Mitochondrial MicroRNAs in Aging and Neurodegenerative Diseases
Abstract
1. Introduction
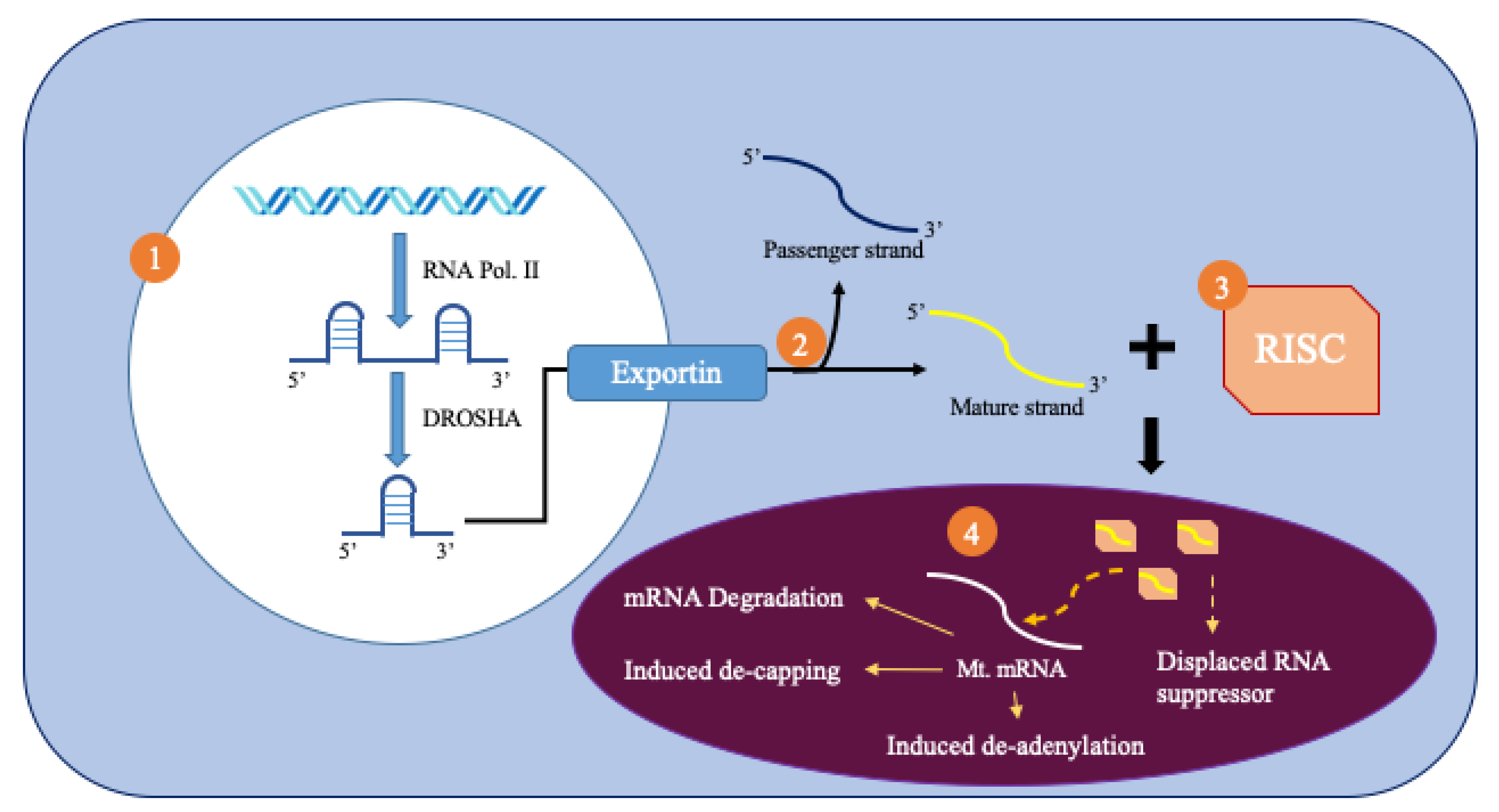
2. MicroRNAs
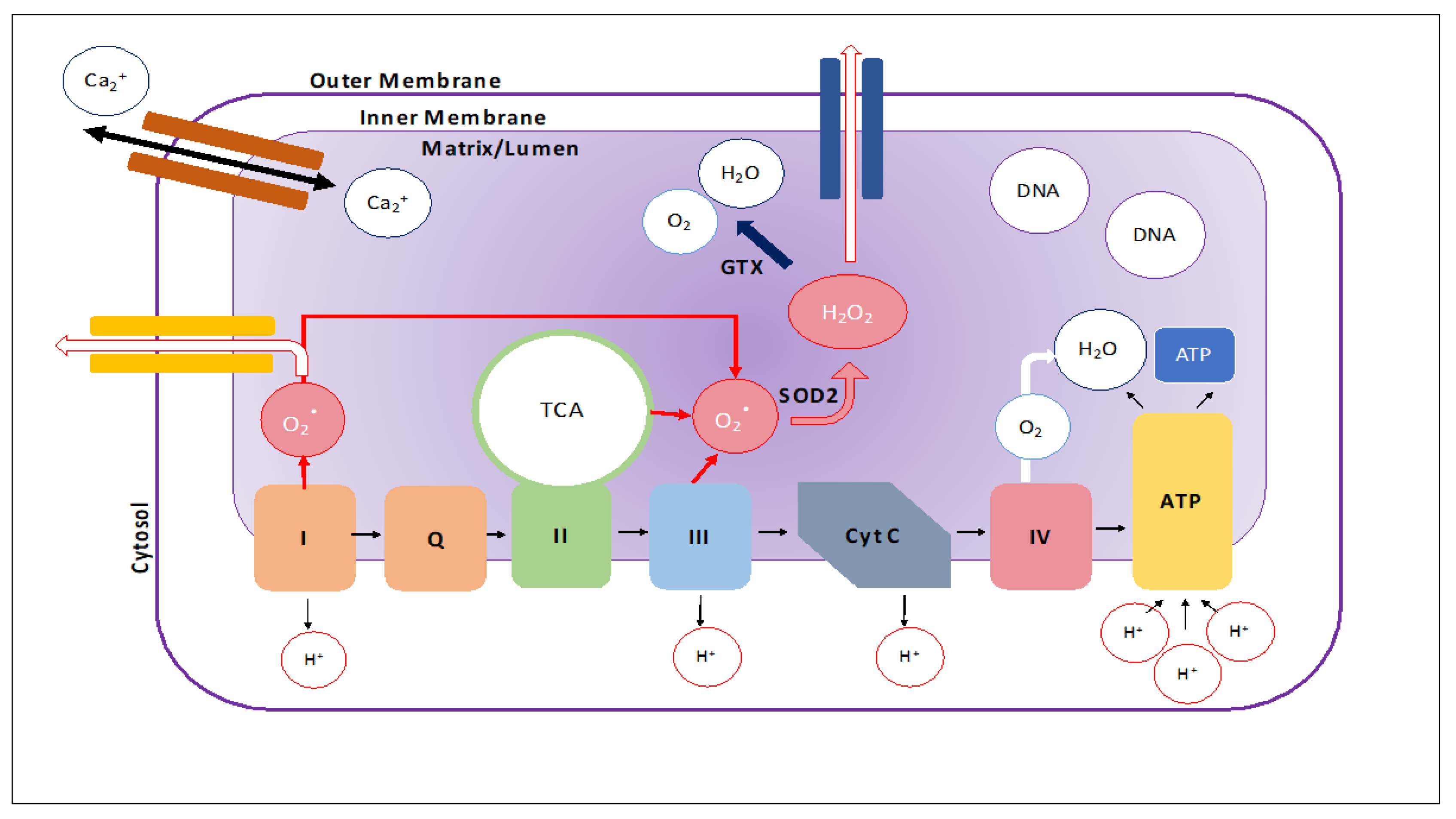
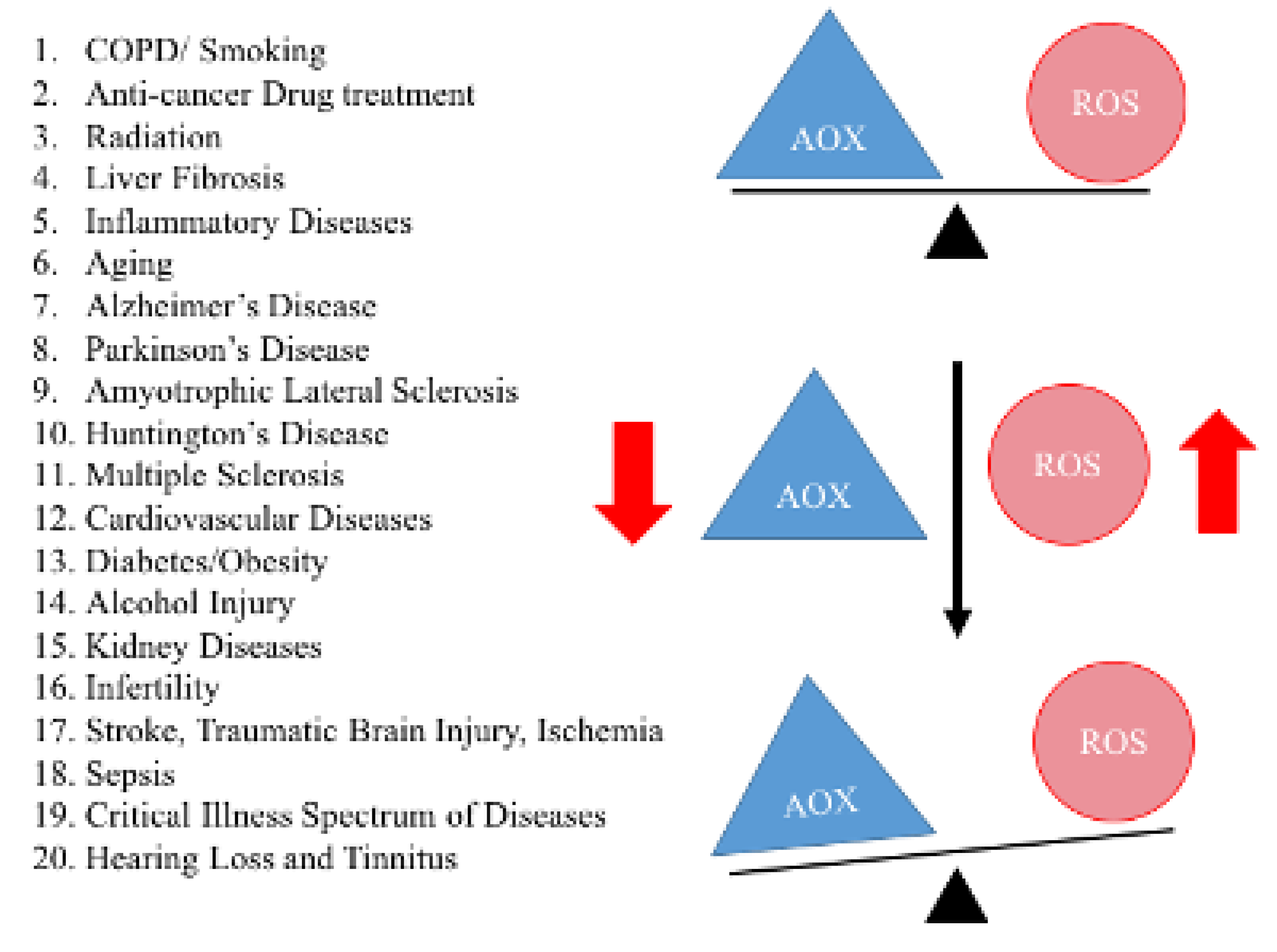
3. Mitochondrial MicroRNAs
4. Alzheimer’s Disease
Mitochondrial microRNAs in Alzheimer’s Disease
5. Parkinson’s Disease
Mitochondrial MicroRNAs in Parkinson’s Disease
6. Huntington’s Disease
Mitochondrial MicroRNAs in Huntington’s Disease
7. Aging
8. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Reddy, P.H.; Beal, M.F. Amyloid beta, mitochondrial dysfunction and synaptic damage: Implications for cognitive decline in aging and Alzheimer’s disease. Trends Mol. Med. 2008, 14, 45–53. [Google Scholar] [CrossRef]
- Wallace, D.C. Mitochondrial diseases in man and mouse. Science 1999, 283, 1482–1488. [Google Scholar] [CrossRef] [PubMed]
- Wallace, D.C. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: A dawn for evolutionary medicine. Annu. Rev. Genet. 2005, 39, 359–407. [Google Scholar] [CrossRef] [PubMed]
- Wallace, D.C. Why do we still have a maternally inherited mitochondrial DNA? Insights from evolutionary medicine. Annu. Rev. Biochem. 2007, 76, 781–821. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.H. Mitochondrial medicine for aging and neurodegenerative diseases. Neuromol. Med. 2008, 10, 291–315. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.H. Mitochondrial dysfunction in aging and Alzheimer’s disease: Strategies to protect neurons. Antioxid. Redox Signal. 2007, 9, 1647–1658. [Google Scholar] [CrossRef]
- Beal, M.F. Mitochondria take center stage in aging and neurodegeneration. Ann. Neurol. 2005, 58, 495–505. [Google Scholar] [CrossRef]
- Kumar, S.; Reddy, P.H. Are circulating microRNAs peripheral biomarkers for Alzheimer’s disease? Biochim. Biophys. Acta 2016, 1862, 1617–1627. [Google Scholar] [CrossRef]
- Macgregor-Das, A.M.; Das, S. A microRNA’s journey to the center of the mitochondria. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H206–H215. [Google Scholar] [CrossRef]
- Vijayan, M.; Reddy, P.H. Non-Coding RNAs Based Molecular Links in Type 2 Diabetes, Ischemic Stroke, and Vascular Dementia. J. Alzheimers Dis. 2020. [Google Scholar] [CrossRef]
- Reddy, P.H.; Tonk, S.; Kumar, S.; Vijayan, M.; Kandimalla, R.; Kuruva, C.S.; Reddy, A.P. A critical evaluation of neuroprotective and neurodegenerative MicroRNAs in Alzheimer’s disease. Biochem. Biophys. Res. Commun. 2017, 483, 1156–1165. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Vijayan, M.; Bhatti, J.S.; Reddy, P.H. MicroRNAs as Peripheral Biomarkers in Aging and Age-Related Diseases. Prog. Mol. Biol. Transl. Sci. 2017, 146, 47–94. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, M.; Reddy, P.H. Peripheral biomarkers of stroke: Focus on circulatory microRNAs. Biochim. Biophys. Acta 2016, 1862, 1984–1993. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Chen, H.W.; Oktay, Y.; Zhang, J.; Allen, E.L.; Smith, G.M.; Fan, K.C.; Hong, J.S.; French, S.W.; McCaffery, J.M.; et al. PNPASE regulates RNA import into mitochondria. Cell 2010, 142, 456–467. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J. Alzheimer’s disease: Genes, proteins, and therapy. Physiol. Rev. 2001, 81, 741–766. [Google Scholar] [CrossRef]
- Reddy, P.H.; Manczak, M.; Mao, P.; Calkins, M.J.; Reddy, A.P.; Shirendeb, U. Amyloid-beta and mitochondria in aging and Alzheimer’s disease: Implications for synaptic damage and cognitive decline. J. Alzheimers Dis. 2010, 20 (Suppl. 2), S499–S512. [Google Scholar] [CrossRef]
- Di Paolo, G.; Kim, T.W. Linking lipids to Alzheimer’s disease: Cholesterol and beyond. Nat. Rev. Neurosci. 2011, 12, 284–296. [Google Scholar] [CrossRef]
- Kumar, S.; Reddy, A.P.; Yin, X.; Reddy, P.H. Novel MicroRNA-455-3p and its protective effects against abnormal APP processing and amyloid beta toxicity in Alzheimer’s disease. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 2428–2440. [Google Scholar] [CrossRef]
- Amakiri, N.; Kubosumi, A.; Tran, J.; Reddy, P.H. Amyloid Beta and MicroRNAs in Alzheimer’s Disease. Front. Neurosci. 2019, 13, 430. [Google Scholar] [CrossRef]
- Wang, M.; Qin, L.; Tang, B. MicroRNAs in Alzheimer’s Disease. Front. Genet. 2019, 10, 153. [Google Scholar] [CrossRef]
- Shu, B.; Zhang, X.; Du, G.; Fu, Q.; Huang, L. MicroRNA-107 prevents amyloid-β-induced neurotoxicity and memory impairment in mice. Int. J. Mol. Med. 2018, 41, 1665–1672. [Google Scholar] [CrossRef] [PubMed]
- Rech, M.; Kuhn, A.R.; Lumens, J.; Carai, P.; van Leeuwen, R.; Verhesen, W.; Verjans, R.; Lecomte, J.; Liu, Y.; Luiken, J.J.F.P.; et al. AntagomiR-103 and -107 Treatment Affects Cardiac Function and Metabolism. Mol. Nucleic Acids 2019, 14, 424–437. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, Z.; Tan, L.; Wang, Y.; Lu, C.; Chen, R.; Zhang, S.; Gao, Y.; Liu, Y.; Yin, Y.; et al. Correcting miR92a-vGAT-Mediated GABAergic Dysfunctions Rescues Human Tau-Induced Anxiety in Mice. Mol. Ther. 2017, 25, 140–152. [Google Scholar] [CrossRef] [PubMed]
- Fisichella, V.; Giurdanella, G.; Platania, C.B.; Romano, G.L.; Leggio, G.M.; Salomone, S.; Drago, F.; Caraci, F.; Bucolo, C. TGF-β1 prevents rat retinal insult induced by amyloid-β (1-42) oligomers. Eur. J. Pharm. 2016, 787, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Liu, L.; Meng, J. MicroRNA-125b promotes neurons cell apoptosis and Tau phosphorylation in Alzheimer’s disease. Neurosci. Lett. 2017, 661, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, R.B.; Mufson, E.J.; Counts, S.E. Evidence for a neuroprotective microRNA pathway in amnestic mild cognitive impairment. Front. Neurosci. 2015, 9, 430. [Google Scholar] [CrossRef]
- Jovicic, A.; Zaldivar Jolissaint, J.F.; Moser, R.; Silva Santos, M.e.F.; Luthi-Carter, R. MicroRNA-22 (miR-22) overexpression is neuroprotective via general anti-apoptotic effects and may also target specific Huntington’s disease-related mechanisms. PLoS ONE 2013, 8, e54222. [Google Scholar] [CrossRef]
- Russell, A.E.; Doll, D.N.; Sarkar, S.N.; Simpkins, J.W. TNF-α and Beyond: Rapid Mitochondrial Dysfunction Mediates TNF-α-Induced Neurotoxicity. J. Clin. Cell Immunol. 2016, 7. [Google Scholar] [CrossRef]
- Shi, Q.; Gibson, G.E. Up-regulation of the mitochondrial malate dehydrogenase by oxidative stress is mediated by miR-743a. J. Neurochem. 2011, 118, 440–448. [Google Scholar] [CrossRef]
- Zhang, R.; Zhou, H.; Jiang, L.; Mao, Y.; Cui, X.; Xie, B.; Cui, D.; Wang, H.; Zhang, Q.; Xu, S. MiR-195 dependent roles of mitofusin2 in the mitochondrial dysfunction of hippocampal neurons in SAMP8 mice. Brain Res. 2016, 1652, 135–143. [Google Scholar] [CrossRef]
- Sørensen, S.S.; Nygaard, A.B.; Christensen, T. miRNA expression profiles in cerebrospinal fluid and blood of patients with Alzheimer’s disease and other types of dementia—An exploratory study. Transl. Neurodegener. 2016, 5, 6. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Jiao, J.; Gao, G.; Prabhakar, B.S. Control of mitochondrial activity by miRNAs. J. Cell Biochem. 2012, 113, 1104–1110. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.L. Sirt1 and the Mitochondria. Mol. Cells 2016, 39, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Romano, G.L.; Platania, C.B.M.; Drago, F.; Salomone, S.; Ragusa, M.; Barbagallo, C.; Di Pietro, C.; Purrello, M.; Reibaldi, M.; Avitabile, T.; et al. Retinal and Circulating miRNAs in Age-Related Macular Degeneration: An In vivo Animal and Human Study . Front. Pharm. 2017, 8, 168. [Google Scholar] [CrossRef]
- Kim, J.; Fiesel, F.C.; Belmonte, K.C.; Hudec, R.; Wang, W.X.; Kim, C.; Nelson, P.T.; Springer, W. miR-27a and miR-27b regulate autophagic clearance of damaged mitochondria by targeting PTEN-induced putative kinase 1 (PINK1). Mol. Neurodegener. 2016, 11, 55. [Google Scholar] [CrossRef]
- Hong, H.; Li, Y.; Su, B. Identification of Circulating miR-125b as a Potential Biomarker of Alzheimer’s Disease in APP/PS1 Transgenic Mouse. J. Alzheimers Dis. 2017, 59, 1449–1458. [Google Scholar] [CrossRef]
- Li, S.P.; Liu, B.; Song, B.; Wang, C.X.; Zhou, Y.C. miR-28 promotes cardiac ischemia by targeting mitochondrial aldehyde dehydrogenase 2 (ALDH2) in mus musculus cardiac myocytes. Eur. Rev. Med. Pharm. Sci. 2015, 19, 752–758. [Google Scholar]
- Sarkar, S.; Engler-Chiurazzi, E.B.; Cavendish, J.Z.; Povroznik, J.M.; Russell, A.E.; Quintana, D.D.; Mathers, P.H.; Simpkins, J.W. Over-expression of miR-34a induces rapid cognitive impairment and Alzheimer’s disease-like pathology. Brain Res. 2019, 1721, 146327. [Google Scholar] [CrossRef]
- Giuliani, A.; Cirilli, I.; Prattichizzo, F.; Mensà, E.; Fulgenzi, G.; Sabbatinelli, J.; Graciotti, L.; Olivieri, F.; Procopio, A.D.; Tiano, L.; et al. The mitomiR/Bcl-2 axis affects mitochondrial function and autophagic vacuole formation in senescent endothelial cells. Aging (Albany NY) 2018, 10, 2855–2873. [Google Scholar] [CrossRef]
- Chen, J.; Qi, Y.; Liu, C.F.; Lu, J.M.; Shi, J.; Shi, Y. MicroRNA expression data analysis to identify key miRNAs associated with Alzheimer’s disease. J. Gene Med. 2018, 20, e3014. [Google Scholar] [CrossRef]
- Frankel, L.B.; Wen, J.; Lees, M.; Høyer-Hansen, M.; Farkas, T.; Krogh, A.; Jäättelä, M.; Lund, A.H. microRNA-101 is a potent inhibitor of autophagy. EMBO J. 2011, 30, 4628–4641. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Noh, H.; Lee, Y.; Jeon, J.; Shanmugavadivu, A.; McPhie, D.L.; Kim, K.S.; Cohen, B.M.; Seo, H.; Sonntag, K.C. MiR-126 Regulates Growth Factor Activities and Vulnerability to Toxic Insult in Neurons. Mol. Neurobiol. 2016, 53, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Tomasetti, M.; Nocchi, L.; Staffolani, S.; Manzella, N.; Amati, M.; Goodwin, J.; Kluckova, K.; Nguyen, M.; Strafella, E.; Bajzikova, M.; et al. MicroRNA-126 suppresses mesothelioma malignancy by targeting IRS1 and interfering with the mitochondrial function. Antioxid. Redox Signal. 2014, 21, 2109–2125. [Google Scholar] [CrossRef] [PubMed]
- Akhter, R.; Shao, Y.; Shaw, M.; Formica, S.; Khrestian, M.; Leverenz, J.B.; Bekris, L.M. Regulation of ADAM10 by miR-140-5p and potential relevance for Alzheimer’s disease. Neurobiol. Aging 2018, 63, 110–119. [Google Scholar] [CrossRef]
- Duarte, F.V.; Palmeira, C.M.; Rolo, A.P. The Role of microRNAs in Mitochondria: Small Players Acting Wide. Genes 2014, 5, 865–886. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, Z. Downregulation of miR143/145 gene cluster expression promotes the aortic media degeneration process via the TGF-β1 signaling pathway. Am. J. Transl. Res. 2019, 11, 370–378. [Google Scholar]
- Rippo, M.R.; Olivieri, F.; Monsurrò, V.; Prattichizzo, F.; Albertini, M.C.; Procopio, A.D. MitomiRs in human inflamm-aging: A hypothesis involving miR-181a, miR-34a and miR-146a. Exp. Gerontol 2014, 56, 154–163. [Google Scholar] [CrossRef]
- Wang, W.X.; Springer, J.E. Role of mitochondria in regulating microRNA activity and its relevance to the central nervous system. Neural. Regen Res. 2015, 10, 1026–1028. [Google Scholar] [CrossRef]
- Rodriguez-Ortiz, C.J.; Prieto, G.A.; Martini, A.C.; Forner, S.; Trujillo-Estrada, L.; LaFerla, F.M.; Baglietto-Vargas, D.; Cotman, C.W.; Kitazawa, M. miR-181a negatively modulates synaptic plasticity in hippocampal cultures and its inhibition rescues memory deficits in a mouse model of Alzheimer’s disease. Aging Cell 2020, 19, e13118. [Google Scholar] [CrossRef]
- Zhu, H.C.; Wang, L.M.; Wang, M.; Song, B.; Tan, S.; Teng, J.F.; Duan, D.X. MicroRNA-195 downregulates Alzheimer’s disease amyloid-β production by targeting BACE1. Brain Res. Bull. 2012, 88, 596–601. [Google Scholar] [CrossRef]
- Yan, K.; An, T.; Zhai, M.; Huang, Y.; Wang, Q.; Wang, Y.; Zhang, R.; Wang, T.; Liu, J.; Zhang, Y.; et al. Mitochondrial miR-762 regulates apoptosis and myocardial infarction by impairing ND2. Cell Death Dis. 2019, 10, 500. [Google Scholar] [CrossRef] [PubMed]
- Siedlecki-Wullich, D.; Català-Solsona, J.; Fábregas, C.; Hernández, I.; Clarimon, J.; Lleó, A.; Boada, M.; Saura, C.A.; Rodríguez-Álvarez, J.; Miñano-Molina, A.J. Altered microRNAs related to synaptic function as potential plasma biomarkers for Alzheimer’s disease. Alzheimers Res. 2019, 11, 46. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, Z.F.; Li, W.; Hong, H.; Chen, J.; Tian, Y.; Liu, Z.Y. Protective effects of microRNA-330 on amyloid β-protein production, oxidative stress, and mitochondrial dysfunction in Alzheimer’s disease by targeting VAV1 via the MAPK signaling pathway. J. Cell Biochem. 2018, 119, 5437–5448. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.X.; Huang, Q.; Hu, Y.; Stromberg, A.J.; Nelson, P.T. Patterns of microRNA expression in normal and early Alzheimer’s disease human temporal cortex: White matter versus gray matter. Acta Neuropathol. 2011, 121, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Ren, R.J.; Zhang, Y.F.; Dammer, E.B.; Zhou, Y.; Wang, L.L.; Liu, X.H.; Feng, B.L.; Jiang, G.X.; Chen, S.D.; Wang, G.; et al. Peripheral Blood MicroRNA Expression Profiles in Alzheimer’s Disease: Screening, Validation, Association with Clinical Phenotype and Implications for Molecular Mechanism. Mol. Neurobiol. 2016, 53, 5772–5781. [Google Scholar] [CrossRef]
- Hu, Y.B.; Zhang, Y.F.; Wang, H.; Ren, R.J.; Cui, H.L.; Huang, W.Y.; Cheng, Q.; Chen, H.Z.; Wang, G. miR-425 deficiency promotes necroptosis and dopaminergic neurodegeneration in Parkinson’s disease. Cell Death Dis. 2019, 10, 589. [Google Scholar] [CrossRef]
- Alexander, G.E. Biology of Parkinson’s disease: Pathogenesis and pathophysiology of a multisystem neurodegenerative disorder. Dialogues Clin. Neurosci. 2004, 6, 259–280. [Google Scholar]
- Pahapill, P.A.; Lozano, A.M. The pedunculopontine nucleus and Parkinson’s disease. Brain 2000, 123, 1767–1783. [Google Scholar] [CrossRef]
- Chen, C.; Turnbull, D.M.; Reeve, A.K. Mitochondrial Dysfunction in Parkinson’s Disease-Cause or Consequence? Biology 2019, 8, 38. [Google Scholar] [CrossRef]
- Geisler, S.; Holmström, K.M.; Skujat, D.; Fiesel, F.C.; Rothfuss, O.C.; Kahle, P.J.; Springer, W. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat. Cell Biol. 2010, 12, 119–131. [Google Scholar] [CrossRef]
- Junn, E.; Lee, K.W.; Jeong, B.S.; Chan, T.W.; Im, J.Y.; Mouradian, M.M. Repression of alpha-synuclein expression and toxicity by microRNA-7. Proc. Natl. Acad. Sci. USA 2009, 106, 13052–13057. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, A.D.; Choi, D.C.; Kabaria, S.; Tran, A.; Junn, E. MicroRNA-7 Regulates the Function of Mitochondrial Permeability Transition Pore by Targeting VDAC1 Expression. J. Biol. Chem. 2016, 291, 6483–6493. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Cheng, Y. miR-16-1 promotes the aberrant α-synuclein accumulation in parkinson disease via targeting heat shock protein 70. Sci. World J. 2014, 2014, 938348. [Google Scholar] [CrossRef]
- Radons, J. The human HSP70 family of chaperones: Where do we stand? Cell Stress Chaperones 2016, 21, 379–404. [Google Scholar] [CrossRef]
- Martinez, B.; Peplow, P.V. MicroRNAs in Parkinson’s disease and emerging therapeutic targets. Neural. Regen. Res. 2017, 12, 1945–1959. [Google Scholar] [CrossRef]
- Zhang, J.G.; Wang, J.J.; Zhao, F.; Liu, Q.; Jiang, K.; Yang, G.H. MicroRNA-21 (miR-21) represses tumor suppressor PTEN and promotes growth and invasion in non-small cell lung cancer (NSCLC). Clin. Chim. Acta 2010, 411, 846–852. [Google Scholar] [CrossRef]
- Lungu, G.; Stoica, G.; Ambrus, A. MicroRNA profiling and the role of microRNA-132 in neurodegeneration using a rat model. Neurosci. Lett. 2013, 553, 153–158. [Google Scholar] [CrossRef]
- Li, D.; Yang, H.; Ma, J.; Luo, S.; Chen, S.; Gu, Q. MicroRNA-30e regulates neuroinflammation in MPTP model of Parkinson’s disease by targeting Nlrp3. Hum. Cell 2018, 31, 106–115. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, D.; Hu, D.; Zhou, X.; Zhou, Y. The role of mitochondria in NLRP3 inflammasome activation. Mol. Immunol. 2018, 103, 115–124. [Google Scholar] [CrossRef]
- Miñones-Moyano, E.; Porta, S.; Escaramís, G.; Rabionet, R.; Iraola, S.; Kagerbauer, B.; Espinosa-Parrilla, Y.; Ferrer, I.; Estivill, X.; Martí, E. MicroRNA profiling of Parkinson’s disease brains identifies early downregulation of miR-34b/c which modulate mitochondrial function. Hum. Mol. Genet. 2011, 20, 3067–3078. [Google Scholar] [CrossRef]
- Wang, H.; Ye, Y.; Zhu, Z.; Mo, L.; Lin, C.; Wang, Q.; Gong, X.; He, X.; Lu, G.; Lu, F.; et al. MiR-124 Regulates Apoptosis and Autophagy Process in MPTP Model of Parkinson’s Disease by Targeting to Bim. Brain Pathol. 2016, 26, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Lee, Y.; McKenna, N.D.; Yi, M.; Simunovic, F.; Wang, Y.; Kong, B.; Rooney, R.J.; Seo, H.; Stephens, R.M.; et al. miR-126 contributes to Parkinson’s disease by dysregulating the insulin-like growth factor/phosphoinositide 3-kinase signaling. Neurobiol. Aging 2014, 35, 1712–1721. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, X.; Zhuang, H.; Chen, H.G.; Chen, Y.; Tian, W.; Wu, W.; Li, Y.; Wang, S.; Zhang, L.; et al. MicroRNA-137 is a novel hypoxia-responsive microRNA that inhibits mitophagy via regulation of two mitophagy receptors FUNDC1 and NIX. J. Biol. Chem. 2014, 289, 10691–10701. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Pan, X.; Zhang, J.; Ma, A.; Yang, S.; Ma, J.; Xie, A. Plasma levels of miR-137 and miR-124 are associated with Parkinson’s disease but not with Parkinson’s disease with depression. Neurol. Sci. 2017, 38, 761–767. [Google Scholar] [CrossRef]
- Cardo, L.F.; Coto, E.; Ribacoba, R.; Mata, I.F.; Moris, G.; Menéndez, M.; Alvarez, V. The screening of the 3’UTR sequence of LRRK2 identified an association between the rs66737902 polymorphism and Parkinson’s disease. J. Hum. Genet. 2014, 59, 346–348. [Google Scholar] [CrossRef]
- Singh, A.; Zhi, L.; Zhang, H. LRRK2 and mitochondria: Recent advances and current views. Brain Res. 2019, 1702, 96–104. [Google Scholar] [CrossRef]
- Li, K.; Zhang, J.; Ji, C.; Wang, L. MiR-144-3p and Its Target Gene β-Amyloid Precursor Protein Regulate 1-Methyl-4-Phenyl-1,2-3,6-Tetrahydropyridine-Induced Mitochondrial Dysfunction. Mol. Cells 2016, 39, 543–549. [Google Scholar] [CrossRef]
- Doxakis, E. Post-transcriptional regulation of alpha-synuclein expression by mir-7 and mir-153. J. Biol. Chem. 2010, 285, 12726–12734. [Google Scholar] [CrossRef]
- Weichhart, T. mTOR as Regulator of Lifespan, Aging, and Cellular Senescence: A Mini-Review. Gerontology 2018, 64, 127–134. [Google Scholar] [CrossRef]
- Caggiu, E.; Paulus, K.; Mameli, G.; Arru, G.; Sechi, G.P.; Sechi, L.A. Differential expression of miRNA 155 and miRNA 146a in Parkinson’s disease patients. eNeurologicalSci. 2018, 13, 1–4. [Google Scholar] [CrossRef]
- Du, C.; Yao, F.; Ren, Y.; Du, Y.; Wei, J.; Wu, H.; Duan, H.; Shi, Y. SOCS-1 is involved in TNF-α-induced mitochondrial dysfunction and apoptosis in renal tubular epithelial cells. Tissue Cell 2017, 49, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.J.; Liu, G.; Jin, S.M.; Parisiadou, L.; Xie, C.; Yu, J.; Sun, L.; Ma, B.; Ding, J.; Vancraenenbroeck, R.; et al. MicroRNA-205 regulates the expression of Parkinson’s disease-related leucine-rich repeat kinase 2 protein. Hum. Mol. Genet. 2013, 22, 608–620. [Google Scholar] [CrossRef] [PubMed]
- Xiong, R.; Wang, Z.; Zhao, Z.; Li, H.; Chen, W.; Zhang, B.; Wang, L.; Wu, L.; Li, W.; Ding, J.; et al. MicroRNA-494 reduces DJ-1 expression and exacerbates neurodegeneration. Neurobiol. Aging 2014, 35, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; van der Walt, J.M.; Mayhew, G.; Li, Y.J.; Züchner, S.; Scott, W.K.; Martin, E.R.; Vance, J.M. Variation in the miRNA-433 binding site of FGF20 confers risk for Parkinson disease by overexpression of alpha-synuclein. Am. J. Hum. Genet. 2008, 82, 283–289. [Google Scholar] [CrossRef]
- Paillusson, S.; Gomez-Suaga, P.; Stoica, R.; Little, D.; Gissen, P.; Devine, M.J.; Noble, W.; Hanger, D.P.; Miller, C.C.J. α-Synuclein binds to the ER-mitochondria tethering protein VAPB to disrupt Ca. Acta Neuropathol. 2017, 134, 129–149. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, C.; Sun, Q.; Pan, H.; Huang, P.; Ding, J.; Chen, S. MicroRNA-4639 Is a Regulator of DJ-1 Expression and a Potential Early Diagnostic Marker for Parkinson’s Disease. Front. Aging Neurosci. 2017, 9, 232. [Google Scholar] [CrossRef]
- Li, S.; Lv, X.; Zhai, K.; Xu, R.; Zhang, Y.; Zhao, S.; Qin, X.; Yin, L.; Lou, J. MicroRNA-7 inhibits neuronal apoptosis in a cellular Parkinson’s disease model by targeting Bax and Sirt2. Am. J. Transl. Res. 2016, 8, 993–1004. [Google Scholar]
- Meng, F.; Henson, R.; Wehbe-Janek, H.; Ghoshal, K.; Jacob, S.T.; Patel, T. MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology 2007, 133, 647–658. [Google Scholar] [CrossRef]
- Thome, A.D.; Harms, A.S.; Volpicelli-Daley, L.A.; Standaert, D.G. microRNA-155 Regulates Alpha-Synuclein-Induced Inflammatory Responses in Models of Parkinson Disease. J. Neurosci. 2016, 36, 2383–2390. [Google Scholar] [CrossRef]
- Yhnell, E. Huntington’s disease: Of mice and men. Oncotarget 2017, 8, 12552–12553. [Google Scholar] [CrossRef]
- Quintanilla, R.A.; Johnson, G.V. Role of mitochondrial dysfunction in the pathogenesis of Huntington’s disease. Brain Res. Bull. 2009, 80, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Cepeda, C.; Tong, X.P. Huntington’s disease: From basic science to therapeutics. CNS Neurosci. 2018, 24, 247–249. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.H.; Shirendeb, U.P. Mutant huntingtin, abnormal mitochondrial dynamics, defective axonal transport of mitochondria, and selective synaptic degeneration in Huntington’s disease. Biochim. Biophys. Acta 2012, 1822, 101–110. [Google Scholar] [CrossRef]
- Reddy, P.H.; Mao, P.; Manczak, M. Mitochondrial structural and functional dynamics in Huntington’s disease. Brain Res. Rev. 2009, 61, 33–48. [Google Scholar] [CrossRef] [PubMed]
- Sawant, N.; Reddy, P.H. Role of Phosphorylated Tau and Glucose Synthase Kinase 3 Beta in Huntington’s Disease Progression. J. Alzheimers Dis. 2019, 72, S177–S191. [Google Scholar] [CrossRef] [PubMed]
- Hoss, A.G.; Labadorf, A.; Latourelle, J.C.; Kartha, V.K.; Hadzi, T.C.; Gusella, J.F.; MacDonald, M.E.; Chen, J.F.; Akbarian, S.; Weng, Z.; et al. miR-10b-5p expression in Huntington’s disease brain relates to age of onset and the extent of striatal involvement. BMC Med. Genom. 2015, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Shi, X.; Wei, S.; Ma, D.; Oyinlade, O.; Lv, S.Q.; Ying, M.; Zhang, Y.A.; Claypool, S.M.; Watkins, P.; et al. Krüppel-like factor 4 (KLF4) induces mitochondrial fusion and increases spare respiratory capacity of human glioblastoma cells. J. Biol. Chem. 2018, 293, 6544–6555. [Google Scholar] [CrossRef]
- Liu, Z.; Li, T.; Zhu, F.; Deng, S.; Li, X.; He, Y. Regulatory roles of miR-22/Redd1-mediated mitochondrial ROS and cellular autophagy in ionizing radiation-induced BMSC injury. Cell Death Dis. 2019, 10, 227. [Google Scholar] [CrossRef]
- Lee, S.T.; Chu, K.; Im, W.S.; Yoon, H.J.; Im, J.Y.; Park, J.E.; Park, K.H.; Jung, K.H.; Lee, S.K.; Kim, M.; et al. Altered microRNA regulation in Huntington’s disease models. Exp. Neurol. 2011, 227, 172–179. [Google Scholar] [CrossRef]
- Holley, A.K.; St Clair, D.K. Watching the watcher: Regulation of p53 by mitochondria. Future Oncol. 2009, 5, 117–130. [Google Scholar] [CrossRef]
- Liu, T.; Im, W.; Mook-Jung, I.; Kim, M. MicroRNA-124 slows down the progression of Huntington’s disease by promoting neurogenesis in the striatum. Neural Regen. Res. 2015, 10, 786–791. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.N.; Johnson, G.V. The interrelationship between mitochondrial dysfunction and transcriptional dysregulation in Huntington disease. J. Bioenerg. Biomembr. 2010, 42, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Kocerha, J.; Xu, Y.; Prucha, M.S.; Zhao, D.; Chan, A.W. microRNA-128a dysregulation in transgenic Huntington’s disease monkeys. Mol. Brain 2014, 7, 46. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, G.C.; Schaan, A.P.; Cabral, G.F.; Santana-da-Silva, M.N.; Pinto, P.; Vidal, A.F.; Ribeiro-Dos-Santos, Â. A Cell’s Fate: An Overview of the Molecular Biology and Genetics of Apoptosis. Int. J. Mol. Sci. 2019, 20, 4133. [Google Scholar] [CrossRef]
- Fukuoka, M.; Takahashi, M.; Fujita, H.; Chiyo, T.; Popiel, H.A.; Watanabe, S.; Furuya, H.; Murata, M.; Wada, K.; Okada, T.; et al. Supplemental Treatment for Huntington’s Disease with miR-132 that Is Deficient in Huntington’s Disease Brain. Mol. Nucleic Acids 2018, 11, 79–90. [Google Scholar] [CrossRef]
- Ghose, J.; Sinha, M.; Das, E.; Jana, N.R.; Bhattacharyya, N.P. Regulation of miR-146a by RelA/NFkB and p53 in STHdh(Q111)/Hdh(Q111) cells, a cell model of Huntington’s disease. PLoS ONE 2011, 6, e23837. [Google Scholar] [CrossRef]
- Zhang, X.; Li, C.F.; Zhang, L.; Wu, C.Y.; Han, L.; Jin, G.; Rezaeian, A.H.; Han, F.; Liu, C.; Xu, C.; et al. TRAF6 Restricts p53 Mitochondrial Translocation, Apoptosis, and Tumor Suppression. Mol. Cell 2016, 64, 803–814. [Google Scholar] [CrossRef]
- Kunkanjanawan, T.; Carter, R.L.; Prucha, M.S.; Yang, J.; Parnpai, R.; Chan, A.W. miR-196a Ameliorates Cytotoxicity and Cellular Phenotype in Transgenic Huntington’s Disease Monkey Neural Cells. PLoS ONE 2016, 11, e0162788. [Google Scholar] [CrossRef]
- Di Rita, A.; Maiorino, T.; Bruqi, K.; Volpicelli, F.; Bellenchi, G.C.; Strappazzon, F. miR-218 Inhibits Mitochondrial Clearance by Targeting PRKN E3 Ubiquitin Ligase. Int. J. Mol. Sci. 2020, 21, 355. [Google Scholar] [CrossRef]
- Díez-Planelles, C.; Sánchez-Lozano, P.; Crespo, M.C.; Gil-Zamorano, J.; Ribacoba, R.; González, N.; Suárez, E.; Martínez-Descals, A.; Martínez-Camblor, P.; Álvarez, V.; et al. Circulating microRNAs in Huntington’s disease: Emerging mediators in metabolic impairment. Pharm. Res. 2016, 108, 102–110. [Google Scholar] [CrossRef]
- Zhao, X.; Song, X.; Bai, X.; Tan, Z.; Ma, X.; Guo, J.; Zhang, Z.; Du, Q.; Huang, Y.; Tong, D. microRNA-222 Attenuates Mitochondrial Dysfunction During Transmissible Gastroenteritis Virus Infection. Mol. Cell Proteom. 2019, 18, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.; Smith, F.; Kumar, S.; Vijayan, M.; Reddy, P.H. Are microRNAs true sensors of ageing and cellular senescence? Ageing Res. Rev. 2017, 35, 350–363. [Google Scholar] [CrossRef] [PubMed]
- Trifunovic, A.; Wredenberg, A.; Falkenberg, M.; Spelbrink, J.N.; Rovio, A.T.; Bruder, C.E.; Bohlooly-Y, M.; Gidlöf, S.; Oldfors, A.; Wibom, R.; et al. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature 2004, 429, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Sato, Y.; Wang, C.; Yue, F.; Kuang, S.; Gavin, T.P. Impaired exercise tolerance, mitochondrial biogenesis, and muscle fiber maintenance in miR-133a-deficient mice. Faseb J. 2016, 30, 3745–3758. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Luo, J.; Zhao, J.; Shang, D.; Lv, Q.; Zang, P. Combined Use of Circulating miR-133a and NT-proBNP Improves Heart Failure Diagnostic Accuracy in Elderly Patients. Med. Sci. Monit. 2018, 24, 8840–8848. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Boopathi, E.; Addya, S.; Phillips, B.; Rigoutsos, I.; Penn, R.B.; Rattan, S. Aging-associated changes in microRNA expression profile of internal anal sphincter smooth muscle: Role of microRNA-133a. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 311, G964–G973. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Sanchez, A.; Rutter, G.A.; Latreille, M. MiRNAs in β-Cell Development, Identity, and Disease. Front. Genet. 2016, 7, 226. [Google Scholar] [CrossRef]
- Indrieri, A.; Carrella, S.; Romano, A.; Spaziano, A.; Marrocco, E.; Fernandez-Vizarra, E.; Barbato, S.; Pizzo, M.; Ezhova, Y.; Golia, F.M.; et al. miR-181a/b downregulation exerts a protective action on mitochondrial disease models. EMBO Mol. Med. 2019, 11. [Google Scholar] [CrossRef]
- Meng, F.; Li, Z.; Zhang, Z.; Yang, Z.; Kang, Y.; Zhao, X.; Long, D.; Hu, S.; Gu, M.; He, S.; et al. MicroRNA-193b-3p regulates chondrogenesis and chondrocyte metabolism by targeting HDAC3. Theranostics 2018, 8, 2862–2883. [Google Scholar] [CrossRef]
- Ukai, T.; Sato, M.; Akutsu, H.; Umezawa, A.; Mochida, J. MicroRNA-199a-3p, microRNA-193b, and microRNA-320c are correlated to aging and regulate human cartilage metabolism. J. Orthop. Res. 2012, 30, 1915–1922. [Google Scholar] [CrossRef]
- Kurtsdotter, I.; Topcic, D.; Karlén, A.; Singla, B.; Hagey, D.W.; Bergsland, M.; Siesjö, P.; Nistér, M.; Carlson, J.W.; Lefebvre, V.; et al. SOX5/6/21 Prevent Oncogene-Driven Transformation of Brain Stem Cells. Cancer Res. 2017, 77, 4985–4997. [Google Scholar] [CrossRef] [PubMed]
| miRNA | Change | Potential Role | Reference(s) |
|---|---|---|---|
| miR-15a | Upregulation | Targets BACE1 | Sørensen et al. (2016) [31] |
| increases mitochondrial dysfunction and unbalances mitochondrial membrane potential | Li et al. (2012) [32] | ||
| miR-23a/23b | Downregulation | Promote SIRT1 | Weinberg et al. (2015) [26] |
| Increased SIRT1 affects mitochondrial biogenesis and turnover. | Tang (2016) [33] | ||
| miR-27a | Upregulation | Targets the TGF-β pathway | Romano et al. (2017) [34] |
| Negatively regulates PINK1 mediated mitochondrial clearance | Kim et al. (2016) [35] | ||
| miR-28-3p | Upregulation | APP/PS1 mice | Hong et al. (2017) [36] |
| Inhibition of Aldehyde dehydrogenase 2 | Li et al. (2015) [37] | ||
| miR-34a | Upregulation | Targets ADAM10, NMDAR 2B, and SIRT1 | Sarkar et al. (2019) [38] |
| Localizes in the mitochondria and downregulates Bcl-2 which increases casp-1 activity. Activates casp-3. Promotes apoptosis and dysfunction of mitochondria | Giuliani et al. (2018) [39] | ||
| miR-101 | Upregulation | Transcription regulation | Chen et al. (2018) [40] |
| Targets STMN1, RAB5A and ATG4D. Inhibits autophagy thus persistence of damaged mitochondria. | Frankel et al. (2011) [41] | ||
| miR-107 | Downregulation | Reduced expression in the hippocampus | Shu et al. (2018) [21] |
| Decreased mitochondrial ETC function and morphological changes | Rech et al. (2019) [22] | ||
| miR-125b | Upregulation | Increased Bax, decreased Bcl-2 | Ma et al. (2017) [25] |
| miR-126 | Upregulation | GF/PI3K/AKT and ERK signaling cascades | Kim et al. (2016) [42] |
| Inhibits complex 1 of mitochondria and reduces aerobic respiration. | Tomasetti et al. (2014) [43] | ||
| miR-132 | Downregulation | Inhibits complex 1 of mitochondria and reduces aerobic respiration. | Weinberg et al. (2015) [26] |
| miR-140 | Upregulation | ADAM10 | Akhter et al. (2018) [44] |
| Promotes mitochondrial fission via Mfn1 | Duarte et al. (2014) [45] | ||
| miR-143 | Downregulation | Increased activation of TGF-β | Zhang and Wang (2019) [46] |
| Increased mitochondrial death as decreased ERK5 pathway | Li et al. (2012) [32] | ||
| miR-146a | Upregulation | Associated with mTOR, TNF α | Romano et al. (2017) [34] |
| Modulates Bcl-2 | Rippo et al. (2014) [47] | ||
| miR-155 | Upregulation | Associated with mTOR, TNF α | Romano et al. (2017) [34] |
| Localizes in the mitochondria | Wang and Springer (2015) [48] | ||
| miR-181a | Upregulation | Upregulation of GluA2 | Rodriguez-Ortiz et al. (2020) [49] |
| Localizes in the mitochondria and downregulates Bcl-2 which increases casp-1 activity. Activates casp-3. Promotes apoptosis and dysfunction of mitochondria | Giuliani et al. (2018) [39] | ||
| miR-181c | Upregulation | Down-regulates Bcl-2 and leads to apoptosis | Fisichella et al. (2016) [24] |
| miR-195 | Downregulation | Reduced targeting of BACE1 leads to an increased Aβ levels. | Zhu et al. (2012) [50] |
| Reduced mitochondrial ATP production | Yan et al. (2019) [51] | ||
| miR-210-3p | Upregulation | Clinical marker for MCI and AD | Siedlecki-Wullich et al. (2019) [52] |
| Targets mitochondrial iron sulfur cluster homologue. Decreasing these clusters can reduce activity of mitochondrial enzymes that require iron sulfur clusters. miR-210 can affect aconitase. | Li et al. (2012) [32] | ||
| miR-212 | Downregulation | Increase SIRT1 in aMCI in the frontal cortex. While it may be protective, sustained downregulation can lead to FOX03a mediated apoptosis. | Weinberg et al. (2015) [26] |
| miR-330 | Downregulation | Affects VAV1 and affects mitochondria through MAPK signaling | Zhou et al. (2018) [53] |
| miR-424 | Upregulation | Cortex white matter | Wang et al. (2011) [54] |
| Suppression of ATP levels and mitochondrial integrity through ADP-ribosylation factor-like 2 mRNA. | Duarte et al. (2014) [45] | ||
| miR-425 | Upregulation | BACE1 protein inhibition | Ren et al. (2016) [55] |
| Via RIPK1 causes mitochondrial dysfunction and increased ROS production. Involved in necroptosis. | Hu et al. (2019) [56] |
| miRNA | Change | Potential Role | References |
|---|---|---|---|
| miR-7 | Downregulation | Increased a-SYN/Substantia Nigra | Junn et al. (2009) [61] |
| Reduced binding to 3′UTR of VDAC1 thus upregulation of anion channel and increased ROS production. | Chaudhuri et al. (2016) [62] | ||
| miR-16-1 | Upregulation | Decrease HSP70 leading to an increased a-SYN | Zhang et al. (2014) [63] |
| HSP70 blocks mitochondrial translocation of Bax, membrane permeabilization, and apoptosis. | Radons (2016) [64] | ||
| miR-21 | Upregulation | Upregulated in midbrain and directly targets 3′UTR of LAMP2A. | Martinez and Peplow (2017) [65] |
| Downregulates PTEN and PINK1, key regulators of mitophagy. | Zhang et al. (2010) [66] | ||
| miR-27a/b | Unknown | Increased accumulation and decreased suppression of PINK1 at 3′UTR. Decreased degradation of damaged mitochondria. | Kim et al. (2016) [35] |
| miR-29a | Upregulation | Mitochondrial voltage dependent anion channel | Lungu et al. (2013) [67] |
| miR-29b | Upregulation | Loss of mitochondrial membrane potential | Lungu et al. (2013) [67] |
| miR-30e | Downregulation | Reduced suppression of Nlrp3 | Li et al. (2018) [68] |
| NLRP3 resides in ER but upon stimulation can interact with mitochondria and cause loss of mitochondrial membrane potential, increase ROS production, and calcium dys-homeostasis. | Liu (2018) [69] | ||
| miR-34b/c | Downregulation | Decreased Parkin/DJ-1 leads to increased ROS production in the mitochondria. Decreased ability to reduce MTT. Ballooning of the mitochondria with decreased ATP production. | Miñones-Moyano et al. (2011) [70] |
| miR-124 | Upregulated | Reduced translocation of Bax into mitochondria due to inhibition of Bim. | Wang et al. (2016) [71] |
| miR-126 | Upregulated | Downregulation of IGF-1/PI3K signaling. It targets TOM1, p85beta, insulin receptor substrate 1, CRK | Kim et al. (2014) [72] |
| miR-137 | Slightly Upregulated | Involved in expression of mitophagy receptors FUNDC1 and NIX. Inhibits mitophagy | Li et al. (2014) [73] |
| Li et al. (2017) [74] | |||
| miR138-2-3p | Downregulation | Increased LRRK2 (lysosomal function in astrocytes) | Cardo et al. (2014) [75] |
| LRRK2 localizes in mitochondria and has regulatory function on mitochondrial fission and fusion. Mutations of LRRK2 leads to increased oxidative stress. | Singh et al. (2019) [76] | ||
| miR-144-3p | Downregulation | Besides inhibiting the expression of APP, miR-144-3p is involved in the mitochondrial gene expression of PGC-1α, NRF-1, TFAM. | Li et al. (2016) [77] |
| miR-153 | Downregulation | Decreased mTOR signaling | Doxakis et al. (2010) [78] |
| Decreased mTOR signaling can lead to reduced clearance of dysfunctional mitochondria (ROS producing) and reduces mitochondrial biogenesis. | Weichhart (2018) [79] | ||
| miR-155 | Upregulation | Suppression of SOCS-1 and SOC-3 (anti-inflammatory molecules) | Caggiu et al. (2018) [80] |
| SOCS-1 suppresses damage to mitochondrial membrane and oxidative stress. | Du et al. (2017) [81] | ||
| miR-205 | Downregulation | Increased LRRK2 | Cho et al. (2013) [82] |
| LRRK2 localizes in mitochondria and has regulatory function on mitochondrial fission and fusion. Mutations of LRRK2 leads to increased oxidative stress. | Singh et al. (2019) [76] | ||
| miR-494 | Upregulation | Decreased DJ-1 (mitochondrial damage) | Xiong et al. (2014) [83] |
| miR-433 | Binding inhibited | Increasing FGF20 (cell death) | Wang et al. (2008) [84] |
| FGF20 increases translation of alpha-synuclein, which in turn disrupts calcium exchange between mitochondria and ER. There is a reduction in mitochondrial ATP production. | Paillusson et al. (2017) [85] | ||
| miR-4639-5p | Upregulation | Decreased DJ-1 leads to mitochondrial fragmentation | Chen et al. (2017) [86] |
| miRNA | Change | Potential Role | References |
|---|---|---|---|
| miR-10b-5p | Upregulation | Targets HOXD10, NF1, KLF4. | Hoss et al. (2015) [96] |
| Overexpression of KLF4 can lead to increased ROS production in mitochondria in already impaired mitochondria. KLF4 can also induce mitochondrial fusion. | Wang et al. (2018) [97] | ||
| miR-22 | Downregulation | Reduced caspase activation and targets HDAC4, Redd1, Rcor1, and Rgs2. | Jovicic et al. (2013) [27] |
| Increased Bcl-xl expression leads to decreased pro-apoptotic proteins thus possibly allowing damaged mitochondria to survive. | Liu et al. (2019) [98] | ||
| miR-29c | Downregulation | Normally upregulates p53 levels by suppressing p85 alpha. | Lee et al. (2011) [99] |
| Reduced levels of p53 may not lead to apoptotic events of damaged mitochondria that have increased ROS production. | Holley and Clair (2009) [100] | ||
| miR-124 | Downregulation | Decreased PGC-1α | Liu et al. (2015) [101] |
| Decreased mitochondrial biogenesis and dysfunction (increased ROS, decreased ATP synthesis) | Jin and Johnson (2010) [102] | ||
| miR-128a | Downregulation | Tumor repression, apoptosis, epileptic seizure repression | Kocerha et al. (2014) [103] |
| Targets FADD. Decreased FADD can prevent apoptosis in damaged mitochondria. | Cavalcante et al. (2019) [104] | ||
| miR-132 | Downregulation | Decreased AGO2 function | Fukuoka et al. (2018) [105] |
| miR-146a | Downregulation | HTT gene downregulates miR-146a | Ghose et al. (2011) [106] |
| Affect IRAK-1 and TRAF-6 which are inflammation mediators. | Rippo et al. (2014) [47] | ||
| TRAF-6 restricts mitochondrial translocation | Zhang et al. (2016) [107] | ||
| miR-196a | Upregulation | Increased BDNF and ANX1A. Increased mitochondrial fusion. Decreased PGC-1α and CBP. | Kunkanjanawan et al. (2016) [108] |
| miR-218 | Downregulation | Normally downregulates PRKN. However, in diseased state upregulates PRKN. There is increased mitochondrial degradation and mitophagy as a result. | Di Rita et al. (2019) [109] |
| miR-222-3p | Upregulation | Targets MMP1, PTEN, SOD2, and other targets | Díez-Planelles et al. (2016) [110] |
| Increase THBS1 thus increasing mitochondrial calcium level. Reduces mitochondrial membrane | Zhao et al. (2019) [111] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
John, A.; Kubosumi, A.; Reddy, P.H. Mitochondrial MicroRNAs in Aging and Neurodegenerative Diseases. Cells 2020, 9, 1345. https://doi.org/10.3390/cells9061345
John A, Kubosumi A, Reddy PH. Mitochondrial MicroRNAs in Aging and Neurodegenerative Diseases. Cells. 2020; 9(6):1345. https://doi.org/10.3390/cells9061345
Chicago/Turabian StyleJohn, Albin, Aaron Kubosumi, and P. Hemachandra Reddy. 2020. "Mitochondrial MicroRNAs in Aging and Neurodegenerative Diseases" Cells 9, no. 6: 1345. https://doi.org/10.3390/cells9061345
APA StyleJohn, A., Kubosumi, A., & Reddy, P. H. (2020). Mitochondrial MicroRNAs in Aging and Neurodegenerative Diseases. Cells, 9(6), 1345. https://doi.org/10.3390/cells9061345






