Cuprizone Affects Hypothermia-Induced Neuroprotection and Enhanced Neuroblast Differentiation in the Gerbil Hippocampus after Ischemia
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
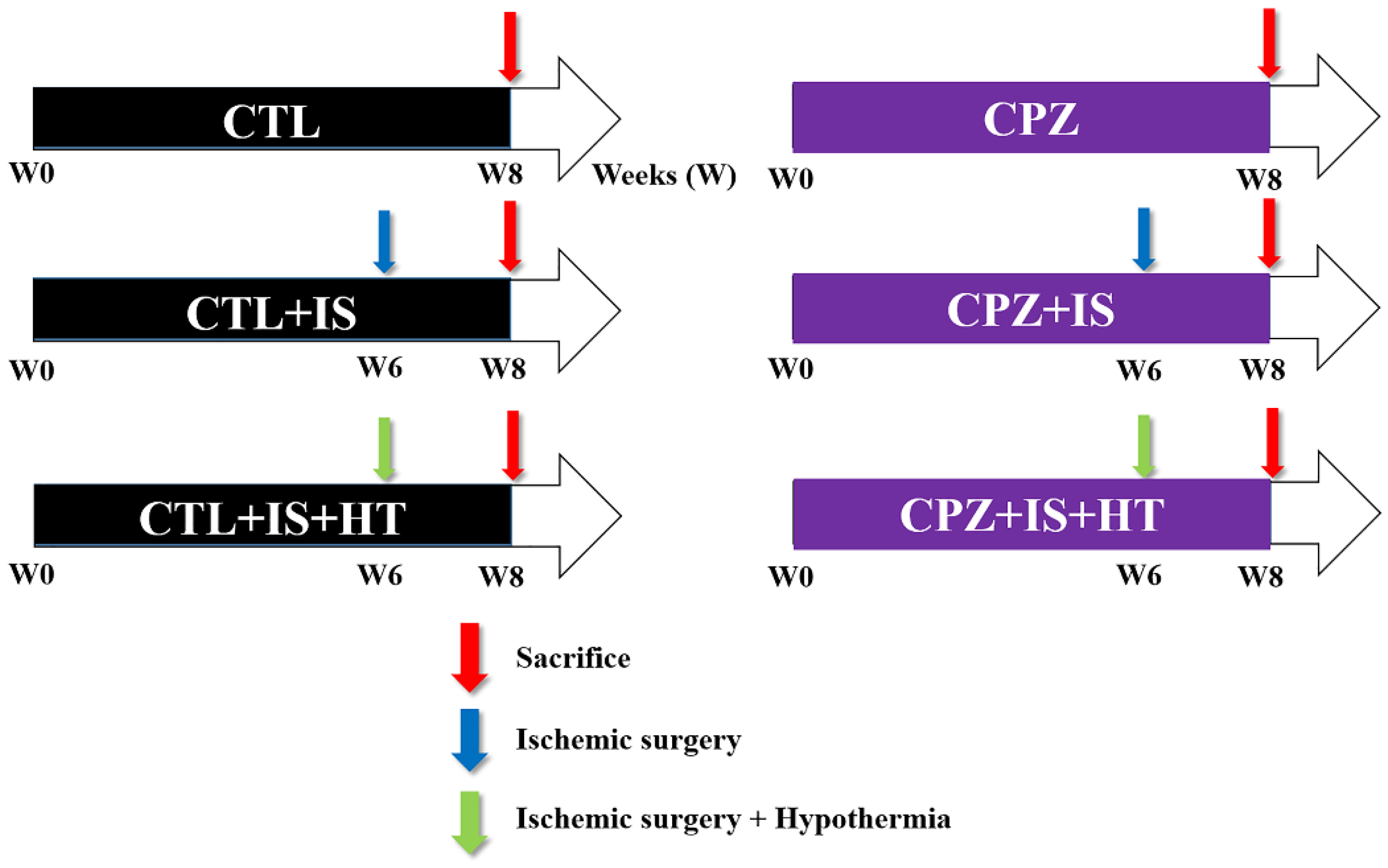
2.2. Experimental Groups and Treatments
2.3. Induction of Transient Forebrain Ischemia
2.4. Tissue Processing
2.5. Cresyl Violet Staining
2.6. Immunohistochemistry for Glial Fibrillary Acidic Protein (GFAP), Ionized Calcium-Binding Adapter Molecule 1 (Iba-1), MBP, Mac3, Ki67, and Doublecortin (DCX)
2.7. Enzyme Immunoassay for Cytokines
2.8. Semi-Quantification of Data
2.9. Statistical Analysis
3. Results
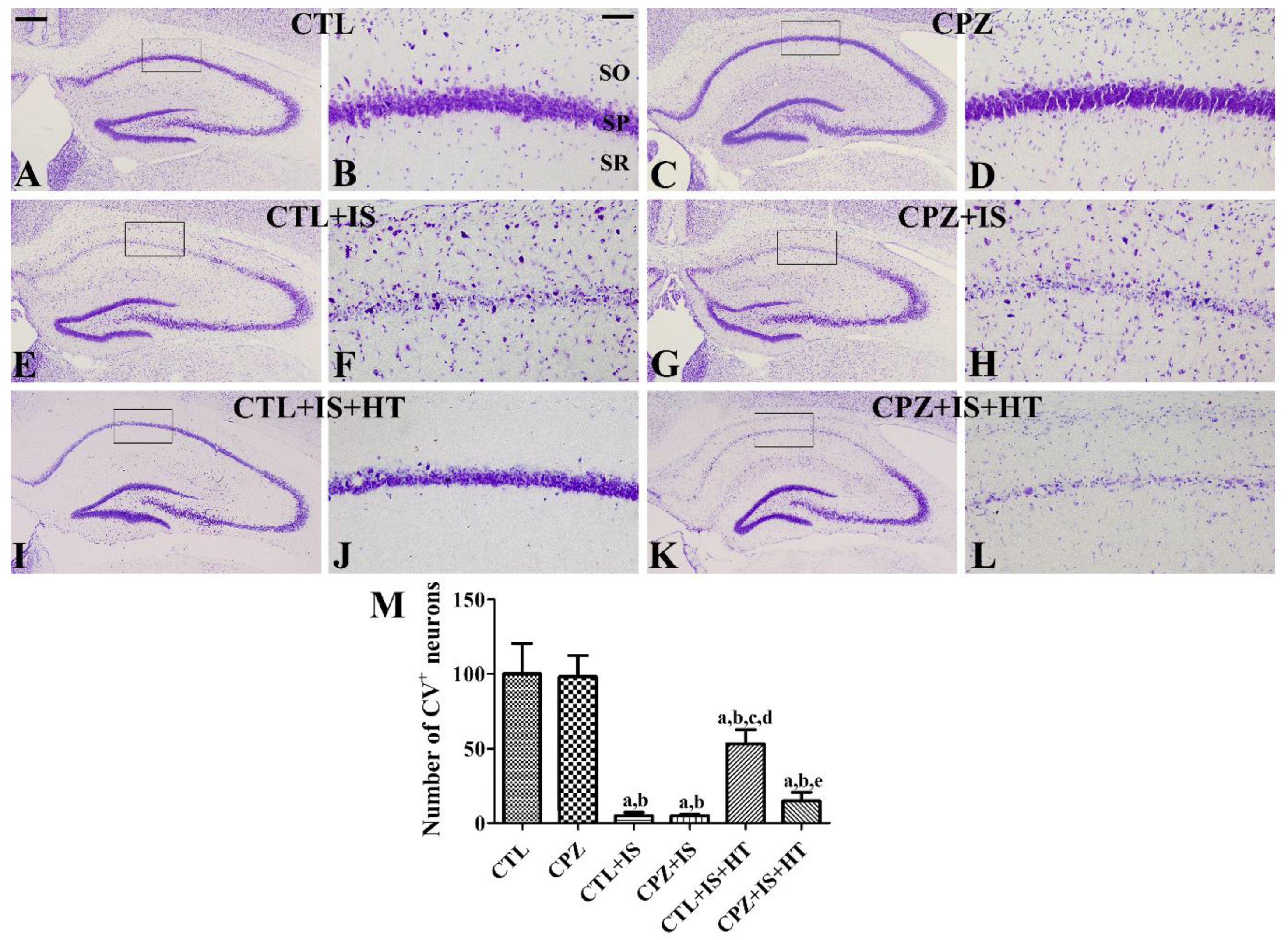
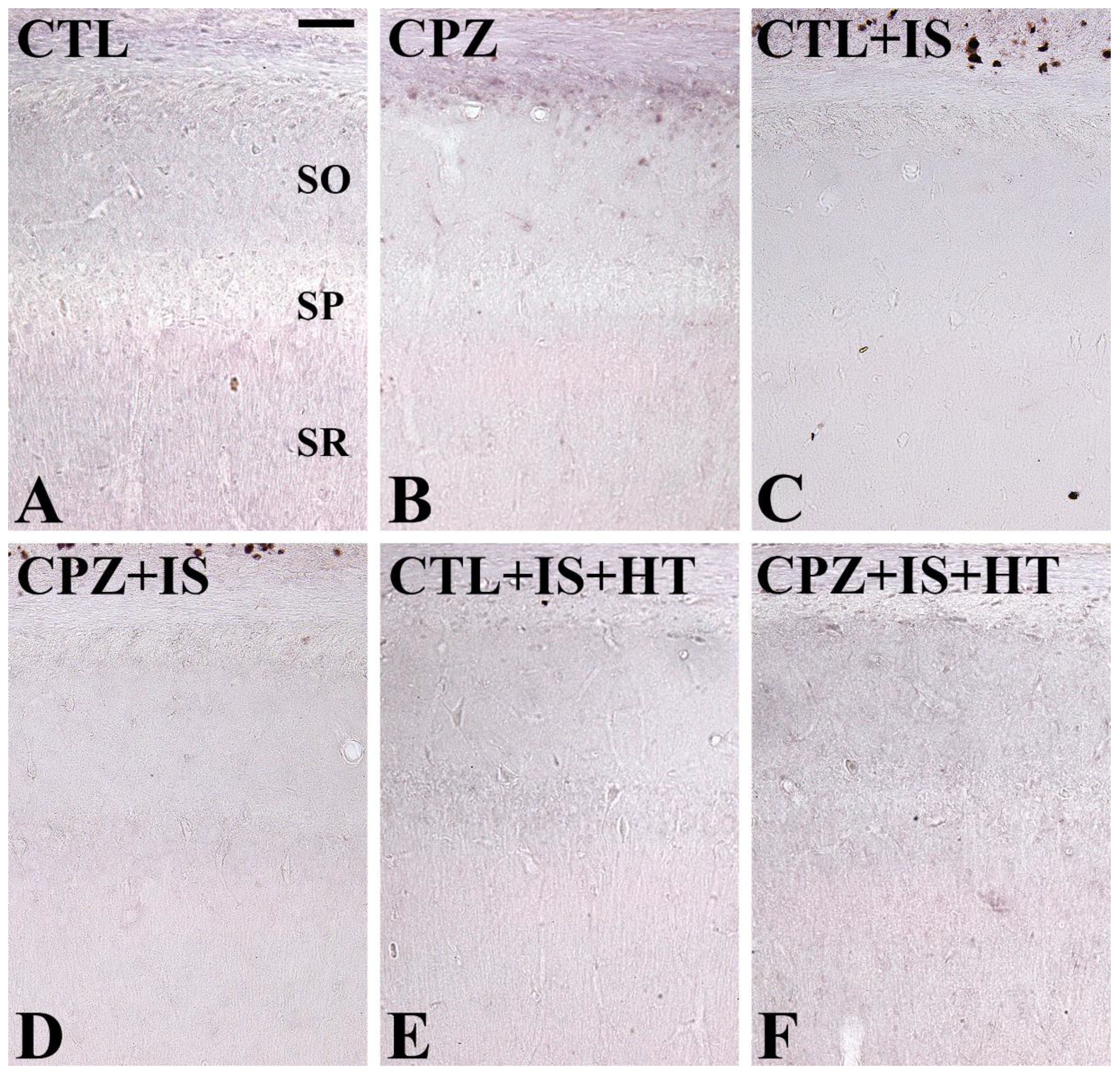
3.1. Effects of Cuprizone on Cell Survival after Ischemia in Normothermic and Hypothermic Gerbils
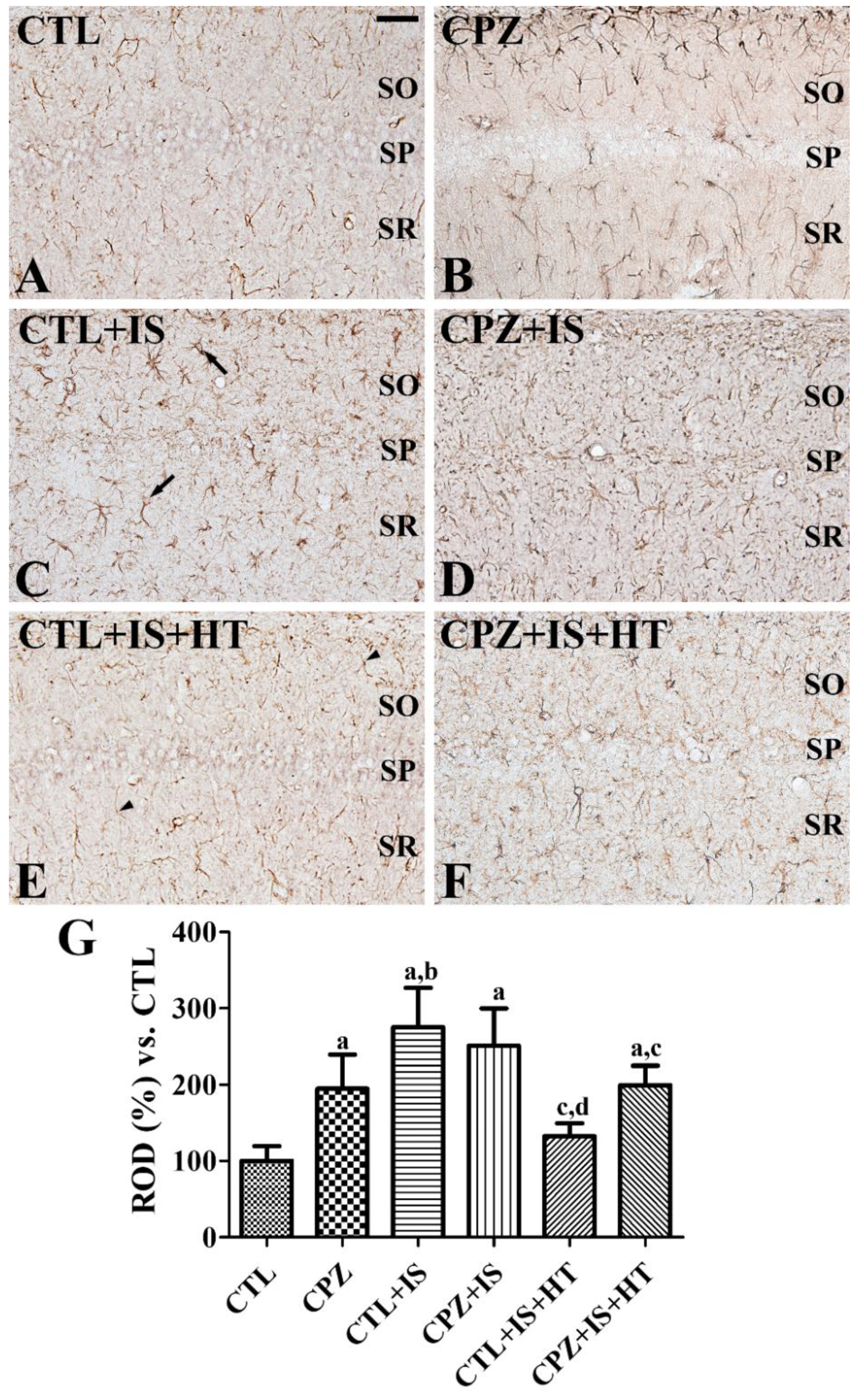
3.2. Effects of Cuprizone on Reactive Astrocytosis after Ischemia in Normothermic and Hypothermic Gerbils
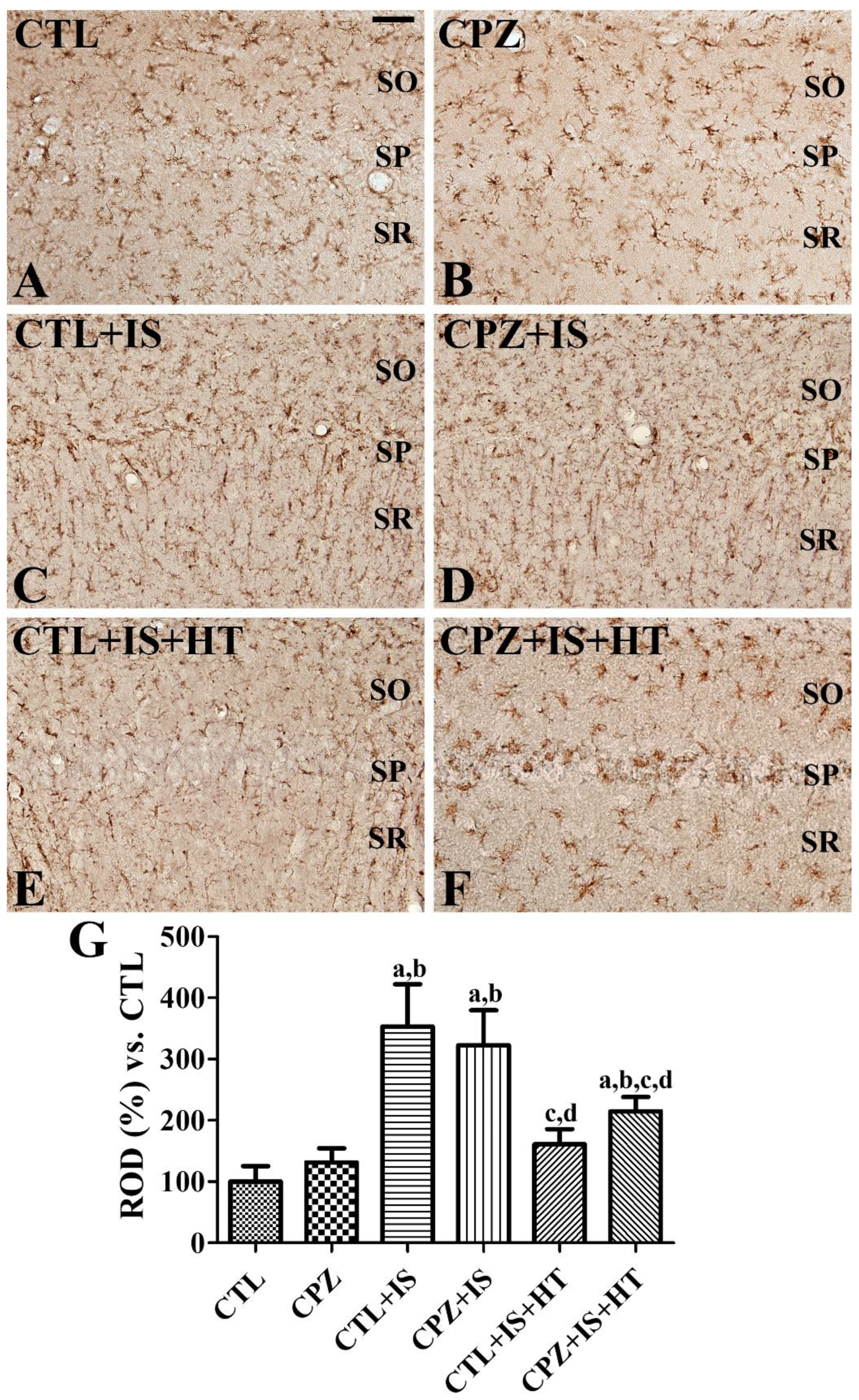
3.3. Effects of Cuprizone on Reactive Microgliosis after Ischemia in Normothermic and Hypothermic Gerbils
3.4. Effects of Cuprizone on Macrophages after Ischemia in Normothermic and Hypothermic Gerbils
3.5. Effects of Cuprizone on MBP Expression after Ischemia in Normothermic and Hypothermic Gerbils
3.6. Effects of Cuprizone on IL-1β and TNF-α after Ischemia in Normothermic and Hypothermic Gerbils
3.7. Effects of Cuprizone on Proliferating Cells after Ischemia in Normothermic and Hypothermic Gerbils
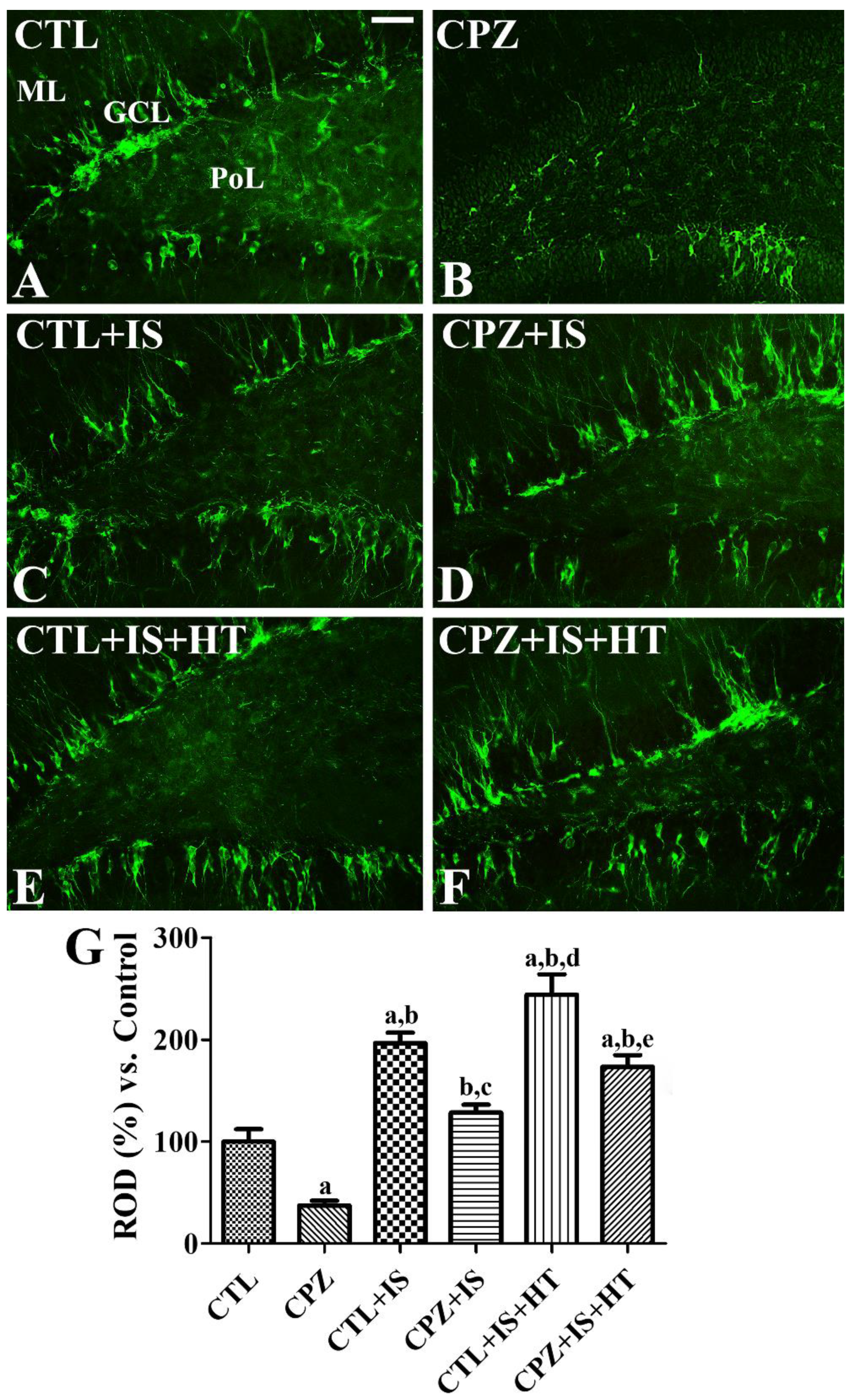
3.8. Effects of Cuprizone on Differentiated Neuroblasts after Ischemia in Normothermic and Hypothermic Gerbils
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Soler, E.P.; Ruiz, V.C. Epidemiology and risk factors of cerebral ischemia and ischemic heart diseases: Similarities and differences. Curr. Cardiol. Rev. 2010, 6, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Kirino, T.; Sano, K. Selective vulnerability in the gerbil hippocampus following transient ischemia. Acta Neuropathol. 1984, 62, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.S.; Polsky, K.; Nadler, J.V.; Crain, B.J. Selective neocortical and thalamic cell death in the gerbil after transient ischemia. Neuroscience 1990, 35, 289–299. [Google Scholar] [CrossRef]
- Petito, C.K.; Torres-Munoz, J.; Roberts, B.; Olarte, J.P.; Nowak, T.S., Jr.; Pulsinelli, W.A. DNA fragmentation follows delayed neuronal death in CA1 neurons exposed to transient global ischemia in the rat. J. Cereb. Blood Flow Metab. 1997, 17, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Moskowitz, M.A.; Lo, E.H.; Iadecola, C. The science of stroke: Mechanisms in search of treatments. Neuron 2010, 67, 181–198. [Google Scholar] [CrossRef] [PubMed]
- Selakovic, V.; Korenic, A.; Radenovic, L. Spatial and temporal patterns of oxidative stress in the brain of gerbils submitted to different duration of global cerebral ischemia. Int. J. Dev. Neurosci. 2011, 29, 645–654. [Google Scholar] [CrossRef]
- Wang, Q.; Tompkins, K.D.; Simonyi, A.; Korthuis, R.J.; Sun, A.Y.; Sun, G.Y. Apocynin protects against global cerebral ischemia-reperfusion-induced oxidative stress and injury in the gerbil hippocampus. Brain Res. 2006, 1090, 182–189. [Google Scholar] [CrossRef]
- Won, M.H.; Kang, T.C.; Jeon, G.S.; Lee, J.C.; Kim, D.Y.; Choi, E.M.; Lee, K.H.; Choi, C.D.; Chung, M.H.; Cho, S.S. Immunohistochemical detection of oxidative DNA damage induced by ischemia-reperfusion insults in gerbil hippocampus in vivo. Brain Res. 1999, 836, 70–78. [Google Scholar] [CrossRef]
- Green, D.R.; Reed, J.C. Mitochondria and apoptosis. Science 1998, 281, 1309–1312. [Google Scholar] [CrossRef]
- Gross, A.; McDonnell, J.M.; Korsmeyer, S.J. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999, 13, 1899–1911. [Google Scholar] [CrossRef]
- Eum, W.S.; Kim, D.W.; Hwang, I.K.; Yoo, K.Y.; Kang, T.C.; Jang, S.H.; Choi, H.S.; Choi, S.H.; Kim, Y.H.; Kim, S.Y.; et al. In vivo protein transduction: Biologically active intact pep-1-superoxide dismutase fusion protein efficiently protects against ischemic insult. Free Radic. Biol. Med. 2004, 37, 1656–1669. [Google Scholar] [PubMed]
- Hwang, I.K.; Eum, W.S.; Yoo, K.Y.; Cho, J.H.; Kim, D.W.; Choi, S.H.; Kang, T.C.; Kwon, O.S.; Kang, J.H.; Choi, S.Y.; et al. Copper chaperone for Cu,Zn-SOD supplement potentiates the Cu,Zn-SOD function of neuroprotective effects against ischemic neuronal damage in the gerbil hippocampus. Free Radic. Biol. Med. 2005, 39, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.J.; Yoo, D.Y.; Kim, D.W.; Yeo, H.J.; Cho, S.B.; Hyeon, J.; Park, J.H.; Park, J.; Eum, W.S.; Hwang, H.S.; et al. Neuroprotective effect of PEP-1-peroxiredoxin2 on CA1 regions in the hippocampus against ischemic insult. Biochim. Biophys. Acta 2014, 1840, 2321–2330. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Hwang, I.K.; Yoo, D.Y.; Eum, W.S.; Kim, D.W.; Shin, M.J.; Ahn, E.H.; Jo, H.S.; Ryu, E.J.; Yong, J.I.; et al. Tat-antioxidant 1 protects against stress-induced hippocampal HT-22 cells death and attenuate ischaemic insult in animal model. J. Cell. Mol. Med. 2015, 19, 1333–1345. [Google Scholar] [CrossRef]
- Colbourne, F.; Grooms, S.Y.; Zukin, R.S.; Buchan, A.M.; Bennett, M.V. Hypothermia rescues hippocampal CA1 neurons and attenuates down-regulation of the AMPA receptor GluR2 subunit after forebrain ischemia. Proc. Natl. Acad. Sci. USA 2003, 100, 2906–2910. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.S.; Asai, S.; Ishikawa, K.; Nishida, Y.; Nagata, T.; Takahashi, Y. Global profiling of influence of intra-ischemic brain temperature on gene expression in rat brain. Brain Res. Rev. 2008, 58, 171–191. [Google Scholar] [CrossRef]
- Noguchi, K.; Matsumoto, N.; Shiozaki, T.; Tasaki, O.; Ogura, H.; Kuwagata, Y.; Sugimoto, H.; Seiyama, A. Effects of timing and duration of hypothermia on survival in an experimental gerbil model of global ischaemia. Resuscitation 2011, 82, 481–486. [Google Scholar] [CrossRef]
- Takeda, Y.; Namba, K.; Higuchi, T.; Hagioka, S.; Takata, K.; Hirakawa, M.; Morita, K. Quantitative evaluation of the neuroprotective effects of hypothermia ranging from 34 °C to 31 °C on brain ischemia in gerbils and determination of the mechanism of neuroprotection. Crit. Care Med. 2003, 31, 255–260. [Google Scholar] [CrossRef]
- Yenari, M.A.; Han, H.S. Neuroprotective mechanisms of hypothermia in brain ischaemia. Nat. Rev. Neurosci. 2012, 13, 267–278. [Google Scholar] [CrossRef]
- Tissier, R.; Chenoune, M.; Pons, S.; Zini, R.; Darbera, L.; Lidouren, F.; Ghaleh, B.; Berdeaux, A.; Morin, D. Mild hypothermia reduces per-ischemic reactive oxygen species production and preserves mitochondrial respiratory complexes. Resuscitation 2013, 84, 249–255. [Google Scholar] [CrossRef]
- Silasi, G.; Colbourne, F. Therapeutic hypothermia influences cell genesis and survival in the rat hippocampus following global ischemia. J. Cereb. Blood Flow Metab. 2011, 31, 1725–1735. [Google Scholar] [CrossRef] [PubMed]
- Xiong, M.; Cheng, G.Q.; Ma, S.M.; Yang, Y.; Shao, X.M.; Zhou, W.H. Post-ischemic hypothermia promotes generation of neural cells and reduces apoptosis by Bcl-2 in the striatum of neonatal rat brain. Neurochem. Int. 2011, 58, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Sandu, R.E.; Uzoni, A.; Ciobanu, O.; Moldovan, M.; Anghel, A.; Radu, E.; Coogan, A.N.; Popa-Wagner, A. Post-stroke gaseous hypothermia increases vascular density but not neurogenesis in the ischemic penumbra of aged rats. Restor. Neurol. Neurosci. 2016, 34, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Praet, J.; Guglielmetti, C.; Berneman, Z.; Van der Linden, A.; Ponsaerts, P. Cellular and molecular neuropathology of the cuprizone mouse model: Clinical relevance for multiple sclerosis. Neurosci. Biobehav. Rev. 2014, 47, 485–505. [Google Scholar] [CrossRef]
- Kim, W.; Hahn, K.R.; Jung, H.Y.; Kwon, H.J.; Nam, S.M.; Kim, J.W.; Park, J.H.; Yoo, D.Y.; Kim, D.W.; Won, M.H.; et al. Melatonin ameliorates cuprizone-induced reduction of hippocampal neurogenesis, brain-derived neurotrophic factor, and phosphorylation of cyclic AMP response element-binding protein in the mouse dentate gyrus. Brain Behav. 2019, 9, e01388. [Google Scholar] [CrossRef]
- Koutsoudaki, P.N.; Skripuletz, T.; Gudi, V.; Moharregh-Khiabani, D.; Hildebrandt, H.; Trebst, C.; Stangel, M. Demyelination of the hippocampus is prominent in the cuprizone model. Neurosci. Lett. 2009, 451, 83–88. [Google Scholar] [CrossRef]
- Venturini, G. Enzymic activities and sodium, potassium and copper concentrations in mouse brain and liver after cuprizone treatment in vivo. J. Neurochem. 1973, 21, 1147–1151. [Google Scholar] [CrossRef]
- Faizi, M.; Salimi, A.; Seydi, E.; Naserzadeh, P.; Kouhnavard, M.; Rahimi, A.; Pourahmad, J. Toxicity of cuprizone a Cu2+ chelating agent on isolated mouse brain mitochondria: A justification for demyelination and subsequent behavioral dysfunction. Toxicol. Mech. Methods 2016, 26, 276–283. [Google Scholar] [CrossRef]
- Sanadgol, N.; Golab, F.; Askari, H.; Moradi, F.; Ajdary, M.; Mehdizadeh, M. Alpha-lipoic acid mitigates toxic-induced demyelination in the corpus callosum by lessening of oxidative stress and stimulation of polydendrocytes proliferation. Metab. Brain Dis. 2018, 33, 27–37. [Google Scholar] [CrossRef]
- Wang, P.; Tian, W.W.; Song, J.; Guan, Y.F.; Miao, C.Y. Deficiency of NG2+ cells contributes to the susceptibility of stroke-prone spontaneously hypertensive rats. CNS Neurosci. Ther. 2011, 17, 327–332. [Google Scholar] [CrossRef]
- Buschmann, J.P.; Berger, K.; Awad, H.; Clarner, T.; Beyer, C.; Kipp, M. Inflammatory response and chemokine expression in the white matter corpus callosum and gray matter cortex region during cuprizone-induced demyelination. J. Mol. Neurosci. 2012, 48, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Yoo, D.Y.; Cho, S.B.; Jung, H.Y.; Kim, W.; Lee, K.Y.; Kim, J.W.; Moon, S.M.; Won, M.H.; Choi, J.H.; Yoon, Y.S.; et al. Protein disulfide-isomerase A3 significantly reduces ischemia-induced damage by reducing oxidative and endoplasmic reticulum stress. Neurochem. Int. 2019, 122, 19–30. [Google Scholar] [CrossRef]
- Loskota, W.J.; Lomax, P.; Verity, M.A. Stereotaxic Atlas of the Mongolian Gerbil Brain (Meriones unguiculatus); Ann Arbor Science Publishers Inc.: Ann Arbor, MI, USA, 1974. [Google Scholar]
- Saito, K.; Suyama, K.; Nishida, K.; Sei, Y.; Basile, A.S. Early increases in TNF-α, IL-6 and IL-1β levels following transient cerebral ischemia in gerbil brain. Neurosci. Lett. 1996, 206, 149–152. [Google Scholar] [CrossRef]
- Moon, S.M.; Choi, G.M.; Yoo, D.Y.; Jung, H.Y.; Yim, H.S.; Kim, D.W.; Hwang, I.K.; Cho, B.M.; Chang, I.B.; Cho, S.M.; et al. Differential effects of pioglitazone in the hippocampal CA1 region following transient forebrain ischemia in low- and high-fat diet-fed gerbils. Neurochem. Res. 2015, 40, 1063–1073. [Google Scholar] [CrossRef]
- Abe, H.; Tanaka, T.; Kimura, M.; Mizukami, S.; Saito, F.; Imatanaka, N.; Akahori, Y.; Yoshida, T.; Shibutani, M. Cuprizone decreases intermediate and late-stage progenitor cells in hippocampal neurogenesis of rats in a framework of 28-day oral dose toxicity study. Toxicol. Appl. Pharmacol. 2015, 287, 210–221. [Google Scholar] [CrossRef] [PubMed]
- Tanuma, N.; Sakuma, H.; Sasaki, A.; Matsumoto, Y. Chemokine expression by astrocytes plays a role in microglia/macrophage activation and subsequent neurodegeneration in secondary progressive multiple sclerosis. Acta Neuropathol. 2006, 112, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Ayers, M.M.; Catmull, D.V.; Hazelwood, L.J.; Bernard, C.C.; Orian, J.M. Astrocyte-associated axonal damage in pre-onset stages of experimental autoimmune encephalomyelitis. Glia 2005, 51, 235–240. [Google Scholar] [CrossRef]
- An, J.; Yin, J.J.; He, Y.; Sui, R.X.; Miao, Q.; Wang, Q.; Yu, J.Z.; Yu, J.W.; Shi, F.D.; Ma, C.G.; et al. Temporal and spatial dynamics of astroglial reaction and immune response in cuprizone-induced demyelination. Neurotox. Res. 2020, 37, 587–601. [Google Scholar] [CrossRef]
- Ohgomori, T.; Jinno, S. Cuprizone-induced demyelination in the mouse hippocampus is alleviated by phytoestrogen genistein. Toxicol. Appl. Pharmacol. 2019, 363, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Nack, A.; Brendel, M.; Nedelcu, J.; Daerr, M.; Nyamoya, S.; Beyer, C.; Focke, C.; Deussing, M.; Hoornaert, C.; Ponsaerts, P.; et al. Expression of translocator protein and [18F]-GE180 ligand uptake in multiple sclerosis animal models. Cells 2019, 8, 94. [Google Scholar] [CrossRef]
- Norkute, A.; Hieble, A.; Braun, A.; Johann, S.; Clarner, T.; Baumgartner, W.; Beyer, C.; Kipp, M. Cuprizone treatment induces demyelination and astrocytosis in the mouse hippocampus. J. Neurosci. Res. 2009, 87, 1343–1355. [Google Scholar] [CrossRef] [PubMed]
- Koo, E.; Sheldon, R.A.; Lee, B.S.; Vexler, Z.S.; Ferriero, D.M. Effects of therapeutic hypothermia on white matter injury from murine neonatal hypoxia-ischemia. Pediatr. Res. 2017, 82, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Webster, C.M.; Kelly, S.; Koike, M.A.; Chock, V.Y.; Giffard, R.G.; Yenari, M.A. Inflammation and NFkappaB activation is decreased by hypothermia following global cerebral ischemia. Neurobiol. Dis. 2009, 33, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Evans, A.T. Effect of hypothermia on microglial reaction in ischemic brain. Neuroreport 1997, 8, 947–950. [Google Scholar] [CrossRef] [PubMed]
- Nitatori, T.; Sato, N.; Waguri, S.; Karasawa, Y.; Araki, H.; Shibanai, K.; Kominami, E.; Uchiyama, Y. Delayed neuronal death in the CA1 pyramidal cell layer of the gerbil hippocampus following transient ischemia is apoptosis. J. Neurosci. 1995, 15, 1001–1011. [Google Scholar] [CrossRef]
- Noorzehi, G.; Pasbakhsh, P.; Borhani-Haghighi, M.; Kashani, I.R.; Madadi, S.; Tahmasebi, F.; Nekoonam, S.; Azizi, M. Microglia polarization by methylprednizolone acetate accelerates cuprizone induced demyelination. J. Mol. Histol. 2018, 49, 471–479. [Google Scholar] [CrossRef]
- Ingersoll, S.A.; Martin, C.B.; Barnum, S.R.; Martin, B.K. CNS-specific expression of C3a and C5a exacerbate demyelination severity in the cuprizone model. Mol. Immunol. 2010, 48, 219–230. [Google Scholar] [CrossRef]
- Zhou, D.; Li, P.; Lin, Y.; Lott, J.M.; Hislop, A.D.; Canaday, D.H.; Brutkiewicz, R.R.; Blum, J.S. Lamp-2a facilitates MHC class II presentation of cytoplasmic antigens. Immunity 2005, 22, 571–581. [Google Scholar] [CrossRef]
- Valdor, R.; Mocholi, E.; Botbol, Y.; Guerrero-Ros, I.; Chandra, D.; Koga, H.; Gravekamp, C.; Cuervo, A.M.; Macian, F. Chaperone-mediated autophagy regulates T cell responses through targeted degradation of negative regulators of T cell activation. Nat. Immunol. 2014, 15, 1046–1054. [Google Scholar] [CrossRef] [PubMed]
- Kramann, N.; Menken, L.; Hayardeny, L.; Hanisch, U.K.; Brück, W. Laquinimod prevents cuprizone-induced demyelination independent of Toll-like receptor signaling. Neurol. Neuroimmunol. Neuroinflamm. 2016, 3, e233. [Google Scholar] [CrossRef]
- Abdullah, A.; Zhang, M.; Frugier, T.; Bedoui, S.; Taylor, J.M.; Crack, P.J. STING-mediated type-I interferons contribute to the neuroinflammatory process and detrimental effects following traumatic brain injury. J. Neuroinflamm. 2018, 15, 323. [Google Scholar] [CrossRef] [PubMed]
- Taoufik, E.; Valable, S.; Müller, G.J.; Roberts, M.L.; Divoux, D.; Tinel, A.; Voulgari-Kokota, A.; Tseveleki, V.; Altruda, F.; Lassmann, H.; et al. FLIPL protects neurons against in vivo ischemia and in vitro glucose deprivation-induced cell death. J. Neurosci. 2007, 27, 6633–6646. [Google Scholar] [CrossRef]
- Kurumatani, T.; Kudo, T.; Ikura, Y.; Takeda, M. White matter changes in the gerbil brain under chronic cerebral hypoperfusion. Stroke 1998, 29, 1058–1062. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Dawson, G. Hypoxia induces synthesis of a novel 22-kDa protein in neonatal rat oligodendrocytes. J. Neurochem. 1992, 59, 1709–1716. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.Q.; Liu, X.R.; Zhao, J.Y.; Yan, F.; Wang, R.L.; Wen, S.H.; Wang, L.; Luo, Y.M.; Ji, X.M. Brain-selective mild hypothermia promotes long-term white matter integrity after ischemic stroke in mice. CNS Neurosci. Ther. 2018, 24, 1275–1285. [Google Scholar] [CrossRef] [PubMed]
- Aryanpour, R.; Pasbakhsh, P.; Zibara, K.; Namjoo, Z.; Beigi Boroujeni, F.; Shahbeigi, S.; Kashani, I.R.; Beyer, C.; Zendehdel, A. Progesterone therapy induces an M1 to M2 switch in microglia phenotype and suppresses NLRP3 inflammasome in a cuprizone-induced demyelination mouse model. Int. Immunopharmacol. 2017, 51, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.J.; He, Y.; An, J.; Miao, Q.; Sui, R.X.; Wang, Q.; Yu, J.Z.; Xiao, B.G.; Ma, C.G. Dynamic balance of microglia and astrocytes involved in the remyelinating effect of ginkgolide B. Front. Cell. Neurosci. 2020, 13, 572. [Google Scholar] [CrossRef]
- Han, H.S.; Yenari, M.A. Cellular targets of brain inflammation in stroke. Curr. Opin. Investig. Drugs 2003, 4, 522–529. [Google Scholar]
- Pinteaux, E.; Rothwell, N.J.; Boutin, H. Neuroprotective actions of endogenous interleukin-1 receptor antagonist (IL-1ra) are mediated by glia. Glia 2006, 53, 551–556. [Google Scholar] [CrossRef]
- Yang, G.Y.; Gong, C.; Qin, Z.; Ye, W.; Mao, Y.; Bertz, A.L. Inhibition of TNFα attenuates infarct volume and ICAM-1 expression in ischemic mouse brain. Neuroreport 1998, 9, 2131–2134. [Google Scholar] [CrossRef]
- Yang, G.Y.; Gong, C.; Qin, Z.; Liu, X.H.; Betz, A.L. Tumor necrosis factor alpha expression produces increased blood-brain barrier permeability following temporary focal cerebral ischemia in mice. Mol. Brain Res. 1999, 69, 135–143. [Google Scholar] [CrossRef]
- Arvidsson, A.; Collin, T.; Kirik, D.; Kokaia, Z.; Lindvall, O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat. Med. 2002, 8, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Lichtenwalner, R.J.; Parent, J.M. Adult neurogenesis and the ischemic forebrain. J. Cereb. Blood Flow Metab. 2006, 26, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Solway, K.; Messing, R.O.; Sharp, F.R. Increased neurogenesis in the dentate gyrus after transient global ischemia in gerbils. J. Neurosci. 1998, 18, 7768–7778. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Kim, Y.; Ro, E.J.; Ho, C.; Lee, D.; Trapp, B.D.; Suh, H. Hippocampal neurogenesis and neural circuit formation in a cuprizone-induced multiple sclerosis mouse model. J. Neurosci. 2020, 40, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.; Zhang, Z.; Barnett, A.; Bellinger, T.J.; Turcato, F.; Schmidt, K.; Luo, Y. Cuprizone-induced demyelination under physiological and post-stroke condition leads to decreased neurogenesis response in adult mouse brain. Exp. Neurol. 2020, 326, 113168. [Google Scholar] [CrossRef]
- Choi, J.H.; Yoo, K.Y.; Lee, C.H.; Park, J.H.; Yan, B.C.; Kwon, S.H.; Seo, J.Y.; Cho, J.H.; Hwang, I.K.; Won, M.H. Comparison of neurogenesis in the dentate gyrus between the adult and aged gerbil following transient global cerebral ischemia. Neurochem. Res. 2012, 37, 802–810. [Google Scholar] [CrossRef]
- Iwai, M.; Sato, K.; Omori, N.; Nagano, I.; Manabe, Y.; Shoji, M.; Abe, K. Three steps of neural stem cells development in gerbil dentate gyrus after transient ischemia. J. Cereb. Blood Flow Metab. 2002, 22, 411–419. [Google Scholar] [CrossRef]
- Spaccapelo, L.; Galantucci, M.; Neri, L.; Contri, M.; Pizzala, R.; D’Amico, R.; Ottani, A.; Sandrini, M.; Zaffe, D.; Giuliani, D.; et al. Up-regulation of the canonical Wnt-3A and Sonic hedgehog signaling underlies melanocortin-induced neurogenesis after cerebral ischemia. Eur. J. Pharmacol. 2013, 707, 78–86. [Google Scholar] [CrossRef]
- Yoo, D.Y.; Kim, W.; Nam, S.M.; Chung, J.Y.; Choi, J.H.; Yoon, Y.S.; Won, M.H.; Hwang, I.K. Chronic effects of pyridoxine in the gerbil hippocampal CA1 region after transient forebrain ischemia. Neurochem. Res. 2012, 37, 1011–1018. [Google Scholar] [CrossRef]
- Abe, H.; Tanaka, T.; Kimura, M.; Mizukami, S.; Imatanaka, N.; Akahori, Y.; Yoshida, T.; Shibutani, M. Developmental exposure to cuprizone reduces intermediate-stage progenitor cells and cholinergic signals in the hippocampal neurogenesis in rat offspring. Toxicol. Lett. 2015, 234, 180–193. [Google Scholar] [CrossRef] [PubMed]
- Lasarzik, I.; Winkelheide, U.; Thal, S.C.; Benz, N.; Lörscher, M.; Jahn-Eimermacher, A.; Werner, C.; Engelhard, K. Mild hypothermia has no long-term impact on postischemic neurogenesis in rats. Anesth. Analg. 2009, 109, 1632–1639. [Google Scholar] [CrossRef] [PubMed]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, W.; Hahn, K.R.; Jung, H.Y.; Kwon, H.J.; Nam, S.M.; Kim, T.H.; Kim, J.W.; Yoo, D.Y.; Kim, D.W.; Choi, J.H.; et al. Cuprizone Affects Hypothermia-Induced Neuroprotection and Enhanced Neuroblast Differentiation in the Gerbil Hippocampus after Ischemia. Cells 2020, 9, 1438. https://doi.org/10.3390/cells9061438
Kim W, Hahn KR, Jung HY, Kwon HJ, Nam SM, Kim TH, Kim JW, Yoo DY, Kim DW, Choi JH, et al. Cuprizone Affects Hypothermia-Induced Neuroprotection and Enhanced Neuroblast Differentiation in the Gerbil Hippocampus after Ischemia. Cells. 2020; 9(6):1438. https://doi.org/10.3390/cells9061438
Chicago/Turabian StyleKim, Woosuk, Kyu Ri Hahn, Hyo Young Jung, Hyun Jung Kwon, Sung Min Nam, Tae Hyeong Kim, Jong Whi Kim, Dae Young Yoo, Dae Won Kim, Jung Hoon Choi, and et al. 2020. "Cuprizone Affects Hypothermia-Induced Neuroprotection and Enhanced Neuroblast Differentiation in the Gerbil Hippocampus after Ischemia" Cells 9, no. 6: 1438. https://doi.org/10.3390/cells9061438
APA StyleKim, W., Hahn, K. R., Jung, H. Y., Kwon, H. J., Nam, S. M., Kim, T. H., Kim, J. W., Yoo, D. Y., Kim, D. W., Choi, J. H., Yoon, Y. S., & Hwang, I. K. (2020). Cuprizone Affects Hypothermia-Induced Neuroprotection and Enhanced Neuroblast Differentiation in the Gerbil Hippocampus after Ischemia. Cells, 9(6), 1438. https://doi.org/10.3390/cells9061438





