RNA-Sequencing-Based Transcriptomic Analysis Reveals a Role for Annexin-A1 in Classical and Influenza A Virus-Induced Autophagy
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Viruses
2.3. Cell Culture
2.4. A549-ΔANXA1 Cell Line Generation
2.5. Total RNA Extraction, Library Construction and RNA-Sequencing and Data Analysis
2.6. Gene Ontology (GO) and Gene Set Enrichment Analysis (GSEA) Pathway Analysis
2.7. Western Blot
2.8. Confocal Microscopy
2.9. Influenza A Virus (IAV) Infection
2.10. Statistical Analysis
3. Results
3.1. Detection of CRISPR-Cas9-Induced ANXA1 Mutations in A549 ∆ANXA1 Cells
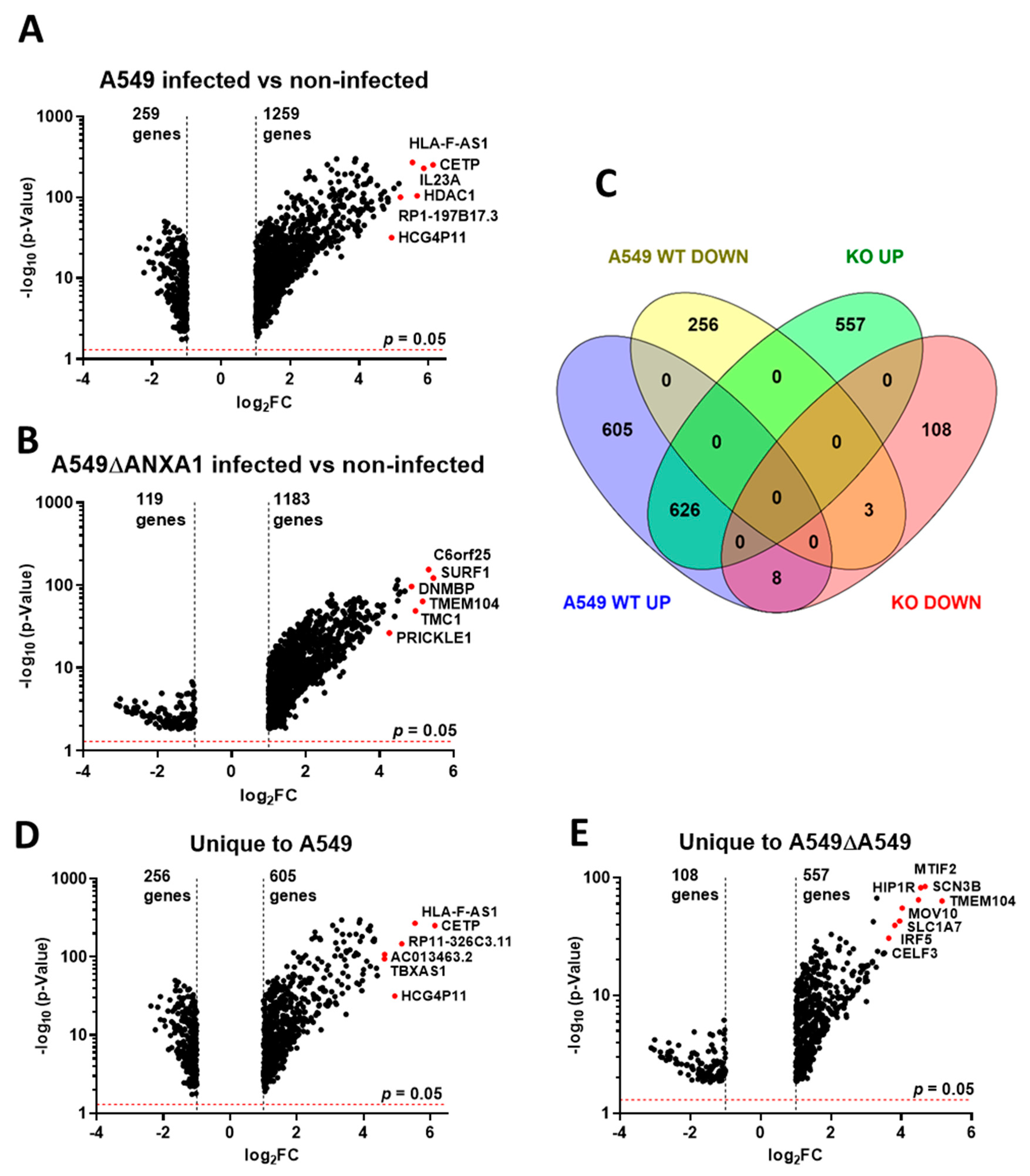
Transcript Expression Profiling in Influenza Virus-Infected A549 Parental and ∆ANXA1 Cells
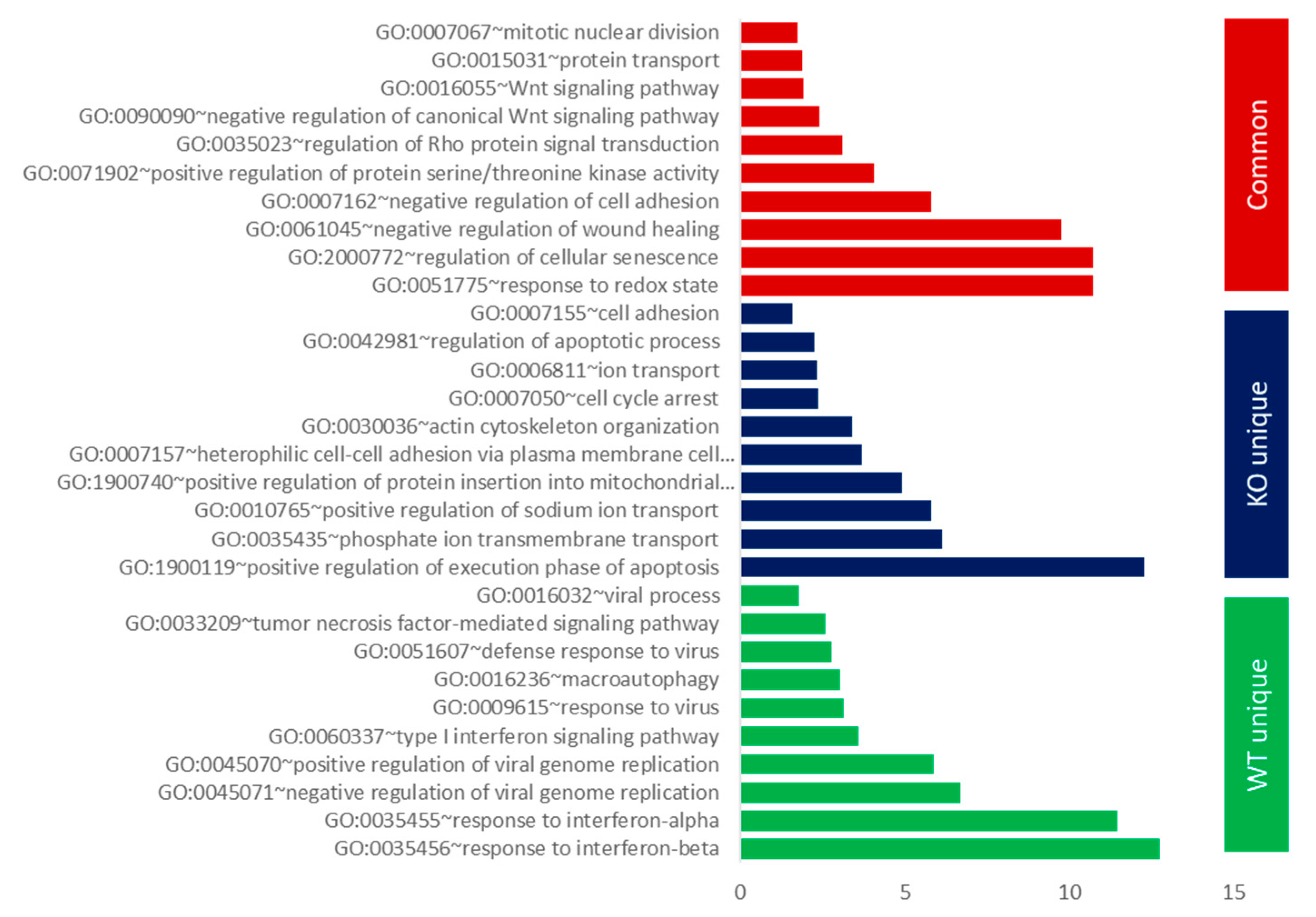
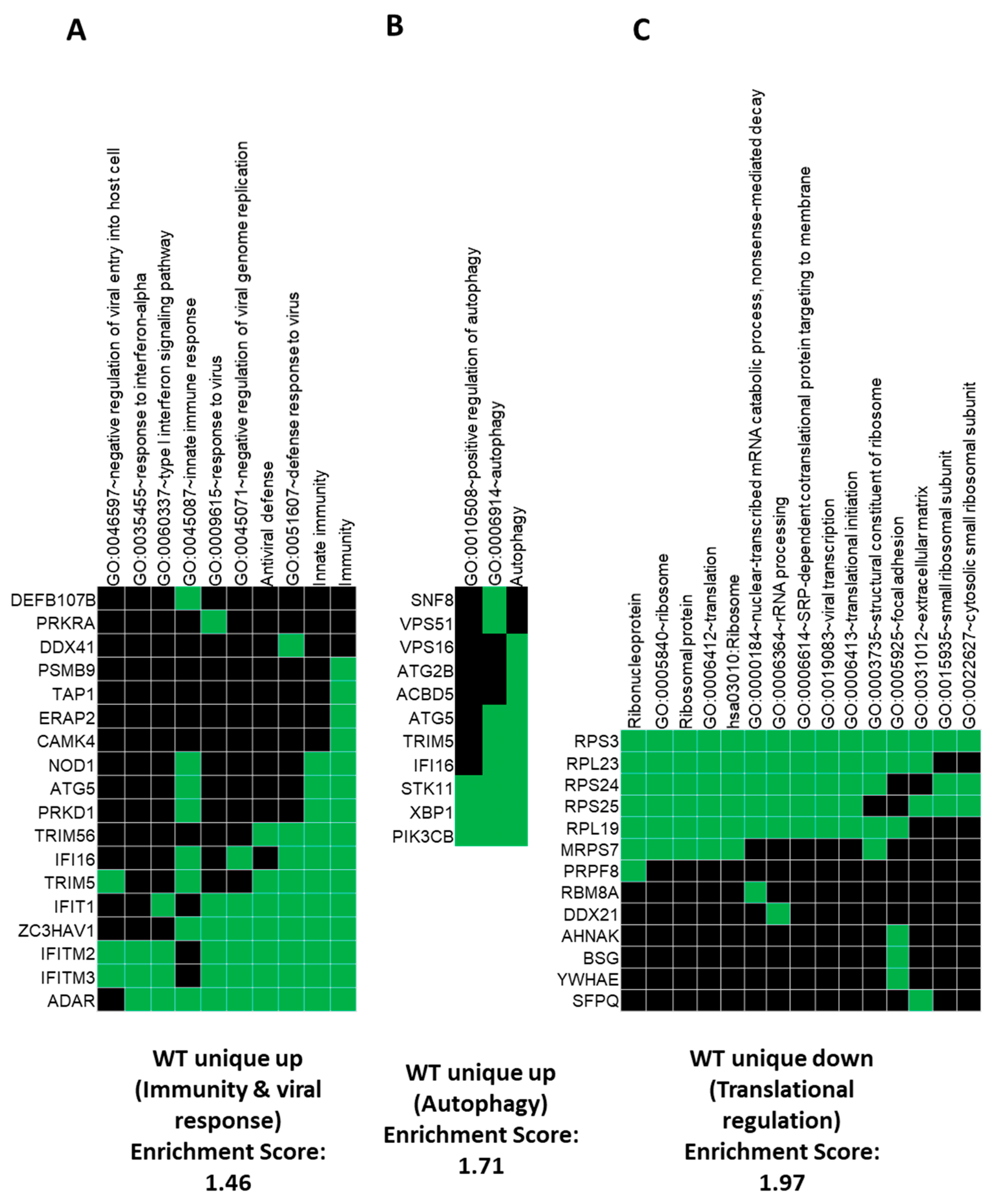
3.2. Gene Ontology (GO) and GSEA Pathway Analysis
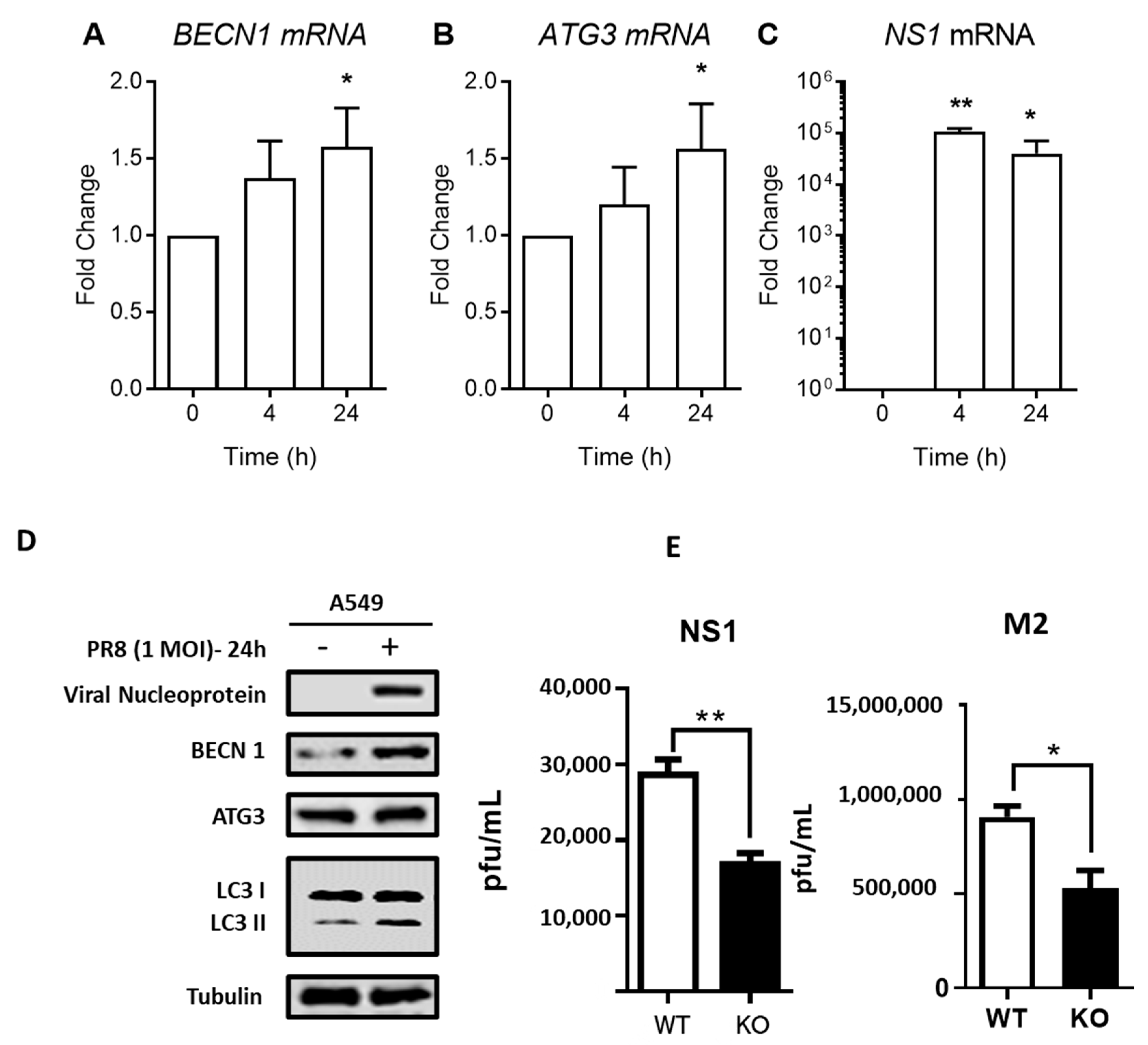
3.3. IAV Enhances Autophagy through Regulation of Autophagic Proteins
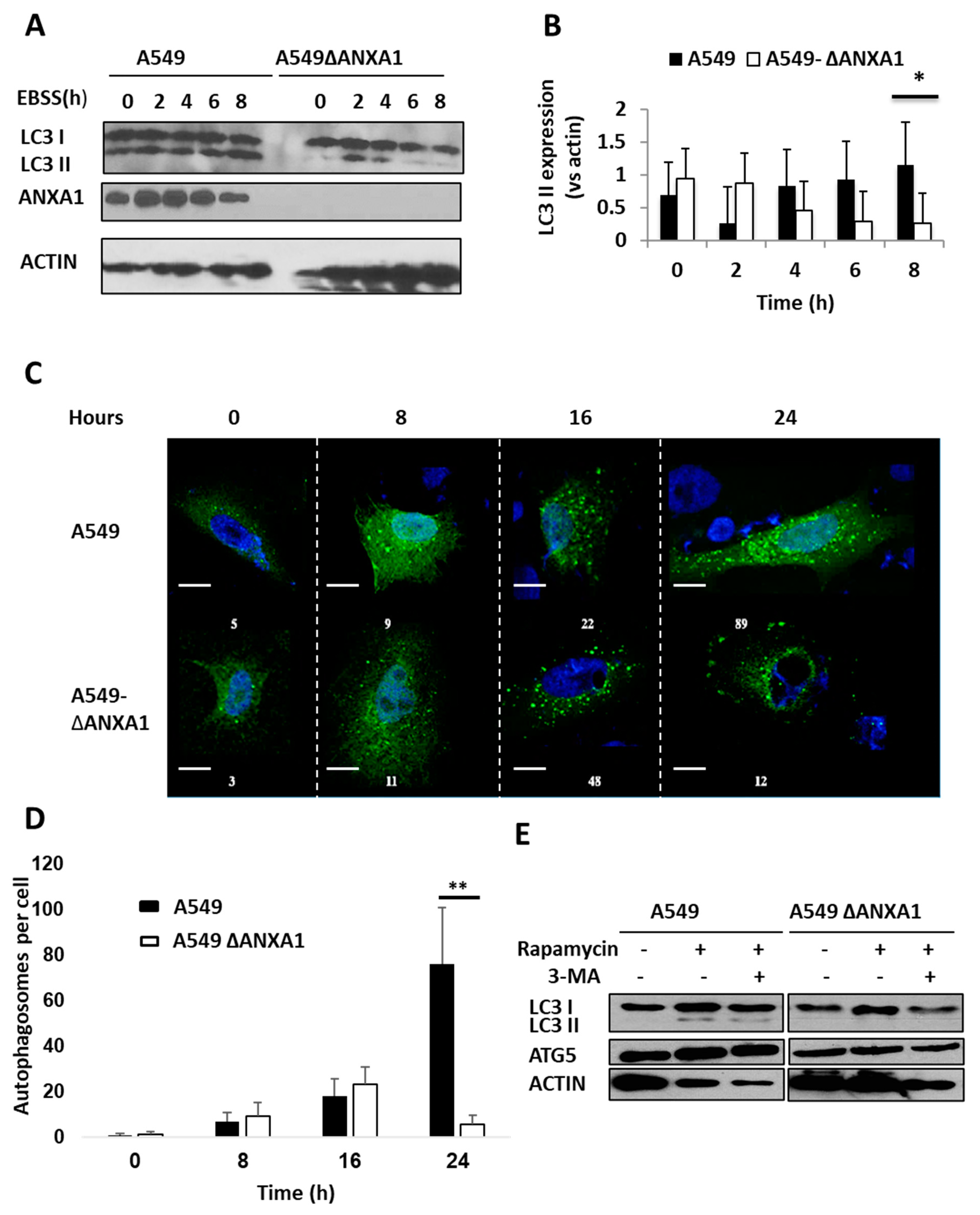
3.4. Silencing ANXA1 Results in the Reduction of Autophagosomes and Autophagy after Starvation and Inhibition of mTOR
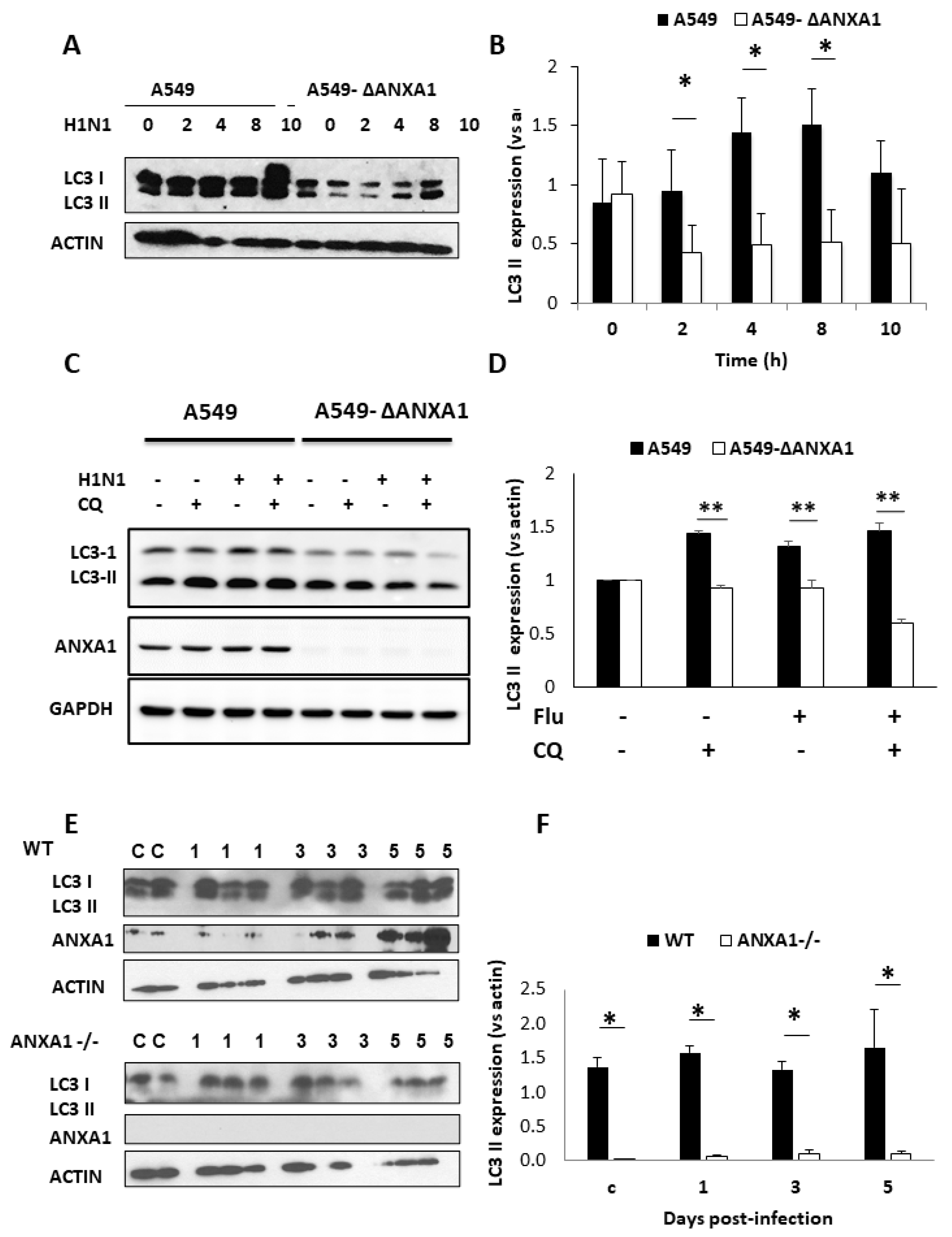
3.5. ANXA1 Is Involved in Autophagy Induced by Influenza Virus In Vitro and In Vivo
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lamb, R. Orthomyxoviridae: The viruses and their replication. Fields Virol. 2001, 1353–1395. [Google Scholar]
- Wang, S.; Li, H.; Chen, Y.; Wei, H.; Gao, G.F.; Liu, H.; Huang, S.; Chen, J.-L. Transport of influenza a virus neuraminidase (na) to host cell surface is regulated by arhgap21 and cdc42. J. Biol. Chem. 2012, 287, 9804–9816. [Google Scholar] [CrossRef] [PubMed]
- Gannagé, M.; Dormann, D.; Albrecht, R.; Dengjel, J.; Torossi, T.; Rämer, P.C.; Lee, M.; Strowig, T.; Arrey, F.; Conenello, G. Matrix protein 2 of influenza a virus blocks autophagosome fusion with lysosomes. Cell Host Microbe 2009, 6, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Hale, B.G.; Randall, R.E.; Ortín, J.; Jackson, D. The multifunctional ns1 protein of influenza a viruses. J. Gen. Virol. 2008, 89, 2359–2376. [Google Scholar] [CrossRef]
- Wei, H.; Wang, S.; Chen, Q.; Chen, Y.; Chi, X.; Zhang, L.; Huang, S.; Gao, G.F.; Chen, J.-L. Suppression of interferon lambda signaling by socs-1 results in their excessive production during influenza virus infection. PLoS Pathog. 2014, 10, e1003845. [Google Scholar] [CrossRef] [PubMed]
- Palese, P.; Shaw, M. Fields Virology, 5th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2006. [Google Scholar]
- Klionsky, D.J.; Abdelmohsen, K.; Abe, A.; Abedin, M.J.; Abeliovich, H.; Acevedo Arozena, A.; Adachi, H.; Adams, C.M.; Adams, P.D.; Adeli, K. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy 2016, 12, 1–222. [Google Scholar] [CrossRef]
- Dreux, M.; Chisari, F.V. Viruses and the autophagy machinery. Cell Cycle 2010, 9, 1295–1307. [Google Scholar] [CrossRef]
- Choi, Y.; Bowman, J.W.; Jung, J.U. Autophagy during viral infection-a double-edged sword. Nat. Rev. Microbiol. 2018, 16, 341–354. [Google Scholar] [CrossRef]
- Abdoli, A.; Alirezaei, M.; Mehrbod, P.; Forouzanfar, F. Autophagy: The multi-purpose bridge in viral infections and host cells. Rev. Med. Virol. 2018, 28, e1973. [Google Scholar] [CrossRef]
- Zhang, R.; Chi, X.; Wang, S.; Qi, B.; Yu, X.; Chen, J.-L. The regulation of autophagy by influenza a virus. Biomed. Res. Int. 2014, 2014. [Google Scholar] [CrossRef]
- Ma, J.; Sun, Q.; Mi, R.; Zhang, H. Avian influenza a virus h5n1 causes autophagy-mediated cell death through suppression of mtor signaling. J. Genet. Genom. 2011, 38, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Beale, R.; Wise, H.; Stuart, A.; Ravenhill, B.J.; Digard, P.; Randow, F. A lc3-interacting motif in the influenza a virus m2 protein is required to subvert autophagy and maintain virion stability. Cell Host Microbe 2014, 15, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Jiang, X.; Liu, D.; Fan, Z.; Hu, X.; Yan, J.; Wang, M.; Gao, G.F. Autophagy is involved in influenza a virus replication. Autophagy 2009, 5, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Klionsky, D.J. Mammalian autophagy: Core molecular machinery and signaling regulation. Curr. Opin. Cell Biol. 2010, 22, 124–131. [Google Scholar] [CrossRef]
- Mizushima, N.; Yoshimori, T.; Levine, B. Methods in mammalian autophagy research. Cell 2010, 140, 313–326. [Google Scholar] [CrossRef]
- Raynal, P.; Pollard, H.B. Annexins: The problem of assessing the biological role for a gene family of multifunctional calcium-and phospholipid-binding proteins. Biochim. Biophys. Acta (Bba)-Rev. Biomembr. 1994, 1197, 63–93. [Google Scholar] [CrossRef]
- Rosengarth, A.; Gerke, V.; Luecke, H. X-ray structure of full-length annexin 1 and implications for membrane aggregation1. J. Mol. Biol. 2001, 306, 489–498. [Google Scholar] [CrossRef]
- Parente, L.; Solito, E. Annexin 1: More than an anti-phospholipase protein. Inflamm. Res. 2004, 53, 125–132. [Google Scholar] [CrossRef]
- Foo, S.L.; Yap, G.; Cui, J.; Lim, L.H.K. Annexin-a1—A blessing or a curse in cancer? Trends Mol. Med. 2019, 25, 315–327. [Google Scholar] [CrossRef]
- Arora, S.; Lim, W.; Bist, P.; Perumalsamy, R.; Lukman, H.M.; Li, F.; Welker, L.B.; Yan, B.; Sethi, G.; Tambyah, P.A.; et al. Influenza a virus enhances its propagation through the modulation of annexin-a1 dependent endosomal trafficking and apoptosis. Cell Death Differ. 2016, 23, 1243. [Google Scholar] [CrossRef]
- Katoh, N. Detection of annexins i and iv in bronchoalveolar lavage fluids from calves inoculated with bovine herpes virus-1. J. Vet. Med. Sci. 2000, 62, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. Star: Ultrafast universal rna-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Anders, S.; Pyl, P.T.; Huber, W. Htseq-a python framework to work with high-throughput sequencing data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. Edger: A bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Cradick, T.J.; Qiu, P.; Lee, C.M.; Fine, E.J.; Bao, G. Cosmid: A web-based tool for identifying and validating crispr/cas off-target sites. Mol. Ther.-Nucleic Acids 2014, 3, e214. [Google Scholar] [CrossRef] [PubMed]
- Mans, L.A.; Querol Cano, L.; van Pelt, J.; Giardoglou, P.; Keune, W.-J.; Haramis, A.-P.G. The tumor suppressor lkb1 regulates starvation-induced autophagy under systemic metabolic stress. Sci. Rep. 2017, 7, 7327. [Google Scholar] [CrossRef]
- Kishino, A.; Hayashi, K.; Hidai, C.; Masuda, T.; Nomura, Y.; Oshima, T. Xbp1-foxo1 interaction regulates er stress-induced autophagy in auditory cells. Sci. Rep. 2017, 7, 4442. [Google Scholar] [CrossRef]
- Kim, J.; Kundu, M.; Viollet, B.; Guan, K.-L. Ampk and mtor regulate autophagy through direct phosphorylation of ulk1. Nat. Cell Biol. 2011, 13, 132. [Google Scholar] [CrossRef]
- Ballou, L.M.; Lin, R.Z. Rapamycin and mtor kinase inhibitors. J. Chem. Biol. 2008, 1, 27–36. [Google Scholar] [CrossRef]
- Shang, L.; Chen, S.; Du, F.; Li, S.; Zhao, L.; Wang, X. Nutrient starvation elicits an acute autophagic response mediated by ulk1 dephosphorylation and its subsequent dissociation from ampk. Proc. Natl. Acad. Sci. USA 2011, 108, 4788–4793. [Google Scholar] [CrossRef]
- Bist, P.; Shu, S.; Lee, H.; Arora, S.; Nair, S.; Lim, J.Y.; Dayalan, J.; Gasser, S.; Biswas, S.K.; Fairhurst, A.M.; et al. Annexin-a1 regulates tlr-mediated ifn-beta production through an interaction with tank-binding kinase 1. J. Immunol. 2013, 191, 4375–4382. [Google Scholar] [CrossRef]
- Feizi, N.; Mehrbod, P.; Romani, B.; Soleimanjahi, H.; Bamdad, T.; Feizi, A.; Jazaeri, E.O.; Targhi, H.S.; Saleh, M.; Jamali, A.; et al. Autophagy induction regulates influenza virus replication in a time-dependent manner. J. Med. Microbiol. 2017, 66, 536–541. [Google Scholar] [CrossRef]
- Yeganeh, B.; Ghavami, S.; Rahim, M.N.; Klonisch, T.; Halayko, A.J.; Coombs, K.M. Autophagy activation is required for influenza a virus-induced apoptosis and replication. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 364–378. [Google Scholar] [CrossRef]
- Wang, R.; Zhu, Y.; Zhao, J.; Ren, C.; Li, P.; Chen, H.; Jin, M.; Zhou, H. Autophagy promotes replication of influenza a virus in vitro. J. Virol. 2019, 93. [Google Scholar] [CrossRef]
- Kuehnl, A.; Musiol, A.; Raabe, C.A.; Rescher, U. Emerging functions as host cell factors—An encyclopedia of annexin-pathogen interactions. Biol. Chem. 2016, 397, 949–959. [Google Scholar] [CrossRef]
- Kang, J.-H.; Li, M.; Chen, X.; Yin, X.-M. Proteomics analysis of starved cells revealed annexin a1 as an important regulator of autophagic degradation. Biochem. Biophys. Res. Commun. 2011, 407, 581–586. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, J.; Morgan, D.; Cheng, D.H.; Foo, S.L.; Yap, G.L.R.; Ampomah, P.B.; Arora, S.; Sachaphibulkij, K.; Periaswamy, B.; Fairhurst, A.-M.; et al. RNA-Sequencing-Based Transcriptomic Analysis Reveals a Role for Annexin-A1 in Classical and Influenza A Virus-Induced Autophagy. Cells 2020, 9, 1399. https://doi.org/10.3390/cells9061399
Cui J, Morgan D, Cheng DH, Foo SL, Yap GLR, Ampomah PB, Arora S, Sachaphibulkij K, Periaswamy B, Fairhurst A-M, et al. RNA-Sequencing-Based Transcriptomic Analysis Reveals a Role for Annexin-A1 in Classical and Influenza A Virus-Induced Autophagy. Cells. 2020; 9(6):1399. https://doi.org/10.3390/cells9061399
Chicago/Turabian StyleCui, Jianzhou, Dhakshayini Morgan, Dao Han Cheng, Sok Lin Foo, Gracemary L. R. Yap, Patrick B. Ampomah, Suruchi Arora, Karishma Sachaphibulkij, Balamurugan Periaswamy, Anna-Marie Fairhurst, and et al. 2020. "RNA-Sequencing-Based Transcriptomic Analysis Reveals a Role for Annexin-A1 in Classical and Influenza A Virus-Induced Autophagy" Cells 9, no. 6: 1399. https://doi.org/10.3390/cells9061399
APA StyleCui, J., Morgan, D., Cheng, D. H., Foo, S. L., Yap, G. L. R., Ampomah, P. B., Arora, S., Sachaphibulkij, K., Periaswamy, B., Fairhurst, A.-M., De Sessions, P. F., & Lim, L. H. K. (2020). RNA-Sequencing-Based Transcriptomic Analysis Reveals a Role for Annexin-A1 in Classical and Influenza A Virus-Induced Autophagy. Cells, 9(6), 1399. https://doi.org/10.3390/cells9061399




