Mesenchymal Stem Cell Derived Extracellular Vesicles for Tissue Engineering and Regenerative Medicine Applications
Abstract
1. Introduction
2. EV Biogenesis and Characterisation
3. EV Isolation Methods
4. MSC-EVs in Tissue Engineering and Regeneration
4.1. Nervous Regeneration
4.2. Cardiac Regeneration
4.3. Bone Regeneration
4.4. Cartilage Regeneration
4.5. Kidney Regeneration
4.6. Liver Regeneration
4.7. Muscle Regeneration
4.8. Wound Healing
4.9. The Regenerative Effect of MSC-EVs on Various Tissues
5. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Black, C.K.; Termanini, K.M.; Aguirre, O.; Hawksworth, J.S.; Sosin, M. Solid organ transplantation in the 21st century. Ann. Transl. Med. 2018, 6, 409. [Google Scholar] [CrossRef]
- Platt, J.; Cascalho, M. New and old technologies for organ replacement. Curr. Opin. Organ Transplant. 2013, 18, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef]
- Fitzsimmons, R.; Mazurek, M.S.; Soos, A.; Simmons, C.A. Mesenchymal stromal/stem cells in regenerative medicine and tissue engineering. Stem Cells Int. 2018, 2018, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Cossu, G.; Birchall, M.; Brown, T.; De Coppi, P.; Culme-Seymour, E.; Gibbon, S.; Hitchcock, J.; Mason, C.; Montgomery, J.; Morris, S.; et al. Lancet Commission: stem cells and regenerative medicine. Lancet 2018, 391, 883–910. [Google Scholar] [CrossRef]
- Howard, D.; Buttery, L.; Shakesheff, K.M.; Roberts, S.J. Tissue engineering: strategies, stem cells and scaffolds. J. Anat. 2008, 213, 66–72. [Google Scholar] [CrossRef]
- Le Blanc, K.; Ringdén, O. Immunobiology of human mesenchymal stem cells and future use in hematopoietic stem cell transplantation. Boil. Blood Marrow Transplant. 2005, 11, 321–334. [Google Scholar] [CrossRef]
- Samsonraj, R.M.; Raghunath, M.; Nurcombe, V.; Hui, J.H.; Van Wijnen, A.J.; Cool, S.M. Concise review: multifaceted characterization of human mesenchymal stem cells for use in regenerative medicine. STEM CELLS Transl. Med. 2017, 6, 2173–2185. [Google Scholar] [CrossRef]
- O’Brien, T.; Creane, M.; Windebank, A.J.; Terzic, A.; Dietz, A.B. Translating stem cell research to the clinic: a primer on translational considerations for your first stem cell protocol. Stem Cell Res. Ther. 2015, 6, 146. [Google Scholar] [CrossRef]
- Amer, M.H.; Rose, F.R.A.J.; Shakesheff, K.M.; Modo, M.; White, L. Translational considerations in injectable cell-based therapeutics for neurological applications: concepts, progress and challenges. NPJ Regen. Med. 2017, 2, 23. [Google Scholar] [CrossRef]
- Volarevic, V.; Markovic, B.S.; Gazdic, M.; Volarevic, A.; Jovicic, N.; Arsenijević, N.; Armstrong, L.; Djonov, V.; Lako, M.; Stojkovic, M. Ethical and safety issues of stem cell-based therapy. Int. J. Med Sci. 2018, 15, 36–45. [Google Scholar] [CrossRef]
- MacPherson, A.; Kimmelman, J. Ethical development of stem-cell-based interventions. Nat. Med. 2019, 25, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Zakrzewski, W.; Dobrzyński, M.; Szymonowicz, M.; Rybak, Z. Stem cells: past, present, and future. Stem Cell Res. Ther. 2019, 10, 68. [Google Scholar] [CrossRef] [PubMed]
- Gould, S.J.; Raposo, G. As we wait: coping with an imperfect nomenclature for extracellular vesicles. J. Extracell. Vesicles 2013, 2, 2892. [Google Scholar] [CrossRef] [PubMed]
- Yuana, Y.; Sturk, A.; Nieuwland, R. Extracellular vesicles in physiological and pathological conditions. Blood Rev. 2013, 27, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, A.-K.; Giebel, B. Exosomes: Small vesicles participating in intercellular communication. Int. J. Biochem. Cell Boil. 2012, 44, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Yáñez-Mó, M.; Siljander, P.; Andreu, Z.; Zavec, A.B.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef]
- Van Der Pol, E.; Böing, A.N.; Harrison, P.; Sturk, A.; Nieuwland, R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol. Rev. 2012, 64, 676–705. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Han, C.; Sun, X.; Liu, L.; Jiang, H.; Shen, Y.; Xu, X.; Li, J.; Zhang, G.; Huang, J.; Lin, Z.; et al. Exosomes and their therapeutic potentials of stem cells. Stem Cells Int. 2015, 2016, 1–11. [Google Scholar] [CrossRef]
- Tolar, J.; Le Blanc, K.; Keating, A.; Blazar, B.R. Concise review: hitting the right spot with mesenchymal stromal cells. STEM CELLS 2010, 28, 1446–1455. [Google Scholar] [CrossRef] [PubMed]
- Lai, R.C.; Yeo, R.W.Y.; Lim, S.K. Mesenchymal stem cell exosomes. Semin. Cell Dev. Boil. 2015, 40, 82–88. [Google Scholar] [CrossRef]
- Lener, T.; Gimona, M.; Aigner, L.; Börger, V.; Buzas, E.; Camussi, G.; Chaput, N.; Chatterjee, D.; Court, F.A.; Del Portillo, H.A.; et al. Applying extracellular vesicles based therapeutics in clinical trials—An ISEV position paper. J. Extracell. Vesicles 2015, 4, 30087. [Google Scholar] [CrossRef] [PubMed]
- Tögel, F.; Weiss, K.; Yang, Y.; Hu, Z.; Zhang, P.; Westenfelder, C. Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. Am. J. Physiol. Physiol. 2007, 292, F1626–F1635. [Google Scholar] [CrossRef] [PubMed]
- Yeo, R.W.Y.; Lai, R.C.; Zhang, B.; Tan, S.S.; Yin, Y.; Teh, B.J.; Lim, S.K. Mesenchymal stem cell: an efficient mass producer of exosomes for drug delivery. Adv. Drug Deliv. Rev. 2013, 65, 336–341. [Google Scholar] [CrossRef]
- Park, K.-S.; Bandeira, E.; Shelke, G.V.; Lässer, C.; Lötvall, J. Enhancement of therapeutic potential of mesenchymal stem cell-derived extracellular vesicles. Stem Cell Res. Ther. 2019, 10, 288. [Google Scholar] [CrossRef]
- Catalano, M.; O’Driscoll, L. Inhibiting extracellular vesicles formation and release: a review of EV inhibitors. J. Extracell. Vesicles 2019, 9, 1703244. [Google Scholar] [CrossRef]
- Shifrin, D.A.; Beckler, M.D.; Coffey, R.J.; Tyska, M.J. Extracellular vesicles: communication, coercion, and conditioning. Mol. Boil. Cell 2013, 24, 1253–1259. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: biogenesis, biologic function and clinical potential. Cell Biosci. 2019, 9, 19. [Google Scholar] [CrossRef]
- Caruso, S.; Poon, I.K.H. Apoptotic cell-derived extracellular vesicles: more than just debris. Front. Immunol. 2018, 9, 1486. [Google Scholar] [CrossRef]
- Mobarrez, F.; Sjövik, C.; Soop, A.; Hållström, L.; Frostell, C.; Pisetsky, D.S.; Wallén, H. CD40L expression in plasma of volunteers following LPS administration: a comparison between assay of CD40L on platelet microvesicles and soluble CD40L. Platelets 2014, 26, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, D.; Fenix, A.M.; Franklin, J.L.; Higginbotham, J.N.; Zhang, Q.; Zimmerman, L.J.; Liebler, D.C.; Ping, J.; Liu, Q.; Evans, R.; et al. Reassessment of exosome composition. Cell 2019, 177, 428–445.e18. [Google Scholar] [CrossRef] [PubMed]
- Crowley, L.; Marfell, B.J.; Scott, A.P.; Waterhouse, N.J. Quantitation of apoptosis and necrosis by annexin v binding, propidium iodide uptake, and flow cytometry. Cold Spring Harb. Protoc. 2016, 2016. [Google Scholar] [CrossRef]
- Record, M.; Silvente-Poirot, S.; Poirot, M.; Wakelam, M. Extracellular vesicles: lipids as key components of their biogenesis and functions. J. Lipid Res. 2018, 59, 1316–1324. [Google Scholar] [CrossRef]
- Daly, M.; O’Driscoll, L. MicroRNA Profiling of exosomes. Adv. Struct. Saf. Stud. 2016, 1509, 37–46. [Google Scholar]
- Trionfini, P.; Benigni, A.; Remuzzi, G. MicroRNAs in kidney physiology and disease. Nat. Rev. Nephrol. 2014, 11, 23–33. [Google Scholar] [CrossRef]
- Pfeifer, P.; Werner, N.; Jansen, F. Role and function of microRNAs in extracellular vesicles in cardiovascular biology. BioMed Res. Int. 2015, 2015, 1–11. [Google Scholar] [CrossRef]
- Lee, Y.S.; Dutta, A. MicroRNAs in cancer. Annu. Rev. Pathol. 2009, 4, 199–227. [Google Scholar] [CrossRef]
- Gardiner, C.; Di Vizio, L.; Sahoo, S.; Théry, C.; Witwer, K.W.; Wauben, M.; Hill, A.F. Techniques used for the isolation and characterization of extracellular vesicles: results of a worldwide survey. J. Extracell. Vesicles 2016, 5, 27066. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, N.; Whiteside, T.L.; Reichert, T. Challenges in exosome isolation and analysis in health and disease. Int. J. Mol. Sci. 2019, 20, 4684. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Jin, K.; Gao, L.; Zhang, Z.; Li, F.; Zhou, F.; Zhang, L. Methods and technologies for exosome isolation and characterization. Small Methods 2018, 2, 1800021. [Google Scholar] [CrossRef]
- Doyle, L.M.; Wang, M.Z. Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef] [PubMed]
- Konoshenko, M.Y.; Lekchnov, E.A.; Vlassov, A.V.; Laktionov, P.P. Isolation of extracellular vesicles: general methodologies and latest trends. BioMed Res. Int. 2018, 2018, 8545347. [Google Scholar] [CrossRef] [PubMed]
- García-Manrique, P.; Matos, M.; Gutierrez, G.; Pazos, C.; Blanco-López, M.C. Therapeutic biomaterials based on extracellular vesicles: classification of bio-engineering and mimetic preparation routes. J. Extracell. Vesicles 2018, 7, 1422676. [Google Scholar] [CrossRef]
- Jang, S.C.; Kim, O.Y.; Yoon, C.M.; Choi, D.-S.; Roh, T.-Y.; Park, J.; Nilsson, J.; Lötvall, J.; Kim, Y.-K.; Gho, Y.S. Bioinspired exosome-mimetic nanovesicles for targeted delivery of chemotherapeutics to malignant tumors. ACS Nano 2013, 7, 7698–7710. [Google Scholar] [CrossRef]
- Jeong, D.; Jo, W.; Yoon, J.; Kim, J.; Gianchandani, S.; Gho, Y.S.; Park, J. Nanovesicles engineered from ES cells for enhanced cell proliferation. Biomaterials 2014, 35, 9302–9310. [Google Scholar] [CrossRef]
- Yoon, J.; Jo, W.; Jeong, D.; Kim, J.; Jeong, H.; Park, J. Generation of nanovesicles with sliced cellular membrane fragments for exogenous material delivery. Biomaterials 2015, 59, 12–20. [Google Scholar] [CrossRef]
- Forterre, A.; Jalabert, A.; Berger, E.; Baudet, M.; Chikh, K.; Errazuriz, E.; De Larichaudy, J.; Chanon, S.; Weiss-Gayet, M.; Hesse, A.M.; et al. Proteomic analysis of C2C12 myoblast and myotube exosome-like vesicles: a new paradigm for myoblast-myotube cross talk? PLoS ONE 2014, 9, e84153. [Google Scholar]
- Boni, R.; Ali, A.; Shavandi, A.; Clarkson, A. Current and novel polymeric biomaterials for neural tissue engineering. J. Biomed. Sci. 2018, 25, 90. [Google Scholar] [CrossRef]
- Ma, Y.; Ge, S.; Zhang, J.; Zhou, D.; Li, L.; Wang, X.; Su, J. Mesenchymal stem cell-derived extracellular vesicles promote nerve regeneration after sciatic nerve crush injury in rats. Int. J. Clin. Exp. Pathol. 2017, 10, 10032–10039. [Google Scholar]
- Ma, Y.; Dong, L.; Zhou, D.; Li, L.; Zhang, W.; Zhen, Y.; Wang, T.; Su, J.; Chen, D.; Mao, C.; et al. Extracellular vesicles from human umbilical cord mesenchymal stem cells improve nerve regeneration after sciatic nerve transection in rats. J. Cell. Mol. Med. 2019, 23, 2822–2835. [Google Scholar] [CrossRef] [PubMed]
- Bucan, V.; Vaslaitis, D.; Peck, C.-T.; Strauss, S.; Vogt, P.M.; Radtke, C. Effect of exosomes from rat adipose-derived mesenchymal stem cells on neurite outgrowth and sciatic nerve regeneration after crush injury. Mol. Neurobiol. 2018, 56, 1812–1824. [Google Scholar] [CrossRef] [PubMed]
- Haertinger, M.; Weiss, T.; Mann, A.; Tabi, A.; Brandel, V.; Radtke, C. Adipose stem cell-derived extracellular vesicles induce proliferation of Schwann cells via internalization. Cells 2020, 9, 163. [Google Scholar] [CrossRef]
- Mao, Q.; Nguyen, P.D.; Shanti, R.M.; Shi, S.; Shakoori, P.; Zhang, Q.; Le, A.D. Gingiva-derived mesenchymal stem cell-extracellular vesicles activate Schwann cell repair phenotype and promote nerve regeneration. Tissue Eng. Part A 2019, 25, 887–900. [Google Scholar] [CrossRef]
- Xin, H.; Li, Y.; Buller, B.; Katakowski, M.; Zhang, Y.; Wang, X.; Shang, X.; Zhang, Z.G.; Chopp, M. Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. STEM CELLS 2012, 30, 1556–1564. [Google Scholar] [CrossRef]
- Couillard-Despres, S.; Winner, B.; Schaubeck, S.; Aigner, R.; Vroemen, M.; Weidner, N.; Bogdahn, U.; Winkler, J.; Kuhn, H.-G.; Aigner, L. Doublecortin expression levels in adult brain reflect neurogenesis. Eur. J. Neurosci. 2005, 21, 1–14. [Google Scholar] [CrossRef]
- Zanetta, L.; Marcus, S.G.; Vasile, J.; Dobryansky, M.; Cohen, H.; Eng, K.; Shamamian, P.; Mignatti, P. Expression of Von Willebrand factor, an endothelial cell marker, is up-regulated by angiogenesis factors: a potential method for objective assessment of tumor angiogenesis. Int. J. Cancer 2000, 85, 281–288. [Google Scholar] [CrossRef]
- Xin, H.; Li, Y.; Cui, Y.; Yang, J.J.; Zhang, Z.G.; Chopp, M. Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. Br. J. Pharmacol. 2013, 33, 1711–1715. [Google Scholar] [CrossRef]
- Zhang, Y.; Chopp, M.; Meng, Y.; Katakowski, M.; Xin, H.; Mahmood, A.; Xiong, Y. Effect of exosomes derived from multipluripotent mesenchymal stromal cells on functional recovery and neurovascular plasticity in rats after traumatic brain injury. J. Neurosurg. 2015, 122, 856–867. [Google Scholar] [CrossRef]
- Cheng, J.; Korte, N.; Nortley, R.; Sethi, H.; Tang, Y.; Attwell, D. Targeting pericytes for therapeutic approaches to neurological disorders. Acta Neuropathol. 2018, 136, 507–523. [Google Scholar] [CrossRef]
- Figley, S.A.; Khosravi, R.; Legasto, J.M.; Tseng, Y.-F.; Fehlings, M.G. Characterization of vascular disruption and blood-spinal cord barrier permeability following traumatic spinal cord injury. J. Neurotrauma 2014, 31, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhou, Y.; Zhang, R.; Wen, L.; Wu, K.; Li, Y.; Yao, Y.; Duan, R.; Jia, Y. Bone mesenchymal stem cell-derived extracellular vesicles promote recovery following spinal cord injury via improvement of the integrity of the blood-spinal cord barrier. Front. Mol. Neurosci. 2019, 13, 209. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Jiao, G.; Wu, W.; Wang, H.; Ren, S.; Zhang, L.; Zhou, H.; Liu, H.; Chen, Y. Exosomes from bone marrow mesenchymal stem cells inhibit neuronal apoptosis and promote motor function recovery via the Wnt/beta-catenin signaling pathway. Cell Transplant 2019, 28, 1373–1383. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhang, P.; Yao, X.; Li, H.; Shen, H.; Li, X.; Wu, J.; Lu, X. Exosomes derived from mir-133b-modified mesenchymal stem cells promote recovery after spinal cord injury. Front. Mol. Neurosci. 2018, 12, 845. [Google Scholar] [CrossRef]
- Clark, K.; Zhang, S.; Barthe, S.; Kumar, P.; Pivetti, C.D.; Kreutzberg, N.; Reed, C.; Wang, Y.; Paxton, Z.J.; Farmer, D.L.; et al. Placental mesenchymal stem cell-derived extracellular vesicles promote myelin regeneration in an animal model of multiple sclerosis. Cells 2019, 8, 1497. [Google Scholar] [CrossRef]
- Riazifar, M.; Mohammadi, M.R.; Pone, E.J.; Yeri, A.; Lässer, C.; Segaliny, A.I.; McIntyre, L.L.; Shelke, G.; Hutchins, E.; Hamamoto, A.; et al. Stem cell-derived exosomes as nanotherapeutics for autoimmune and neurodegenerative disorders. ACS Nano 2019, 13, 6670–6688. [Google Scholar] [CrossRef]
- Canales-Aguirre, A.A.; Reza-Zaldivar, E.E.; Sapiéns, M.A.H.; Gutiérrez-Mercado, Y.K.; Sandoval-Ávila, S.; Gomez-Pinedo, U.; Márquez-Aguirre, A.L.; Vazquez-Mendez, E.; Padilla-Camberos, E. Mesenchymal stem cell-derived exosomes promote neurogenesis and cognitive function recovery in a mouse model of Alzheimer’s disease. Neural Regen. Res. 2019, 14, 1626–1634. [Google Scholar] [CrossRef]
- Madeddu, P.; Urbanek, K.; Kajstura, J.; Yan, S.; Finato, N.; Bussani, R.; Nadal-Ginard, B.; Silvestri, F.; Leri, A.; Beltrami, C.A.; et al. Evidence that human cardiac myocytes divide after myocardial infarction. New Engl. J. Med. 2001, 344, 1750–1757. [Google Scholar]
- Senyo, S.; Steinhauser, M.L.; Pizzimenti, C.L.; Yang, V.K.; Cai, L.; Wang, M.; Wu, T.-D.; Guerquin-Kern, J.-L.; Lechene, C.P.; Lee, R.T. Mammalian heart renewal by pre-existing cardiomyocytes. Nature 2012, 493, 433–436. [Google Scholar] [CrossRef]
- Malliaras, K.; Zhang, Y.; Seinfeld, J.; Galang, G.; Tseliou, E.; Cheng, K.; Sun, B.; Aminzadeh, M.; Marbán, E. Cardiomyocyte proliferation and progenitor cell recruitment underlie therapeutic regeneration after myocardial infarction in the adult mouse heart. EMBO Mol. Med. 2013, 5, 191–209. [Google Scholar] [CrossRef]
- Lai, R.C.; Arslan, F.; Lee, M.M.; Sze, N.S.K.; Choo, A.; Chen, T.S.; Salto-Tellez, M.; Timmers, L.; Lee, C.N.; El Oakley, R.M.; et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010, 4, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Arslan, F.; Lai, R.C.; Smeets, M.B.; Akeroyd, L.; Choo, A.; Aguor, E.N.E.; Timmers, L.; Van Rijen, H.V.; Doevendans, P.A.; Pasterkamp, G.; et al. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res. 2013, 10, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Ballen, K.; Zhao, Y.; Sun, L.; Sun, X.; Zhao, X.; Sun, X.; Qian, H.; Xu, W.; Zhu, W. Faculty of 1000 evaluation for exosomes derived from akt-modified human umbilical cord mesenchymal stem cells improve cardiac regeneration and promote angiogenesis via activating platelet-derived growth factor D. Stem cells Transl. 2017, 6, 51–59. [Google Scholar]
- Adamiak, M.; Cheng, G.; Bobis-Wozowicz, S.; Zhao, L.; Kedracka-Krok, S.; Samanta, A.; Karnas, E.; Xuan, Y.-T.; Skupien-Rabian, B.; Chen, X.; et al. Induced pluripotent stem cell (iPSC)–derived extracellular vesicles are safer and more effective for cardiac repair than iPSCs. Circ. Res. 2018, 122, 296–309. [Google Scholar] [CrossRef]
- Balbi, C.; Lodder, K.; Costa, A.; Moimas, S.; Moccia, F.; Van Herwaarden, T.; Rosti, V.; Campagnoli, F.; Palmeri, A.; De Biasio, P.; et al. Reactivating endogenous mechanisms of cardiac regeneration via paracrine boosting using the human amniotic fluid stem cell secretome. Int. J. Cardiol. 2019, 287, 87–95. [Google Scholar] [CrossRef]
- Huang, P.; Wang, L.; Li, Q.; Xu, J.; Xu, J.; Xiong, Y.; Chen, G.; Qian, H.; Jin, C.; Yu, Y.; et al. Combinatorial treatment of acute myocardial infarction using stem cells and their derived exosomes resulted in improved heart performance. Stem Cell Res. Ther. 2019, 10, 300–312. [Google Scholar] [CrossRef]
- Hu, X.; Wu, R.; Shehadeh, L.A.; Zhou, Q.; Jiang, C.; Huang, X.; Zhang, L.; Gao, F.; Liu, X.-B.; Yu, H.; et al. Severe hypoxia exerts parallel and cell-specific regulation of gene expression and alternative splicing in human mesenchymal stem cells. BMC Genom. 2014, 15, 303. [Google Scholar] [CrossRef]
- Hu, X.; Xu, Y.; Zhong, Z.; Wu, Y.; Zhao, J.; Wang, Y.; Cheng, H.; Kong, M.; Zhang, F.; Chen, Q.; et al. A large-scale investigation of hypoxia-preconditioned allogeneic mesenchymal stem cells for myocardial repair in nonhuman primatesnovelty and significance. Circ. Res. 2016, 118, 970–983. [Google Scholar] [CrossRef]
- Bian, S.; Zhang, L.; Duan, L.; Wang, X.; Min, Y.; Yu, H. Extracellular vesicles derived from human bone marrow mesenchymal stem cells promote angiogenesis in a rat myocardial infarction model. J. Mol. Med. 2013, 92, 387–397. [Google Scholar] [CrossRef]
- Zhu, L.-P.; Tian, T.; Wang, J.-Y.; He, J.-N.; Chen, T.; Pan, M.; Xu, L.; Zhang, H.-X.; Qiu, X.-T.; Li, C.-C.; et al. Hypoxia-elicited mesenchymal stem cell-derived exosomes facilitates cardiac repair through miR-125b-mediated prevention of cell death in myocardial infarction. Theranostics 2018, 8, 6163–6177. [Google Scholar] [CrossRef]
- Zhu, J.; Lu, K.; Zhang, N.; Zhao, Y.; Ma, Q.; Shen, J.; Lin, Y.; Xiang, P.; Tang, Y.; Hu, X.; et al. Myocardial reparative functions of exosomes from mesenchymal stem cells are enhanced by hypoxia treatment of the cells via transferring microRNA-210 in an nSMase2-dependent way. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1659–1670. [Google Scholar] [PubMed]
- Han, C.; Zhou, J.; Liang, C.; Liu, B.; Pan, X.; Zhang, Y.; Wang, Y.; Yan, B.; Xie, W.; Liu, F.; et al. Human umbilical cord mesenchymal stem cell derived exosomes encapsulated in functional peptide hydrogels promote cardiac repair. Biomater. Sci. 2019, 7, 2920–2933. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, L.; Gao, Z.; Chen, G.; Zhang, C. Bone marrow stromal/stem cell-derived extracellular vesicles regulate osteoblast activity and differentiation in vitro and promote bone regeneration in vivo. Sci. Rep. 2016, 6, 21961. [Google Scholar] [CrossRef]
- Qi, X.; Zhang, J.; Yuan, H.; Xu, Z.; Li, Q.; Niu, X.; Hu, B.; Wang, Y.; Li, X.-L. Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells repair critical-sized bone defects through enhanced angiogenesis and osteogenesis in osteoporotic rats. Int. J. Boil. Sci. 2016, 12, 836–849. [Google Scholar] [CrossRef]
- Liang, B.; Liang, J.-M.; Ding, J.-N.; Xu, J.; Xu, J.-G.; Chai, Y. Dimethyloxaloylglycine-stimulated human bone marrow mesenchymal stem cell-derived exosomes enhance bone regeneration through angiogenesis by targeting the AKT/mTOR pathway. Stem Cell Res. Ther. 2019, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Chen, Y.; Dunstan, C.; Roohani-Esfahani, S.-I.; Zreiqat, H. Priming adipose stem cells with tumor necrosis factor-alpha preconditioning potentiates their exosome efficacy for bone regeneration. Tissue Eng. Part A 2017, 23, 1212–1220. [Google Scholar] [CrossRef] [PubMed]
- Furuta, T.; Miyaki, S.; Ishitobi, H.; Ogura, T.; Kato, Y.; Kamei, N.; Miyado, K.; Higashi, Y.; Ochi, M. Mesenchymal stem cell-derived exosomes promote fracture healing in a mouse model. STEM CELLS Transl. Med. 2016, 5, 1620–1630. [Google Scholar] [CrossRef]
- Huang, C.-C.; Narayanan, R.; Alapati, S.; Ravindran, S. Exosomes as biomimetic tools for stem cell differentiation: Applications in dental pulp tissue regeneration. Biomaterial 2016, 111, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Chew, J.R.J.; Chuah, S.J.; Teo, K.Y.W.; Zhang, S.; Lai, R.C.; Fu, J.H.; Lim, L.P.; Lim, S.K.; Toh, W.S. Mesenchymal stem cell exosomes enhance periodontal ligament cell functions and promote periodontal regeneration. Acta Biomater. 2019, 89, 252–264. [Google Scholar] [CrossRef]
- Li, W.; Liu, Y.; Zhang, P.; Tang, Y.; Zhou, M.; Jiang, W.; Zhang, X.; Wu, G.; Zhou, Y. Tissue-engineered bone immobilized with human adipose stem cells-derived exosomes promotes bone regeneration. ACS Appl. Mater. Interfaces 2018, 10, 5240–5254. [Google Scholar] [CrossRef]
- Chen, L.; Mou, S.; Li, F.; Zeng, Y.; Sun, Y.; Horch, R.E.; Wei, W.; Wang, Z.; Sun, J. Self-assembled human adipose-derived stem cell-derived extracellular vesicle-functionalized biotin-doped polypyrrole titanium with long-term stability and potential osteoinductive ability. ACS Appl. Mater. Interfaces 2019, 11, 46183–46196. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, X.; Li, H.; Chen, C.-Y.; Hu, B.; Niu, X.; Li, Q.; Zhao, B.; Xie, Z.; Wang, Y. Exosomes/tricalcium phosphate combination scaffolds can enhance bone regeneration by activating the PI3K/Akt signaling pathway. Stem Cell Res. Ther. 2016, 7, 136. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Wang, Z.; Zhang, L.; Lei, Q.; Zhao, A.; Wang, H.; Li, Q.; Cao, Y.; Zhang, W.J.; Chen, Z.-C. Extracellular vesicle-functionalized decalcified bone matrix scaffolds with enhanced pro-angiogenic and pro-bone regeneration activities. Sci. Rep. 2017, 7, 45622. [Google Scholar] [CrossRef]
- Diomede, F.; Gugliandolo, A.; Cardelli, P.; Merciaro, I.; Ettorre, V.; Traini, T.; Bedini, R.; Scionti, D.; Bramanti, A.; Nanci, A.; et al. Three-dimensional printed PLA scaffold and human gingival stem cell-derived extracellular vesicles: a new tool for bone defect repair. Stem Cell Res. Ther. 2018, 9, 104. [Google Scholar] [CrossRef]
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M.B. Osteoarthritis: A disease of the joint as an organ. Arthritis Rheum. 2012, 64, 1697–1707. [Google Scholar] [CrossRef]
- Zavatti, M.; Beretti, F.; Casciaro, F.; Bertucci, E.; Maraldi, T. Comparison of the therapeutic effect of amniotic fluid stem cells and their exosomes on monoiodoacetate-induced animal model of osteoarthritis. BioFactors 2019, 46, 106–117. [Google Scholar] [CrossRef]
- Vonk, L.A.; Van Dooremalen, S.F.J.; Liv, N.; Klumperman, J.; Coffer, P.J.; Saris, D.B.; Lorenowicz, M.J. Mesenchymal stromal/stem cell-derived extracellular vesicles promote human cartilage regeneration in vitro. Theranostics 2018, 8, 906–920. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, S.; Huang, J.; Guo, W.; Chen, J.; Zhang, L.; Zhao, B.; Peng, J.; Wang, A.; Wang, Y.; et al. The ECM-cell interaction of cartilage extracellular matrix on chondrocytes. BioMed Res. Int. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Hosseini-Farahabadi, S.; Geetha-Loganathan, P.; Fu, K.; Nimmagadda, S.; Yang, H.J.; Richman, J.M. Dual functions for WNT5A during cartilage development and in disease. Matrix Boil. 2013, 32, 252–264. [Google Scholar] [CrossRef]
- Mao, G.; Zhang, Z.; Hu, S.; Zhang, Z.; Chang, Z.; Huang, Z.; Liao, W.; Kang, Y. Exosomes derived from miR-92a-3p-overexpressing human mesenchymal stem cells enhance chondrogenesis and suppress cartilage degradation via targeting WNT5A. Stem Cell Res. Ther. 2018, 9, 247. [Google Scholar] [CrossRef]
- Wang, R.; Xu, B.; Xu, H. TGF-beta1 promoted chondrocyte proliferation by regulating Sp1 through MSC-exosomes derived miR-135b. Cell Cycle 2018, 17. [Google Scholar] [CrossRef]
- Zhang, S.; Chuah, S.J.; Lai, R.C.; Hui, J.H.P.; Lim, S.K.; Toh, W.S. MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterial 2018, 156, 16–27. [Google Scholar] [CrossRef]
- Zhang, S.; Chu, W.C.; Lai, R.C.; Lim, S.K.; Hui, J.H.P.; Toh, W.S. Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration. Osteoarthr. Cartil. 2016, 24, 2135–2140. [Google Scholar] [CrossRef]
- Zhang, S.; Teo, K.Y.W.; Chuah, S.J.; Lai, R.C.; Lim, S.K.; Toh, W.S. MSC exosomes alleviate temporomandibular joint osteoarthritis by attenuating inflammation and restoring matrix homeostasis. Biomaterial 2019, 200, 35–47. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, D.; Liu, Z.; Zhou, F.; Dai, J.; Wu, B.; Zhou, J.; Heng, B.C.; Zou, X.H.; Ouyang, H.; et al. Exosomes from embryonic mesenchymal stem cells alleviate osteoarthritis through balancing synthesis and degradation of cartilage extracellular matrix. Stem Cell Res. Ther. 2017, 8, 189. [Google Scholar] [CrossRef]
- Yan, L.; Wu, X. Exosomes produced from 3D cultures of umbilical cord mesenchymal stem cells in a hollow-fiber bioreactor show improved osteochondral regeneration activity. Cell Boil. Toxicol. 2019, 1–14. [Google Scholar] [CrossRef]
- Liu, X.; Yang, Y.; Li, Y.; Niu, X.; Zhao, B.; Wang, Y.; Bao, C.; Xie, Z.; Lin, Q.; Zhu, L. Integration of stem cell-derived exosomes with in situ hydrogel glue as a promising tissue patch for articular cartilage regeneration. Nanoscale 2017, 9, 4430–4438. [Google Scholar] [CrossRef]
- Bishop, E.; Mostafa, S.; Pakvasa, M.; Luu, H.H.; Lee, M.J.; Wolf, J.M.; Ameer, G.A.; He, T.-C.; Reid, R.R. 3-D bioprinting technologies in tissue engineering and regenerative medicine: Current and future trends. Genes Dis. 2017, 4, 185–195. [Google Scholar] [CrossRef]
- Chen, P.; Zheng, L.; Wang, Y.; Tao, M.; Xie, Z.; Xia, C.; Gu, C.; Chen, J.; Qiu, P.; Mei, S.; et al. Desktop-stereolithography 3D printing of a radially oriented extracellular matrix/mesenchymal stem cell exosome bioink for osteochondral defect regeneration. Theranostics 2019, 9, 2439–2459. [Google Scholar] [CrossRef]
- Bruno, S.; Grange, C.; Deregibus, M.C.; Calogero, R.A.; Saviozzi, S.; Collino, F.; Morando, L.; Busca, A.; Falda, M.; Bussolati, B.; et al. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J. Am. Soc. Nephrol. 2009, 20, 1053–1067. [Google Scholar] [CrossRef]
- Zhao, L.; Li, L.; Zhang, P.; Jiang, H.; Chen, J. Genetic communication by extracellular vesicles is an important mechanism underlying stem cell-based therapy-mediated protection against acute kidney injury. Stem Cell Res. Ther. 2019, 10, 119. [Google Scholar] [CrossRef]
- Wang, S.-Y.; Hong, Q.; Zhang, C.-Y.; Yang, Y.-J.; Cai, G.; Chen, X.-M. miRNAs in stem cell-derived extracellular vesicles for acute kidney injury treatment: comprehensive review of preclinical studies. Stem Cell Res. Ther. 2019, 10, 281–287. [Google Scholar] [CrossRef]
- Tomasoni, S.; Longaretti, L.; Rota, C.; Morigi, M.; Conti, S.; Gotti, E.; Capelli, C.; Introna, M.; Remuzzi, G.; Benigni, A. Transfer of growth factor receptor mRNA via exosomes unravels the regenerative effect of mesenchymal stem cells. Stem Cells Dev. 2012, 22, 772–780. [Google Scholar] [CrossRef]
- Gatti, S.; Bruno, S.; Deregibus, M.C.; Sordi, A.; Cantaluppi, V.; Tetta, C.; Camussi, G. Microvesicles derived from human adult mesenchymal stem cells protect against ischaemia-reperfusion-induced acute and chronic kidney injury. Nephrol. Dial. Transplant. 2011, 26, 1474–1483. [Google Scholar] [CrossRef]
- Shen, B.; Liu, J.; Zhang, F.; Wang, Y.; Qin, Y.; Zhou, Z.; Qiu, J.; Fan, Y. CCR2 Positive Exosome released by mesenchymal stem cells suppresses macrophage functions and alleviates ischemia/reperfusion-induced renal injury. Stem Cells Int. 2016, 2016, 1–9. [Google Scholar] [CrossRef]
- Bruno, S.; Grange, C.; Collino, F.; Deregibus, M.C.; Cantaluppi, V.; Biancone, L.; Tetta, C.; Camussi, G. Microvesicles derived from mesenchymal stem cells enhance survival in a lethal model of acute kidney injury. PLoS ONE 2012, 7, e33115. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, S.; Zhao, M.; Wang, C.; Li, L.; Yuan, Y.; Li, L.; Liao, G.; Bresette, W.; Zhang, J.; et al. Injectable extracellular vesicle-released self-assembling peptide nanofiber hydrogel as an enhanced cell-free therapy for tissue regeneration. J. Control. Release 2019, 316, 93–104. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, H.; Xu, W.; Wang, B.; Wu, H.; Tao, Y.; Zhang, B.; Wang, M.; Mao, F.; Yan, Y.; et al. Exosomes released by human umbilical cord mesenchymal stem cells protect against cisplatin-induced renal oxidative stress and apoptosis in vivo and in vitro. Stem Cell Res. Ther. 2013, 4, 34. [Google Scholar] [CrossRef]
- Ju, G.-Q.; Cheng, J.; Zhong, L.; Wu, S.; Zou, X.-Y.; Zhang, G.-Y.; Gu, D.; Miao, S.; Zhu, Y.-J.; Sun, J.; et al. Microvesicles derived from human umbilical cord mesenchymal stem cells facilitate tubular epithelial cell dedifferentiation and growth via hepatocyte growth factor induction. PLoS ONE 2015, 10, e0121534. [Google Scholar] [CrossRef]
- Gu, D.; Zou, X.; Ju, G.; Zhang, G.; Bao, E.; Zhu, Y. Mesenchymal stromal cells derived extracellular vesicles ameliorate acute renal ischemia reperfusion injury by inhibition of mitochondrial fission through miR-30. Stem Cells Int. 2016, 2016, 1–12. [Google Scholar] [CrossRef]
- Zou, X.; Zhang, G.; Cheng, Z.; Yin, D.; Du, T.; Ju, G.; Miao, S.; Liu, G.-H.; Lu, M.; Zhu, Y. Microvesicles derived from human Wharton’s Jelly mesenchymal stromal cells ameliorate renal ischemia-reperfusion injury in rats by suppressing CX3CL1. Stem Cell Res. Ther. 2014, 5, 40. [Google Scholar] [CrossRef]
- Ranghino, A.; Bruno, S.; Bussolati, B.; Moggio, A.; DiMuccio, V.; Tapparo, M.; Biancone, L.; Gontero, P.; Frea, B.; Camussi, G. The effects of glomerular and tubular renal progenitors and derived extracellular vesicles on recovery from acute kidney injury. Stem Cell Res. Ther. 2017, 8, 24. [Google Scholar] [CrossRef]
- Sanchez, M.B.H.; Bruno, S.; Grange, C.; Tapparo, M.; Cantaluppi, V.; Tetta, C.; Camussi, G. Human liver stem cells and derived extracellular vesicles improve recovery in a murine model of acute kidney injury. Stem Cell Res. Ther. 2014, 5, 124. [Google Scholar] [CrossRef]
- Piccoli, G.B.; Grassi, G.; Cabiddu, G.; Nazha, M.; Roggero, S.; Capizzi, I.; De Pascale, A.; Priola, A.M.; Di Vico, C.; Maxia, S.; et al. Diabetic kidney disease: a syndrome rather than a single disease. Rev. Diabet. Stud. 2015, 12, 87–109. [Google Scholar] [CrossRef]
- Jiang, Z.-Z.; Liu, Y.-M.; Niu, X.; Yin, J.-Y.; Hu, B.; Guo, S.-C.; Fan, Y.; Wang, Y.; Wang, N. Exosomes secreted by human urine-derived stem cells could prevent kidney complications from type I diabetes in rats. Stem Cell Res. Ther. 2016, 7, 24. [Google Scholar] [CrossRef]
- Nagaishi, K.; Mizue, Y.; Chikenji, T.S.; Otani, M.; Nakano, M.; Konari, N.; Fujimiya, M. Mesenchymal stem cell therapy ameliorates diabetic nephropathy via the paracrine effect of renal trophic factors including exosomes. Sci. Rep. 2016, 6, 34842. [Google Scholar] [CrossRef]
- Grange, C.; Tritta, S.; Tapparo, M.; Cedrino, M.; Tetta, C.; Camussi, G.; Brizzi, M.F. Stem cell-derived extracellular vesicles inhibit and revert fibrosis progression in a mouse model of diabetic nephropathy. Sci. Rep. 2019, 9, 4468. [Google Scholar] [CrossRef]
- Kholia, S.; Sanchez, M.B.H.; Cedrino, M.; Papadimitriou, E.; Tapparo, M.; Deregibus, M.C.; Brizzi, M.F.; Tetta, C.; Camussi, G. Human liver stem cell-derived extracellular vesicles prevent aristolochic acid-induced kidney fibrosis. Front. Immunol. 2018, 9, 9. [Google Scholar] [CrossRef]
- He, J.; Wang, Y.; Sun, S.; Yu, M.; Wang, C.; Pei, X.; Zhu, B.; Wu, J.; Zhao, W. Bone marrow stem cells-derived microvesicles protect against renal injury in the mouse remnant kidney model. Nephrology 2012, 17, 493–500. [Google Scholar] [CrossRef]
- He, J.; Wang, Y.; Lu, X.; Zhu, B.; Pei, X.; Wu, J.; Zhao, W. Micro-vesicles derived from bone marrow stem cells protect the kidney both in vivo and in vitro by microRNA-dependent repairing. Nephrology 2015, 20, 591–600. [Google Scholar] [CrossRef]
- Matsukura, T.; Inaba, C.; Weygant, E.A.; Kitamura, D.; Janknecht, R.; Matsumoto, H.; Hyink, D.P.; Kashiwada, S.; Obara, T. Extracellular vesicles from human bone marrow mesenchymal stem cells repair organ damage caused by cadmium poisoning in a medaka model. Physiol. Rep. 2019, 7, e14172. [Google Scholar] [CrossRef] [PubMed]
- Nassar, W.; El-Ansary, M.; Sabry, D.; Fayad, T.; Kotb, E.; Temraz, M.; Saad, A.-N.; Essa, W.; Adel, H.; A Mostafa, M. Umbilical cord mesenchymal stem cells derived extracellular vesicles can safely ameliorate the progression of chronic kidney diseases. Biomater. Res. 2016, 20, 21. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.Y.; Lai, R.C.; Wong, W.; Dan, Y.Y.; Lim, S.K.; Ho, H.K. Mesenchymal stem cell-derived exosomes promote hepatic regeneration in drug-induced liver injury models. Stem Cell Res. Ther. 2014, 5, 76. [Google Scholar] [CrossRef] [PubMed]
- Nong, K.; Wang, W.; Niu, X.; Hu, B.; Ma, C.; Bai, Y.; Wu, B.; Wang, Y.; Ai, K. Hepatoprotective effect of exosomes from human-induced pluripotent stem cell–derived mesenchymal stromal cells against hepatic ischemia-reperfusion injury in rats. Cytotherapy 2016, 18, 1548–1559. [Google Scholar] [CrossRef]
- Du, Y.; Li, D.; Han, C.; Wu, H.; Xu, L.; Zhang, M.; Zhang, J.; Chen, X. Exosomes from human-induced pluripotent stem cell–derived mesenchymal stromal cells (hiPSC-MSCs) protect liver against hepatic ischemia/ reperfusion injury via activating sphingosine kinase and sphingosine-1-phosphate signaling pathway. Cell. Physiol. Biochem. 2017, 43, 611–625. [Google Scholar] [CrossRef]
- Cannavo, A.; Liccardo, D.; Komici, K.; Corbi, G.; De Lucia, C.; Femminella, G.D.; Elia, A.; Bencivenga, L.; Ferrara, N.; Koch, W.J.; et al. Sphingosine kinases and sphingosine 1-phosphate receptors: signaling and actions in the cardiovascular system. Front. Pharmacol. 2017, 8. [Google Scholar] [CrossRef]
- Blaho, V.A.; Hla, T. Regulation of mammalian physiology, development, and disease by the sphingosine 1-phosphate and lysophosphatidic acid receptors. Chem. Rev. 2011, 111, 6299–6320. [Google Scholar] [CrossRef]
- Ng, M.L.; Yarla, N.S.; Menschikowski, M.; Sukocheva, O. Regulatory role of sphingosine kinase and sphingosine-1-phosphate receptor signaling in progenitor/stem cells. World J. Stem Cells 2018, 10, 119–133. [Google Scholar] [CrossRef]
- Yao, J.; Zheng, J.; Cai, J.; Zeng, K.; Zhou, C.; Zhang, J.; Li, S.; Li, H.; Chen, L.; He, L.; et al. Extracellular vesicles derived from human umbilical cord mesenchymal stem cells alleviate rat hepatic ischemia-reperfusion injury by suppressing oxidative stress and neutrophil inflammatory response. FASEB J. 2018, 33, 1695–1710. [Google Scholar] [CrossRef]
- Mardpour, S.; Ghanian, M.H.; Abandansari, H.S.; Mardpour, S.; Nazari, A.; Shekari, F.; Baharvand, H. Hydrogel-mediated sustained systemic delivery of mesenchymal stem cell-derived extracellular vesicles improves hepatic regeneration in chronic liver failure. ACS Appl. Mater. Interfaces 2019, 11, 37421–37433. [Google Scholar] [CrossRef]
- Nakamura, Y.; Miyaki, S.; Ishitobi, H.; Matsuyama, S.; Nakasa, T.; Kamei, N.; Akimoto, T.; Higashi, Y.; Ochi, M. Mesenchymal-stem-cell-derived exosomes accelerate skeletal muscle regeneration. FEBS Lett. 2015, 589, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- Mellows, B.; Mitchell, R.; Antonioli, M.; Kretz, O.; Chambers, D.; Zeuner, M.-T.; Denecke, B.; Musante, L.; Ramachandra, D.L.; Debacq-Chainiaux, F.; et al. Protein and molecular characterization of a clinically compliant amniotic fluid stem cell-derived extracellular vesicle fraction capable of accelerating muscle regeneration through enhancement of angiogenesis. Stem Cells Dev. 2017, 26, 1316–1333. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, R.; Mellows, B.; Sheard, J.; Antonioli, M.; Kretz, O.; Chambers, D.; Zeuner, M.-T.; Tomkins, J.E.; Denecke, B.; Musante, L.; et al. Secretome of adipose-derived mesenchymal stem cells promotes skeletal muscle regeneration through synergistic action of extracellular vesicle cargo and soluble proteins. Stem Cell Res. Ther. 2019, 10, 116. [Google Scholar] [CrossRef]
- Wang, C.; Song, W.; Chen, B.; Liu, X.; He, Y. Exosomes isolated from adipose-derived stem cells: a new cell-free approach to prevent the muscle degeneration associated with torn rotator cuffs. Am. J. Sports Med. 2019, 47, 3247–3255. [Google Scholar] [CrossRef]
- Wu, R.; Huang, C.; Wu, Q.; Jia, X.; Liu, M.; Xue, Z.; Qiu, Y.; Niu, X.; Wang, Y. Exosomes secreted by urine-derived stem cells improve stress urinary incontinence by promoting repair of pubococcygeus muscle injury in rats. Stem Cell Res. Ther. 2019, 10, 80. [Google Scholar] [CrossRef]
- Figliolini, F.; Ranghino, A.; Grange, C.; Cedrino, M.; Tapparo, M.; Cavallari, C.; Rossi, A.; Togliatto, G.; Femminò, S.; Gugliuzza, M.V.; et al. Extracellular vesicles from adipose stem cells prevent muscle damage and inflammation in a mouse model of hind limb ischemia: role of Neuregulin-1. Arter. Thromb. Vasc. Boil. 2019, 40, 239–254. [Google Scholar] [CrossRef]
- Velnar, T.; Bailey, T.; Smrkolj, V. The wound healing process: an overview of the cellular and molecular mechanisms. J. Int. Med Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef]
- Zhao, R.; Liang, H.; Clarke, E.; Jackson, C.; Xue, M. Inflammation in Chronic Wounds. Int. J. Mol. Sci. 2016, 17, 2085. [Google Scholar] [CrossRef]
- Shabbir, A.; Cox, A.; Rodriguez-Menocal, L.; Salgado, M.; Van Badiavas, E. Mesenchymal stem cell exosomes induce proliferation and migration of normal and chronic wound fibroblasts, and enhance angiogenesis in vitro. Stem Cells Dev. 2015, 24, 1635–1647. [Google Scholar] [CrossRef]
- Zhang, J.; Guan, J.; Niu, X.; Hu, G.-W.; Guo, S.-C.; Li, Q.; Xie, Z.; Zhang, C.; Wang, Y. Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis. J. Transl. Med. 2015, 13, 49. [Google Scholar] [CrossRef]
- Choi, J.S.; Cho, W.L.; Choi, Y.J.; Kim, J.D.; Park, H.-A.; Kim, S.Y.; Park, J.H.; Jo, D.-G.; Cho, Y.W. Functional recovery in photo-damaged human dermal fibroblasts by human adipose-derived stem cell extracellular vesicles. J. Extracell. Vesicles 2019, 8, 1565885. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Wang, J.; Zhou, X.; Xiong, Z.; Zhao, J.; Yu, R.; Huang, F.; Zhang, H.; Chen, L. Exosomes derived from human adipose mensenchymal stem cells accelerates cutaneous wound healing via optimizing the characteristics of fibroblasts. Sci. Rep. 2016, 6, 32993. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.D.F.; Cunha, P.D.S.; Carregal, V.; Silva, P.D.C.D.; De Miranda, M.C.; Kunrath-Lima, M.; De Melo, M.I.A.; Faraco, C.C.F.; Barbosa, J.L.; Frézard, F.; et al. Extracellular vesicles from adipose-derived mesenchymal stem/stromal cells accelerate migration and activate akt pathway in human keratinocytes and fibroblasts independently of miR-205 activity. Stem Cells Int. 2017, 2017, 9841035. [Google Scholar] [CrossRef] [PubMed]
- Cooper, D.; Wang, C.; Patel, R.; Trujillo, A.; Patel, N.A.; Prather, J.; Gould, L.J.; Wu, M.H. Human adipose-derived stem cell conditioned media and exosomes containing MALAT1 promote human dermal fibroblast migration and ischemic wound healing. Adv. Wound Care 2018, 7, 299–308. [Google Scholar] [CrossRef]
- Pelizzo, G.; Avanzini, M.; Cornaglia, A.I.; Silvestri, A.; Mantelli, M.; Travaglino, P.; Croce, S.; Romano, P.; Avolio, L.; Iacob, G.; et al. Extracellular vesicles derived from mesenchymal cells: perspective treatment for cutaneous wound healing in pediatrics. Regen. Med. 2018, 13, 385–394. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, M.; Gong, A.; Zhang, X.; Wu, X.; Zhu, Y.; Shi, H.; Wu, L.; Zhu, W.; Qian, H.; et al. HucMSC-exosome mediated-Wnt4 signaling is required for cutaneous wound healing. STEM CELLS 2015, 33, 2158–2168. [Google Scholar] [CrossRef]
- Zhang, B.; Wu, X.; Zhang, X.; Sun, Y.; Yan, Y.; Shi, H.; Zhu, Y.; Wu, L.; Pan, Z.; Zhu, W.; et al. Human umbilical cord mesenchymal stem cell exosomes enhance angiogenesis through the Wnt4/beta-catenin pathway. Stem Cells Transl. Med. 2015, 4, 513–522. [Google Scholar] [CrossRef]
- Zhang, B.; Shi, Y.; Gong, A.; Pan, Z.; Shi, H.; Yang, H.; Fu, H.; Yan, Y.; Zhang, X.; Wang, M.; et al. HucMSC exosome-delivered 14-3-3zeta orchestrates self-control of the Wnt response via modulation of YAP during cutaneous regeneration. Stem Cells 2016, 34, 2485–2500. [Google Scholar] [CrossRef]
- Fang, S.; Xu, C.; Zhang, Y.; Xue, C.; Yang, C.; Bi, H.; Qian, X.; Wu, M.; Ji, K.; Zhao, Y.; et al. Umbilical cord-derived mesenchymal stem cell-derived exosomal microRNAs suppress myofibroblast differentiation by inhibiting the transforming growth factor-beta/SMAD2 pathway during wound healing. Stem Cells Transl. Med. 2016, 5, 1425–1439. [Google Scholar] [CrossRef]
- Kim, S.Y.; Joglekar, M.V.; Hardikar, A.A.; Phan, T.H.; Khanal, D.; Tharkar, P.; Limantoro, C.; Johnson, J.; Kalionis, B.; Chrzanowski, W. Placenta stem/stromal cell-derived extracellular vesicles for potential use in lung repair. Proteom. 2019, 19, e1800166. [Google Scholar] [CrossRef]
- Khatri, M.; Richardson, L.A.; Meulia, T. Mesenchymal stem cell-derived extracellular vesicles attenuate influenza virus-induced acute lung injury in a pig model. Stem Cell Res. Ther. 2018, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Potter, D.R.; Miyazawa, B.Y.; Gibb, S.L.; Deng, X.; Togaratti, P.P.; Croze, R.H.; Srivastava, A.K.; Trivedi, A.; Matthay, M.; Holcomb, J.B.; et al. Mesenchymal stem cell-derived extracellular vesicles attenuate pulmonary vascular permeability and lung injury induced by hemorrhagic shock and trauma. J. Trauma Acute Care Surg. 2018, 84, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Wu, Y.; Zhao, R.; Zhang, K.; Midgley, A.C.; Kong, D.; Li, Z.; Zhao, Q. MSC-derived sEVs enhance patency and inhibit calcification of synthetic vascular grafts by immunomodulation in a rat model of hyperlipidemia. Biomaterial 2019, 204, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Almeria, C.; Weiss, R.; Roy, M.; Tripisciano, C.; Kasper, C.; Weber, V.; Egger, D. Hypoxia conditioned mesenchymal stem cell-derived extracellular vesicles induce increased vascular tube formation in vitro. Front. Bioeng. Biotechnol. 2019, 7, 292. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Li, Q.; Niu, X.; Zhang, G.; Ling, X.; Zhang, J.; Wang, Y.; Deng, Z. Extracellular vesicles secreted by human urine-derived stem cells promote ischemia repair in a mouse model of hind-limb ischemia. Cell. Physiol. Biochem. 2018, 47, 1181–1192. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.K.A.; Perretta, S.; Perrod, G.; Pidial, L.; Lindner, V.; Carn, F.; Lemieux, S.; Alloyeau, D.; Boucenna, I.; Menasché, P.; et al. Thermoresponsive gel embedded with adipose stem-cell-derived extracellular vesicles promotes esophageal fistula healing in a thermo-actuated delivery strategy. ACS Nano 2018, 12, 9800–9814. [Google Scholar] [CrossRef]
- Cao, L.; Xu, H.; Wang, G.; Liu, M.; Tian, D.; Yuan, Z. Extracellular vesicles derived from bone marrow mesenchymal stem cells attenuate dextran sodium sulfate-induced ulcerative colitis by promoting M2 macrophage polarization. Int. Immunopharmacol. 2019, 72, 264–274. [Google Scholar] [CrossRef]
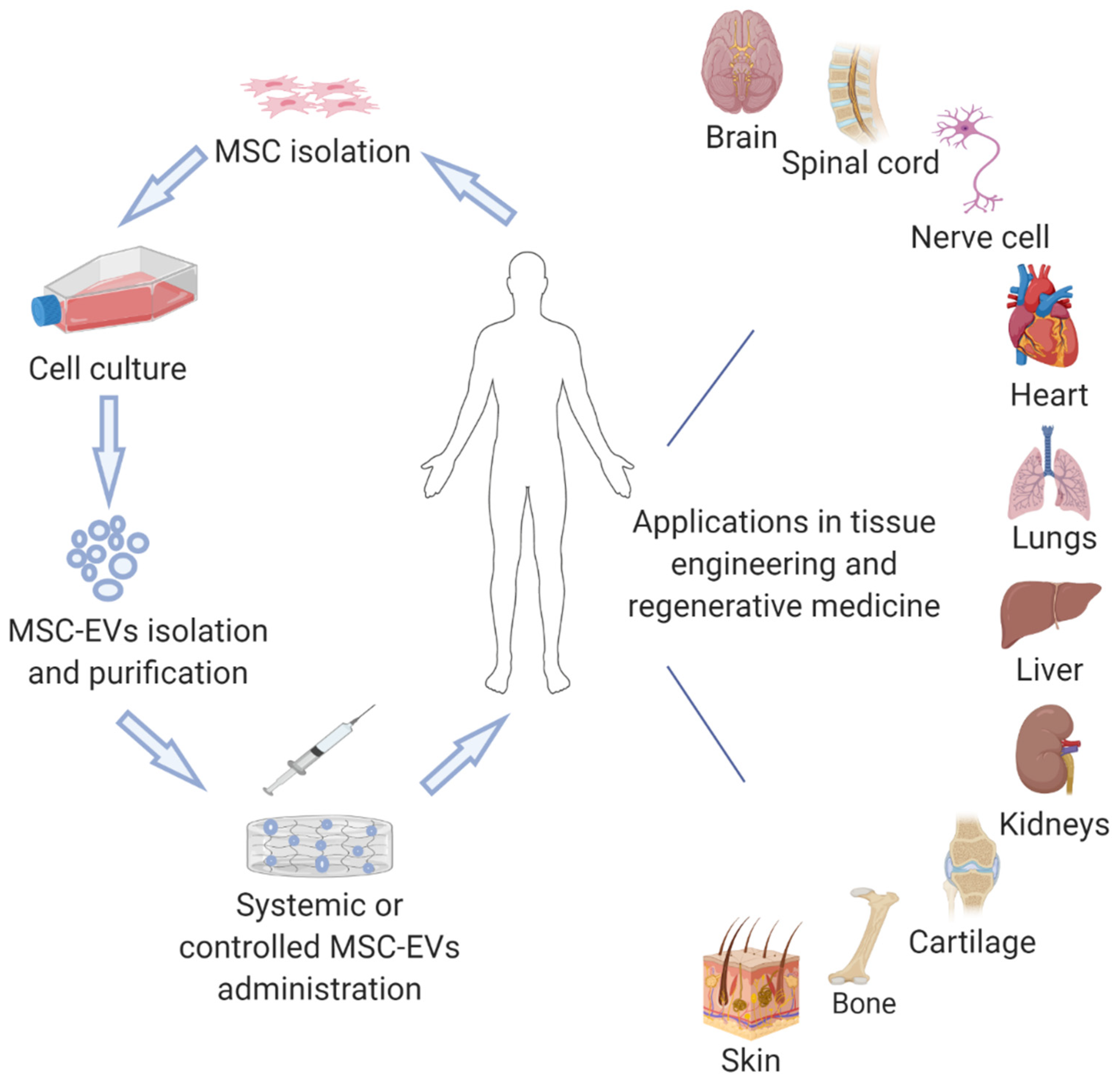
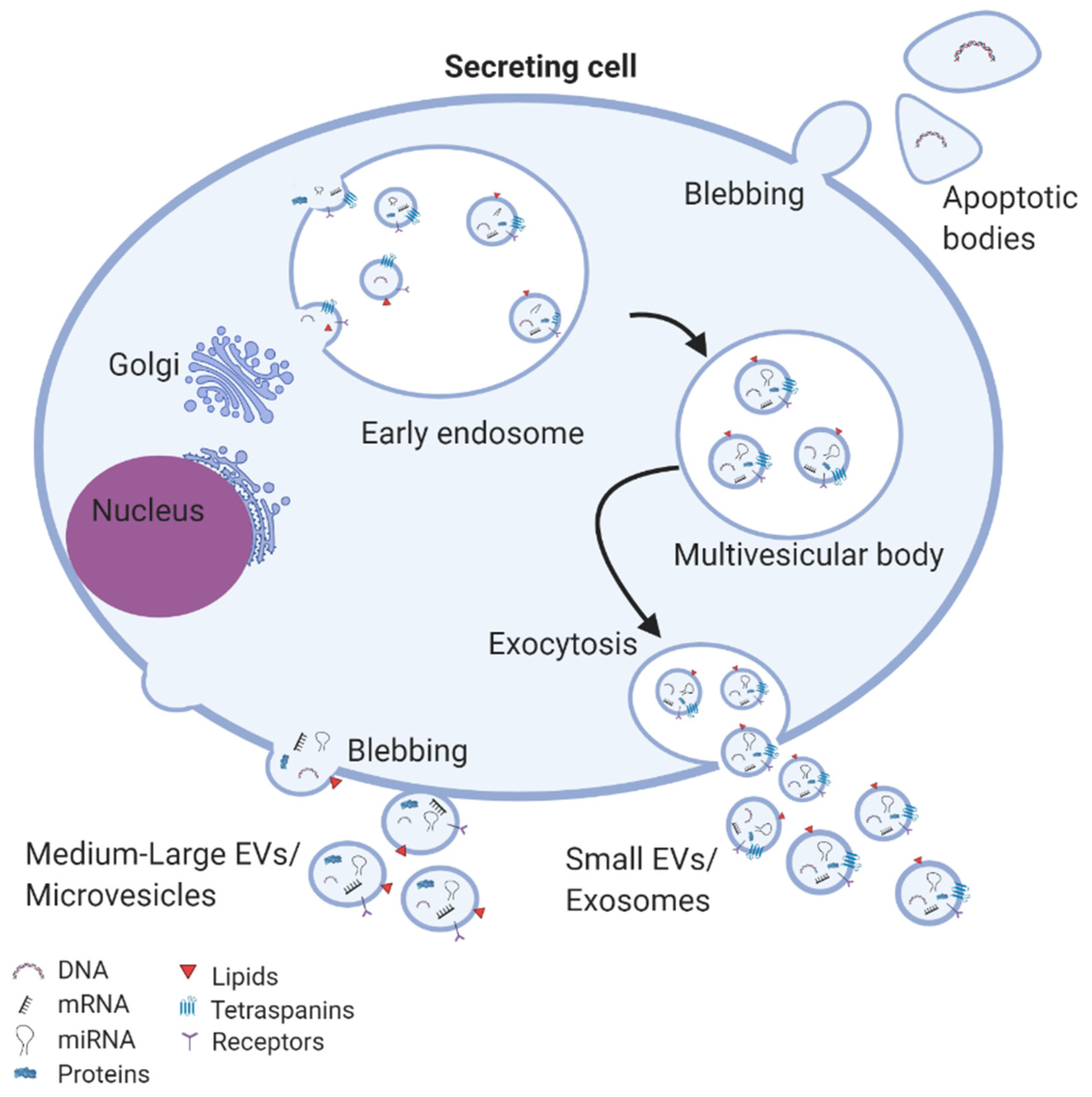
| Injury/Damage | Cell Source | Isolation Method | Administration Route/Quantity | Main Findings from Studies Evaluating These EVs |
|---|---|---|---|---|
| Nervous System | ||||
| Sciatic peripheral nerve crush | Rat BMMSCs | Ultracentrifugation (100,000× g) | Injection/45 μg total EV protein in 30 μL PBS | Improved sciatic function index, enhanced histomorphometric repair in nerve regeneration and increased GAP-43 expression [50] |
| Sciatic peripheral nerve crush | Human UCMSCs | Differential centrifugation and ultracentrifugation (100,000× g) | Injection/100 μg total EV protein | Generation of axons and Schwann cells, reduction of denervated muscle atrophy and modulation of inflammation [51] |
| Sciatic peripheral nerve crush | Rat ADMSCs | Differential centrifugation and ultracentrifugation (100,000× g) | Injection/details not provided | Peripheral nerve regeneration and neurite growth in sciatic nerve defects through Schwann cell (SC) modulation [52] |
| Peripheral nerve injury (in vitro) | Rat ADMSCs | Total Exosome isolation reagent kit (Invitrogen) | 2 and 8 µg total EV protein | Enhanced proliferation of Schwann cells in the site of peripheral nerve injuries via internalisation [53] |
| Sciatic peripheral nerve crush | Human gingiva MSCs | ExoQuick-TC kit (System Biosciences) | EV-scaffold transplantation/40 μg total EV protein in 20 μL PBS | Enhanced proliferation and migration of Schwann cells via the activation JNK pathway and the up-regulation of c-JUN, Notch1, GFAP and SOX2 [54] |
| Cerebral artery stroke (in vitro) | Rat BMMSCs | Sucrose gradient ultracentrifugation (100,000× g) | Details not provided | Neurite outgrowth by transfer of miR-133b to neural cells [55] |
| Cerebral artery stroke | Rat BMMSCs | Sucrose gradient ultracentrifugation (100,000× g) | Injection/100 μg total EV protein in 500 μL PBS | Increased axonal density and synaptophysin-positive areas along the ischemic boundary zone of the cortex and striatum [58] |
| TBI | Rat BMMSCs | ExoQuick-TC kit (System Biosciences) | Injection/100 μg total EV protein in 500 μL PBS | Improved recovery of brain function by increasing the number of number of neurons and endothelial cells in the lesion boundary zone and dentate gyrus [59] |
| SCI | Mouse BMMSCs | Differential centrifugation and sequential ultracentrifugation (up to 110,000× g) | Injection/200 μL derived from 1 × 106 MSCs post-SCI and 200 μL derived from 1 × 106 MSCs at 1-day post-injury | Reduced migration of pericytes and improved structural integrity of the BSCB [62] |
| SCI | Rat BMMSCs | Differential centrifugation and ultracentrifugation (100,000× g) | Injection/200 μg total EV protein in 200 μL PBS | Reduced neuronal apoptosis through the activation of the Wnt/β-catenin signalling pathway [63] |
| SCI | Rat BMMSCs | ExoQuick-TC kit (System Biosciences) | Injection/100 μg total EV protein in 500 μL PBS | Modification of rat BMMSC-EVs with miR-133b activated the ERK1/2, STAT3 pathway, which resulted to enhanced neuron preservation [64] |
| MS | Human placental MSCs | Differential centrifugation and ultracentrifugation (112,700× g) | Injection/1 × 107 or 1 × 1010 particles in 200 μL PBS | Increased myelination in the spinal cord of treated mice and improved motor function [65] |
| EAE | Human BMMSCs | Differential centrifugation and ultracentrifugation (120,000× g) | Injection/150 μg of total EV protein | Reduced neuroinflammation, demyelination and improved motor function |
| Alzheimer’s disease | Details not included | Differential centrifugation and ultracentrifugation (110,000× g) | Injection/10 µg total EV protein in 2 µL PBS | Enhanced neurogenesis and cognitive function recovery [67] |
| Cardiac | ||||
| MI | Human embryonic MSCs | Sucrose gradient ultracentrifugation (200,000× g) | Injection/3 µg total EV protein in 200 µL PBS | Reduced infarct size [71] |
| MI | Human embryonic MSCs | Tangential flow filtration | Infusion/0.4 μg total EV protein in 1 mL PBS | Increased myocardial viability and inhibited adverse remodelling [72] |
| MI | Human UCMSCs | Sucrose gradient ultracentrifugation (100,000× g) | Infusion/400 µg of total EV protein | Akt-modified MSC-EVs promoted endothelial cell proliferation, migration, tube-like structure formation, and blood vessel formation [73] |
| MI | Murine iPSCs | Differential centrifugation and ultracentrifugation (110,000× g) | Injection/100 µg of total EV protein in 30 μL PBS | Increased cardiac repair, left ventricular function, vascularization, and reduced apoptosis and hypertrophy [74] |
| MI | Human amniotic fluid-derived MSCs | Serial ultracentrifugation | Injection/4.5 μg total EV protein | Improved cardiac regeneration via paracrine modulation of endogenous mechanisms [75] |
| MI | Rat BMMSCs | Differential centrifugation and ultracentrifugation (120,000× g) | Injection/10 μg total EV protein in 100 μL PBS | BMMSCs and their derived EVs synergistically improved cardiac function, reduced infarct size, and increased neovascularization [76] |
| MI | Human BMMSCs | Ultracentrifugation (100,000× g) | Injection/80 μg total EV protein | Hypoxia-elicited BMMSC-EVs showed higher cardiac regeneration as compared to MSC-EVs isolated in normoxia [79] |
| MI | Murine BMMSCs | Differential centrifugation and ultracentrifugation (140,000× g) | Injection/200 µg of total EV protein in 200 μL PBS | Hypoxia-elicited BMMSC-EVs prevented cardiomyocyte apoptosis through the enrichment of miR-125b-5p [80] |
| MI | Rat BMMSCs | Repeated ultracentrifugation | Injection/EVs derived from 2 × 107 MSCs, in 30 μL PBS | Hypoxia-elicited BMMSC-EVs enhanced the cardioprotective actions of EVs [81] |
| MI | Human UCMSCs | Total exosome isolation kit (Life Technologies) | EV-hydrogel transplantation/20 µg of total EV protein in 20 μL PBS | Sustain delivery of MSC-EVs improved myocardial function by reducing inflammation, fibrosis and apoptosis, and by promoting angiogenesis [82] |
| Bone | ||||
| Critical-sized calvarial defect | Human BMMSCs | Differential centrifugation and ultracentrifugation (120,000× g) | EV-hydrogel transplantation/100 μg of total EV protein in 50 μl PBS | Increased angiogenesis and bone formation [83] |
| Critical-sized calvarial defect | Human iPSCs | Ultrafiltration and gradient ultracentrifugation (100,000× g) | EV-scaffold transplantation/100 μg or 200 μg of total EV protein | Increased angiogenesis, osteogenesis and bone formation [84] |
| Critical-sized calvarial defect | Human BMMSCs | Differential centrifugation and ultracentrifugation (110,000× g) | Injection to implanted scaffolds/100 μg of total EV protein in 200 μL PBS | BMMSC-EVs modified with dimethyloxaloylglycin enhanced bone regeneration through Akt/mTOR pathway activation and angiogenesis stimulation [85] |
| Bone regeneration (in vitro) | Human ADMSCs | Ultracentrifugation (100,000× g) | Details not provided | TNFα-primed ADMSC-EVs enhanced proliferation and differentiation of osteoblastic cells by increasing the expression of Wnt-3a [86] |
| Femoral shaft fracture(CD9−/− and wild types) | Human BMMSCs | Ultracentrifugation (180,000× g) | Injection / details not provided | Improved fracture healing in CD9−/− mice, and accelerated bone repair in wild types [87] |
| Tooth root slice | Human dental pulp MSCs | ExoQuick-TC kit (System Biosciences) | Injection/details not provided | Increased odontogenic differentiation regeneration of dental pulp-like tissue [88] |
| Periodontal defect | Unknown | Tangential flow filtration | EV-scaffold transplantation/40 μg of total EV protein | Enhanced periodontal ligament cell migration, proliferation and periodontal regeneration [89] |
| Critical-sized skull defect | Human ADMSCs | Differential centrifugation and ultracentrifugation (100,000× g) | EV-scaffold transplantation/1 μg of total EV protein in 1 μL PBS | MSC-EVs immobilised in poly(lactic-co-glycolic acid) stimulated the controlled release of EVs promoting MSC migration and homing in the bone defects [90] |
| Ectopic bone formation | Human ADMSCs | Differential centrifugation | EV-scaffold transplantation/10 μg of total EV protein in 1 mL PBS | MSC-EV immobilised constructs showed higher osteo-inductive ability and long-term stability for bone graft modification [91] |
| Critical-sized calvarial defects | Human BMMSCs | Differential centrifugation and sucrose gradient ultracentrifugation (100,000× g) | EV-scaffold transplantation/5 × 1011 or 1 × 1012 particles in 1 mL PBS | BMMSC-EVs loaded into tricalcium phosphate scaffolds promoted osteogenesis activity and bone regeneration [92] |
| Ectopic bone formation | Rat BMMSCs | Differential centrifugation | EV-scaffold transplantation/20 μg of total EV protein in 20 μL PBS | MSC-EVs loaded into decalcified bone matrix scaffolds stimulated neo-vascularization and bone formation [93] |
| Cortical calvaria bone defect | Human gingival MSCs | ExoQuick-TC kit (System Biosciences) | EV-scaffold transplantation/details not provided | MSC-EVs loaded in three-dimensional polylactic acid scaffolds enhanced osteogenic properties and improved bone healing [94] |
| Cartilage | ||||
| OA | Human amniotic fluid MSCs | Total exosome isolation kit (Life Technologies) | Injection/100 μg of total EV protein | EV-treated defects showed superior pain tolerance level and improved histological scores than the MSC-treated defects [96] |
| Cartilage regeneration (in vitro) | Human BMMSCs | Differential centrifugation and ultracentrifugation (100,000× g) | Details not provided | Increased production of collagen type II and proteoglycans in chondrocytes isolated from OA patients [97] |
| OA | Human BMMSCs | Differential centrifugation and sucrose gradient ultracentrifugation (100,000× g) | Injection/500 μg of total EV protein in 1 mL PBS | BMMSC-EVs modified with miR-92a-3p, suppressed cartilage degradation by targeting the WNT5A and promoted cartilage repair [100] |
| OA | Rat MSCs | Tangential flow filtration | Injection/1 × 1011 particles in 1 mL PBS | TGFβ primed MSC-EVs promoted cartilage tissue repair through Sp1 regulation [101] |
| OA | Human embryonic MSCs | Tangential flow filtration | Injection/100 µg of total EV protein in 100 µL PBS | Increased chondrocyte proliferation, reduced apoptosis, regulated inflammation and matrix homeostasis [102,103,104] |
| OA | Human embryonic MSCs | Differential centrifugation and ultracentrifugation (100,000× g) | Injection/details not provided | Improved osteoarthritis through balancing the synthesis and degradation of cartilage ECM [105] |
| Cartilage defect | Human UCMSCs | Differential centrifugation and ultracentrifugation (110,000× g) | Injection/1 × 1010 particles in 1 mL PBS | UCMSCs cultured in a bioreactor resulted in a higher yield of EVs and superior therapeutic efficiency [106] |
| Articular cartilage defect | Human iPSCs | Differential centrifugation, ultracentrifugation (100,000× g) and ultrafiltration | EV-scaffold transplantation/1 × 1011 particles in 1 mL PBS | iPSC-EVs incorporated with in situ hydrogel glue could integrate with native cartilage matrix and promote cell deposition at cartilage defect [107] |
| Osteochondral defect | BMMSCs | Ultrafiltration and sucrose gradient ultracentrifugation (100,000× g) | EV-scaffold transplantation/200 µg of total EV protein in 200 µL PBS | BMMSC-EVs together with cartilage ECM/ gelatin methacrylate hydrogel have been used to create a 3D printed device, which favoured cartilage regeneration [109] |
| Kidney | ||||
| AKI | Human BMMSCs | Ultracentrifugation (100,000× g) | Injection/15 µg of total EV protein | Enhanced recovery of injured tubular cells, enhanced tubular cell proliferation, reduced apoptosis [110] |
| AKI (in vitro) | Human BMMSCs | Ultracentrifugation (100,000× g) | Details not provided | Increased renoprotection activity mediated by the transfer of the mRNA for IGF-1 receptor to tubular cells through the MSC-EVs [113] |
| AKI | Human BMMSCs | Ultracentrifugation (100,000× g) | Injection/30 µg of total EV protein | Enhanced tubular epithelial cell proliferation, reduced cell apoptosis [114] |
| AKI | Mice BMMSCs | Sequential ultracentrifugation (up to 110,000× g) | Injection/200 μg of total EV protein in 20 μL PBS | Enhanced tubular epithelial cell proliferation, reduced cell apoptosis [115] |
| AKI | Human BMMSCs | Ultracentrifugation (100,000× g) | Injection/Single dose of 100 μg of total EV protein or multiple doses of 100 μg of total EV protein after cisplatin administration and 50 μg of total EV protein after 2, 6, 10, 14, and 18 days | Upregulated expression of anti-apoptotic genes in cisplatin-treated human tubular epithelial cells and down-regulated expression of cell-apoptotic genes [116] |
| AKI | Mice BMMSCs | Differential centrifugation and ultracentrifugation (100,000× g) | EV or EV-hydrogel injection/80 μg of total EV protein in 15 μL PBS or 80 μg of total EV protein in 15 μL of hydrogel solution | BMMSC-EVs loaded to self-assembling peptide nanofiber hydrogel, showed better EV efficacy and improved renal function [117] |
| AKI | Human UCMSCs | Differential centrifugation and sucrose gradient centrifugation (100,000× g) | Injection/200 μg of total EV protein | Reduced oxidative stress and renal tubular cell apoptosis, increased renal cell proliferation [118] |
| AKI | Human UCMSCs | Ultracentrifugation (100,000× g) | Injection/30 μg of total EV protein | Increased tubular cell proliferation and dedifferentiation [119] |
| AKI | Human Wharton’s Jelly MSCs | Ultracentrifugation (100,000× g) | Injection/100 μg of total EV protein in 1 mL medium 199 | Improved renal function by enhancing tubular cell proliferation and reduced inflammation and apoptosis via mitochondrial fission [120,121] |
| AKI | Human glomerular MSCs | Ultracentrifugation (100,000× g) | Injection/EVs derived from 1 × 105 cells in 120 μL PBS ((T-CD133+-EVs: 480 × 106/mouse; MSC-EVs: 400 × 106/mouse; MSC-EV-float: 400 × 106/mouse; Fibroblasts-EVs: 230 × 106/mouse) | Enhanced renal recovery [122] |
| AKI | Human liver MSCs | Ultracentrifugation (100,000× g) | Injection/EVs derived from 3.5 × 105 or 10 × 105 MSCs | Enhanced renal function through increased tubular cell proliferation and inhibition of apoptosis [123] |
| CDK | Human urinary MSCs | Ultracentrifugation (100,000× g) followed by sucrose gradient ultracentrifugation (100,000× g) | Injection/100 μg of total EV protein in 200 μL PBS | Reduced CKD progression by inhibiting podocyte apoptosis and promoting vascular regeneration and cell survival [125] |
| CDK | Rat urinary MSCs | Total exosome isolation kit (Life Technologies) | Injection/5.3 × 107 particles in 200 μL PBS | Improved renal morphology through the anti-apoptotic behaviour of tubular epithelial cells [126] |
| CDK | Human BMMSCs and human liver MSCs | Ultracentrifugation (100,000× g) | Injection/1 × 1010 particles for each injection once a week for 4 weeks (5 injections) | Reduced CDK progression through miRNAs capable of down-regulating profibrotic genes [127] |
| CDK | Human liver MSCs | Sucrose gradient ultracentrifugation (350,000× g) and ultracentrifugation (100,000× g) | Injection/1 × 1010 particles in 1 mL PBS for each injection once a week for 4 weeks | Reduced CDK progression through miRNAs capable of down-regulating profibrotic genes [128] |
| CDK | Mice BMMSC | Ultracentrifugation (100,000× g) | Injection/30 μg of total EV protein (3 doses) | Protected renal injury via microRNA-dependent repairing [129,130] |
| CDK | Human BMMSCs | ExoQuick-TC ULTRA (EQULTRA-20TC-1, SBI) | Injection/4 × 107 particles in 2 μL PBS | Improved renal function by repairing the damage to apical and basolateral membranes and mitochondria of kidney proximal tubules [131] |
| Grade III-IV CKD (clinical trial) | Human UCMSCs | Ultracentrifugation (100,000× g) | Injection/100 μg of total EV protein per kg per dose (2 doses) | Reduced the progression of CDK [132] |
| Liver | ||||
| Carbon tetrachloride (CCl4)-induced liver injury | Human embryonic MSCs | Tangential flow filtration and chromatography | Injection/0.4 μg of total EV protein in 100 μL PBS | Increased hepatocyte proliferation and reduced apoptosis [133] |
| Hepatic ischemia-reperfusion injury | Human iPSCs | Differential centrifugation and ultrafiltration | Injection/600 μg of total EV protein in 400 μL PBS | Enhanced hepatic regeneration via inhibition of apoptosis of hepatic cells, suppression of inflammatory and attenuation of the oxidative stress response [134] |
| Hepatic ischemia-reperfusion injury | Human iPSCs | ExoQuick Exosome Precipitation Solution (SBI Systems Biosciences) | Injection/2.5 × 1012 particles in 500 μL PBS | Increased hepatocyte proliferation in vitro and in vivo in a dose-dependent manner [135] |
| Hepatic ischemia-reperfusion injury | Human UCMSCs | Ultracentrifugation (100,000× g) | Injection/10 mg of total EV protein per kg | Reduced infiltration of neutrophils and oxidative stress in hepatic tissue [139] |
| Chronic hepatic fibrosis | Human embryonic MSCs | Differential centrifugation and ultracentrifugation (100,000× g) | EV-hydrogel injection/350 μg of total EV protein in 400 μL PEG hydrogel solution | Sustained release of MSC-EVs from PEG hydrogels protected against hepatic failure [140] |
| Muscle | ||||
| Cardiotoxin-induced muscle injury | Human BMMSCs | Sequential ultracentrifugation (110,000× g) | Injection/details not provided | Enhanced muscle regeneration through increased myogenesis and angiogenesis [141] |
| Cardiotoxin-induced muscle injury | Human amniotic fluid MSCs | Ultracentrifugation (200,000× g) | Injection/details not provided | Enhanced muscle regeneration through regulation of inflammation and angiogenesis [142] |
| Tibialis anterior muscle damage | Human ADMSCs | Ultracentrifugation (200,000× g) | Injection/EVs derived from 1 × 106 cells | Enhanced muscle regeneration through the synergistic effect of EVs and soluble proteins [143] |
| Muscle degeneration associated to torn rotator cuffs | Human ADMSCs | Differential centrifugation and ultracentrifugation (100,000× g) | Injection/1 × 1011 particles in 20 μL PBS | Enhanced myofiber regeneration and biomechanical properties of muscles in rotator cuffs [144] |
| Pubococcygeus muscle injury | Human urine-derived MSCs | Differential centrifugation and ultracentrifugation (100,000× g) | Injection/1 × 1010 particles in 1 mL PBS | Enhanced muscle regeneration through the activation, proliferation, and differentiation of muscle satellite cells [145] |
| Muscle damage associated to critical limb ischemia | Human ADMSCs | Ultracentrifugation (100,000× g) | Injection/1 × 1010 particles intravenously or intramuscularly after intervention, 0.5 × 1010 via intramuscular injection after day 1 and 2 | Enhanced muscle regeneration/protection through NRG1-mediated signals [146] |
| Wounds | ||||
| Fibroblasts from normal and chronic wound patients (in vitro) | Human BMMSCs | Differential centrifugation and ultracentrifugation (100,000× g) | 0.1, 1, and 10 μg total EV protein in 1 mL PBS | Enhanced proliferation and migration of fibroblasts and tube formation by endothelial cells [149] |
| Skin wound | Human iPSC | Differential centrifugation and ultracentrifugation (100,000× g) | Injection/160 μg total EV protein in 160 μL PBS at wound sites and 40 μg total EV protein in 40 μL PBS at wound beds | Increased collagen synthesis and angiogenesis [150] |
| Photo-damaged dermal fibroblasts in vitro) | Human ADMSCs | Tangential flow filtration | 1 × 108 particles in 1 mL PBS | Increased collagen and elastin synthesis and decreased metalloproteinases activity [151] |
| Skin wound | Human ADMSCs | Ultrafiltration and ExoQuick-TC kit (System Biosciences) | Injection/200 μg of total EV protein in 200 μL PBS | Enhanced wound healing by regulating the collagen distribution secreted by fibroblasts in the early and late stage of wound healing [152] |
| Excisional skin wound | Human ADMSCs | Differential centrifugation and ultracentrifugation (100,000× g) | EV-gel transplantation/1.9 × 108 particles in hydroxyethyl cellulose aqueous gel | Enhanced wound healing through increased migration and proliferation of keratinocytes and fibroblasts [153] |
| Ischemic wound | Human ADMSCs | ExoQuick-TC kit (System Biosciences) | Injection/details not provided | Enhanced wound healing through migration and proliferation of dermal fibroblasts [154] |
| Cutaneous wound | Rabbit ADMSCs and BMMSCs | Ultracentrifugation (100,000× g) | Injection/EVs derived from 10 × 106 MSCs | Significant better wound healing upon treatment with ADMSC-EVs [155] |
| Second-degree burn wound | Human UCMSCs | Differential centrifugation and sucrose gradient ultracentrifugation (100,000× g) | Injection/200 μg of total EV protein in 200 μL PBS | Enhanced wound healing through increased fibroblasts proliferation and angiogenesis and reduced skin cell apoptosis [156,157,158] |
| Skin wound | Human UCMSCs | Differential centrifugation and ultracentrifugation (120,000× g) | EV-scaffold transplantation/100 μg of total EV protein in 100 μL PBS | Enhanced wound healing and reduced scar formation through inhibition of myofibroblast differentiation [159] |
| Other tissues/organs | ||||
| Injuries in lung cells (in vitro) | Human placenta MSC | Differential centrifugation and ultracentrifugation (100,000× g) | 6 × 105 particles in 20 µL serum-free media | Enhanced cell migration, reduced oxidative cell stress and inflammation [160] |
| Influenza virus-induced acute lung injury | Swine BMMSCs | Ultracentrifugation (25,000 rpm) | Intratracheally/EVs produced by 10 × 106 MSCs | Reduced virus shedding, production of proinflammatory cytokines, and influenza virus-induced lung lesions [161] |
| lung injury induced by haemorrhagic shock and trauma | Human BMMSs | Differential centrifugation and ultracentrifugation (100,000× g) | Injection/30 μg of total EV protein in 200 μL PBS | Improved pulmonary vascular permeability through the activation of proteins and pathways linked to cytoskeletal rearrangement [162] |
| Hyperlipidaemia | Human placenta MSC | Differential centrifugation and ultracentrifugation (100,000× g) | EV-scaffold transplantation/100 μg of total EV protein in 1 mL PBS | Reduced calcification of synthetic vascular grafts by immunomodulation and improved vascular function [163] |
| Neointimal hyperplasia (in vitro) | Human ADMSCs | Differential centrifugation and ultracentrifugation (100,000× g) | 100 μg of total EV protein in 1 mL PBS | Reduced abnormal proliferation and migration of vascular smooth muscle cell [164] |
| Hind-limb ischemia | Human UCMSCs | Differential centrifugation and ultracentrifugation (100,000× g) | Injection/2 × 1010 particles in 1 mL PBS | Enhanced angiogenesis and muscle regeneration, and ischemic limp function [165] |
| Tracheoesophageal fistulas | Porcine ADMSCs | Ultracentrifugation (100,000× g) | EV-gel injection/1.3 × 1011 particles in 1 ml of pluronic F127 gel | Enhanced oesophageal fistula healing through targeted delivery of EVs embedded in thermo-responsive hydrogels [166] |
| Ulcerative colitis | Mouse BMMSCs | Differential centrifugation and ultracentrifugation (100,000× g) | Injection/50 μg of total EV protein per day for 7 days | Improved symptoms through stimulating M2 macrophage polarization and negative inflammatory response [167] |
| Tissue Injury/Disease | Condition | Treatment | Trial Phase | Trial ID |
|---|---|---|---|---|
| Lung injury | Healthy | Aerosol inhalation of allogenic ADMSC-EVs (2 × 108 particles/3 ml or 1 × 108 particles/3 ml) | Phase I | NCT04313647 |
| Lung disease (pneumonia) | Coronavirus disease-19 | Aerosol inhalation of allogenic ADMSC-EVs (2 × 108 particles/3 ml) for 5 days | Phase I | NCT04276987 |
| Chronic lung disease | Bronchopulmonary dysplasia | Intravenous infusion of BMMSC-EVs (20 or 60 or 200 pmol phospholid/kg body weight) | Phase I | NCT03857841 |
| Cartilage injury | Osteoarthritis | Osteochondral explants from arthroplasty patients treated with ADMSC-EVs | Phase I | NCT04223622 |
| Brain injury | Acute ischemic stroke | Administration of allogenic MSC-EVs enriched with miR-124 (200 μg total EV protein) via Stereotaxis | Phase II | NCT03384433 |
| Skin injury | Dystrophic Epidermolysis Bullosa | Allogenic BMMSC-EVs locally administrated | Phase II | NCT04173650 |
| Kidney disease | CDK | Injection of allogenic UMMSC-EVS (100 μg of total EV protein per kg per dose) | Phase II/III (completed) | [132] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsiapalis, D.; O’Driscoll, L. Mesenchymal Stem Cell Derived Extracellular Vesicles for Tissue Engineering and Regenerative Medicine Applications. Cells 2020, 9, 991. https://doi.org/10.3390/cells9040991
Tsiapalis D, O’Driscoll L. Mesenchymal Stem Cell Derived Extracellular Vesicles for Tissue Engineering and Regenerative Medicine Applications. Cells. 2020; 9(4):991. https://doi.org/10.3390/cells9040991
Chicago/Turabian StyleTsiapalis, Dimitrios, and Lorraine O’Driscoll. 2020. "Mesenchymal Stem Cell Derived Extracellular Vesicles for Tissue Engineering and Regenerative Medicine Applications" Cells 9, no. 4: 991. https://doi.org/10.3390/cells9040991
APA StyleTsiapalis, D., & O’Driscoll, L. (2020). Mesenchymal Stem Cell Derived Extracellular Vesicles for Tissue Engineering and Regenerative Medicine Applications. Cells, 9(4), 991. https://doi.org/10.3390/cells9040991





