K.; Chakrabarti, S.; Andrews, N.W.; Simon, S.M. Synaptotagmin VII restricts fusion pore expansion during lysosomal exocytosis. PLoS Biol. 2004, 2, E233. [Google Scholar] [CrossRef]
- Turvey, M.R.; Thorn, P. Lysine-fixable dye tracing of exocytosis shows F-actin coating is a step that follows granule fusion in pancreatic acinar cells. Pflugers Arch. 2004, 448, 552–555. [Google Scholar] [CrossRef]
- Thorn, P. New insights into the control of secretion. Commun. Integr. Biol. 2009, 2, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Bretou, M.; Jouannot, O.; Fanget, I.; Pierobon, P.; Larochette, N.; Gestraud, P.; Guillon, M.; Emiliani, V.; Gasman, S.; Desnos, C.; et al. Cdc42 controls the dilation of the exocytotic fusion pore by regulating membrane tension. Mol. Biol. Cell 2014, 25, 3195–3209. [Google Scholar] [CrossRef] [PubMed]
- Quevedo, M.F.; Lucchesi, O.; Bustos, M.A.; Pocognoni, C.A.; De La Iglesia, P.X.; Tomes, C.N. The rab3A-22A chimera prevents sperm exocytosis by stabilizing open fusion pores. J. Biol. Chem. 2016, 291, 23101–23111. [Google Scholar] [CrossRef] [PubMed]
- Südhof, T.C. THE SYNAPTIC VESICLE CYCLE. Annu. Rev. Neurosci. 2004, 27, 509–547. [Google Scholar] [CrossRef]
- Heuser, J.E.; Reese, T.S. Evidence for recycling of synaptic vesicle membrane during transmitter release at the frog neuromuscular junction. J. Cell Biol. 1973, 57, 315–344. [Google Scholar] [CrossRef]
- Sharma, S.; Lindau, M. The fusion pore, 60 years after the first cartoon. FEBS Lett. 2018, 592, 3542–3562. [Google Scholar] [CrossRef]
- Miklavc, P.; Albrecht, S.; Wittekindt, O.H.; Schullian, P.; Haller, T.; Dietl, P. Existence of exocytotic hemifusion intermediates with a lifetime of up to seconds in type II pneumocytes. Biochem. J. 2009, 424, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Miklavc, P.; Wittekindt, O.H.; Felder, E.; Dietl, P. Ca2+-dependent actin coating of lamellar bodies after exocytotic fusion: A prerequisite for content release or kiss-and-run. Ann. N. Y. Acad. Sci. 2009, 1152, 43–52. [Google Scholar] [CrossRef]
- Chiang, H.C.; Shin, W.; Zhao, W.D.; Hamid, E.; Sheng, J.; Baydyuk, M.; Wen, P.J.; Jin, A.; Momboisse, F.; Wu, L.G. Post-fusion structural changes and their roles in exocytosis and endocytosis of dense-core vesicles. Nat. Commun. 2014, 5, 1–15. [Google Scholar] [CrossRef]
- Wen, P.J.; Grenklo, S.; Arpino, G.; Tan, X.; Liao, H.-S.; Heureaux, J.; Peng, S.-Y.; Chiang, H.-C.; Hamid, E.; Zhao, W.-D.; et al. Actin dynamics provides membrane tension to merge fusing vesicles into the plasma membrane. Nat. Commun. 2016, 7, 1–14. [Google Scholar] [CrossRef]
- Gasman, S.; Chasserot-Golaz, S.; Malacombe, M.; Way, M.; Bader, M.F. Regulated Exocytosis in Neuroendocrine Cells: A Role for Subplasmalemmal Cdc42/N-WASP-induced Actin Filaments. Mol. Biol. Cell 2004, 15, 520–531. [Google Scholar] [CrossRef] [PubMed]
- Berberian, K.; Torres, A.J.; Fang, Q.; Kisler, K.; Lindau, M. F-actin and myosin II accelerate catecholamine release from chromaffin granules. J. Neurosci. 2009, 29, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Segawa, A.; Yamashina, S. Roles of Microfilaments in Exocytosis: A New Hypothesis. Cell Struct. Funct. 1989, 14, 531–544. [Google Scholar] [CrossRef] [PubMed]
- Tsilibary, E.; Williams, M. Actin Structural in Peripheral Changes Rat Induced Lung: By S, Labeling Cytochalasin form accepted. J. Histochem. Cytochem. 1983, 31, 1289–1297. [Google Scholar] [CrossRef] [PubMed]
- Sokac, A.M.; Co, C.; Taunton, J.; Bement, W. Cdc42-dependent actin polymerization during compensatory endocytosis in Xenopus eggs. Nat. Cell Biol. 2003, 5, 727–732. [Google Scholar] [CrossRef]
- Nemoto, T.; Kojima, T.; Oshima, A.; Bito, H.; Kasai, H. Stabilization of exocytosis by dynamic F-actin coating of zymogen granules in pancreatic acini. J. Biol. Chem. 2004, 279, 37544–37550. [Google Scholar] [CrossRef]
- Masedunskas, A.; Sramkova, M.; Parente, L.; Sales, K.U.; Amornphimoltham, P.; Bugge, T.H.; Weigert, R. Role for the actomyosin complex in regulated exocytosis revealed by intravital microscopy. Proc. Natl. Acad. Sci. USA 2011, 108, 13552–13557. [Google Scholar] [CrossRef]
- Sokac, A.M.; Bement, W.M. Kiss-and-coat and compartment mixing: Coupling exocytosis to signal generation and local actin assembly. Mol. Biol. Cell 2006, 17, 1495–1502. [Google Scholar] [CrossRef]
- Yu, H.Y.E.; Bement, W.M. Control of local actin assembly by membrane fusion-dependent compartment mixing. Nat. Cell Biol. 2007, 9, 149–159. [Google Scholar] [CrossRef]
- Rousso, T.; Schejter, E.D.; Shilo, B.Z. Orchestrated content release from Drosophila glue-protein vesicles by a contractile actomyosin network. Nat. Cell Biol. 2016, 18, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Shitara, A.; Malec, L.; Ebrahim, S.; Chen, D.; Bleck, C.; Hoffman, M.P.; Weigert, R. Cdc42 negatively regulates endocytosis during apical membrane maintenance in live animals. Mol. Biol. Cell 2019, 30, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Nightingale, T.D.; Cutler, D.F.; Cramer, L.P. Actin coats and rings promote regulated exocytosis. Trends Cell Biol. 2012, 22, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Otomo, T.; Tomchick, D.R.; Otomo, C.; Panchal, S.C.; Machius, M.; Rosen, M.K. Structural basis of actin filament nucleation and processive capping by a formin homology 2 domain. Nature 2005, 433, 488–494. [Google Scholar] [CrossRef]
- Romero, S.; Clainche, C.L.; Didry, D.; Egile, C.; Pantaloni, D.; Carlier, M.F. Formin is a processive motor that requires profilin to accelerate actin assembly and associated ATP hydrolysis. Cell 2004, 119, 419–429. [Google Scholar] [CrossRef]
- Vavylonis, D.; Kovar, D.R.; O’Shaughnessy, B.; Pollard, T.D. Model of formin-associated actin filament elongation. Mol. Cell 2006, 21, 455–466. [Google Scholar] [CrossRef]
- Yu, H.-Y.E.; Bement, W.M. Multiple myosins are required to coordinate actin assembly with coat compression during compensatory endocytosis. Mol. Biol. Cell 2007, 18, 4096–4105. [Google Scholar] [CrossRef]
- Nightingale, T.D.; McCormack, J.J.; Grimes, W.; Robinson, C.; da Silva, M.L.; White, I.J.; Vaughan, A.; Cramer, L.P.; Cutler, D.F. Tuning the endothelial response: Differential release of exocytic cargos from Weibel-Palade bodies. J. Thromb. Haemost. 2018, 16, 1873–1886. [Google Scholar] [CrossRef]
- Milberg, O.; Shitara, A.; Ebrahim, S.; Masedunskas, A.; Tora, M.; Tran, D.T.; Chen, Y.; Conti, M.A.; Adelstein, R.S.; Hagen, K.G.T.; et al. Concerted actions of distinct nonmuscle myosin II isoforms drive intracellular membrane remodeling in live animals. J. Cell Biol. 2017, 216, 1925–1936. [Google Scholar] [CrossRef]
- Miklavc, P.; Ehinger, K.; Sultan, A.; Felder, T.; Paul, P.; Gottschalk, K.-E.E.; Frick, M. Actin depolymerisation and crosslinking join forces with myosin II to contract actin coats on fused secretory vesicles. J. Cell Sci. 2015, 128, 1193–1203. [Google Scholar] [CrossRef]
- Ebrahim, S.; Chen, D.; Weiss, M.; Malec, L.; Ng, Y.; Rebustini, I.; Krystofiak, E.; Hu, L.; Liu, J.; Masedunskas, A.; et al. Dynamic polyhedral actomyosin lattices remodel micron-scale curved membranes during exocytosis in live mice. Nat. Cell Biol. 2019, 21, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Ebrahim, S.; Liu, J.; Weigert, R. The Actomyosin Cytoskeleton Drives Micron-Scale Membrane Remodeling In Vivo Via the Generation of Mechanical Forces to Balance Membrane Tension Gradients. BioEssays 2018, 40, 1–7. [Google Scholar] [CrossRef]
- Sokac, A.M.; Schietroma, C.; Gundersen, C.B.; Bement, W.M. Myosin-1c couples assembling actin to membranes to drive compensatory endocytosis. Dev. Cell 2006, 11, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Giardini, P.A.; Fletcher, D.A.; Theriot, J.A. Compression forces generated by actin comet tails on lipid vesicles. Proc. Natl. Acad. Sci. USA 2003, 100, 6493–6498. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.; Soekmadji, C.; Mitchell, J.M.; Thomas, W.G.; Thorn, P. Real-time measurement of f-actin remodelling during exocytosis using lifeact-EGFP transgenic animals. PLoS ONE 2012, 7, e39815. [Google Scholar] [CrossRef] [PubMed]
- Engisch, K.L.; Nowycky, M.C. Compensatory and excess retrieval: Two types of endocytosis following single step depolarizations in bovine adrenal chromaffin cells. J. Physiol. 1998, 506, 591–608. [Google Scholar] [CrossRef]
- Mooren, O.L.; Galletta, B.J.; Cooper, J.A. Roles for Actin Assembly in Endocytosis. Annu. Rev. Biochem. 2012, 81, 661–686. [Google Scholar] [CrossRef]
- Gómez-Elías, M.D.; Fissore, R.A.; Cuasnicú, P.S.; Cohen, D.J. Compensatory endocytosis occurs after cortical granule exocytosis in mouse eggs. J. Cell. Physiol. 2020, 235, 4351–4360. [Google Scholar] [CrossRef]
- Thorn, P.; Fogarty, K.E.; Parker, I. Zymogen granule exocytosis is characterized by long fusion pore openings and preservation of vesicle lipid identity. Proc. Natl. Acad. Sci. USA 2004, 101, 6774–6779. [Google Scholar] [CrossRef]
- Shitara, A.; Weigert, R. Imaging membrane remodeling during regulated exocytosis in live mice. Exp. Cell Res. 2015, 337, 219–225. [Google Scholar] [CrossRef]
- Sramkova, M.; Masedunskas, A.; Weigert, R. Plasmid DNA is internalized from the apical plasma membrane of the salivary gland epithelium in live animals. Histochem. Cell Biol. 2012, 138, 201–213. [Google Scholar] [CrossRef]
- Stevenson, N.L.; White, I.J.; McCormack, J.J.; Robinson, C.; Cutler, D.F.; Nightingale, T.D. Clathrin-mediated post-fusion membrane retrieval influences the exocytic mode of endothelial Weibel-Palade bodies. J. Cell Sci. 2017, 130, 2591–2605. [Google Scholar] [CrossRef] [PubMed]
- Mair, N.; Haller, T.; Dietl, P. Exocytosis in alveolar type II cells revealed by cell capacitance and fluorescence measurements. Am. J. Physiol.-Lung Cell. Mol. Physiol. 1999, 276, L376–L382. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Leong, N.T.; Wong, T.; Drubin, D.G. A Pan1/End3/Sla1 complex links Arp2/3-mediated actin assembly to sites of clathrinmediated endocytosis. Mol. Biol. Cell 2015, 26, 3841–3856. [Google Scholar] [CrossRef] [PubMed]
- Almeida-Souza, L.; Frank, R.A.W.; García-Nafría, J.; Colussi, A.; Gunawardana, N.; Johnson, C.M.; Yu, M.; Howard, G.; Andrews, B.; Vallis, Y.; et al. A Flat BAR Protein Promotes Actin Polymerization at the Base of Clathrin-Coated Pits. Cell 2018, 174, 325–337. [Google Scholar] [CrossRef]
- Sun, Y.; Jaldin-Fincati, J.; Liu, Z.; Bilan, P.J.; Klip, A. A complex of Rab13 with MICAL-L2 and α-actinin-4 is essential for insulin-dependent GLUT4 exocytosis. Mol. Biol. Cell 2016, 27, 75–89. [Google Scholar] [CrossRef]
- Wang, Z.; Oh, E.; Thurmond, D.C. Glucose-stimulated Cdc42 signaling is essential for the second phase of insulin secretion. J. Biol. Chem. 2007, 282, 9536–9546. [Google Scholar] [CrossRef]
- Megnagi, B.; Finkelstein, M.; Shabtay, O.; Breitbart, H. The role and importance of cofilin in human sperm capacitation and the acrosome reaction. Cell Tissue Res. 2015, 362, 665–675. [Google Scholar] [CrossRef]
- Gawden-Bone, C.M.; Frazer, G.L.; Richard, A.C.; Ma, C.Y.; Strege, K.; Griffiths, G.M. PIP5 Kinases Regulate Membrane Phosphoinositide and Actin Composition for Targeted Granule Secretion by Cytotoxic Lymphocytes. Immunity 2018, 49, 427–437.e4. [Google Scholar] [CrossRef]
- Reid, A.T.; Lord, T.; Stanger, S.J.; Roman, S.D.; McCluskey, A.; Robinson, P.J.; Aitken, R.J.; Nixon, B. Dynamin regulates specific membrane fusion events necessary for acrosomal exocytosis in mouse spermatozoa. J. Biol. Chem. 2012, 287, 37659–37672. [Google Scholar] [CrossRef]
- Zhou, W.; Anderson, A.L.; Turner, A.P.; De Iuliis, G.N.; McCluskey, A.; McLaughlin, E.A.; Nixon, B. Characterization of a novel role for the dynamin mechanoenzymes in the regulation of human sperm acrosomal exocytosis. Mol. Hum. Reprod. 2017, 23, 657–673. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.; Ji, C.; Wu, Y.; Ferguson, S.M.; Tamarina, N.; Philipson, L.H.; Lou, X. Dynamin 2 regulates biphasic insulin secretion and plasma glucose homeostasis. J. Clin. Investig. 2015, 125, 4026–4041. [Google Scholar] [CrossRef]
- Ye, D.; Wang, X.; Wei, C.; He, M.; Wang, H.; Wang, Y.; Zhu, Z.; Sun, Y. Marcksb plays a key role in the secretory pathway of zebrafish Bmp2b. PLoS Genet. 2019, 15, 1–28. [Google Scholar] [CrossRef]
- Satoh, K.; Narita, T.; Katsumata-Kato, O.; Sugiya, H.; Seo, Y. Involvement of myristoylated alanine-rich C kinase substrate phosphorylation and translocation in cholecystokinin-induced amylase release in rat pancreatic acini. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 310, G399–G409. [Google Scholar] [CrossRef] [PubMed]
- Robinson, C.L.; Evans, R.D.; Sivarasa, K.; Ramalho, J.S.; Briggs, D.A.; Hume, A.N. The adaptor protein melanophilin regulates dynamic myosin-Va:cargo interaction and dendrite development in melanocytes. Mol. Biol. Cell 2019, 30, 742–752. [Google Scholar] [CrossRef] [PubMed]
- Oberhofer, A.; Spieler, P.; Rosenfeld, Y.; Stepp, W.L.; Cleetus, A.; Hume, A.N.; Mueller-Planitz, F.; Ökten, Z. Myosin Va’s adaptor protein melanophilin enforces track selection on the microtubule and actin networks in vitro. Proc. Natl. Acad. Sci. USA 2017, 114, E4714–E4723. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wei, G.; Cao, Y.; Deng, Q.; Han, X.; Huang, X.; Huo, Y.; He, Y.; Chen, L.; Luo, J. Myosin IIa is critical for cAMP-mediated endothelial secretion of von Willebrand factor. Blood 2018, 131, 686–698. [Google Scholar] [CrossRef]
- Vogel, G.F.; Klee, K.M.C.; Janecke, A.R.; Müller, T.; Hess, M.W.; Huber, L.A. Cargo-selective apical exocytosis in epithelial cells is conducted by Myo5B, Slp4a, Vamp7, and Syntaxin 3. J. Cell Biol. 2015, 211, 587–604. [Google Scholar] [CrossRef]
- Ritter, A.T.; Kapnick, S.M.; Murugesan, S.; Schwartzberg, P.L.; Griffiths, G.M.; Lippincott-Schwartz, J. Cortical actin recovery at the immunological synapse leads to termination of lytic granule secretion in cytotoxic T lymphocytes. Proc. Natl. Acad. Sci. USA 2017, 114, E6585–E6594. [Google Scholar] [CrossRef]
- Wang, H.H.; Cui, Q.; Zhang, T.; Wang, Z.B.; Ouyang, Y.C.; Shen, W.; Ma, J.Y.; Schatten, H.; Sun, Q.Y. Rab3A, Rab27A, and Rab35 regulate different events during mouse oocyte meiotic maturation and activation. Histochem. Cell Biol. 2016, 145, 647–657. [Google Scholar] [CrossRef]
- Quevedo, M.F.; Bustos, M.A.; Masone, D.; Roggero, C.M.; Bustos, D.M.; Tomes, C.N. Grab recruitment by Rab27A-Rabphilin3a triggers Rab3A activation in human sperm exocytosis. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 612–622. [Google Scholar] [CrossRef]
- Ramadass, M.; Johnson, J.L.; Catz, S.D. Rab27a regulates GM-CSF-dependent priming of neutrophil exocytosis. J. Leukoc. Biol. 2017, 101, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, K.; Taoka, M.; Isobe, T.; Izumi, T. Rab2a and Rab27a cooperatively regulate the transition from granule maturation to exocytosis through the dual effector Noc2. J. Cell Sci. 2017, 130, 541–550. [Google Scholar] [CrossRef]
- Sheshachalam, A.; Baier, A.; Eitzen, G. The effect of Rho drugs on mast cell activation and degranulation. J. Leukoc. Biol. 2017, 102, 71–81. [Google Scholar] [CrossRef]
- Mietkowska, M.; Schuberth, C.; Wedlich-Söldner, R.; Gerke, V. Actin dynamics during Ca2+ -dependent exocytosis of endothelial Weibel-Palade bodies. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 1218–1229. [Google Scholar] [CrossRef]
- Bierings, R.; Hellen, N.; Kiskin, N.; Knipe, L.; Fonseca, A.V.; Patel, B.; Meli, A.; Rose, M.; Hannah, M.J.; Carter, T. The interplay between the Rab27A effectors Slp4-a and MyRIP controls hormone-evoked Weibel-Palade body exocytosis. Blood 2012, 120, 2757–2767. [Google Scholar] [CrossRef] [PubMed]
- Masedunskas, A.; Appaduray, M.A.; Lucas, C.A.; Cagigas, M.L.; Heydecker, M.; Holliday, M.; Meiring, J.C.M.; Hook, J.; Kee, A.; White, M.; et al. Parallel assembly of actin and tropomyosin, but not myosin II, during de novo actin filament formation in live mice. J. Cell Sci. 2018, 131, 1–8. [Google Scholar] [CrossRef] [PubMed]
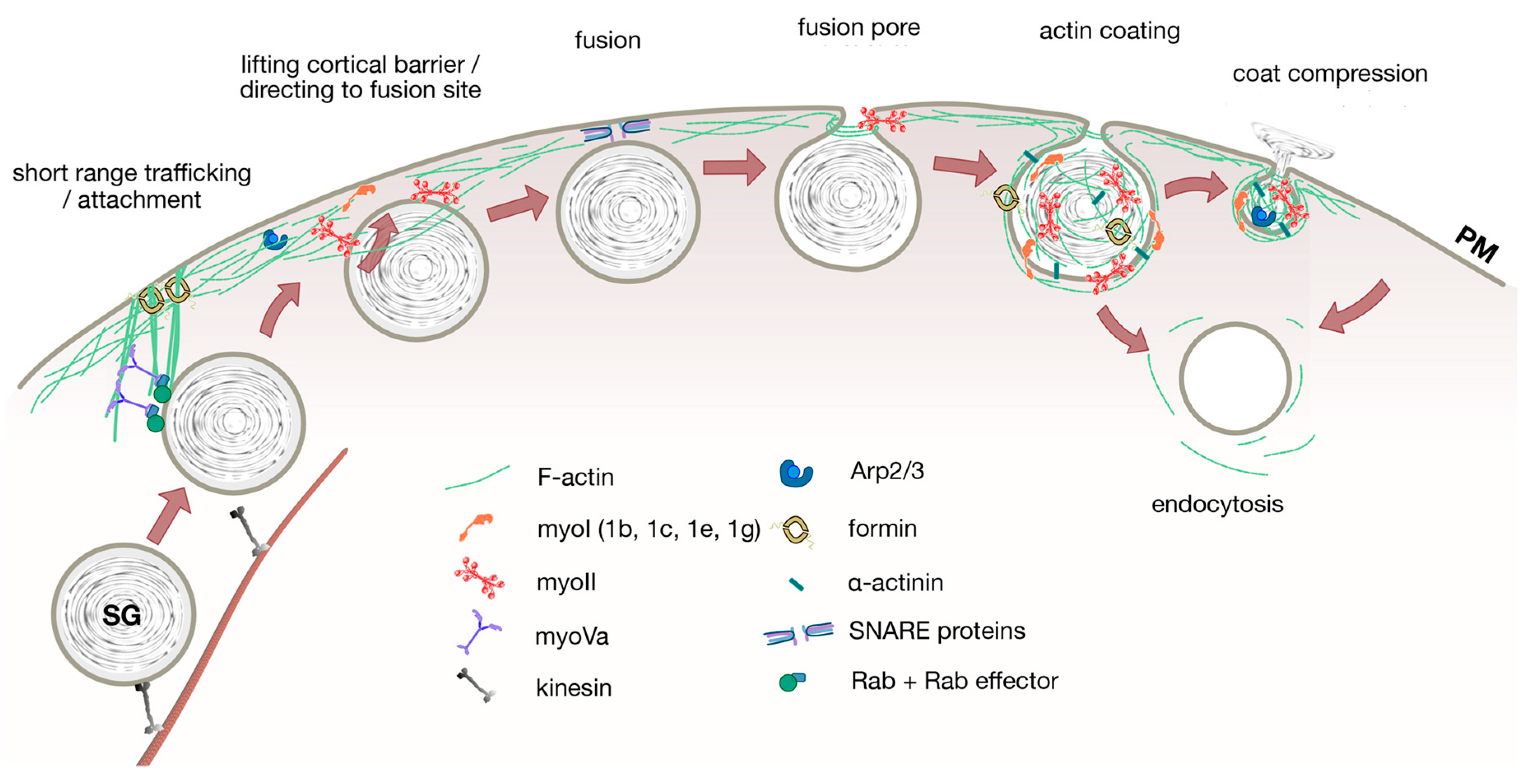
| Pre-Fusion | Post-Fusion | ||||
|---|---|---|---|---|---|
| Trafficking | Cortical attachment/actin remodeling | Fusion pore dynamics | Actin coat formation, stabilization, contraction | Endocytosis | |
| α-Actinin | Muscle cells (R.n.) [136] | Endothelial cells (H.s.) [75] Alveolar type II cells (R.n.) [120] | |||
| Annexin | Alveolar type II cells (R.n.) [71] Endothelial cells (H.s.) [72,73] | ||||
| Arp2/3 | NK cells (H.s.) [46] | Salivary gland acinar cells (D.m.) [64] Beta cells (M.m.) [48] | |||
| Cdc42 | Beta cells (M.m.) [137] | Oocytes (X.l.) [106,110] | |||
| Clathrin | Beta cells (M.m.) [48] Endothelial cells (H.s.) [132] | ||||
| Cofilin | Sperm (H.s.) [138] | Alveolar type II cells (R.n.) [120] | |||
| DAG | CT lymphocytes (M.m.) [139] | Oocytes (X.l.) [110] | |||
| Dynamin | Sperm (M.m.; H.s.) [140,141] | Beta cells (M.m.) [48,142] Endothelial cells (H.s.) [132] | |||
| Formins | Epithelial cells (D.m.) [15] Pancreatic acinar cells (M.m.) [14] | Mast cells (M.m.) [45] | Beta cells (M.m.) [48] | Alveolar type II cells (R.n.) [80] Beta cells (M.m.) [48] Salivary gland acinar cells (D.m.) [111] | |
| MARCKS | Airway epithelial cells (M.m.) [60] Embryonic cells (D.r.) [143] Pancreatic acinar cells (R.n.) [144] | ||||
| Melanophilin | Melanocytes (M.m.) [29,145,146] | Melanocytes (M.m.) [29] | |||
| MLCK | Renin-secreting cells (M.m.) [51] | Alveolar type II cells (R.n.) [120] Salivary gland acinar cells (M.m.) [119] | |||
| MyoI | Fibroblasts (M.m.) [52] Alveolar type II cells (R.n.) [53] B lymphocytes (M.m.) [54] | Alveolar type II cells (R.n.) [53] Oocytes (X.l.) [123] Oocytes (X.l.) [117] | Oocytes (X.l.) [123] | ||
| MyoII | NK cells (H.s.) [46] Renin-secreting cells (M.m.) [51] Endothelial cells (H.s.) [147] | Pancreatic acinar cells (M.m.) [88] | Alveolar type II cells (R.n.) [120] Salivary gland acinar cells (M.m.; D.m.) [108,111] Oocytes (X.l.) [117] Lacrimal gland acinar cells (O.c.) [47] Endothelial cells (H.s.) [43,147] | ||
| MyoV | Endothelial cells (H.s.) [26] Melanocytes (M.m.) [29,145] Epithelial cells (H.s.) [148] Beta cells (M.m.) [66] | Endothelial cells (H.s.) [3] Beta cells (M.m.) [66] Mast cells (M.m.) [44] | |||
| MyoVII | Beta cells (M.m.) [68] | ||||
| MyRIP (Exophilin 8) | Endothelial cells (H.s.) [12,26] | Endothelial cells (H.s.) [12,26] Beta cells (M.m.) [68] | |||
| PIP2 | CT lymphocytes (M.m) [139,149] | Salivary gland acinar cells (D.m.) [64] | |||
| PKC | Oocytes (X.l.) [110] Endothelial cells (H.s.) [118] | ||||
| Rab3 | Sperm (H.s.) [28] Endothelial cells (H.s.) [72] Oocytes (M.m.) [150] | Epithelial cells (H.s.) [69] Sperm (H.s.) [151] | Sperm (H.s.) [94] | ||
| Rab11 | Epithelial cells (H.s.) [148] Oocytes (M.m.) [27] | Mast cells (M.m.) [77] | |||
| Rab27 | Endothelial cells (H.s.) [26] Oocytes (M.m.) [27] Sperm (H.s.) [28] Melanocytes (M.m.) [29,145] | Endothelial cells (H.s.) [26] Sperm (H.s.) [28,151] Oocytes (M.m.) [150] Melanocytes (M.m.) [29] CT lymphocytes (M.m.) [30,149] Mast cells (M.m.) [44] Neutrophils (M.m.) [152] Beta cells (M.m.) [153] | |||
| Rho | Mast cells (R.n.) [154] | Oocytes (X.l.) [117] Alveolar type II cells (R.n.) [80] Salivary gland acinar cells (D.m.) [111] Pancreatic acinar cells (M.m.) [107] Endothelial cells (H.s.) [155] | |||
| ROCK | Alveolar type II cells (R.n.) [120] | ||||
| SLP4 (Granuphilin) | Endothelial cells (H.s.) [156] Epithelial cells (H.s.) [69,148] | ||||
| Tropomyosin | Salivary gland acinar cells (M.m.) [157] | ||||
| UNC-45 | NK cells (H.s.) [50] | ||||
| WASP | Salivary gland (D.m.) [64] Oocytes (X.l.) [106] | ||||
| Zyxin | Endothelial cells (H.s.) [75] | Endothelial cells (H.s.) [75] | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miklavc, P.; Frick, M. Actin and Myosin in Non-Neuronal Exocytosis. Cells 2020, 9, 1455. https://doi.org/10.3390/cells9061455
Miklavc P, Frick M. Actin and Myosin in Non-Neuronal Exocytosis. Cells. 2020; 9(6):1455. https://doi.org/10.3390/cells9061455
Chicago/Turabian StyleMiklavc, Pika, and Manfred Frick. 2020. "Actin and Myosin in Non-Neuronal Exocytosis" Cells 9, no. 6: 1455. https://doi.org/10.3390/cells9061455
APA StyleMiklavc, P., & Frick, M. (2020). Actin and Myosin in Non-Neuronal Exocytosis. Cells, 9(6), 1455. https://doi.org/10.3390/cells9061455





