Contribution of a GATA4-Expressing Hematopoietic Progenitor Lineage to the Adult Mouse Endothelium
Abstract
1. Introduction
2. Materials and Methods
2.1. Analytical Flow Cytometry
2.2. Immunofluorescence and Confocal Microscopy
2.3. Placenta/Fetal Liver Chimaeras
2.4. Labeling of the SCL Lineage
3. Results
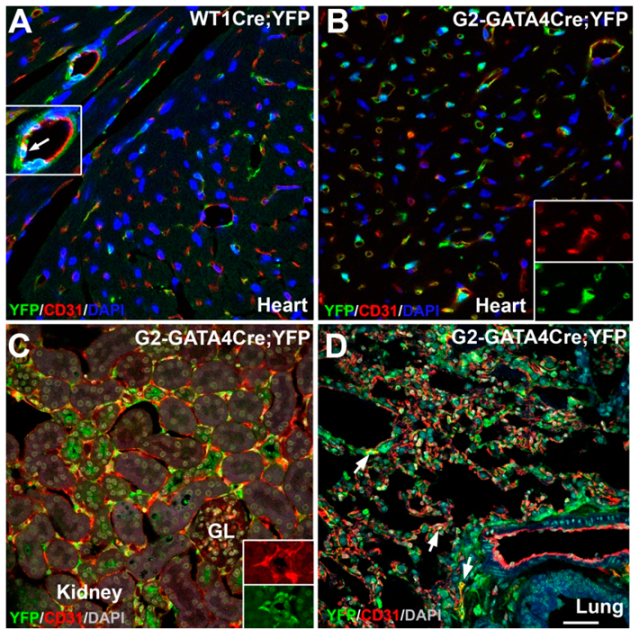
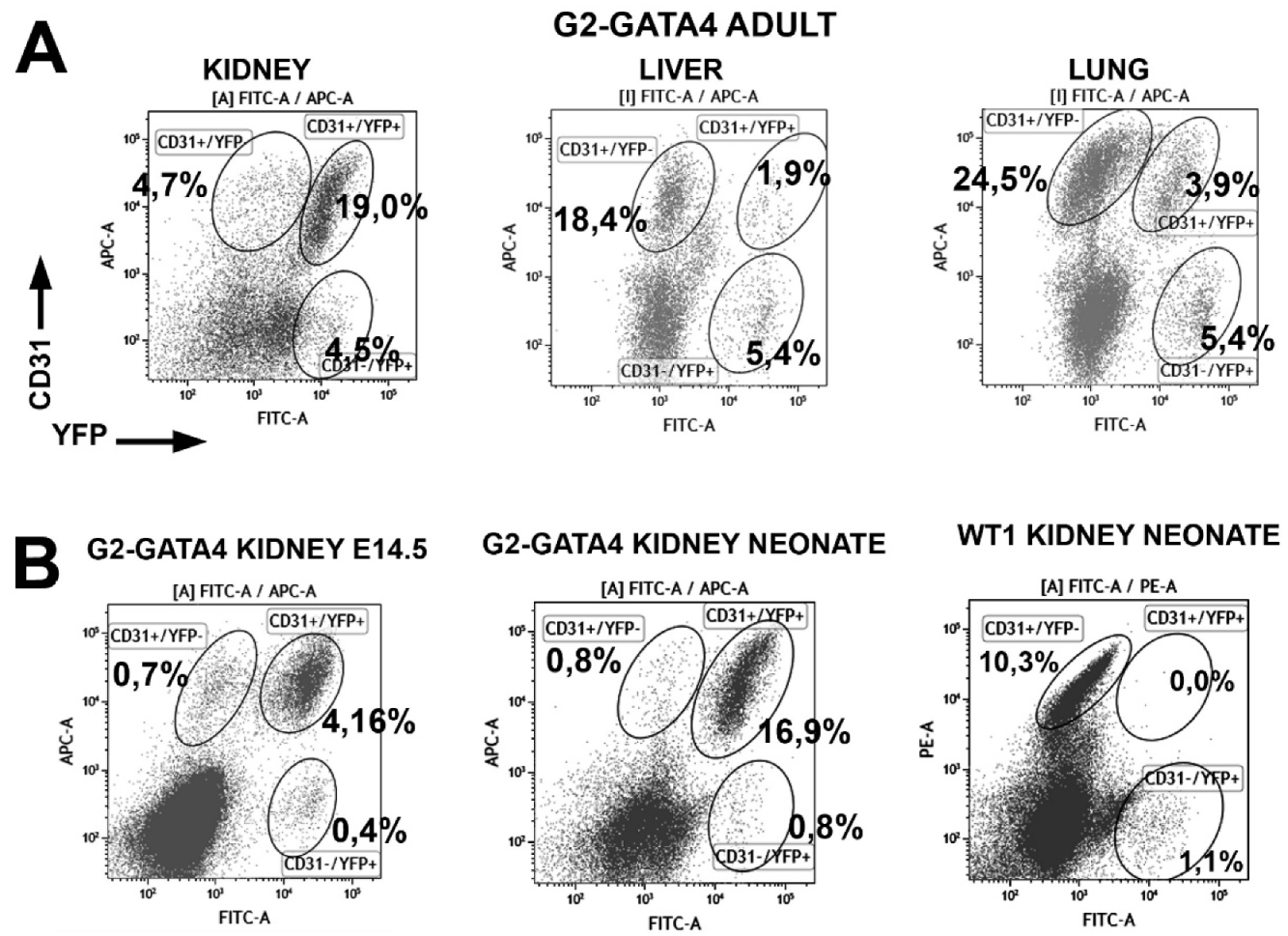
3.1. The G2-GATA4 Cell Lineage Constitutes a Major Part of the Adult Cardiac and Kidney Endothelium
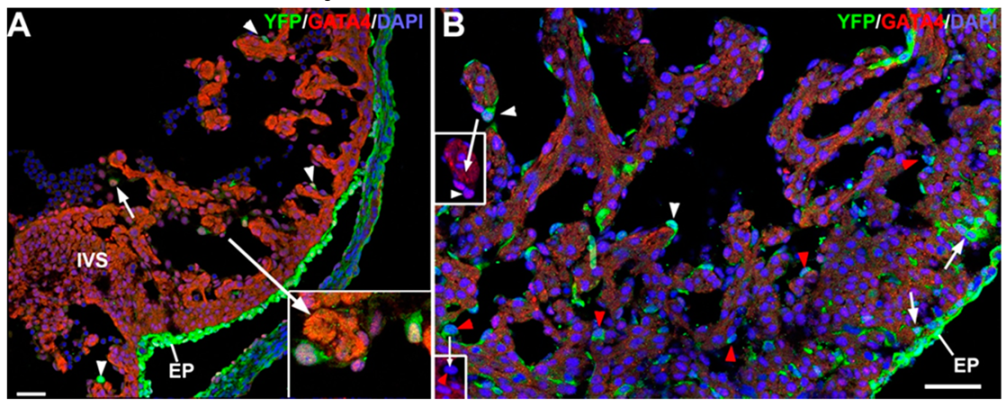
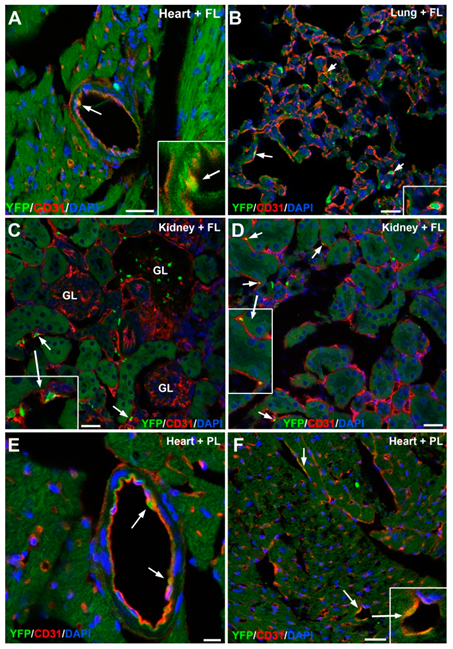
3.2. The G2-GATA4 Cell Lineage Contribution to the Cardiac Vasculature Starts During the Earliest Stages of Coronary Development and it is Related with Endocardium
3.3. The G2-GATA4 Cell Lineage Contribution to the Embryonic Endothelium of the Kidney Derives from the Lateral Mesoderm
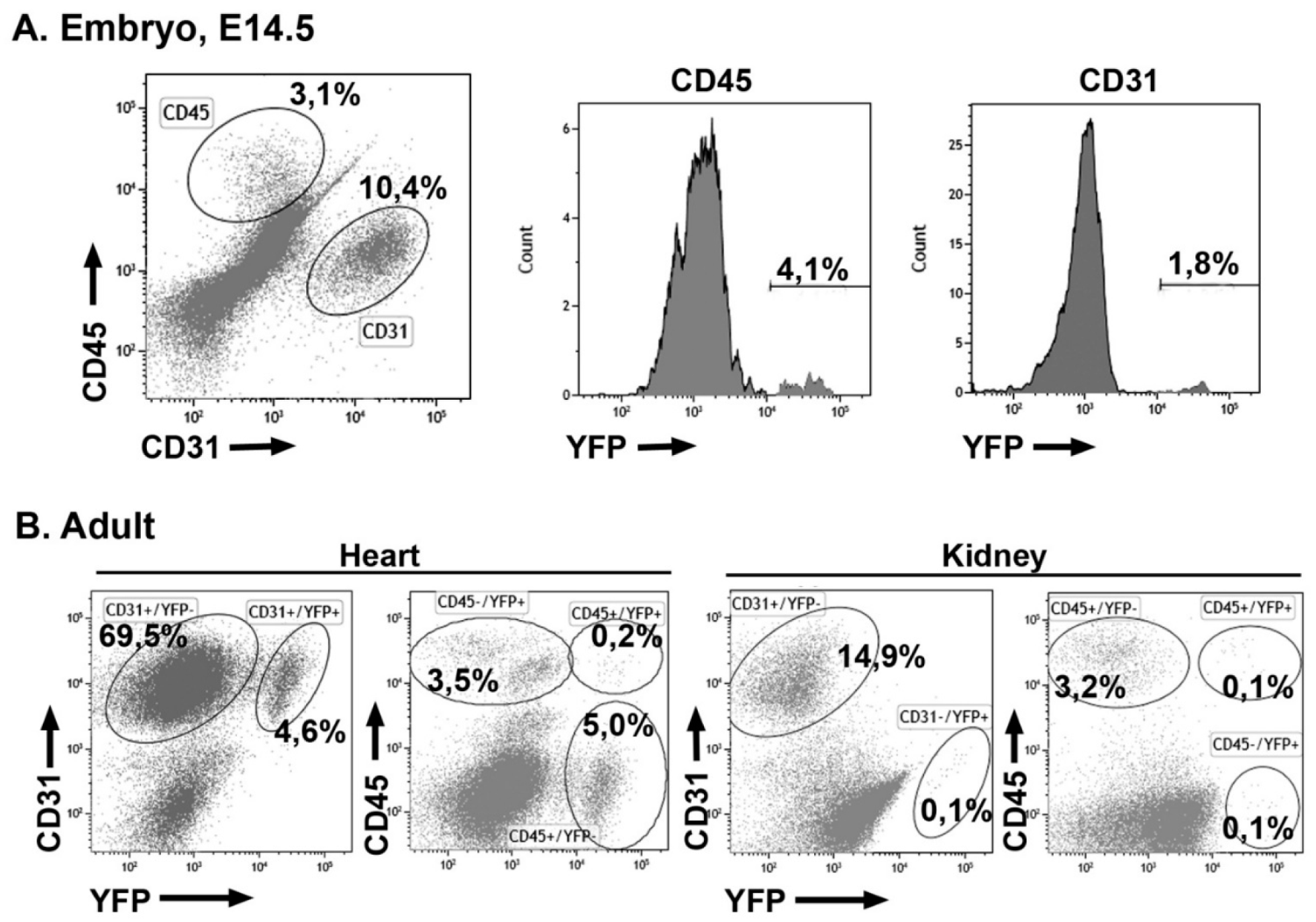
3.4. Early Hematopoietic Progenitors Contribute to the Coronary Endothelium
3.5. Embryonic G2-GATA4 Lineage Circulating Progenitors can be Recruited into the Endothelium in Postnatal Stages
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brand-Saberi, B.; Seifert, R.; Grim, M.; Wilting, J.; Kühlewein, M.; Christ, B. Blood vessel formation in the avian limb bud involves angioblastic and angiotrophic growth. Dev. Dyn. 1995, 202, 181–194. [Google Scholar] [CrossRef]
- Plein, A.; Fantin, A.; Denti, L.; Pollard, J.W.; Ruhrberg, C. Erythro-myeloid progenitors contribute endothelial cells to blood vessels. Nature 2018, 562, 223–228. [Google Scholar] [CrossRef]
- Asahara, T.; Kawamoto, A.; Masuda, H. Concise review: Circulating endothelial progenitor cells for vascular medicine. Stem Cells 2011, 29, 1650–1655. [Google Scholar] [CrossRef]
- Cañete, A.; Comaills, V.; Prados, I.; Castro, A.M.; Hammad, S.; Ybot-Gonzalez, P.; Bockamp, E.; Hengstler, J.G.; Gottgens, B.; Sánchez, M.J. Characterization of a fetal liver cell population endowed with long-term multiorgan endothelial reconstitution potential. Stem Cells 2017, 35, 507–521. [Google Scholar] [CrossRef]
- Cañete, A.; Carmona, R.; Ariza, L.; Sánchez, M.J.; Rojas, A.; Muñoz-Chápuli, R. A population of hematopoietic stem cells derives from GATA4-expressing progenitors located in the placenta and lateral mesoderm of mice. Haematologica 2017, 102, 647–655. [Google Scholar] [CrossRef][Green Version]
- Caprioli, A.; Jaffredo, T.; Gautier, R.; Dubourg, C.; Dieterlen-Lièvre, F. Blood-borne seeding by hematopoietic and endothelial precursors from the allantois. Proc. Natl. Acad. Sci. USA 1998, 17, 1641–1646. [Google Scholar] [CrossRef]
- Pardanaud, L.; Eichmann, A. Identification, emergence and mobilization of circulating endothelial cells or progenitors in the embryo. Development 2006, 133, 2527–2537. [Google Scholar] [CrossRef][Green Version]
- Katz, T.C.; Singh, M.K.; Degenhardt, K.; Rivera-Feliciano, J.; Johnson, R.L.; Epstein, J.A.; Tabin, C. Distinct compartments of the proepicardial organ give rise to coronary vascular endothelial cells. Dev. Cell 2012, 22, 639–650. [Google Scholar] [CrossRef]
- Cano, E.; Carmona, R.; Ruiz-Villalba, A.; Rojas, A.; Chau, Y.; Hastie, N.D.; Muñoz-Chápuli, R.; Pérez-Pomares, J.M. Proepicardial contribution to mammalian coronary endothelium patterns coronary blood vessels. Proc. Natl. Acad. Sci. USA 2016, 113, 656–661. [Google Scholar] [CrossRef]
- Wu, B.; Zhang, Z.; Lui, W.; Chen, X.; Wang, Y.; Chamberlain, A.A.; Moreno-Rodriguez, R.A.; Markwald, R.R.; O’Rourke, B.P.; Sharp, D.J.; et al. Endocardial cells form the coronary arteries by angiogenesis through myocardial-endocardial VEGF signaling. Cell 2012, 151, 1083–1096. [Google Scholar] [CrossRef]
- Red-Horse, K.; Ueno, H.; Weissman, I.L.; Krasnow, M.A. Coronary arteries form by developmental reprogramming of venous cells. Nature 2010, 464, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Hu, T.; Zhang, H.; He, L.; Huang, X.; Liu, Q.; Yu, W.; He, L.; Yang, Z.; Zhang, Z.; et al. Subepicardial endothelial cells invade the embryonic ventricle wall to form coronary arteries. Cell Res. 2013, 23, 1075–1090. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Pu, W.; Li, G.; Huang, X.; He, L.; Tian, X.; Liu, Q.; Zhang, L.; Wu, S.M.; Sucov, H.M.; et al. Endocardium minimally contributes to coronary endothelium in the embryonic ventricular free walls. Circ. Res. 2016, 118, 1880–1893. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.I.; Sharma, B.; Akerberg, B.N.; Numi, H.J.; Kivelä, R.; Saharinen, P.; Aghajanian, H.; McKay, A.S.; Bogard, P.E.; Chang, A.H.; et al. The sinus venosus contributes to coronary vasculature through VEGFC-stimulated angiogenesis. Development 2014, 141, 4500–4512. [Google Scholar] [CrossRef]
- Tian, X.; Pu, W.T.; Zhou, B. Cellular origin and developmental program of coronary angiogenesis. Circ. Res. 2015, 116, 515–530. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Chang, A.; Red-Horse, K. Coronary artery development: Progenitor cells and differentiation pathways. Annu. Rev. Physiol. 2017, 79, 1–19. [Google Scholar] [CrossRef]
- Carmona, R.; Barrena, S.; López Gambero, A.J.; Rojas, A.; Muñoz-Chápuli, R. Epicardial cell lineages and the origin of the coronary endothelium. FASEB J. 2020, 34. [Google Scholar] [CrossRef]
- Del Monte, G.; Chamorro, J.; Guadix, J.A.; MacGrogan, D.; Burch, J.; Muñoz-Chápuli, R.; Pérez-Pomares, J.M.; de la Pompa, J.L. Differential notch signalling in the epicardium is required for cardiac inflow development and coronary vessel morphogenesis. Circ. Res. 2011, 108, 824–836. [Google Scholar] [CrossRef]
- Wessels, A.; van den Hoff, M.J.; Adamo, R.F.; Phelps, A.L.; Lockhart, M.M.; Sauls, K.; Briggs, L.E.; Norris, R.A.; van Wijk, B.; Pérez-Pomares, J.M.; et al. Epicardially derived fibroblasts preferentially contribute to the parietal leaflets of the atrioventricular valves in the murine heart. Dev. Biol. 2012, 366, 111–124. [Google Scholar] [CrossRef]
- Casanova, J.C.; Travisano, S.; de la Pompa, J.L. Epithelial-to-mesenchymal transition in epicardium is independent of Snail1. Genesis 2013, 51, 32–40. [Google Scholar] [CrossRef]
- Cano, E.; Carmona, R.; Muñoz-Chápuli, R. WT1-expressing progenitors contribute to multiple tissues in the developing lung. Am. J. Physiol. Lung Cell Mol. Physiol. 2013, 305, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Rojas, A.; De Val, S.; Heidt, A.B.; Xu, S.M.; Bristow, J.; Black, B.L. Gata4 expression in lateral mesoderm is downstream of BMP4 and is activated directly by Forkhead and GATA transcription factors through a distal enhancer element. Development 2005, 132, 3405–3417. [Google Scholar] [CrossRef] [PubMed]
- Delgado, I.; Carrasco, M.; Cano, E.; Carmona, R.; García-Carbonero, R.; Marín-Gómez, L.M.; Soria, B.; Martín, F.; Cano, D.A.; Muñoz-Chápuli, R.; et al. GATA4 loss in the septum transversum mesenchyme promotes liver fibrosis in mice. Hepatology 2014, 59, 2358–2370. [Google Scholar] [CrossRef] [PubMed]
- Merki, E.; Zamora, M.; Raya, A.; Kawakami, Y.; Wang, J.; Zhang, X.; Burch, J.; Kubalak, S.W.; Kaliman, P.; Izpisua Belmonte, J.C.; et al. Epicardial retinoid X receptor alpha is required for myocardial growth and coronary artery formation. Proc. Natl. Acad. Sci. USA 2005, 102, 18455–18460. [Google Scholar] [CrossRef]
- Jiao, K.; Kulessa, H.; Tompkins, K.; Zhou, Y.; Batts, L.; Baldwin, H.S.; Hogan, B.L. An essential role of Bmp4 in the atrioventricular septation of the mouse heart. Genes Dev. 2003, 17, 2362–2367. [Google Scholar] [CrossRef]
- Eley, L.; Alqahtani, A.M.; MacGrogan, D.; Richardson, R.V.; Murphy, L.; Salguero-Jimenez, A.; Sintes Rodriguez San Pedro, M.; Tiurma, S.; McCutcheon, L.; Gilmore, A.; et al. A novel source of arterial valve cells linked to bicuspid a ortic valve without raphe in mice. Elife 2018, 7, e34110. [Google Scholar] [CrossRef]
- Mifflin, J.J.; Dupuis, L.E.; Alcala, N.E.; Russell, L.G.; Kern, C.B. Intercalated cushion cells within the cardiac outflow tract are derived from the myocardial troponin T type 2 (Tnnt2) Cre lineage. Dev. Dyn. 2018, 247, 1005–1017. [Google Scholar] [CrossRef]
- Peake, K.; Manning, J.; Lewis, C.A.; Barr, C.; Rossi, F.; Krieger, C.J. Busulfan as a myelosuppressive agent for generating stable high-level bone marrow chimerism in mice. Vis. Exp. 2015, 1, e52553. [Google Scholar] [CrossRef]
- Göthert, J.R.; Gustin, S.E.; Hall, M.A.; Green, A.R.; Göttgens, B.; Izon, D.J.; Begley, C.G. In vivo fate-tracing studies using the Scl stem cell enhancer: Embryonic hematopoietic stem cells significantly contribute to adult hematopoiesis. Blood 2005, 105, 2724–2732. [Google Scholar] [CrossRef]
- Tomanek, R.J.; Zheng, W. Role of growth factors in coronary morphogenesis. Tex. Heart Inst. J. 2002, 29, 250–254. [Google Scholar]
- Hu, Y.; Li, M.; Göthert, J.R.; Gomez, R.; Sequeira-Lopez, M.L. Hemovascular Progenitors in the Kidney Require Sphingosine-1-Phosphate Receptor 1 for Vascular Development. J. Am. Soc. Nephrol. 2016, 27, 1984–1995. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, T.; Sequeira-Lopez, M.L.S. Development of the renal vasculature. Semin. Cell Dev. Biol. 2019, 91, 132–146. [Google Scholar] [CrossRef] [PubMed]
- Munro, D.A.D.; Hohenstein, P.; Davies, J.A. Cycles of vascular plexus formation within the nephrogenic zone of the developing mouse kidney. Sci. Rep. 2017, 7, 3273. [Google Scholar] [CrossRef]
- Halt, K.J.; Pärssinen, H.E.; Junttila, S.M.; Saarela, U.; Sims-Lucas, S.; Koivunen, P.; Myllyharju, J.; Quaggin, S.; Skovorodkin, I.N.; Vainio, S.J. CD146(+) cells are essential for kidney vasculature development. Kidney Int. 2016, 90, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Aird, W.C. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ. Res. 2007, 100, 174–190. [Google Scholar] [CrossRef]
- Aird, W.C. Phenotypic heterogeneity of the endothelium: II. Representative vascular beds. Circ. Res. 2007, 100, 158–173. [Google Scholar] [CrossRef]
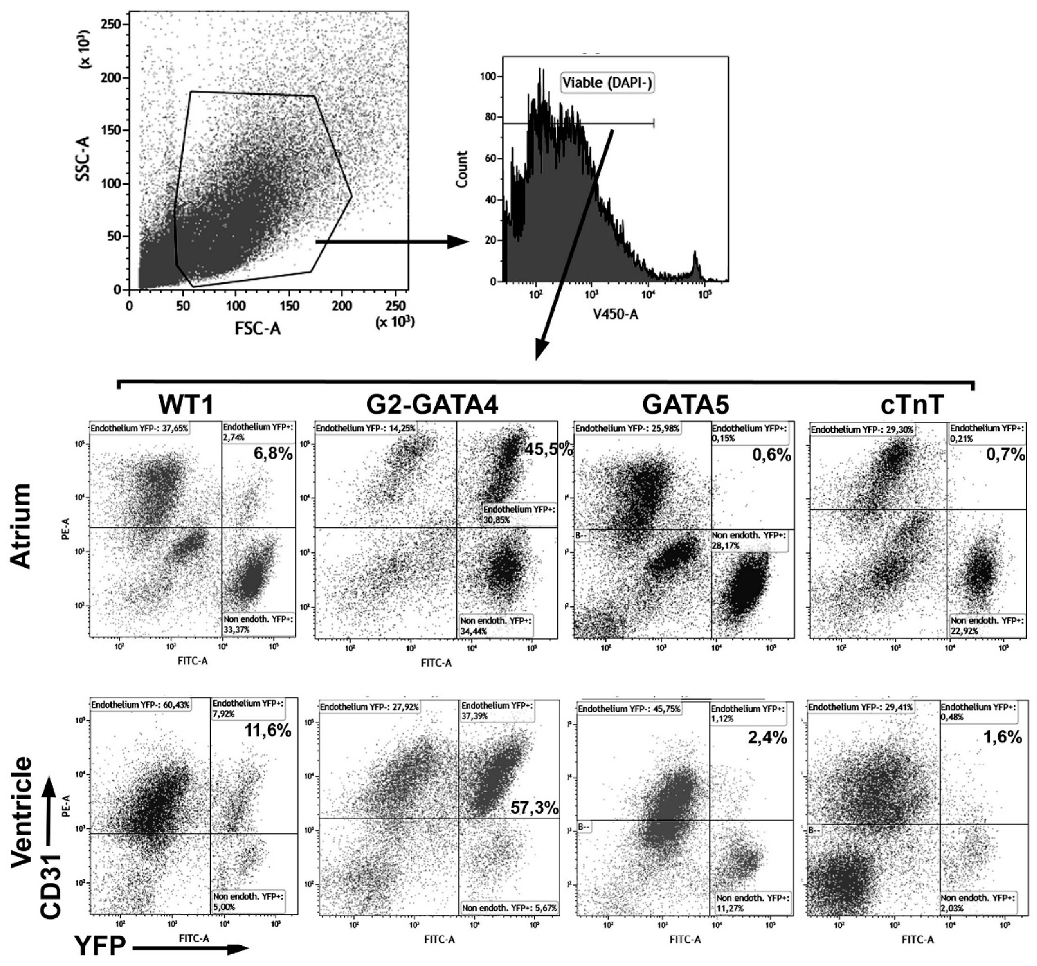
| Lineage | WT1 | G2-GATA4 | GATA5 | cTnT | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CD31 | CD90 | CD140a | CD31 | CD90 | CD140a | CD31 | CD90 | CD140a | CD31 | CD90 | CD140a | ||
| Atrium | % | 22.7 ± 4.1 (n = 8) | 10.1 ± 2.2 (n = 6) | 20.5 ± 4.2 (n = 6) | 29.9 ± 3.3 (n = 9) | 9.1 ± 1.5 (n = 8) | 19.9 ± 3.9 (n = 8) | 30.6 ± 3.2 (n = 4) | 7.7 ± 0.8 (n = 3) | 21.8 ± 6.1 (n = 2) | 26.4 ± 4.1 (n = 4) | 10.1 ± 1.2 (n = 3) | 18.6 ± 4.6 (n = 3) |
| %YFP | 7.9 ± 1.1 | 23.3 ± 4.3 | 56.0 ± 7.8 | 55.2 ± 2.8 | 52.2 ± 6.2 | 60.9 ± 10.9 | 0.67 ± 0.16 | 50.3 ± 6.8 | 38.4 ± 9.7 | 0.58 ± 0.11 | 20.4 ± 0.71 | 18.9 ± 8.1 | |
| Ventricle | % | 60.7 ± 3.8 (n = 8) | 6.1 ± 0.5 (n = 8) | 6.7 ± 1.1 (n = 8) | 72.7 ± 2.1 (n = 9) | 6.5 ± 0.6 (n = 8) | 4.4 ± 0.5 (n = 8) | 58.3 ± 6.3 (n = 4) | 6.6 ± 1.5 (n = 4) | 3.6 ± 0.6 (n = 4) | 60.6 ± 4.0 (n = 4) | 6.8 ± 1.0 (n = 4) | 6.9 ± 0.8 (n = 4) |
| %YFP | 9.9 ± 0.7 | 37.4 ± 2.7 | 31.4 ± 4.7 | 53.3 ± 3.1 | 57.4 ± 6.8 | 52.3 ± 5.9 | 1.6 ± 0.74 | 63.8 ± 2.5 | 38.7 ± 4.1 | 0.97 ± 0.092 | 35.1 ± 6.6 | 14.0 ± 2.7 | |
| Ventricle | Lung | Liver | Kidney | ||
|---|---|---|---|---|---|
| WT1 Neonates | % CD31 | 11.3 ± 2.6 (n = 3) | 6.2 ± 2.3 (n = 3) | 8.1 ± 0.3 (n = 3) | 15.6 ± 2.1 (n = 3) |
| % CD31+/YFP+ | 10.4 ± 1.3 | 2.9 ± 0.9 | 1.0 ± 0.6 | 0.03 ± 0.03 | |
| WT1 Adults | % CD31 | 60.7 ± 3.8 (n = 8) | 35.6 ± 0.9 (n = 3) | 28.5 ± 1.09 (n = 3) | 22.6 ± 3.8 (n = 3) |
| % CD31+/YFP+ | 9.9 ± 0.7 | 4.1 ± 1.2 | 5.6 ± 1.5 | 0.46 ± 0.07 | |
| G2-GATA4 Neonates | % CD31 | 17.7 ± 1.6 (n = 12) | 5.2 ± 0.8 (n = 7) | 8.9 ± 2.3 (n = 7) | 16.9 ± 2.1 (n = 5) |
| % CD31+/YFP+ | 34.5 ± 2.3 | 10.8 ± 1.4 | 9.6 ± 2.1 | 92.7 ± 1.0 | |
| G2-GATA4 Adults | % CD31 | 72.7 ± 2.1 (n = 9) | 37.8 ± 2.69 (n = 5) | 34.5 ± 5.49 (n = 3) | 17.8 ± 6.2 (n = 3) |
| % CD31+/YFP+ | 53.3 ± 3.1 | 15.6 ± 1.8 | 9.9 ± 4.3 | 86.7 ± 4.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carmona, R.; Díaz del Moral, S.; Barrena, S.; Muñoz-Chápuli, R. Contribution of a GATA4-Expressing Hematopoietic Progenitor Lineage to the Adult Mouse Endothelium. Cells 2020, 9, 1257. https://doi.org/10.3390/cells9051257
Carmona R, Díaz del Moral S, Barrena S, Muñoz-Chápuli R. Contribution of a GATA4-Expressing Hematopoietic Progenitor Lineage to the Adult Mouse Endothelium. Cells. 2020; 9(5):1257. https://doi.org/10.3390/cells9051257
Chicago/Turabian StyleCarmona, Rita, Sandra Díaz del Moral, Silvia Barrena, and Ramón Muñoz-Chápuli. 2020. "Contribution of a GATA4-Expressing Hematopoietic Progenitor Lineage to the Adult Mouse Endothelium" Cells 9, no. 5: 1257. https://doi.org/10.3390/cells9051257
APA StyleCarmona, R., Díaz del Moral, S., Barrena, S., & Muñoz-Chápuli, R. (2020). Contribution of a GATA4-Expressing Hematopoietic Progenitor Lineage to the Adult Mouse Endothelium. Cells, 9(5), 1257. https://doi.org/10.3390/cells9051257






