Veterinary Regenerative Medicine for Musculoskeletal Disorders: Can Mesenchymal Stem/Stromal Cells and Their Secretome Be the New Frontier?
Abstract
1. Introduction
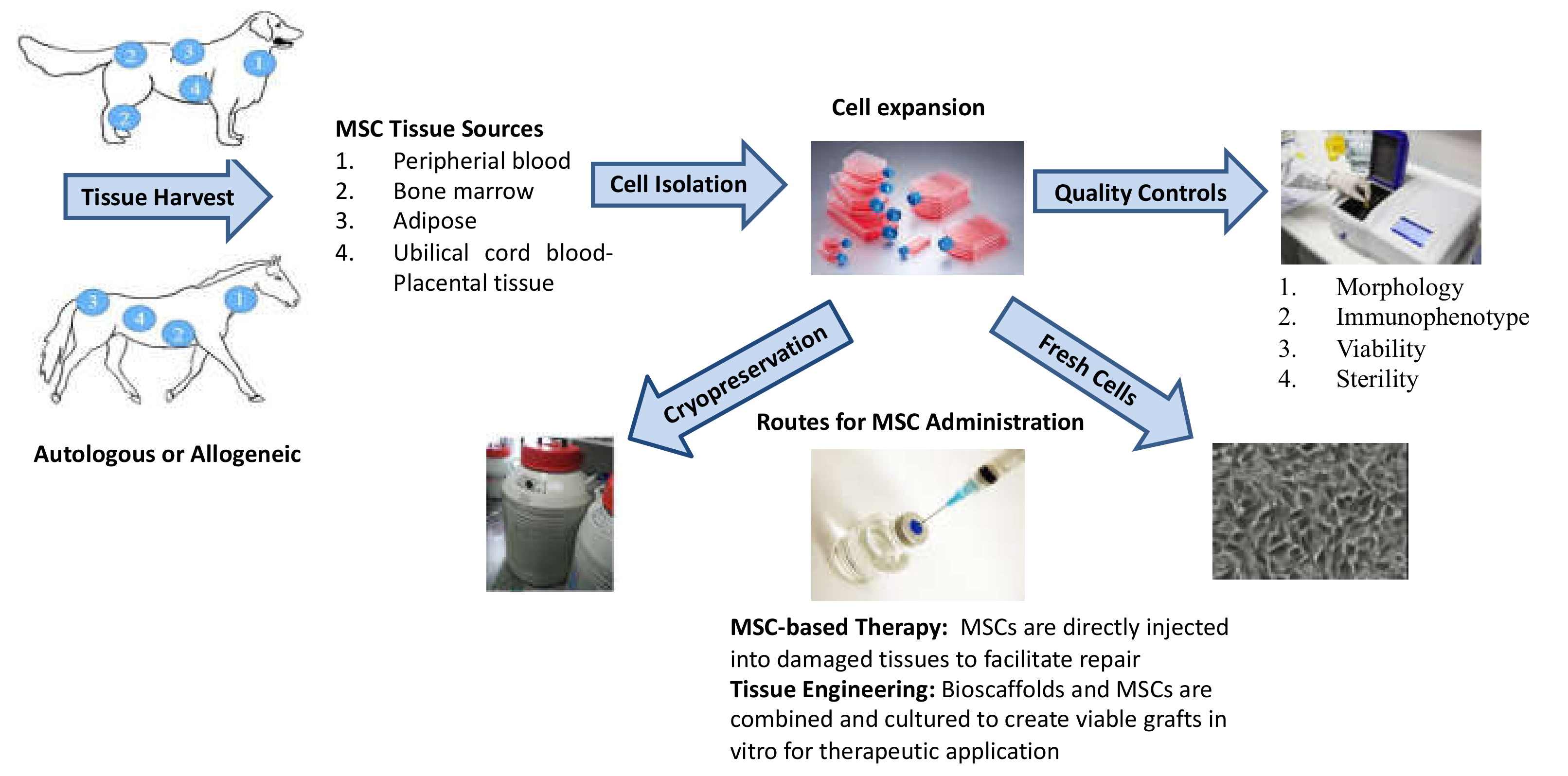
2. Mesenchymal Stromal Cells and Veterinary Regenerative Medicine: Main Features, Sources, Isolation, and Cryopreservation Procedures
2.1. MSC Isolation Tissue
2.2. MSC Isolation Procedures
2.3. MSC Culturing Conditions
2.4. Cryopreservation
2.5. Quality Controls
2.6. Routes for MSC Administration
2.7. Autologous vs Allogeneic MSCs
3. MSCs in the Veterinary Field: Disease Targets
3.1. Osteoarthritis
3.2. Tendon Ligament Injury
3.3. Intervertebral Disk Degeneration
4. Animal Spontaneous Pathologies as Potential Preclinical Models for Human Therapy
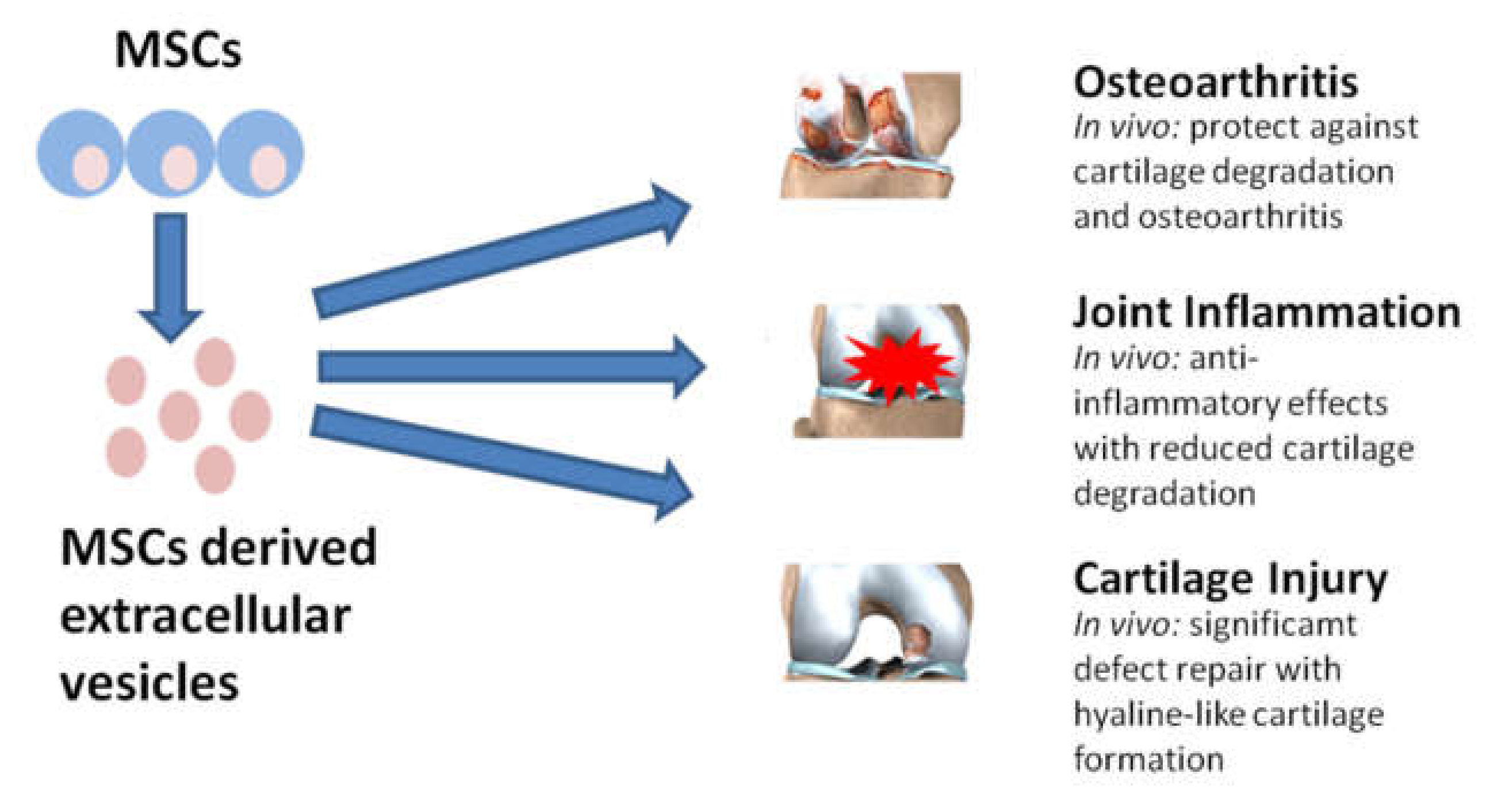
5. Secretome and Extracellular Vesicles as a Potential Therapy for Different Disease Areas
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Girolamo, L.; Lucarelli, E.; Alessandri, G.; Avanzini, M.A.; Bernardo, M.E.; Biagi, E.; Brini, A.T.; D’Amico, G.; Fagioli, F.; Ferrero, I.; et al. Mesenchymal Stem/Stromal Cells: A New “Cells as Drugs” Paradigm. Efficacy and Critical Aspects in Cell Therapy. Curr. Pharm. Des. 2013, 19, 2459–2473. [Google Scholar] [CrossRef]
- Bari, E.; Perteghella, S.; Di Silvestre, D.; Sorlini, M.; Catenacci, L.; Sorrenti, M.; Marrubini, G.; Rossi, R.; Tripodo, G.; Mauri, P.; et al. Pilot Production of Mesenchymal Stem/Stromal Freeze-Dried Secretome for Cell-Free Regenerative Nanomedicine: A Validated GMP-Compliant Process. Cells 2018, 7, 190. [Google Scholar] [CrossRef]
- Robey, P. “Mesenchymal stem cells”: Fact or fiction, and implications in their therapeutic use. F1000Research 2017, 6. [Google Scholar] [CrossRef]
- Katsuda, T.; Kosaka, N.; Takeshita, F.; Ochiya, T. The therapeutic potential of mesenchymal stem cell-derived extracellular vesicles. Proteomics 2013, 13, 1637–1653. [Google Scholar] [CrossRef]
- Kidd, S.; Spaeth, E.; Dembinski, J.L.; Dietrich, M.; Watson, K.; Klopp, A.; Battula, V.L.; Weil, M.; Andreeff, M.; Marini, F.C. Direct Evidence of Mesenchymal Stem Cell Tropism for Tumor and Wounding Microenvironments Using In Vivo Bioluminescent Imaging. Stem Cells 2009, 27, 2614–2623. [Google Scholar] [CrossRef]
- Da Silva Meirelles, L.; Chagastelles, P.C.; Nardi, N.B. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J. Cell Sci. 2006, 119, 2204–2213. [Google Scholar] [CrossRef] [PubMed]
- Tessier, L.; Bienzle, D.; Williams, L.B.; Koch, T.G. Phenotypic and Immunomodulatory Properties of Equine Cord Blood-Derived Mesenchymal Stromal Cells. PLoS ONE 2015, 10, e0122954. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.; Lopez, M.J.; Hicok, K. Adult multipotent stromal cell cryopreservation: Pluses and pitfalls. Vet. Surg. 2018, 47, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Barrachina, L.; Romero, A.; Zaragoza, P.; Rodellar, C.; Vazquez, F.J. Practical considerations for clinical use of mesenchymal stem cells: From the laboratory to the horse. Vet. J. 2018, 238, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Orbay, H.; Tobita, M.; Mizuno, H. Mesenchymal Stem Cells Isolated from Adipose and Other Tissues: Basic Biological Properties and Clinical Applications. Stem Cells Int. 2012. [Google Scholar] [CrossRef] [PubMed]
- Sacchetti, B.; Funari, A.; Remoli, C.; Giannicola, G.; Kogler, G.; Liedtke, S.; Cossu, G.; Serafini, M.; Sampaolesi, M.; Tagliafico, E.; et al. No Identical “Mesenchymal Stem Cells” at Different Times and Sites: Human Committed Progenitors of Distinct Origin and Differentiation Potential Are Incorporated as Adventitial Cells in Microvessels. Stem Cell Rep. 2016, 6, 897–913. [Google Scholar] [CrossRef] [PubMed]
- Iacono, E.; Merlo, B.; Romagnoli, N.; Rossi, B.; Ricci, F.; Spadari, A. Equine Bone Marrow and Adipose Tissue Mesenchymal Stem Cells: Cytofluorimetric Characterization, In Vitro Differentiation, and Clinical Application. J. Equine Vet. Sci. 2015, 35, 130–140. [Google Scholar] [CrossRef]
- Bearden, R.N.; Huggins, S.S.; Cummings, K.J.; Smith, R.; Gregory, C.A.; Saunders, W.B. In-vitro characterization of canine multipotent stromal cells isolated from synovium, bone marrow, and adipose tissue: A donor-matched comparative study. Stem Cell Res. Ther. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Toupadakis, C.A.; Wong, A.; Genetos, D.C.; Cheung, W.K.; Borjesson, D.L.; Ferraro, G.L.; Galuppo, L.D.; Leach, J.K.; Owens, S.D.; Yellowley, C.E. Comparison of the osteogenic potential of equine mesenchymal stem cells from bone marrow, adipose tissue, umbilical cord blood, and umbilical cord tissue. Am. J. Vet. Res. 2010, 71, 1237–1245. [Google Scholar] [CrossRef]
- Vidal, M.A.; Kilroy, G.E.; Lopez, M.J.; Johnson, J.R.; Moore, R.M.; Gimble, J.M. Characterization of equine adipose tissue-derived stromal cells: Adipogenic and osteogenic capacity and comparison with bone marrow-derived mesenchymal stromal cells. Vet. Surg. 2007, 36, 613–622. [Google Scholar] [CrossRef]
- Giovannini, S.; Brehm, W.; Mainil-Varlet, P.; Nesic, D. Multilineage differentiation potential of equine blood-derived fibroblast-like cells. Differentiation 2008, 76, 118–129. [Google Scholar] [CrossRef]
- Del Bue, M.; Ricco, S.; Ramoni, R.; Conti, V.; Gnudi, G.; Grolli, S. Equine adipose-tissue derived mesenchymal stem cells and platelet concentrates: Their association in vitro and in vivo. Vet. Res. Commun. 2008, 32, S51–S55. [Google Scholar] [CrossRef]
- Martinello, T.; Bronzini, I.; Maccatrozzo, L.; Mollo, A.; Sampaolesi, M.; Mascarello, F.; Decaminada, M.; Patruno, M. Canine adipose-derived-mesenchymal stem cells do not lose stem features after a long-term cryopreservation. Res. Vet. Sci. 2011, 91, 18–24. [Google Scholar] [CrossRef]
- Jezierska-Wozniak, K.; Nosarzewska, D.; Tutas, A.; Mikolajczyk, A.; Oklinski, M.; Jurkowski, M.K. Use of adipose tissue as a source of mesenchymal stem cells. Postepy Hig. Med. Dosw. 2010, 64, 326–332. [Google Scholar]
- Nava, S.; Sordi, V.; Pascucci, L.; Tremolada, C.; Ciusani, E.; Zeira, O.; Cadei, M.; Soldati, G.; Pessina, A.; Parati, E.; et al. Long-Lasting Anti-Inflammatory Activity of Human Microfragmented Adipose Tissue. Stem Cells Int. 2019, 2019, 5901479. [Google Scholar] [CrossRef]
- You, D.; Jang, M.J.; Kim, B.H.; Song, G.; Lee, C.; Suh, N.; Jeong, I.G.; Ahn, T.Y.; Kim, C.-S. Comparative Study of Autologous Stromal Vascular Fraction and Adipose-Derived Stem Cells for Erectile Function Recovery in a Rat Model of Cavernous Nerve Injury. Stem Cells Transl. Med. 2015, 4, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Kemilew, J.; Sobczyska-Rak, A.; Zyliska, B.; Szponder, T.; Nowicka, B.; Urban, B. The Use of Allogenic Stromal Vascular Fraction (SVF) Cells in Degenerative Joint Disease of the Spine in Dogs. In Vivo 2019, 33, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Minteer, D.; Marra, K.G.; Rubin, J.P. Adipose-Derived Mesenchymal Stem Cells: Biology and Potential Applications. Mesenchymal Stem Cells Basics Clin. Appl. I 2013, 129, 59–71. [Google Scholar] [CrossRef]
- Kuznetsov, S.A.; Mankani, M.H.; Gronthos, S.; Satomura, K.; Bianco, P.; Robey, P.G. Circulating skeletal stem cells. J. Cell Biol. 2001, 153, 1133–1139. [Google Scholar] [CrossRef] [PubMed]
- Roufosse, C.A.; Direkze, N.C.; Otto, W.R.; Wright, N.A. Circulating mesenchymal stem cells. Int. J. Biochem. Cell Biol. 2004, 36, 585–597. [Google Scholar] [CrossRef]
- Koerner, J.; Nesic, D.; Romero, J.D.; Brehm, W.; Mainil-Varlet, P.; Grogan, S.P. Equine peripheral blood-derived progenitors in comparison to bone marrow-derived mesenchymal stem cells. Stem Cells 2006, 24, 1613–1619. [Google Scholar] [CrossRef]
- Santos, V.H.; Pfeifer, J.P.H.; de Souza, J.B.; Milani, B.H.G.; de Oliveira, R.A.; Assis, M.G.; Deffune, E.; Moroz, A.; Alves, A.L.G. Culture of mesenchymal stem cells derived from equine synovial membrane in alginate hydrogel microcapsules. BMC Vet. Res. 2018, 14. [Google Scholar] [CrossRef]
- Kern, S.; Eichler, H.; Stoeve, J.; Klueter, H.; Bieback, K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 2006, 24, 1294–1301. [Google Scholar] [CrossRef]
- Schuh, E.M.; Friedman, M.S.; Carrade, D.D.; Li, J.; Heeke, D.; Oyserman, S.M.; Galuppo, L.D.; Lara, D.J.; Walker, N.J.; Ferraro, G.L.; et al. Identification of variables that optimize isolation and culture of multipotent mesenchymal stem cells from equine umbilical-cord blood. Am. J. Vet. Res. 2009, 70, 1526–1535. [Google Scholar] [CrossRef]
- Iacono, E.; Lanci, A.; Merlo, B.; Ricci, F.; Pirrone, A.; Antonelli, C.; Mariella, J.; Castagnetti, C. Effects of amniotic fluid mesenchymal stem cells in carboxymethyl cellulose gel on healing of spontaneous pressure sores: Clinical outcome in seven hospitalized neonatal foals. Turk. J. Biol. 2016, 40, 484–492. [Google Scholar] [CrossRef]
- Iacono, E.; Brunori, L.; Pirrone, A.; Pagliaro, P.P.; Ricci, F.; Tazzari, P.L.; Merlo, B. Isolation, characterization and differentiation of mesenchymal stem cells from amniotic fluid, umbilical cord blood and Wharton’s jelly in the horse. Reproduction 2012, 143, 455–468. [Google Scholar] [CrossRef] [PubMed]
- Passeri, S.; Nocchi, F.; Lamanna, R.; Lapi, S.; Miragliotta, V.; Giannessi, E.; Abramo, F.; Stornelli, M.R.; Matarazzo, M.; Plenteda, D.; et al. Isolation and expansion of equine umbilical cord-derived matrix cells (EUCMCs). Cell Biol. Int. 2009, 33, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Ranera, B.; Lyahyai, J.; Romero, A.; Jose Vazquez, F.; Rosa Remacha, A.; Luisa Bernal, M.; Zaragoza, P.; Rodellar, C.; Martin-Burriel, I. Immunophenotype and gene expression profiles of cell surface markers of mesenchymal stem cells derived from equine bone marrow and adipose tissue. Vet. Immunol. Immunopathol. 2011, 144, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Vidal, M.A.; Walker, N.J.; Napoli, E.; Borjesson, D.L. Evaluation of Senescence in Mesenchymal Stem Cells Isolated from Equine Bone Marrow, Adipose Tissue, and Umbilical Cord Tissue. Stem Cells Dev. 2012, 21, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Naskou, M.C.; Sumner, S.M.; Chocallo, A.; Kemelmakher, H.; Thoresen, M.; Copland, I.; Galipeau, J.; Peroni, J.F. Platelet lysate as a novel serum-free media supplement for the culture of equine bone marrow-derived mesenchymal stem cells. Stem Cell Res. Ther. 2018, 9. [Google Scholar] [CrossRef]
- Russell, K.A.; Koch, T.G. Equine platelet lysate as an alternative to fetal bovine serum in equine mesenchymal stromal cell culture—Too much of a good thing? Equine Vet. J. 2016, 48, 261–264. [Google Scholar] [CrossRef]
- Bari, E.; Perteghella, S.; Farago, S.; Torre, M.L. Association of silk sericin and platelet lysate: Premises for the formulation of wound healing active medications. Int. J. Biol. Macromol. 2018, 119, 37–47. [Google Scholar] [CrossRef]
- Verdanova, M.; Pytlik, R.; Kalbacova, M.H. Evaluation of Sericin as a Fetal Bovine Serum-Replacing Cryoprotectant During Freezing of Human Mesenchymal Stromal Cells and Human Osteoblast-Like Cells. Biopreserv. Biobank. 2014, 12, 99–105. [Google Scholar] [CrossRef]
- Espina, M.; Juelke, H.; Brehm, W.; Ribitsch, I.; Winter, K.; Delling, U. Evaluation of transport conditions for autologous bone marrow-derived mesenchymal stromal cells for therapeutic application in horses. PeerJ 2016, 4. [Google Scholar] [CrossRef]
- Garvican, E.R.; Cree, S.; Bull, L.; Smith, R.K.W.; Dudhia, J. Viability of equine mesenchymal stem cells during transport and implantation (vol 5, 94, 2014). Stem Cell Res. Ther. 2016, 7. [Google Scholar] [CrossRef]
- Broeckx, S.; Suls, M.; Beerts, C.; Vandenberghe, A.; Seys, B.; Wuertz-Kozak, K.; Duchateau, L.; Spaas, J.H. Allogenic Mesenchymal Stem Cells as a Treatment for Equine Degenerative Joint Disease: A Pilot Study. Curr. Stem Cell Res. Ther. 2014, 9, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Mercati, F.; Pascucci, L.; Curina, G.; Scocco, P.; Tardella, F.M.; Dall’Aglio, C.; Marini, C.; Ceccarelli, P. Evaluation of storage conditions on equine adipose tissue-derived multipotent mesenchymal stromal cells. Vet. J. 2014, 200, 339–342. [Google Scholar] [CrossRef]
- Bronzini, I.; Patruno, M.; Iacopetti, I.; Martinello, T. Influence of temperature, time and different media on mesenchymal stromal cells shipped for clinical application. Vet. J. 2012, 194, 121–123. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Dietrich, M.A.; Lopez, M.J. Therapeutic Doses of Multipotent Stromal Cells from Minimal Adipose Tissue. Stem Cell Rev. Rep. 2014, 10, 600–611. [Google Scholar] [CrossRef]
- Martinello, T.; Bronzini, I.; Maccatrozzo, L.; Iacopetti, I.; Sampaolesi, M.; Mascarello, F.; Patruno, M. Cryopreservation Does Not Affect the Stem Characteristics of Multipotent Cells Isolated from Equine Peripheral Blood. Tissue Eng. Part C Methods 2010, 16, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Torre, M.L.; Lucarelli, E.; Guidi, S.; Ferrari, M.; Alessandri, G.; De Girolamo, L.; Pessina, A.; Ferrero, I.; Gism. Ex Vivo Expanded Mesenchymal Stromal Cell Minimal Quality Requirements for Clinical Application. Stem Cells Dev. 2015, 24, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.B.; Russell, K.A.; Koenig, J.B.; Koch, T.G. Aspiration, but not injection, decreases cultured equine mesenchymal stromal cell viability. BMC Vet. Res. 2016, 12. [Google Scholar] [CrossRef][Green Version]
- Schnabel, L.V.; Fortier, L.A.; McIlwraith, C.W.; Nobert, K.M. Therapeutic use of stem cells in horses: Which type, how, and when? Vet. J. 2013, 197, 570–577. [Google Scholar] [CrossRef]
- Sole, A.; Spriet, M.; Galuppo, L.D.; Padgett, K.A.; Borjesson, D.L.; Wisner, E.R.; Brosnan, R.J.; Vidal, M.A. Scintigraphic evaluation of intra-arterial and intravenous regional limb perfusion of allogeneic bone marrow-derived mesenchymal stem cells in the normal equine distal limb using 99mTc-HMPAO. Equine Vet. J. 2012, 44, 594–599. [Google Scholar] [CrossRef]
- Siegel, G.; Kluba, T.; Hermanutz-Klein, U.; Bieback, K.; Northoff, H.; Schaefer, R. Phenotype, donor age and gender affect function of human bone marrow-derived mesenchymal stromal cells. BMC Med. 2013, 11. [Google Scholar] [CrossRef]
- Carrade, D.D.; Owens, S.D.; Galuppo, L.D.; Vidal, M.A.; Ferraro, G.L.; Librach, F.; Buerchler, S.; Friedman, M.S.; Walker, N.J.; Borjesson, D.L. Clinicopathologic findings following intra-articular injection of autologous and allogeneic placentally derived equine mesenchymal stem cells in horses. Cytotherapy 2011, 13, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.; Drury, T.; Roic, I.; Hansen, P.; Malin, M.; Boyd, R.; Sumer, H.; Ferguson, R. Outcome of Allogeneic Adult Stem Cell Therapy in Dogs Suffering from Osteoarthritis and Other Joint Defects. Stem Cells Int. 2018, 2018, 7309201. [Google Scholar] [CrossRef] [PubMed]
- Guest, D.J.; Smith, M.R.W.; Allen, W.R. Monitoring the fate of autologous and allogeneic mesenchymal progenitor cells injected into the superficial digital flexor tendon of horses: Preliminary study. Equine Vet. J. 2008, 40, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Brandao, J.S.; Alvarenga, M.L.; Hubbe Pfeifer, J.P.; dos Santos, V.H.; Fonseca-Alves, C.E.; Rodrigues, M.; Laufer-Amorim, R.; Lucas Castillo, J.A.; Garcia Alves, A.L. Allogeneic mesenchymal stem cell transplantation in healthy equine superficial digital flexor tendon: A study of the local inflammatory response. Res. Vet. Sci. 2018, 118, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Brehm, W.; Burk, J.; Delling, U.; Gittel, C.; Ribitsch, I. Stem cell-based tissue engineering in veterinary orthopaedics. Cell Tissue Res. 2012, 347, 677–688. [Google Scholar] [CrossRef]
- Gala, K.; Burdzinska, A.; Idziak, M.; Wilczek, E.; Paczek, L. Transplantation of mesenchymal stem cells into the skeletal muscle induces cytokine generation. Cytokine 2013, 64, 243–250. [Google Scholar] [CrossRef]
- Zayed, M.; Adair, S.; Ursini, T.; Schumacher, J.; Misk, N.; Dhar, M. Concepts and challenges in the use of mesenchymal stem cells as a treatment for cartilage damage in the horse. Res. Vet. Sci. 2018, 118, 317–323. [Google Scholar] [CrossRef]
- Litzke, L.F.; Wagner, E.; Baumgaertner, W.; Hetzel, U.; Josimovic-Alasevic, O.; Libera, J. Repair of extensive articular cartilage defects in horses by autologous chondrocyte transplantation. Ann. Biomed. Eng. 2004, 32, 57–69. [Google Scholar] [CrossRef]
- Sasaki, A.; Mizuno, M.; Ozeki, N.; Katano, H.; Otabe, K.; Tsuji, K.; Koga, H.; Mochizuki, M.; Sekiya, I. Canine mesenchymal stem cells from synovium have a higher chondrogenic potential than those from infrapatellar fat pad, adipose tissue, and bone marrow. PLoS ONE 2018, 13, e0202922. [Google Scholar] [CrossRef]
- Lim, J.K.; Hui, J.; Li, L.; Thambyah, A.; Goh, J.; Lee, E.H. Enhancement of tendon graft osteointegration using mesenchymal stem cells in a rabbit model of anterior cruciate ligament reconstruction. Arthrosc. J. Arthrosc. Relat. Surg. 2004, 20, 899–910. [Google Scholar] [CrossRef]
- Zeira, O.; Scaccia, S.; Pettinari, L.; Ghezzi, E.; Asiag, N.; Martinelli, L.; Zahirpour, D.; Dumas, M.P.; Konar, M.; Lupi, D.M.; et al. Intra-Articular Administration of Autologous Micro-Fragmented Adipose Tissue in Dogs with Spontaneous Osteoarthritis: Safety, Feasibility, and Clinical Outcomes. Stem Cells Transl. Med. 2018, 7, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Guercio, A.; Di Marco, P.; Casella, S.; Cannella, V.; Russotto, L.; Purpari, G.; Di Bella, S.; Piccione, G. Production of canine mesenchymal stem cells from adipose tissue and their application in dogs with chronic osteoarthritis of the humeroradial joints. Cell Biol. Int. 2012, 36, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.M.; Dow, S.W. Concise Review: Stem Cell Trials Using Companion Animal Disease Models. Stem Cells 2016, 34, 1709–1729. [Google Scholar] [CrossRef] [PubMed]
- Vilar, J.M.; Morales, M.; Santana, A.; Spinella, G.; Rubio, M.; Cuervo, B.; Cugat, R.; Carrillo, J.M. Controlled, blinded force platform analysis of the effect of intraarticular injection of autologous adipose-derived mesenchymal stem cells associated to PRGF-Endoret in osteoarthritic dogs. BMC Vet. Res. 2013, 9. [Google Scholar] [CrossRef]
- Vilar, J.M.; Batista, M.; Morales, M.; Santana, A.; Cuervo, B.; Rubio, M.; Cugat, R.; Sopena, J.; Carrillo, J.M. Assessment of the effect of intraarticular injection of autologous adipose-derived mesenchymal stem cells in osteoarthritic dogs using a double blinded force platform analysis. BMC Vet. Res. 2014, 10. [Google Scholar] [CrossRef]
- Amable, P.R.; Vieira Carias, R.B.; Telles Teixeira, M.V.; Pacheco, I.d.C.; Farias Correa do Amaral, R.J.; Granjeiro, J.M.; Borojevic, R. Platelet-rich plasma preparation for regenerative medicine: Optimization and quantification of cytokines and growth factors. Stem Cell Res. Ther. 2013, 4. [Google Scholar] [CrossRef]
- Cuervo, B.; Rubio, M.; Sopena, J.; Manuel Dominguez, J.; Vilar, J.; Morales, M.; Cugat, R.; Maria Carrillo, J. Hip Osteoarthritis in Dogs: A Randomized Study Using Mesenchymal Stem Cells from Adipose Tissue and Plasma Rich in Growth Factors. Int. J. Mol. Sci. 2014, 15, 13437–13460. [Google Scholar] [CrossRef]
- Black, L.L.; Gaynor, J.; Gahring, D.; Adams, C.; Aron, D.; Harman, S.; Gingerich, D.A.; Harman, R. Effect of adipose-derived mesenchymal stem and regenerative cells on lameness in dogs with chronic osteoarthritis of the coxofemoral joints: A randomized, double-blinded, multicenter, controlled trial. Vet. Ther. 2007, 8, 272–284. [Google Scholar]
- Black, L.; Gaynor, J.; Adams, C.; Dhupa, S.; Sams, A.; Taylor, R.; Harman, S.; Gingerich, D.; Harman, R. Effect of Intraarticular Injection of Autologous Adipose-Derived Mesenchymal Stem and Regenerative Cells on Clinical Signs of Chronic Osteoarthritis of the Elbow Joint in Dogs. Vet. Ther. 2008, 9, 192–200. [Google Scholar]
- Bogers, S.H. Cell-Based Therapies for Joint Disease in Veterinary Medicine: What We Have Learned and What We Need to Know. Front. Vet. Sci. 2018, 5. [Google Scholar] [CrossRef]
- McIlwraith, C.W.; Frisbie, D.D.; Rodkey, W.G.; Kisiday, J.D.; Werpy, N.M.; Kawcak, C.E.; Steadman, J.R. Evaluation of Intra-Articular Mesenchymal Stem Cells to Augment Healing of Microfractured Chondral Defects. Arthrosc. J. Arthrosc. Relat. Surg. 2011, 27, 1552–1561. [Google Scholar] [CrossRef] [PubMed]
- Ferris, D.J.; Frisbie, D.D.; Kisiday, J.D.; McIlwraith, C.W.; Hague, B.A.; Major, M.D.; Schneider, R.K.; Zubrod, C.J.; Kawcak, C.E.; Goodrich, L.R. Clinical Outcome After Intra-Articular Administration of Bone Marrow Derived Mesenchymal Stem Cells in 33 Horses With Stifle Injury. Vet. Surg. 2014, 43, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, X.; Cao, W.; Shi, Y. Plasticity of mesenchymal stem cells in immunomodulation: Pathological and therapeutic implications. Nat. Immunol. 2014, 15, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Nicpon, J.; Marycz, K.; Grzesiak, J. Therapeutic effect of adipose-derived mesenchymal stem cell injection in horses suffering from bone spavin. Pol. J. Vet. Sci. 2013, 16, 753–754. [Google Scholar] [CrossRef]
- Broeckx, S.Y.; Seys, B.; Suls, M.; Vandenberghe, A.; Marien, T.; Adriaensen, E.; Declercq, J.; Van Hecke, L.; Braun, G.; Hellmann, K.; et al. Equine Allogeneic Chondrogenic Induced Mesenchymal Stem Cells Are an Effective Treatment for Degenerative Joint Disease in Horses. Stem Cells Dev. 2019, 28, 410–422. [Google Scholar] [CrossRef]
- Smith, R.; McIlwraith, W.; Schweitzer, R.; Kadler, K.; Cook, J.; Caterson, B.; Dakin, S.; Heinegard, D.; Screen, H.; Stover, S.; et al. Advances in the understanding of tendinopathies: A report on the Second Havemeyer Workshop on equine tendon disease. Equine Vet. J. 2014, 46, 4–9. [Google Scholar] [CrossRef]
- Dowling, B.A.; Dart, A.J.; Hodgson, D.R.; Smith, R.K.W. Superficial digital flexor tendonitis in the horse. Equine Vet. J. 2000, 32, 369–378. [Google Scholar] [CrossRef]
- Chong, A.K.S.; Chang, J.; Go, J.C.H. Mesenchymal stem cells and tendon healing. Front. Biosci. 2009, 14, 4598–4605. [Google Scholar] [CrossRef]
- Richardson, L.E.; Dudhia, J.; Clegg, P.D.; Smith, R. Stem cells in veterinary medicine—Attempts at regenerating equine tendon after injury. Trends Biotechnol. 2007, 25, 409–416. [Google Scholar] [CrossRef]
- Carvalho, A.d.M.; Badial, P.R.; Cisneros Alvarez, L.E.; Miluzzi Yamada, A.L.; Borges, A.S.; Deffune, E.; Hussni, C.A.; Garcia Alves, A.L. Equine tendonitis therapy using mesenchymal stem cells and platelet concentrates: A randomized controlled trial. Stem Cell Res. Ther. 2013, 4. [Google Scholar] [CrossRef]
- Smith, R.K.W.; Werling, N.J.; Dakin, S.G.; Alam, R.; Goodship, A.E.; Dudhia, J. Beneficial Effects of Autologous Bone Marrow-Derived Mesenchymal Stem Cells in Naturally Occurring Tendinopathy. PLoS ONE 2013, 8, e0075697. [Google Scholar] [CrossRef]
- Smith, R.K.W.; Korda, M.; Blunn, G.W.; Goodship, A.E. Isolation and implantation of autologous equine mesenchymal stem cells from bone marrow into the superficial digital flexor tendon as a potential novel treatment. Equine Vet. J. 2003, 35, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.G.L.; Stewart, A.A.; Dudhia, J.; Kasashima, Y.; Goodship, A.E.; Smith, R.K.W. Cell-based Therapies for Tendon and Ligament Injuries. Vet. Clin. North Am. Equine Pract. 2011, 27, 315–333. [Google Scholar] [CrossRef]
- Godwin, E.E.; Young, N.J.; Dudhia, J.; Beamish, I.C.; Smith, R.K.W. Implantation of bone marrow-derived mesenchymal stem cells demonstrates improved outcome in horses with overstrain injury of the superficial digital flexor tendon. Equine Vet. J. 2012, 44, 25–32. [Google Scholar] [CrossRef]
- Kol, A.; Wood, J.A.; Holt, D.D.C.; Gillette, J.A.; Bohannon-Worsley, L.K.; Puchalski, S.M.; Walker, N.J.; Clark, K.C.; Watson, J.L.; Borjesson, D.L. Multiple intravenous injections of allogeneic equine mesenchymal stem cells do not induce a systemic inflammatory response but do alter lymphocyte subsets in healthy horses. Stem Cell Res. Ther. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Barberini, D.J.; Aleman, M.; Aristizabal, F.; Spriet, M.; Clark, K.C.; Walker, N.J.; Galuppo, L.D.; Amorim, R.M.; Woolard, K.D.; Borjesson, D.L. Safety and tracking of intrathecal allogeneic mesenchymal stem cell transplantation in healthy and diseased horses. Stem Cell Res. Ther. 2018, 9. [Google Scholar] [CrossRef]
- Beerts, C.; Suls, M.; Broeckx, S.Y.; Seys, B.; Vandenberghe, A.; Declercq, J.; Duchateau, L.; Vidal, M.A.; Spaas, J.H. Tenogenically induced allogeneic Peripheral Blood Mesenchymal stem cells in allogeneic Platelet-Rich Plasma: 2-Year Follow-up after Tendon or ligament Treatment in horses. Front. Vet. Sci. 2017, 4. [Google Scholar] [CrossRef]
- Romero, A.; Barrachina, L.; Ranera, B.; Remacha, A.R.; Moreno, B.; de Blas, I.; Sanz, A.; Vazquez, F.J.; Vitoria, A.; Junquera, C.; et al. Comparison of autologous bone marrow and adipose tissue derived mesenchymal stem cells, and platelet rich plasma, for treating surgically induced lesions of the equine superficial digital flexor tendon. Vet. J. 2017, 224, 76–84. [Google Scholar] [CrossRef]
- Ricco, S.; Renzi, S.; Del Bue, M.; Conti, V.; Merli, E.; Ramoni, R.; Lucarelli, E.; Gnudi, G.; Ferrari, M.; Grolli, S. Allogeneic Adipose Tissue-Derived Mesenchymal Stem Cells in Combination with Platelet Rich Plasma are Safe and Effective in the Therapy of Superficial Digital Flexor Tendonitis in the horse. Int. J. Immunopathol. Pharmacol. 2013, 26, 61–68. [Google Scholar] [CrossRef]
- Dyson, S.J. Medical management of superficial digital flexor tendonitis: A comparative study in 219 horses (1992–2000). Equine Vet. J. 2004, 36, 415–419. [Google Scholar] [CrossRef]
- Pacini, S.; Spinabella, S.; Trombi, L.; Fazzi, R.; Galimberti, S.; Dini, F.; Carlucci, F.; Petrini, M. Suspension of bone marrow-derived undifferentiated mesenchymal stromal cells for repair of superficial digital flexor tendon in race horses. Tissue Eng. 2007, 13, 2949–2955. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.K.W. Mesenchymal stem cell therapy for equine tendinopathy. Disabil. Rehabil. 2008, 30, 1752–1758. [Google Scholar] [CrossRef] [PubMed]
- Canapp, S.O.; Canapp, D.A.; Ibrahim, V.; Carr, B.J.; Cox, C.; Barrett, J.G. The Use of Adipose-Derived Progenitor Cells and Platelet-Rich Plasma Combination for the Treatment of Supraspinatus Tendinopathy in 55 Dogs: A Retrospective Study. Front. Vet. Sci. 2016, 3. [Google Scholar] [CrossRef] [PubMed]
- Canapp, S.O.; Leasure, C.S.; Cox, C.; Ibrahim, V.; Carr, B.J. Partial Cranial Cruciate Ligament Tears Treated with Stem Cell and Platelet-Rich Plasma Combination Therapy in 36 Dogs: A Retrospective Study. Front. Vet. Sci. 2016, 3. [Google Scholar] [CrossRef] [PubMed]
- Gibson, M.A.; Brown, S.G.; Brown, N.O. Semitendinosus myopathy and treatment with adipose-derived stem cells in working German shepherd police dogs. Can. Vet. J. Rev. Vet. Can. 2017, 58, 241–246. [Google Scholar]
- McDougall, R.A.; Canapp, S.O.; Canapp, D.A. Ultrasonographic Findings in 41 Dogs Treated with Bone Marrow Aspirate Concentrate and Platelet-Rich Plasma for a Supraspinatus Tendinopathy: A Retrospective Study. Front. Vet. Sci. 2018, 5. [Google Scholar] [CrossRef]
- Bari, E.; Perteghella, S.; Marrubini, G.; Sorrenti, M.; Catenacci, L.; Tripodo, G.; Mastrogiacomo, M.; Mandracchia, D.; Trapani, A.; Farago, S.; et al. In vitro efficacy of silk sericin microparticles and platelet lysate for intervertebral disk regeneration. Int. J. Biol. Macromol. 2018, 118, 792–799. [Google Scholar] [CrossRef]
- Steffen, F.; Smolders, L.A.; Roentgen, A.M.; Bertolo, A.; Stoyanov, J. Bone Marrow-Derived Mesenchymal Stem Cells as Autologous Therapy in Dogs with Naturally Occurring Intervertebral Disc Disease: Feasibility, Safety, and Preliminary Results. Tissue Eng. Part C Methods 2017, 23, 643–651. [Google Scholar] [CrossRef]
- Steffen, F.; Bertolo, A.; Affentranger, R.; Ferguson, S.J.; Stoyanov, J. Treatment of Naturally Degenerated Canine Lumbosacral Intervertebral Discs with Autologous Mesenchymal Stromal Cells and Collagen Microcarriers: A Prospective Clinical Study. Cell Transplant. 2019, 28, 201–211. [Google Scholar] [CrossRef]
- Sarmento, C.A.P.; Rodrigues, M.N.; Bocabello, R.Z.; Mess, A.M.; Miglino, M.A. Pilot study: Bone marrow stem cells as a treatment for dogs with chronic spinal cord injury. Regen. Med. Res. 2014, 2, 9. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, S.H.; Kim, W.H.; Kweon, O.-K. Transplantation of adipose derived mesenchymal stem cells for acute thoracolumbar disc disease with no deep pain perception in dogs. J. Vet. Sci. 2016, 17, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Besalti, O.; Can, P.; Akpinar, E.; Aktas, Z.; Elcin, A.E.; Elcin, Y.M. Intraspinal Transplantation of Autologous Neurogenically-Induced Bone Marrow-Derived Mesenchymal Stem Cells in the Treatment of Paraplegic Dogs without Deep Pain Perception Secondary to Intervertebral Disk Disease. Turk. Neurosurg. 2015, 25, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Russell, W.M.S.; Burch, R.L. The Principles of Humane Experimental Technique; Methuen: Wheathampstead, UK, 1959. [Google Scholar]
- Reiner, A.T.; Witwer, K.W.; van Balkom, B.W.M.; de Beer, J.; Brodie, C.; Corteling, R.L.; Gabrielsson, S.; Gimona, M.; Ibrahim, A.G.; de Kleijn, D.; et al. Concise Review: Developing Best-Practice Models for the Therapeutic Use of Extracellular Vesicles. Stem Cells Transl. Med. 2017, 6, 1730–1739. [Google Scholar] [CrossRef] [PubMed]
- Laible, G.; Wei, J.; Wagner, S. Improving livestock for agriculture—Technological progress from random transgenesis to precision genome editing heralds a new era. Biotechnol. J. 2015, 10, 109–120. [Google Scholar] [CrossRef]
- Van Weeren, P.R.; Tryfonidou, M.A. Musculoskeletal health from the “One Medicine” perspective—What can we learn from large and.small animal models (with emphasis on articular.cartilage)? BMC Musculoskelet. Disord. 2015, 16, S6. [Google Scholar] [CrossRef][Green Version]
- Crivelli, B.; Chlapanidas, T.; Perteghella, S.; Lucarelli, E.; Pascucci, L.; Brini, A.T.; Ferrero, I.; Marazzi, M.; Pessina, A.; Torre, M.L.; et al. Mesenchymal stem/stromal cell extracellular vesicles: From active principle to next generation drug delivery system. J. Control. Release 2017, 262, 104–117. [Google Scholar] [CrossRef]
- Bari, E.; Ferrarotti, I.; Torre, M.L.; Corsico, A.G.; Perteghella, S. Mesenchymal stem/stromal cell secretome for lung regeneration: The long way through “pharmaceuticalization” for the best formulation. J. Control. Release 2019, 309, 11–24. [Google Scholar] [CrossRef]
- Bari, E.; Perteghella, S.; Catenacci, L.; Sorlini, M.; Croce, S.; Mantelli, M.; Avanzini, M.A.; Sorrenti, M.; Torre, M.L. Freeze-dried and GMP-compliant pharmaceuticals containing exosomes for acellular mesenchymal stromal cell immunomodulant therapy. Nanomedicine 2019, 14, 753–765. [Google Scholar] [CrossRef]
- Del Fattore, A.; Luciano, R.; Pascucci, L.; Goffredo, B.M.; Giorda, E.; Scapaticci, M.; Fierabracci, A.; Muraca, M. Immunoregulatory Effects of Mesenchymal Stem Cell-Derived Extracellular Vesicles on T Lymphocytes. Cell Transplant. 2015, 24, 2615–2627. [Google Scholar] [CrossRef]
- Capomaccio, S.; Cappelli, K.; Bazzucchi, C.; Coletti, M.; Gialletti, R.; Moriconi, F.; Passamonti, F.; Pepe, M.; Petrini, S.; Mecocci, S.; et al. Equine Adipose-Derived Mesenchymal Stromal Cells Release Extracellular Vesicles Enclosing Different Subsets of Small RNAs. Stem Cells Int. 2019, 2019, 4957806. [Google Scholar] [CrossRef]
- Malda, J.; Boere, J.; van de Lest, C.H.A.; van Weeren, P.R.; Wauben, A.H.M. Extracellular vesicles—New tool for joint repair and regeneration. Nat. Rev. Rheumatol. 2016, 12, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Pascucci, L.; Cocce, V.; Bonomi, A.; Ami, D.; Ceccarelli, P.; Ciusani, E.; Vigano, L.; Locatelli, A.; Sisto, F.; Doglia, S.M.; et al. Paclitaxel is incorporated by mesenchymal stromal cells and released in exosomes that inhibit in vitro tumor growth: A new approach for drug delivery. J. Control. Release 2014, 192, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Phinney, D.G.; Pittenger, M.F. Concise Review: MSC-Derived Exosomes for Cell-Free Therapy. Stem Cells 2017, 35, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Villatoro, A.J.; Alcoholado, C.; Martin-Astorga, M.C.; Fernandez, V.; Cifuentes, M.; Becerra, J. Comparative analysis and characterization of soluble factors and exosomes from cultured adipose tissue and bone marrow mesenchymal stem cells in canine species. Vet. Immunol. Immunopathol. 2019, 208, 6–15. [Google Scholar] [CrossRef]
- El-Tookhy, O.S.; Shamaa, A.A.; Shehab, G.G.; Abdallah, A.N.; Azzam, O.M. Histological Evaluation of Experimentally Induced Critical Size Defect Skin Wounds Using Exosomal Solution of Mesenchymal Stem Cells Derived Microvesicles. Int. J. Stem Cells 2017, 10, 144–153. [Google Scholar] [CrossRef]
- Lange-Consiglio, A.; Rossi, D.; Tassan, S.; Perego, R.; Cremonesi, F.; Parolini, O. Conditioned Medium from Horse Amniotic Membrane-Derived Multipotent Progenitor Cells: Immunomodulatory Activity In Vitro and First Clinical Application in Tendon and Ligament Injuries In Vivo. Stem Cells Dev. 2013, 22, 3015–3024. [Google Scholar] [CrossRef]
- Lange-Consiglio, A.; Romele, P.; Magatti, M.; Silini, A.; Idda, A.; Martino, N.A.; Cremonesi, F.; Parolini, O. Priming with inflammatory cytokines is not a prerequisite to increase immune-suppressive effects and responsiveness of equine amniotic mesenchymal stromal cells. Stem Cell Res. Ther. 2020, 11. [Google Scholar] [CrossRef]
- Bari, E.; Ferrarotti, I.; Di Silvestre, D.; Grisoli, P.; Barzon, V.; Balderacchi, A.; Torre, M.L.; Rossi, R.; Mauri, P.; Corsico, A.G.; et al. Adipose Mesenchymal Extracellular Vesicles as Alpha-1-Antitrypsin Physiological Delivery Systems for Lung Regeneration. Cells 2019, 8, 965. [Google Scholar] [CrossRef]
- Li, J.J.; Hosseini-Beheshti, E.; Grau, G.E.; Zreiqat, H.; Little, C.B. Stem Cell-Derived Extracellular Vesicles for Treating Joint Injury and Osteoarthritis. Nanomaterials 2019, 9, 261. [Google Scholar] [CrossRef]
- Ranganath, S.H.; Levy, O.; Inamdar, M.S.; Karp, J.M. Harnessing the Mesenchymal Stem Cell Secretome for the Treatment of Cardiovascular Disease. Cell Stem Cell 2012, 10, 244–258. [Google Scholar] [CrossRef]
- Han, C.; Sun, X.; Liu, L.; Jiang, H.; Shen, Y.; Xu, X.; Li, J.; Zhang, G.; Huang, J.; Lin, Z.; et al. Exosomes and Their Therapeutic Potentials of Stem Cells. Stem Cells Int. 2016, 2016, 7653489. [Google Scholar] [CrossRef] [PubMed]
- Pawitan, J.A. Prospect of Stem Cell Conditioned Medium in Regenerative Medicine. Biomed Res. Int. 2014, 2014, 965849. [Google Scholar] [CrossRef] [PubMed]
| Study | Animal | Study Design | Time | Outcome | Dosage |
|---|---|---|---|---|---|
| Carrade et al. [51] | Horses | Autologous vs Allogeneic placenta-derived MSCs | 0–72 h post-injection | Allogeneic MSCs did not provoke a systemic response, and the minimal inflammatory reaction was found to be similar to the autologous effect | 7.5 × 106 in 2 mL sterile injectable 0.9% NaCl |
| Shah et al. [52] | Dogs | Allogeneic adipose tissue (AD)-MSC | 10 weeks | Better quality of life also demonstrating the safety of the allogeneic treatment | Data not recorded |
| Guest et al. [53] | Horses | Autologous and Allogeneic progenitor cells (MPCs) purified from bone marrow And bone marrow supernatant alone | 10 or 34 days | Post-mortem examinations showed no visible cell-mediated immune response to allogeneic MPCs in any of the treated horses | 1 × 106 cells suspended in 0.5 mL of autologous bone marrow supernatant |
| Brandão et al. [54] | Horses | Autologous and allogeneic AD-MSCs and only PBS as a control group | 6 days | All groups presented mild pain sensitivity, there were no significant differences among the groups in the physical, morphological, thermography, and ultrasonography analyses. Also, the lameness analysis presented similar behaviour between the two cell-treated groups. Both allogeneic and autologous AD-MSCs did not induce a significant inflammatory response, although a higher number of T lymphocytes have been found in the allogeneic treatment. | 1 × 107 cells for each application resuspended in PBS. |
| Disease | Animal | Treatment | Route and Dosage | Outcome | Ref. |
|---|---|---|---|---|---|
| AO- hip | Dog (n = 8) | Autologous AD-MSCs in combination with plasma rich in growth factor (PRGF-Endoret) | Intra-articular injection of over 30 × 106 AD-MSC | Reduced of lameness and absence of side effects for all the period (six months). | [64] |
| AO- hip | Dogs (n = 15) | Autologous AD-MSCs alone | Intra-articular injection of over 30 × 106 AD-MSC | Reduced of lameness only in the first month (less than three months). | [65] |
| AO- hip | Dogs (n = 39) | Comparison between AD-MSCs versus PRGF | Intra-articular injection of 30 × 106 AD-MSC | Dog’s pain was reduced, physical function was improved, and no side effects were found. AD-MSC showed better results in the period considered (six months). | [67] |
| AO- hip (coxofemoral joints) | Dogs (n = 4) | Autologous AD-MSCs in phosphate-buffered saline (PBS) | Dogs received intra-articular injection of either suspension of 4.2–5 × 106 (depending on cell yield) AD-MSCs in 0.6 mL PBSor only 0.6 mL of PBS as a control group | Significant improvement in lameness compared to the control group in the considered period (three months). | [68] |
| AO- humeroradial (elbow) joints | Dogs (n = 14) | Autologous AD-MSCs in phosphate-buffered saline (PBS) | Dogs received an intra-articular injection of 3–5 × 106 (depending on cell yield) AD-MSC in 0.6 mL PBS | Significant improvement in lameness, range of motion, and pain on manipulation over time (six months). | [69] |
| OA-stifle injury (femoral condyles) | Horses (n = 10) | Autologous bone marrow (BM)-MSCs in hyaluronan (HA) | Intra-articular injection of either 20 × 106 BMSCs with 22 mg of HA or 22 mg of HA alone | In the period of 12 months: no evidence of clinically significant improvement but arthroscopic evaluation confirmed a significant increase in tissue repair. Immunohistochemical analysis demonstrated more aggrecan levels in the repaired tissue treated with BM-MSC. | [71] |
| OA | Horses (n = 16) | Comparison between autologous AD-MSC versus steroid drugs (Betamethasone) | Intra-articular injection of 3 groups: (1): 1 mL of AD-MSC in normal saline, at a concentration of 5 × 106 cells/mL (2): 1 mL of betamethasone (3): control untreated | No change in lameness at 30 days but reduced at 60 days. At the period of 180 days, improvement remained in AD-MSC group but not in the steroid group. In the control group, the level of lameness did not change. | [74] |
| OA- degenerated stifle, fetlock, pastern and coffin joints | Horses (n = 165) In detail: stifle (n = 30), fetlock (n = 58), pastern (n = 34) and coffin (n = 43) joints | Allogenic peripheral blood MSCs with or without chondrogenic induction in combination with PRP | Intra-articular injection. Dosage not stated | Considering 180 weeks period: no adverse effects were noticed, except for three patients. Already after six weeks, 45% (native MSCs) and 60% (chondrogenic-induced MSCs) of the treated patients returned to normality, and the beneficial effects further increased after 18 weeks (78% for native MSCs and 86% for chondrogenic induced MSCs). | [41] |
| OA-stifle injury (femorotibial lesions (meniscal, cartilage or ligamentous) | Horses (n = 33) | Autologous BM-MSCs post-surgery (arthroscopy) | Intra-articular injection of 15–20 × 106 BM-MSC in autologous serum/5% DMSO + HA compared to surgery alone | Considering 24 months of follow up: Improvements in ability were realised with BMSC treatment compared to surgery alone. | [72] |
| OA- degenerative fetlock joint disease | Horses (n = 75) | Allogeneic chondrogenic induced MSCs added to allogeneic plasma (EAP) | Intra-articular injection of 2 × 106 allogeneic chondrogenic induced MSCs with EAP | After long-term follow-up (one year), horses were returned to their previous level of work. | [75] |
| Disease | Animal | Treatment | Route and Dosage | Outcome | Ref. |
|---|---|---|---|---|---|
| TLI- superficial digital flexor tendon (SDFT) | Horses (n = 8) | AD-MSC suspended in platelet concentrate (PC) | Intralesional administration of 10 × 106 AD-MSC in in 1 mL of PC. 1 mL of PBS was used as a control group | After 16 weeks improvements were reported for AD-MSC group. In detail: decrease of the lesion progression and inflammatory reaction, better organization of collagen fibres, an increase of blood flow. No difference in terms of gene expression was found. | [80] |
| TLI- SDFT | Horses (n = 141, racehorses) | BM-MSC resuspended in their bone marrow supernatant | Intralesional BM-MSC injection was performed resuspending cells in their bone marrow supernatant at the concentration of 5 × 106 cells/mL. | Two years follow up: no side effects; the need for reinjury was lower than other published works. | [84] |
| TLI | Horses (n = 6) | Allogeneic AD-MSCs | Injection of 100 × 106 allogeneic AD-MSCs via atlanto-occipital (AO) and lumbosacral (LS) injection | AD-MSCs administration was safe. No alterations in blood and neurological examinations at any time (30 days) either with AO or LS injections. OA had better distribution. | [86] |
| TLI | Horses (n = 10) | Allogeneic AD-MSC compared with BM-MSC | Intravenous injections of three doses of 25 × 106 allogeneic AD-MSC and BM-MSC respectively | After the first injection, horses were followed up for 35 days. Evaluation was made on the inflammatory and immune response showing that repeated BM-MSC injection increased blood CD8+ T-cell numbers. | [85] |
| TLI- SDFT (forelimbs) | Horses (n = 12) | Comparison between autologous AD-MSC, BM-MSC, and platelet-rich plasma (PRP) | Injury injections of 20 × 106 BM-MSCs or AD-MSCs suspended in 7 mL of lactated Ringer’s solution (LRS), and 7 mL of PRP. 7mL of LRS was used as control | After 45 weeks, all treatments had beneficial effects, but in detail, data suggest BM-MSCs might be the better approach for tendon healing. | [88] |
| TLI- suspensory ligament (SL)or superficial digital flexor tendon (SDFT) lesion | Horses (SL n = 68) (SDFT n = 36) | Tenogenically induced allogeneic peripheral blood (PB)MSCs combined with PRP | intralesional injection of 1ml of PB-derived MSCs (containing 2-–3 × 106) with 1 mL of PRP | In two years, no adverse effects have been observed. At 12 weeks, results were convincing in lesions improvement where about 80% of both SL and SDFT groups went back to their previous performance. | [87] |
| TLI-SDFT | Horses (n = 11) | Autologous BM-MSC | Injections of at least 1 × 106 of BM-MSCs were re-suspended in 1.5 mL of autologous serum | Patients were back to their sports activities, without having suffered a re-injury | [91] |
| TLI- SDFT | Horses (n = 12) | Autologous BM-MSC suspended in 2 mL of BM supernatant | Implantation of 10 × 106 BM-MSCs were suspended in 2 mL of citrated BM supernatant. 2 mL of PBS were used as a control group | In six months, there were significant benefits in terms of safety and healing tendon process (reduced stiffness, histological showed better organization and reduction in re-injury rate). | [81] |
| TLI- supraspinatus tendinopathy | Dogs (n = 41) | BM-MSCs in combination with PRP | Ultrasound-guided intratendineous injection of BM-MSCs with PRP (1:1 ratio) | On 90 days post-treatment: in most cases, the fibre pattern and echogenicity have improved, while only a minority resolved fibre pattern and echogenicity abnormalities. | [96] |
| TLI- partial cranial cruciate ligament tear | Dogs (n = 36) 19 cases received BM-MSC while 17 cases received AD-MSC | Autologous BM-MSCs vs AD-MSCs were combined with platelet-rich plasma (PRP) in 1:1 ratio when injected. | 2–4 mL of BM-MSCs + PRP or 1–2 mL of AD-MSCs + PRP was injected intra-articularly into the stifle (volume depended on the dog’s size) | Neither treatment was superior to the other in terms of outcome (90 days). | [94] |
| Disease | Animal | Treatment | Route and dosage | Outcome | Ref. |
|---|---|---|---|---|---|
| IVDD | Dogs (n = 6) | Autologous BM-MSCs | Intradiscal injection of 2 × 106 BM-MSCs suspended in 1 mL 10% autologous plasma in PBS. Only PSB solution was used for group control | Twelve months after treatment: even if the injection was well tolerated with no side effects, no successful treatment was found in any dogs. | [98] |
| IVDD- lumbosacral | Dogs (n = 20) | Autologous BM-MSCs | Intradiscal injection of 3 × 106 was applied in three different groups—(1) intradiscal injection of MSC-microcarriers (n = 11), (2) MSC-TGF-β1-microcarriers (n = 6), and (3) microcarriers only during a decompressing spinal surgery (n = 3) | Ten months after treatment: injection was successful in all dogs; thus, they returned to normality. Schmorl’s nodes were found as side effects. | [99] |
| IVDD-chronic spinal cord injury | Dogs (n = 7) | Allogeneic foetal BM-MSCs | Intramedullary injection of 1 × 106 allogeneic BM-MSCs | Ninety days evaluation showed no side effects, increased movement of the hind limbs, and increased locomotory function. | [100] |
| IVDD- Thoracolumbar intervertebral disc disease | Dogs (n = 34) | Allogeneic AD-MSCs + surgery | Intraoperative intraspinal allogeneic AD-MSCs of 1 × 107 cells | Six months after treatment: Improvement in the neurological exam and better endpoint with AD-MSCs application rather than surgery only. | [101] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mocchi, M.; Dotti, S.; Del Bue, M.; Villa, R.; Bari, E.; Perteghella, S.; Torre, M.L.; Grolli, S. Veterinary Regenerative Medicine for Musculoskeletal Disorders: Can Mesenchymal Stem/Stromal Cells and Their Secretome Be the New Frontier? Cells 2020, 9, 1453. https://doi.org/10.3390/cells9061453
Mocchi M, Dotti S, Del Bue M, Villa R, Bari E, Perteghella S, Torre ML, Grolli S. Veterinary Regenerative Medicine for Musculoskeletal Disorders: Can Mesenchymal Stem/Stromal Cells and Their Secretome Be the New Frontier? Cells. 2020; 9(6):1453. https://doi.org/10.3390/cells9061453
Chicago/Turabian StyleMocchi, Michela, Silvia Dotti, Maurizio Del Bue, Riccardo Villa, Elia Bari, Sara Perteghella, Maria Luisa Torre, and Stefano Grolli. 2020. "Veterinary Regenerative Medicine for Musculoskeletal Disorders: Can Mesenchymal Stem/Stromal Cells and Their Secretome Be the New Frontier?" Cells 9, no. 6: 1453. https://doi.org/10.3390/cells9061453
APA StyleMocchi, M., Dotti, S., Del Bue, M., Villa, R., Bari, E., Perteghella, S., Torre, M. L., & Grolli, S. (2020). Veterinary Regenerative Medicine for Musculoskeletal Disorders: Can Mesenchymal Stem/Stromal Cells and Their Secretome Be the New Frontier? Cells, 9(6), 1453. https://doi.org/10.3390/cells9061453







