Abstract
Mesenchymal stem cells (MSCs) are being extensively investigated for their potential in tissue engineering and regenerative medicine. However, recent evidence suggests that the beneficial effects of MSCs may be manifest by their released extracellular vesicles (EVs); typically not requiring the administration of MSCs. This evidence, predominantly from pre-clinical in vitro and in vivo studies, suggests that MSC-EVs may exhibit substantial therapeutic properties in many pathophysiological conditions, potentially restoring an extensive range of damaged or diseased tissues and organs. These benefits of MSC EVs are apparently found, regardless of the anatomical or body fluid origin of the MSCs (and include e.g., bone marrow, adipose tissue, umbilical cord, urine, etc). Furthermore, early indications suggest that the favourable effects of MSC-EVs could be further enhanced by modifying the way in which the donor MSCs are cultured (for example, in hypoxic compared to normoxic conditions, in 3D compared to 2D culture formats) and/or if the EVs are subsequently bio-engineered (for example, loaded with specific cargo). So far, few human clinical trials of MSC-EVs have been conducted and questions remain unanswered on whether the heterogeneous population of EVs is beneficial or some specific sub-populations, how best we can culture and scale-up MSC-EV production and isolation for clinical utility, and in what format they should be administered. However, as reviewed here, there is now substantial evidence supporting the use of MSC-EVs in tissue engineering and regenerative medicine and further research to establish how best to exploit this approach for societal and economic benefit is warranted.
1. Introduction
For some patients with end-organ dysfunction, whole organ transplantation is an established treatment option [1]. However, the limited availability of suitable autologous tissues, the risk of immune-mediated rejection, the required chronic immunosuppression treatment, and the possibility of disease transmission, highlight the need of new therapeutic approaches [2]. Tissue engineering and regenerative medicine strategies have triggered intense attention due to the potential to develop remedies for damaged, malfunctioning, or injured tissues [3]. Cell-based therapies, in their natural form or modified/engineered for a specific purpose, hold much promise in this regard. Indeed, in light of their multiple sources as well as therapeutic versatility, mesenchymal stem cells (MSCs) have been proposed as the most appropriate cell source for these applications [4,5]. As stem cells, they exhibit beneficial characteristics as compared to terminally differentiated cells, including the potential to circumvent immuno-reaction in vitro and in vivo and to differentiate towards a broad range of specific cell lineages [6,7]. MSCs can be isolated from various tissue types including bone marrow, adipose, umbilical cord, peripheral blood, liver, periodontal ligament, lung and many others [8]. However, despite their potential and promise, MSCs face many challenges, such as their variability, scalability and delivery, as well as ethical considerations and safety issues [9,10,11,12,13], which challenge their clinical utility.
EVs are heterogeneous lipid bilayer-surrounded vesicles secreted by all cell types, not only MSCs, and act as mediators of intercellular communication. EVs are involved in numerous physiological and pathophysiological biological processes, including modulating immune responses, homeostasis maintenance, coagulation, inflammation, angiogenesis, and cancer progression [14,15,16,17]. According to their size, dimension and origin, they can be classified in many ways, with the preferred terms now being small EVs, medium-sized EVs, and large EVs [18,19].
There is increasing evidence that many, if not all, of the beneficial effects of MSCs may be attributed to their paracrine action via the release of extracellular vehicles (EVs), rather than cellular engraftment and response to the site of injury [20,21,22]; suggesting that MSC-EVs can produce any therapeutic benefits of MSCs [23]. Added to their attractiveness, compared to the original MSCs, MSC-EVs cannot self-replicating, preventing safety concerns associated with cell therapy, such as uncontrolled cell division and cellular contamination with tumorigenic cells [24]. Moreover, as MSCs often require invasive procedures in order to be isolated, approaches that only require them to be cultured in vitro and their released product used (i.e., EVs) gives hope for increased scalability and yield per MSC batch [25], with filtration suggested to be suitable sterilisation because of their small size [26].
Herein, we review current advancements of the therapeutic potential of MSC-EVs in tissue engineering and regenerative medicine (Figure 1), considering the molecular mechanisms suggested for the MSC-EV action where possible.
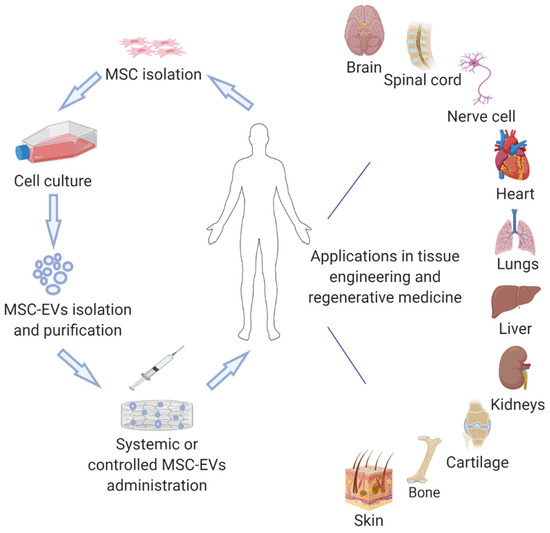
Figure 1.
Examples of potential applications of MSC-EVs in tissue engineering and regenerative medicine.
2. EV Biogenesis and Characterisation
Small-size EVs, most of which were previously termed as exosomes, are vesicles ranging from 30–150 nm. The biogenesis of the small-size EVs occurs initially with the formation of early endosomes from endocytoses of the cell membrane (Figure 2). During the process of maturation, the early endosomes become endosomes or multivesicular bodies and they begin to accumulate intraluminal vesicles, which either degraded by lysosomes or are released as exosomes in the extracellular space [27]. Medium- and large-size EVs, previously described as microvesicles or ectosomes, are large vesicles between 100–1000 nm diameter. Their biogenesis occurs via the direct budding of the cell membrane and releasing into the extracellular space [28,29]. While apoptotic bodies are also large-size vesicles, ranging from 1–5 mm, they originate specifically from apoptotic cells [30].
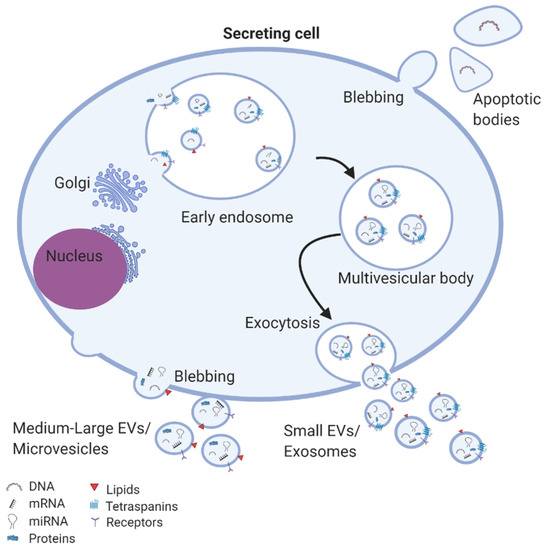
Figure 2.
EV biogenesis and secretion: exosomes are assembled in multivesicular bodies where specific cargos are sorted into exosomes and subsequently released in the extracellular space. Microvesicles are formed from budding of the cell membrane. Apoptotic bodies are generated from apoptotic cells.
As extensively review by the EV research community, according to their cellular origin and the mechanism of secretion, EVs carry somewhat distinct surface markers [21]. Tetraspanin proteins such as CD9, CD81, and CD63 are enriched in the membrane of exosomes and so they are often used to help quality small EVs as exosomes. Furthermore, small EVs may be distinguished by the presence of proteins involved in their biogenesis, including annexin, flotillin, auxiliary proteins (ALIX, TSG101, VPS4), components of the endosomal sorting complex required for transport (ESCRT), GTPase and heat shock proteins (HSP70 and HSP90) [19]. In contrast, CD40 ligand and annexin A1 are associated with medium- and large-size EVs [31,32], whereas apoptotic bodies carry annexin V [33]. Additionally, EV sub-populations contain a range of forms of lipids: cholesterols, diglycerides, sphingolipids (including sphingomyelin and ceramide), phospholipids, and glycerophospholipids, which are key components for the structure, function and biogenesis of EVs [34]. It has been well established that EVs also carry nucleic acids that can be transferred to secondary cells, affecting their cellular processes [17]. Among these, for example, the mRNA content of some EVs has been suggested to significantly influence the biological function of neighbouring cells, such as cell differentiation, transcription, cell proliferation and immune regulation. Similarly, many studies have reported miRNA within EVs being transferred to recipient cells and subsequently altering the gene expression and phenotype of those cells e.g., modulating cycle, apoptosis, migration, inflammation, and neo-angiogenesis [35,36,37,38].
While these studies related to EV biogenesis and characterisation are not all focussed on MSCs, it seems that MSC-EVs are also heterogeneous, carry cargo such as proteins, nucleic acids and lipids, and are involved in cell-to-cell communication.
3. EV Isolation Methods
A challenge for MSC-EV in regenerative medicine, particularly when considering towards clinical utility, is the lack of standardisation in EV isolation methods. Of course, this is not unique to MSC-EVs; there are many options but no standardised method for the isolation and purification of EVs. A world-wide survey by the International Society for Extracellular Vesicles (ISEV) [39], considering EV isolation from any and all sourced showed ultracentrifugation-based methods to be most commonly used, although a range of other approaches have been taken to overcome challenges regarding ultracentrifugation including the need for an ultracentrifuge, low-throughput of samples, and potential damage to EVs caused by high-speed centrifugation [40,41]. To this end, alternative methods such as filtration/ultrafiltration, size exclusion chromatography, immunoprecipitation, and precipitation with reagents such as PEG have been utilised, with varying degrees of efficacy in terms of purity and quantity [42]. Thus, combinations of two or more isolation methods are gaining popularity in order to increase EV purity [43]. Moreover, EVs can be modified to enhance their therapeutic potential. Thus, EVs can be incorporated with elements including drugs, antibodies, proteins and RNA for targeted delivery and with molecules such as lipophilic dyes and amino-reactive fluorophores for in vitro and in vivo traceability. In that cases, novel EV-like approaches have been generated such as bio-engineered EVs, EV-mimetic nanovesicles and EV-based semi-synthetic vesicles, as described and reviewed by others [44,45,46,47,48]. Specifically in relation to MSC-EVs, a range of methods of EV isolation from different sources and for different applications to address clinical problems (in the nervous system, heart, bone, cartilage, kidney, liver, muscle, wounds, and other tissues/organs) have been reported, as summarised in Table 1.

Table 1.
MSC-EVs in tissue engineering and regenerative medicine applications.
4. MSC-EVs in Tissue Engineering and Regeneration
4.1. Nervous Regeneration
The nervous system is a crucial component of the body and any injury or a disease to it can cause serious or potentially lethal consequences. Nerve repair has long remained a significant objective in regenerative medicine, due to the physiological system complexity and the limited healing capacity [49]. A plethora of strategies have been employed to correct peripheral nerve injuries. In one such study, EVs derived from rat bone marrow mesenchymal stem cells (BMMSCs), were reported to stimulate nerve regeneration after sciatic peripheral nerve crush injury in a rat model. The benefit from the EV was manifest as improved sciatic function index after injury, enhanced histomorphometric repair in nerve regeneration, and increased expression of growth associated protein 43 (GAP43), a marker of axon regeneration [50]. Similarly, peripheral nerve recovery was noticed when EV from human umbilical cord mesenchymal stem cells (UCMSCs) were applied in the site of sciatic nerve defect in rats. EVs aggregated at the site of the nerve injury, prompted the generation of axons and Schwann cells that surrounded individual axons, reduced denervated muscle atrophy and modulated inflammation via down-regulation of pro-inflammatory cytokines (interleukin [IL]-6 and IL-1β) and up-regulation anti-inflammatory cytokines (IL-10) [51]. Another study suggested that EVs from rat adipose-derived mesenchymal stem cells (ADMSCs) promoted peripheral nerve regeneration and neurite growth in sciatic nerve defects, assumed to be through Schwann cell (SC) modulation [52]. Mechanistically, Schwann cells stimulation and proliferation in the damaged neurons relies on the perinuclear location of ADSC-EVs and their accumulation in vesicular-like structures within the Schwann, which indicates an endocytosis-mediated internalization pathway [53]. A more detailed mechanism of peripheral nerve regeneration upon treatment with gingiva MSC-EVs illustrated that the proliferation and migration of Schwann cells occur mainly via the activation of c-Jun N-terminal Kinase (JNK) pathway and the up-regulation of c-Jun, Notch1, glial fibrillary acidic protein (GFAP), and SRY (sex determining region Y)-box 2 (Sox2) characteristic genes of de-differentiation or repair phenotype of Schwann cells [54].
Research efforts showed also the therapeutic efficacy of MSC-EVs in the context of central nervous system (CNS) repair. In a model of middle cerebral artery stroke (MCA), EVs derived from rat BMMSCs led to neurite outgrowth by transfer of miR-133b to neural cells [55]. Systemic administration of rat BMMSC-EVs increased the axonal density and synaptophysin-positive areas along the ischemic boundary zone of the cortex and striatum in MCA rats. This was accompanied by enhanced expression of newly synthesised doublecortin (a marker of neuroblasts [56]) and von Willebrand factor (a marker of endothelial cells [57]) as compared to the untreated controls, suggesting thus neurite remodelling, neurogenesis and angiogenesis as a novel treatment for stroke [58].
With respect to brain damage, rat BMMSC-EVs have been assessed in traumatic brain injury (TBI) rat models. Overall, EV treatment improved recovery of brain function after TBI by increasing the number of newly-generated immature and mature neurons in the dentate gyrus as well as the number of newly-generated endothelial cells in the lesion boundary zone and dentate gyrus [59].
The impact of MSC-EVs has also been investigated in spinal cord injuries (SCI). SCI is frequently associated with microvascular stability disruption and an increase in blood-spinal cord barrier (BSCB) permeability, mainly caused by abnormal migration of pericytes [60,61]. Notably, treatment with mouse BMMSC-EVs inhibited the migration of pericytes and thereby improved the structural integrity of the BSCB and, in turn, the motor function in a SCI rat model [62]. Another potent mechanism of spinal cord recovery upon BMMSC-EVs treatment suggested the prevention of neuronal apoptosis through the activation of the Wnt/β-catenin signalling pathway [63]. Furthermore, in an SCI model, modification of rat BMMSC-EVs with miR-133b caused activation of the ERK1/2, STAT3 pathway. This resulted in enhanced neuron preservation, axon regeneration, and locomotor function, when compared to treatment with BMMSC-EVs that had not been modified to carry miR-133b [64].
MSC-EVs from human placenta have been tested in a multiple sclerosis mouse (MS) model. Here, MSC-EVs induced myelin regeneration in vitro by endogenous oligodendrocyte precursor cells differentiation into mature myelinating oligodendrocytes and increased myelination in the spinal cord of treated mice, following by improved motor function outcomes [65]. Furthermore, EVs from human BMMSCs that had been stimulated with interferon-gamma (IFN-γ), reduced neuroinflammation and demyelination improving the motor skills in a MS mouse autoimmune encephalomyelitis (EAE) model [66].
Additionally, in a mouse model of Alzheimer’s disease, MSC-EVs (the source of the MSCs was not detailed) promoted neurogenesis and cognitive function recovery [67].
4.2. Cardiac Regeneration
Endogenous myocardial repair after damage is very slow and is dependent on the limited self-division of pre-existing cardiomyocytes and the recruitment and differentiation of resident cardiac stem cells [68,69,70]. The exogenous cell-free approach of using MSC-EVs therapeutically has emerged to address the insufficient responses to myocardial damage typically achievable by endogenous mechanisms. Initial studies established that human embryonic-derived MSC-EVs could reduce infarct size in a mouse model of myocardial ischemia/reperfusion injury (MI) [71], through the activation of the PI3K/Akt signalling pathway, which increased myocardial viability and inhibited adverse remodelling [72]. Taken into consideration this observation, human UCMSCs were subsequently transfected with Akt, which was found to be at high levels in their released EVs. Compared to the non-modified human UCMSC-EVs, this Akt-carrying human UCMSC-EVs complex further accelerated endothelial cell proliferation, migration and tube-like structure formation in vitro and blood vessel formation in vivo [73].
In this setting, some studies have done direct comparisons of MSCs versus MSC-EVs and as a result have highlighted the added beneficial effects of EVs compared to their MSCs of origin. In one such study, murine induced pluripotent mesenchymal stem cells (iPSCs)-EVs and human amniotic fluid-derived mesenchymal stem cells (hAFS) were found to improve cardiac repair in MI murine models and trigger cardiac regeneration via paracrine modulation of endogenous mechanisms. The administered EVs also exhibiting a safer profile when compared to the administration of their cells of origin [74,75]. However, in another MI rat model study, combinatorial treatment with both rat BMMSCs and their derived EVs further improved cardiac function, reduced infarct size, and increased neovascularisation when compared to experimental groups treated with either BMMSCs or BMMSC-EVs alone [76].
Hypoxia pre-conditioning of human BMMSCs has been reported to enhance cells’ biological activities in vitro [77], whilst showed to improve the effectiveness of Cynomolgous monkey BMMSCs when implanted as a treatment of MI in monkeys [78]. Interestingly, hypoxia positively influenced the therapeutic efficacy of the secreted EVs. Hypoxia-elicited human BMMSC-EVs (1% O2 for 72 h) showed higher cardiac regeneration in a rat MI model than BMMSCs-EVs isolated under normoxia conditions; the mechanism reported as responsible was by increasing angiogenesis in the site of infract region [79]. Moreover, hypoxia-reconditioning murine and rat BMMSC-EVs (at either 1% O2 for 72 h or 0.5% O2 for 24 h) prevented cardiomyocyte apoptosis through the enrichment of miR-125b-5p-EVs and miR-210-EVs. The associated mechanism here was suppression of pro-apoptotic genes p53 and BCL2-antagonist/killer 1 (BAK1) and increased recruitment of cardiac progenitor cells in the infarcted heart [80,81].
As an alternative approach to injecting MSC-EVs to the cardiac defect, they can be encapsulated to hydrogels for controlled and targeted administration. Hence, sustain release profile and increased cardiac regeneration was noticed when human UCMSC-EVs were loaded in functional peptide hydrogels. Specifically, the EV/hydrogel complex improved the myocardial function by reducing inflammation, fibrosis and apoptosis, and by promoting angiogenesis in infarcted border zone of rat hearts [82].
4.3. Bone Regeneration
The paracrine effects of MSCs —through the use of their EVs— also offer a potential alternative approach for skeletal regeneration. To this end, EVs from different sources of MSCs are being investigated for bone reconstruction after injury. It was noted that human BMMSC-EVs and human iPSCs-EVs stimulated osteogenic differentiation of BMMSCs in vitro, and bone formation and angiogenesis BMMSCs in vivo in rat models with critical-sized calvarial defects [83,84]. To enhance the bone healing, the human BMMSC-EVs were modified with dimethyloxaloylglycin, which resulted in further stimulation of angiogenesis through the Akt/mTOR pathway [85]. The efficacy of EVs derived from human ADMSCs towards bone regeneration was also enhanced by pre-conditioning the MSCs with the cytokine tumour necrosis factor-alpha (TNF-α), as was evidenced by increased proliferation and osteogenic differentiation of osteoblastic cells in vitro [86]. Furthermore, in a femoral shaft fracture model of CD9−/− mice whose bone healing capacity is impaired compared to that of wild-type equivalents, administration of human BMMSC-EVs rescued the delay in fracture healing, and accelerated bone repair in wild types [87].
For dental regeneration, the addition of EVs derived from human dental pulp MSCs resulted in odontogenic differentiation in vitro as a results of endocytosis of the EVs, subsequent activation of the P38 mitogen activated protein kinase (MAPK) pathway, and regeneration of dental pulp-like tissue in a tooth root slice model [88]. Moreover, MSC-EVs promoted periodontal ligament cell migration and proliferation through CD73-mediated adenosine receptor activation of pro-survival Akt and ERK signalling and periodontal regeneration in a rat periodontal defect model [89].
With the aim of augmenting scaffold performance and thereby improving bone healing, tissue engineered-constructs have been combined with MSC-EVs. Thus, human ADMSC-EVs were immobilised to poly (lactic-co-glycolic acid) and biotin-doped polypyrrole titanium scaffolds. In vitro studies of scaffolds functionalised with the EVs versus unmodified scaffolds showed the former to results in higher osteo-inductivity of BMMSCs and osteoblasts, while in vivo studies using murine models of bone defects showed significantly greater bone tissue and mature collagen formation [90,91]. Additionally, human BMMSC-EVs loaded into tricalcium phosphate scaffolds enhanced bone healing of calvarial defects by activation PI3K/Akt signalling pathway [92], whilst rat BMMSC-EVs encapsulated into decalcified bone matrix scaffolds stimulated bone regeneration by promoting vascularisation in the grafts [93]. Moreover, three-dimensional polylactic acid scaffolds functionalised with human gingival MSCs have been used as a therapeutic tool for bone tissue engineering, with promising osteogenic properties and response in rat models of cortical calvaria bone damage [94].
4.4. Cartilage Regeneration
Articular cartilage has limited intrinsic regenerative capacity upon injury and if poorly healed may lead to osteoarthritis (OA); a severe disease accompanied with loss of joint function and devastating pain [95]. MSC-EVs from various cell sources provide new insights for the development of cell-free therapies for the treatment of cartilage injuries and OA. The therapeutic efficacy of MSC-EVs over their cells of origin for the treatment of OA was highlighted in a comparison study using amniotic fluid MSCs and their derived EVs. EV-treated defects showed superior pain tolerance level and improved histological scores than the MSC-treated defects [96]. Furthermore, human BMMSC-EVs have been reported to promote in vitro cartilage regeneration by triggering the production of collagen type II and proteoglycans of chondrocytes isolated from OA patients [97]; both of which are extracellular matrix (ECM) components essential for the proper cartilage repair [98]. It is widely accepted that OA is associated with cartilage degradation mediated mainly by Wnt5A, a non-canonical Wnt protein, which can activate matrix metalloproteinases (MMPs) and reduce the formation of cartilage ECM [99]. Human BMMSC-EVs modified to be enriched with miR-92a-3p have been reported to suppress cartilage degradation and promote cartilage repair in vitro and in an in vivo OA mouse model, as a result of miR-92a-3p targeting Wnt5A [100]. Furthermore, pre-conditioning of rat MSCs with transforming growth factor beta (TGFβ) enhanced the quantities of miR-135b in the resulting EVs, which stimulated chondrocyte proliferation in vitro through specificity protein 1 (Sp1) regulation and cartilage tissue repair in a rat model of OA [101].
In studies applying human embryonic MSC-EVs in rat and mouse models with osteochondral defects, it was noted that osteochondral regeneration was mediated through distinct well-orchestrated mechanisms, such as by enhancing chondrocyte proliferation; attenuating apoptosis; and regulating immune reactivity in the site of the injury; by balancing the synthesis and degradation of cartilage ECM and by restoring matrix homeostasis [102,103,104,105].
In addition to enriching EVs with specific miRNA as summarised above, other strategies have been used to augment the potency of MSC-EVs in cartilage repair. For instance, three-dimensional culture of UCMSCs in a hollow-fibre bioreactor resulted in a higher yield of EVs and superior therapeutic efficacy in a rabbit cartilage defect model, compared to MSC-EVs from conventional 2D cultures [106]. To ensure that MSC-EVs are retained at the site cartilage injury, human iPSC-EVs have been incorporated with in situ hydrogel glue. This acellular tissue patch was found to integrate with native cartilage matrix and promote cell deposition at cartilage defect sites, resulting in functional cartilage repair [107]. 3D printing has been described as the next generation of fabrication techniques in tissue engineering that enable the development of complex forms with high precision [108]. Interestingly, BMMSC-EVs together with cartilage ECM/gelatin methacrylate hydrogel have been used as bio-inks for the design of a bio-scaffold. The resulting 3D printed device promoted the targeted delivery of EVs, preventing mitochondrial dysfunction in degenerative chondrocytes in vitro, and facilitated the cartilage regeneration in an in vivo osteochondral defect rabbit model [109].
4.5. Kidney Regeneration
The number of studies using MSC-EVs for the treatment of acute kidney injuries (AKI) and chronic kidney damage (CDK) is continuously increasing, suggesting this strategy may be a promising approach for kidney regeneration. Initially it was established that human BMMSC-EVs accelerated the recovery of injured tubular cells, stimulated cell proliferation, prevented apoptosis and supported functional recovery of glycerol-induced AKI [110]. The suggested route of MSC-EV action involved the delivery of genetic material—such as mRNAs and miRNAs—to injured renal cells, contributing to anti-inflammatory, anti-apoptotic, anti-fibrotic, and pro-angiogenesis effects on AKI [111,112]. Another reported mechanism of renal repair in response to BMMSC-EVs treatment includes the horizontal transfer of human IGF-1 receptor mRNA, which is present in MSC-EVs, to tubular cells [113]. Moreover, administration of human and mice BMMSC-EVs in rat and mice AKI models, respectively, protected the animals from AKI and improved renal function; by inhibiting apoptosis and stimulating tubular epithelial cell proliferation [114,115]. In a cisplatin-induced toxic AKI mouse model, human BMMSC-EVs ameliorated renal function and morphology, and improved survival. Considering the mechanism responsible for this in vitro in cisplatin-treated human tubular epithelial cells, it was shown that the EVs up-regulated anti-apoptotic genes (B-cell lymphoma extra-large B-cell lymphoma 2 and baculoviral IAP repeat containing 8) and down-regulated genes that contribute in the execution-phase of cell apoptosis (caspase-1, caspase-8 and lymphotoxin alpha) [116]. A significant better EV efficacy followed by improved renal function was noticed when mice BMMSC-EVs were loaded to self-assembling peptide nanofiber hydrogels, for control and targeted release of EVs on the site of mice AKI models after ischaemia-reperfusion [117]. Aside from bone marrow, MSC-EVs from other tissues have been isolated and evaluated for renal regeneration. In one such study, human UCMSC-EVs induced in vitro and in vivo renal repair in rat AKI models (cisplatin-induced and unilateral), through reducing oxidative stress and cell apoptosis, promoting cell proliferation, and tubular cells de-differentiation [118,119]. Similar renal regeneration was observed when EVs derived from human Wharton’s Jelly MSCs were applied in AKI rat models. It was also reported that EVs improved renal function by enhancing tubular cell proliferation and reducing inflammation and apoptosis via mitochondrial fission [120,121]. EVs isolated from human glomerular MSCs and liver MSCs have also been credited with stimulating recovery after AKI [122,123].
Several studies have assessed the influence of MSC-EVs in models of chronic kidney damage (CDK) mainly caused by diabetes [124]. Human urinary MSC-EVs have been reported to prevent CKD progression, by inhibiting podocyte apoptosis and promoting vascular regeneration and cell survival in a rat model of streptozotocin-induced diabetic nephropathy [125]. Another study revealed an improvement of renal morphology with profound anti-apoptotic behaviour of tubular epithelial cells when urinary MSC-EVs were injected into diabetic mice [126]. Human BMMSC-EVs and human liver MSC-EVs inhibited fibrosis and prevented its progression in a mouse model of diabetic nephropathy mediated by miRNAs capable of down-regulating profibrotic genes [127]. Analogous observations were noticed upon administration of human liver MSC-EVs in a CKD model induced by aristolochic acid [128]. Murine BMMSC-EVs protected against renal injury both in vitro and in vivo by microRNA-dependent repair in CKD mice models of surgical 5/6 nephrectomy of the kidney tissue [129,130]. Furthermore, injection of human BMMSC-EVs repaired the damage to apical and basolateral membranes and mitochondria of kidney proximal tubules, and improved renal function in a cadmium medaka model resembling CKD due to long-term environmental exposure to heavy metal [131]. In a clinical trial in forty CKD patients stage III and IV (n = 20 administered MSC-EVs, n = 20 administered placebo) it was observed that MSC-EVs derived from umbilical cord are safe and were able to ameliorate the progression of CDK in grade III-IV CKD patients [132].
4.6. Liver Regeneration
Evaluating the potential benefits of MSC-EVs in relation to liver disease, in a carbon tetrachloride (CCl4)-induced liver injury mouse model human embryonic MSC-EVs were found to promote hepatic regeneration, by increasing hepatocyte proliferation and reduced hepatocyte apoptosis [133]. Moreover, human iPSC-EVs enhanced hepatic regeneration in hepatic ischemia-reperfusion injury rat models, by inhibiting apoptosis of hepatic cells, suppressing inflammatory responses, and attenuating the oxidative stress response [134]. Human iPSC-EVs were also reported to induce hepatocyte proliferation in vitro and in vivo in a dose-dependent manner, which is related to the activation of sphingosine kinase and sphingosine-1-phosphate signalling pathway [135], known to promote cell proliferation in various cell types [136,137,138]. Similarly, treatment with human UCMSC-EVs has been shown to ameliorate the infiltration of neutrophils and diminish oxidative stress in hepatic tissue; therefore protecting against hepatic apoptosis [139]. To further enhance the benefits of EVs, human embryonic MSC-EVs were encapsulated in PEG hydrogels for sustain systemic delivery against hepatic failure. Here, EVs accumulated in the liver of the rat model of chronic hepatic fibrosis for prolonged time, exerting superior anti-apoptosis, anti-fibrosis and regenerative properties as compared to conventional EV injection [140].
4.7. Muscle Regeneration
The influence of MSC-EVs have been also assessed in skeletal muscle regeneration. For example, human BMMSC-EVs were found to augment myogenesis and angiogenesis in vitro (mediated by miRNAs such as miR-494) and to enhanced muscle regeneration [141]. Moreover, it was noted that EVs derived from amniotic fluid MSCs contain a spectrum of proteins and miRNAs capable of regulating inflammation and angiogenesis which, in turn, underpin skeletal muscle regeneration [142]. Bioinformatic (miRNA profile and proteomics) analysis of a study assessing the regenerative effect of human ADMSC-EVs on muscle injury showed that repair was mediated by factors distributed both within MSC-EVs and the soluble fraction of the secretome [143].
As a preventative measure, EVs isolated from human ADMSCs have been tested as a means to prevent muscle injuries related to torn rotator cuffs. Here, MSC-EV treatment prevented the atrophy, fatty infiltration, inflammation, and vascularisation of muscles in a rat model of torn rotator cuffs and, also, increased the myofiber regeneration and biomechanical properties of the muscles in rotator cuffs [144]. Furthermore, human urine-derived MSC-EVs promoted repair of pubococcygeus muscle injury in rat models of stress urinary incontinence, through stimulating phosphorylation of extracellular-regulated protein kinases and the activation, proliferation, and differentiation of muscle satellite cells [145]. Additionally, human ASC-EVs have recently been shown to prevent muscle damage in a mouse model of critical hindlimb ischemia, mainly through neuregulin 1 protein (NRG1)-mediated signals playing a crucial role in angiogenesis, prevention of inflammation, and muscle protection [146].
4.8. Wound Healing
Wound healing is a dynamic process that requires a complex of molecular and cellular events, including cellular migration, proliferation, angiogenesis, ECM deposition, and tissue remodelling [147]. Wounds that exhibit impaired or improper healing have failed to progress through the normal stages of healing i.e., homeostasis, inflammation, proliferation, and remodelling; leading to the formation of excessive scars [148]. Several studies have demonstrated the beneficial activities of MSC-EVs for various chronic wounds. In one such study, BMMSC-EVs enhanced, in a dose-dependent manner, the ex vivo proliferation and migration of fibroblasts from healthy donors and chronic wound patients. These EVs also mediated tube formation by endothelial cells, through the activation of pathways (Akt, ERK, and STAT3) involved in wound healing [149]. Another in vitro study suggested that cutaneous wound healing could be facilitated by increasing collagen synthesis and angiogenesis following treatment with human iPSC-EVs [150]. Higher collagen and elastin synthesis was also noted when human ADMSC-EVs were added to photo-damaged human dermal fibroblasts in vitro [151]. In vivo using a mouse skin incision model, injection of human ADMSC-EVs accelerated wound healing via modifying the phenotypic characteristics of fibroblasts. Specifically, collagen I and III distributions secreted by fibroblasts were increased in the early stage of wound healing while, in the late stage, collagen synthesis was diminished to reduce scar formation [152]. EVs from the same source were reported to trigger the migration and proliferation of keratinocytes and fibroblasts in vitro and in vivo in excisional wound-splinting rat models, by a mechanism involving the activation of Akt pathway [153]. lncRNA metastasis-associated lung adenocarcinoma transcript 1 found in human ADMSC-EVs contributed significantly to the migration and proliferation of dermal fibroblasts, promoting wound closure in a rat model of ischemic wound healing [154]. In a comparison study examining the effect of rabbit ADMSC-EVs and BMMSC-EVs on rat cutaneous wound models, treatment with ADMSC-EVs showed significant better healing [155].
Moreover, human UCMSC-EVs have been reported to intensively promote the healing of second-degree burn wounds in vivo, mediated mainly by the activation of Wnt/β-catenin signalling pathway and subsequent increased dermal fibroblasts proliferation, angiogenesis, and reduced skin cell apoptosis [156,157,158]. Wound healing and suppressed scar formation have been facilitated by inhibiting myofibroblast differentiation at the site of skin defects by treating with human UCMSC-EVs. This therapeutic benefit of EVs has been particularly credited to the activities of specific microRNAs (namely, miR-21, -23a, -125b, and -145) [159].
4.9. The Regenerative Effect of MSC-EVs on Various Tissues
Aside from their reported beneficial therapeutic effect on the mentioned tissues and organs, MSC-EVs have been examined for their ability to restore or, indeed, improve the performance of many other organs and body systems -such as lungs, blood vessels, oesophagus and bowel- in case of injuries or diseases. For instance, it was reported that human placenta MSC-EVs could attenuate injuries (upon lipopolysaccharide stimulation) caused in lung cells in vitro [160]. In vivo, swine BMMSC-EVs improved lung function in a pig model of influenza virus-induced acute lung injury [161]. Furthermore, human BMMSC-EVs have been shown to alleviate pulmonary vascular permeability and lung injury induced by haemorrhagic shock and trauma in a mouse model, through the activation of proteins and pathways linked to cytoskeletal rearrangement of vascular permeability [162].
Additionally, human placenta MSC-EVs have been shown to inhibit calcification of synthetic vascular grafts by immunomodulation and improving vascular performance and functionality and in a rat model of hyperlipidaemia [163]. Likewise, EVs isolated from ADMSCs limited the abnormal proliferation and migration of vascular smooth muscle cells, followed by neointimal hyperplasia in the setting of vein graft bypass surgery [164]. Administration of human UCMSC-EVs in a mouse model of hind-limb ischemia ameliorated severe ischemic injury, as revealed by increased limb perfusion and function [165]. With respect to tracheoesophageal diseases such as fistulas, ADMSC-EVs embedded in thermo-responsive hydrogels ensured the targeted delivery of EVs at the site of porcine oesophageal fistula; this, in turn, augmented healing. The proposed mechanism here involved the inhibition of myofibroblast proliferation and fibrosis, the decline of inflammatory response, and the enhancement of angiogenesis [166]. Finally, administration of mouse BMMSC-EVs improved the symptoms of ulcerative colitis in a dextran sodium sulfate-induced mouse model, by stimulating M2 macrophage polarisation and blocking inflammatory response [167].
5. Conclusions and Future Perspectives
As reviewed here, due to their intrinsic therapeutic capacity EVs derived from MSCs—of a range of origins— represent a powerful tool for the treatment of many injuries and diseases, opening multiple novel avenues for tissue engineering and regenerative medicine strategies. While the number of clinical studies is limited to date (Table 2), there is evidence to show that the beneficial effects of MSC-EVs could be further enhanced by bioengineering and genetic modification; stimulation with a variety of different biophysical and biochemical stimuli; drug encapsulation; and nanomaterial science.

Table 2.
List of clinical trials using MSC-EVs against tissue injuries *.
However, there are still challenges to the development of MSC-EVs for clinical use. From a practical point-of-view, a major challenge is establishing the optimal reliably, reproducible and robust methodologies for isolation and purification of the therapeutic EVs and their large-scale production at cGMP standard for clinical utility. Moreover, maybe yet from a more fundamental research point-of-view is to establish which sub-populations (if not all) of the heterogenous EV populations are therapeutically beneficially. The clear classification into different subtypes is still under investigation. Further research is also warranted to establish suitable therapeutic doses and optimal route(s) of administration for clinical utility in the future.
Author Contributions
Conceptualization, D.T. and L.O.; writing—original draft preparation, D.T.; writing—review and editing, L.O. and D.T.; funding acquisition, L.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the EU Commission as Horizon 2020 project EVPRO [H2020-NMBP-TR-IND-2018] and the Irish Research Council Advanced Laureate Award, EVIC [IRCLA/2019/49].
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Black, C.K.; Termanini, K.M.; Aguirre, O.; Hawksworth, J.S.; Sosin, M. Solid organ transplantation in the 21st century. Ann. Transl. Med. 2018, 6, 409. [Google Scholar] [CrossRef]
- Platt, J.; Cascalho, M. New and old technologies for organ replacement. Curr. Opin. Organ Transplant. 2013, 18, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef]
- Fitzsimmons, R.; Mazurek, M.S.; Soos, A.; Simmons, C.A. Mesenchymal stromal/stem cells in regenerative medicine and tissue engineering. Stem Cells Int. 2018, 2018, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Cossu, G.; Birchall, M.; Brown, T.; De Coppi, P.; Culme-Seymour, E.; Gibbon, S.; Hitchcock, J.; Mason, C.; Montgomery, J.; Morris, S.; et al. Lancet Commission: stem cells and regenerative medicine. Lancet 2018, 391, 883–910. [Google Scholar] [CrossRef]
- Howard, D.; Buttery, L.; Shakesheff, K.M.; Roberts, S.J. Tissue engineering: strategies, stem cells and scaffolds. J. Anat. 2008, 213, 66–72. [Google Scholar] [CrossRef]
- Le Blanc, K.; Ringdén, O. Immunobiology of human mesenchymal stem cells and future use in hematopoietic stem cell transplantation. Boil. Blood Marrow Transplant. 2005, 11, 321–334. [Google Scholar] [CrossRef]
- Samsonraj, R.M.; Raghunath, M.; Nurcombe, V.; Hui, J.H.; Van Wijnen, A.J.; Cool, S.M. Concise review: multifaceted characterization of human mesenchymal stem cells for use in regenerative medicine. STEM CELLS Transl. Med. 2017, 6, 2173–2185. [Google Scholar] [CrossRef]
- O’Brien, T.; Creane, M.; Windebank, A.J.; Terzic, A.; Dietz, A.B. Translating stem cell research to the clinic: a primer on translational considerations for your first stem cell protocol. Stem Cell Res. Ther. 2015, 6, 146. [Google Scholar] [CrossRef]
- Amer, M.H.; Rose, F.R.A.J.; Shakesheff, K.M.; Modo, M.; White, L. Translational considerations in injectable cell-based therapeutics for neurological applications: concepts, progress and challenges. NPJ Regen. Med. 2017, 2, 23. [Google Scholar] [CrossRef]
- Volarevic, V.; Markovic, B.S.; Gazdic, M.; Volarevic, A.; Jovicic, N.; Arsenijević, N.; Armstrong, L.; Djonov, V.; Lako, M.; Stojkovic, M. Ethical and safety issues of stem cell-based therapy. Int. J. Med Sci. 2018, 15, 36–45. [Google Scholar] [CrossRef]
- MacPherson, A.; Kimmelman, J. Ethical development of stem-cell-based interventions. Nat. Med. 2019, 25, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Zakrzewski, W.; Dobrzyński, M.; Szymonowicz, M.; Rybak, Z. Stem cells: past, present, and future. Stem Cell Res. Ther. 2019, 10, 68. [Google Scholar] [CrossRef] [PubMed]
- Gould, S.J.; Raposo, G. As we wait: coping with an imperfect nomenclature for extracellular vesicles. J. Extracell. Vesicles 2013, 2, 2892. [Google Scholar] [CrossRef] [PubMed]
- Yuana, Y.; Sturk, A.; Nieuwland, R. Extracellular vesicles in physiological and pathological conditions. Blood Rev. 2013, 27, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, A.-K.; Giebel, B. Exosomes: Small vesicles participating in intercellular communication. Int. J. Biochem. Cell Boil. 2012, 44, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Yáñez-Mó, M.; Siljander, P.; Andreu, Z.; Zavec, A.B.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef]
- Van Der Pol, E.; Böing, A.N.; Harrison, P.; Sturk, A.; Nieuwland, R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol. Rev. 2012, 64, 676–705. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Han, C.; Sun, X.; Liu, L.; Jiang, H.; Shen, Y.; Xu, X.; Li, J.; Zhang, G.; Huang, J.; Lin, Z.; et al. Exosomes and their therapeutic potentials of stem cells. Stem Cells Int. 2015, 2016, 1–11. [Google Scholar] [CrossRef]
- Tolar, J.; Le Blanc, K.; Keating, A.; Blazar, B.R. Concise review: hitting the right spot with mesenchymal stromal cells. STEM CELLS 2010, 28, 1446–1455. [Google Scholar] [CrossRef] [PubMed]
- Lai, R.C.; Yeo, R.W.Y.; Lim, S.K. Mesenchymal stem cell exosomes. Semin. Cell Dev. Boil. 2015, 40, 82–88. [Google Scholar] [CrossRef]
- Lener, T.; Gimona, M.; Aigner, L.; Börger, V.; Buzas, E.; Camussi, G.; Chaput, N.; Chatterjee, D.; Court, F.A.; Del Portillo, H.A.; et al. Applying extracellular vesicles based therapeutics in clinical trials—An ISEV position paper. J. Extracell. Vesicles 2015, 4, 30087. [Google Scholar] [CrossRef] [PubMed]
- Tögel, F.; Weiss, K.; Yang, Y.; Hu, Z.; Zhang, P.; Westenfelder, C. Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. Am. J. Physiol. Physiol. 2007, 292, F1626–F1635. [Google Scholar] [CrossRef] [PubMed]
- Yeo, R.W.Y.; Lai, R.C.; Zhang, B.; Tan, S.S.; Yin, Y.; Teh, B.J.; Lim, S.K. Mesenchymal stem cell: an efficient mass producer of exosomes for drug delivery. Adv. Drug Deliv. Rev. 2013, 65, 336–341. [Google Scholar] [CrossRef]
- Park, K.-S.; Bandeira, E.; Shelke, G.V.; Lässer, C.; Lötvall, J. Enhancement of therapeutic potential of mesenchymal stem cell-derived extracellular vesicles. Stem Cell Res. Ther. 2019, 10, 288. [Google Scholar] [CrossRef]
- Catalano, M.; O’Driscoll, L. Inhibiting extracellular vesicles formation and release: a review of EV inhibitors. J. Extracell. Vesicles 2019, 9, 1703244. [Google Scholar] [CrossRef]
- Shifrin, D.A.; Beckler, M.D.; Coffey, R.J.; Tyska, M.J. Extracellular vesicles: communication, coercion, and conditioning. Mol. Boil. Cell 2013, 24, 1253–1259. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: biogenesis, biologic function and clinical potential. Cell Biosci. 2019, 9, 19. [Google Scholar] [CrossRef]
- Caruso, S.; Poon, I.K.H. Apoptotic cell-derived extracellular vesicles: more than just debris. Front. Immunol. 2018, 9, 1486. [Google Scholar] [CrossRef]
- Mobarrez, F.; Sjövik, C.; Soop, A.; Hållström, L.; Frostell, C.; Pisetsky, D.S.; Wallén, H. CD40L expression in plasma of volunteers following LPS administration: a comparison between assay of CD40L on platelet microvesicles and soluble CD40L. Platelets 2014, 26, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, D.; Fenix, A.M.; Franklin, J.L.; Higginbotham, J.N.; Zhang, Q.; Zimmerman, L.J.; Liebler, D.C.; Ping, J.; Liu, Q.; Evans, R.; et al. Reassessment of exosome composition. Cell 2019, 177, 428–445.e18. [Google Scholar] [CrossRef] [PubMed]
- Crowley, L.; Marfell, B.J.; Scott, A.P.; Waterhouse, N.J. Quantitation of apoptosis and necrosis by annexin v binding, propidium iodide uptake, and flow cytometry. Cold Spring Harb. Protoc. 2016, 2016. [Google Scholar] [CrossRef]
- Record, M.; Silvente-Poirot, S.; Poirot, M.; Wakelam, M. Extracellular vesicles: lipids as key components of their biogenesis and functions. J. Lipid Res. 2018, 59, 1316–1324. [Google Scholar] [CrossRef]
- Daly, M.; O’Driscoll, L. MicroRNA Profiling of exosomes. Adv. Struct. Saf. Stud. 2016, 1509, 37–46. [Google Scholar]
- Trionfini, P.; Benigni, A.; Remuzzi, G. MicroRNAs in kidney physiology and disease. Nat. Rev. Nephrol. 2014, 11, 23–33. [Google Scholar] [CrossRef]
- Pfeifer, P.; Werner, N.; Jansen, F. Role and function of microRNAs in extracellular vesicles in cardiovascular biology. BioMed Res. Int. 2015, 2015, 1–11. [Google Scholar] [CrossRef]
- Lee, Y.S.; Dutta, A. MicroRNAs in cancer. Annu. Rev. Pathol. 2009, 4, 199–227. [Google Scholar] [CrossRef]
- Gardiner, C.; Di Vizio, L.; Sahoo, S.; Théry, C.; Witwer, K.W.; Wauben, M.; Hill, A.F. Techniques used for the isolation and characterization of extracellular vesicles: results of a worldwide survey. J. Extracell. Vesicles 2016, 5, 27066. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, N.; Whiteside, T.L.; Reichert, T. Challenges in exosome isolation and analysis in health and disease. Int. J. Mol. Sci. 2019, 20, 4684. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Jin, K.; Gao, L.; Zhang, Z.; Li, F.; Zhou, F.; Zhang, L. Methods and technologies for exosome isolation and characterization. Small Methods 2018, 2, 1800021. [Google Scholar] [CrossRef]
- Doyle, L.M.; Wang, M.Z. Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef] [PubMed]
- Konoshenko, M.Y.; Lekchnov, E.A.; Vlassov, A.V.; Laktionov, P.P. Isolation of extracellular vesicles: general methodologies and latest trends. BioMed Res. Int. 2018, 2018, 8545347. [Google Scholar] [CrossRef] [PubMed]
- García-Manrique, P.; Matos, M.; Gutierrez, G.; Pazos, C.; Blanco-López, M.C. Therapeutic biomaterials based on extracellular vesicles: classification of bio-engineering and mimetic preparation routes. J. Extracell. Vesicles 2018, 7, 1422676. [Google Scholar] [CrossRef]
- Jang, S.C.; Kim, O.Y.; Yoon, C.M.; Choi, D.-S.; Roh, T.-Y.; Park, J.; Nilsson, J.; Lötvall, J.; Kim, Y.-K.; Gho, Y.S. Bioinspired exosome-mimetic nanovesicles for targeted delivery of chemotherapeutics to malignant tumors. ACS Nano 2013, 7, 7698–7710. [Google Scholar] [CrossRef]
- Jeong, D.; Jo, W.; Yoon, J.; Kim, J.; Gianchandani, S.; Gho, Y.S.; Park, J. Nanovesicles engineered from ES cells for enhanced cell proliferation. Biomaterials 2014, 35, 9302–9310. [Google Scholar] [CrossRef]
- Yoon, J.; Jo, W.; Jeong, D.; Kim, J.; Jeong, H.; Park, J. Generation of nanovesicles with sliced cellular membrane fragments for exogenous material delivery. Biomaterials 2015, 59, 12–20. [Google Scholar] [CrossRef]
- Forterre, A.; Jalabert, A.; Berger, E.; Baudet, M.; Chikh, K.; Errazuriz, E.; De Larichaudy, J.; Chanon, S.; Weiss-Gayet, M.; Hesse, A.M.; et al. Proteomic analysis of C2C12 myoblast and myotube exosome-like vesicles: a new paradigm for myoblast-myotube cross talk? PLoS ONE 2014, 9, e84153. [Google Scholar]
- Boni, R.; Ali, A.; Shavandi, A.; Clarkson, A. Current and novel polymeric biomaterials for neural tissue engineering. J. Biomed. Sci. 2018, 25, 90. [Google Scholar] [CrossRef]
- Ma, Y.; Ge, S.; Zhang, J.; Zhou, D.; Li, L.; Wang, X.; Su, J. Mesenchymal stem cell-derived extracellular vesicles promote nerve regeneration after sciatic nerve crush injury in rats. Int. J. Clin. Exp. Pathol. 2017, 10, 10032–10039. [Google Scholar]
- Ma, Y.; Dong, L.; Zhou, D.; Li, L.; Zhang, W.; Zhen, Y.; Wang, T.; Su, J.; Chen, D.; Mao, C.; et al. Extracellular vesicles from human umbilical cord mesenchymal stem cells improve nerve regeneration after sciatic nerve transection in rats. J. Cell. Mol. Med. 2019, 23, 2822–2835. [Google Scholar] [CrossRef] [PubMed]
- Bucan, V.; Vaslaitis, D.; Peck, C.-T.; Strauss, S.; Vogt, P.M.; Radtke, C. Effect of exosomes from rat adipose-derived mesenchymal stem cells on neurite outgrowth and sciatic nerve regeneration after crush injury. Mol. Neurobiol. 2018, 56, 1812–1824. [Google Scholar] [CrossRef] [PubMed]
- Haertinger, M.; Weiss, T.; Mann, A.; Tabi, A.; Brandel, V.; Radtke, C. Adipose stem cell-derived extracellular vesicles induce proliferation of Schwann cells via internalization. Cells 2020, 9, 163. [Google Scholar] [CrossRef]
- Mao, Q.; Nguyen, P.D.; Shanti, R.M.; Shi, S.; Shakoori, P.; Zhang, Q.; Le, A.D. Gingiva-derived mesenchymal stem cell-extracellular vesicles activate Schwann cell repair phenotype and promote nerve regeneration. Tissue Eng. Part A 2019, 25, 887–900. [Google Scholar] [CrossRef]
- Xin, H.; Li, Y.; Buller, B.; Katakowski, M.; Zhang, Y.; Wang, X.; Shang, X.; Zhang, Z.G.; Chopp, M. Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. STEM CELLS 2012, 30, 1556–1564. [Google Scholar] [CrossRef]
- Couillard-Despres, S.; Winner, B.; Schaubeck, S.; Aigner, R.; Vroemen, M.; Weidner, N.; Bogdahn, U.; Winkler, J.; Kuhn, H.-G.; Aigner, L. Doublecortin expression levels in adult brain reflect neurogenesis. Eur. J. Neurosci. 2005, 21, 1–14. [Google Scholar] [CrossRef]
- Zanetta, L.; Marcus, S.G.; Vasile, J.; Dobryansky, M.; Cohen, H.; Eng, K.; Shamamian, P.; Mignatti, P. Expression of Von Willebrand factor, an endothelial cell marker, is up-regulated by angiogenesis factors: a potential method for objective assessment of tumor angiogenesis. Int. J. Cancer 2000, 85, 281–288. [Google Scholar] [CrossRef]
- Xin, H.; Li, Y.; Cui, Y.; Yang, J.J.; Zhang, Z.G.; Chopp, M. Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. Br. J. Pharmacol. 2013, 33, 1711–1715. [Google Scholar] [CrossRef]
- Zhang, Y.; Chopp, M.; Meng, Y.; Katakowski, M.; Xin, H.; Mahmood, A.; Xiong, Y. Effect of exosomes derived from multipluripotent mesenchymal stromal cells on functional recovery and neurovascular plasticity in rats after traumatic brain injury. J. Neurosurg. 2015, 122, 856–867. [Google Scholar] [CrossRef]
- Cheng, J.; Korte, N.; Nortley, R.; Sethi, H.; Tang, Y.; Attwell, D. Targeting pericytes for therapeutic approaches to neurological disorders. Acta Neuropathol. 2018, 136, 507–523. [Google Scholar] [CrossRef]
- Figley, S.A.; Khosravi, R.; Legasto, J.M.; Tseng, Y.-F.; Fehlings, M.G. Characterization of vascular disruption and blood-spinal cord barrier permeability following traumatic spinal cord injury. J. Neurotrauma 2014, 31, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhou, Y.; Zhang, R.; Wen, L.; Wu, K.; Li, Y.; Yao, Y.; Duan, R.; Jia, Y. Bone mesenchymal stem cell-derived extracellular vesicles promote recovery following spinal cord injury via improvement of the integrity of the blood-spinal cord barrier. Front. Mol. Neurosci. 2019, 13, 209. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Jiao, G.; Wu, W.; Wang, H.; Ren, S.; Zhang, L.; Zhou, H.; Liu, H.; Chen, Y. Exosomes from bone marrow mesenchymal stem cells inhibit neuronal apoptosis and promote motor function recovery via the Wnt/beta-catenin signaling pathway. Cell Transplant 2019, 28, 1373–1383. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhang, P.; Yao, X.; Li, H.; Shen, H.; Li, X.; Wu, J.; Lu, X. Exosomes derived from mir-133b-modified mesenchymal stem cells promote recovery after spinal cord injury. Front. Mol. Neurosci. 2018, 12, 845. [Google Scholar] [CrossRef]
- Clark, K.; Zhang, S.; Barthe, S.; Kumar, P.; Pivetti, C.D.; Kreutzberg, N.; Reed, C.; Wang, Y.; Paxton, Z.J.; Farmer, D.L.; et al. Placental mesenchymal stem cell-derived extracellular vesicles promote myelin regeneration in an animal model of multiple sclerosis. Cells 2019, 8, 1497. [Google Scholar] [CrossRef]
- Riazifar, M.; Mohammadi, M.R.; Pone, E.J.; Yeri, A.; Lässer, C.; Segaliny, A.I.; McIntyre, L.L.; Shelke, G.; Hutchins, E.; Hamamoto, A.; et al. Stem cell-derived exosomes as nanotherapeutics for autoimmune and neurodegenerative disorders. ACS Nano 2019, 13, 6670–6688. [Google Scholar] [CrossRef]
- Canales-Aguirre, A.A.; Reza-Zaldivar, E.E.; Sapiéns, M.A.H.; Gutiérrez-Mercado, Y.K.; Sandoval-Ávila, S.; Gomez-Pinedo, U.; Márquez-Aguirre, A.L.; Vazquez-Mendez, E.; Padilla-Camberos, E. Mesenchymal stem cell-derived exosomes promote neurogenesis and cognitive function recovery in a mouse model of Alzheimer’s disease. Neural Regen. Res. 2019, 14, 1626–1634. [Google Scholar] [CrossRef]
- Madeddu, P.; Urbanek, K.; Kajstura, J.; Yan, S.; Finato, N.; Bussani, R.; Nadal-Ginard, B.; Silvestri, F.; Leri, A.; Beltrami, C.A.; et al. Evidence that human cardiac myocytes divide after myocardial infarction. New Engl. J. Med. 2001, 344, 1750–1757. [Google Scholar]
- Senyo, S.; Steinhauser, M.L.; Pizzimenti, C.L.; Yang, V.K.; Cai, L.; Wang, M.; Wu, T.-D.; Guerquin-Kern, J.-L.; Lechene, C.P.; Lee, R.T. Mammalian heart renewal by pre-existing cardiomyocytes. Nature 2012, 493, 433–436. [Google Scholar] [CrossRef]
- Malliaras, K.; Zhang, Y.; Seinfeld, J.; Galang, G.; Tseliou, E.; Cheng, K.; Sun, B.; Aminzadeh, M.; Marbán, E. Cardiomyocyte proliferation and progenitor cell recruitment underlie therapeutic regeneration after myocardial infarction in the adult mouse heart. EMBO Mol. Med. 2013, 5, 191–209. [Google Scholar] [CrossRef]
- Lai, R.C.; Arslan, F.; Lee, M.M.; Sze, N.S.K.; Choo, A.; Chen, T.S.; Salto-Tellez, M.; Timmers, L.; Lee, C.N.; El Oakley, R.M.; et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010, 4, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Arslan, F.; Lai, R.C.; Smeets, M.B.; Akeroyd, L.; Choo, A.; Aguor, E.N.E.; Timmers, L.; Van Rijen, H.V.; Doevendans, P.A.; Pasterkamp, G.; et al. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res. 2013, 10, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Ballen, K.; Zhao, Y.; Sun, L.; Sun, X.; Zhao, X.; Sun, X.; Qian, H.; Xu, W.; Zhu, W. Faculty of 1000 evaluation for exosomes derived from akt-modified human umbilical cord mesenchymal stem cells improve cardiac regeneration and promote angiogenesis via activating platelet-derived growth factor D. Stem cells Transl. 2017, 6, 51–59. [Google Scholar]
- Adamiak, M.; Cheng, G.; Bobis-Wozowicz, S.; Zhao, L.; Kedracka-Krok, S.; Samanta, A.; Karnas, E.; Xuan, Y.-T.; Skupien-Rabian, B.; Chen, X.; et al. Induced pluripotent stem cell (iPSC)–derived extracellular vesicles are safer and more effective for cardiac repair than iPSCs. Circ. Res. 2018, 122, 296–309. [Google Scholar] [CrossRef]
- Balbi, C.; Lodder, K.; Costa, A.; Moimas, S.; Moccia, F.; Van Herwaarden, T.; Rosti, V.; Campagnoli, F.; Palmeri, A.; De Biasio, P.; et al. Reactivating endogenous mechanisms of cardiac regeneration via paracrine boosting using the human amniotic fluid stem cell secretome. Int. J. Cardiol. 2019, 287, 87–95. [Google Scholar] [CrossRef]
- Huang, P.; Wang, L.; Li, Q.; Xu, J.; Xu, J.; Xiong, Y.; Chen, G.; Qian, H.; Jin, C.; Yu, Y.; et al. Combinatorial treatment of acute myocardial infarction using stem cells and their derived exosomes resulted in improved heart performance. Stem Cell Res. Ther. 2019, 10, 300–312. [Google Scholar] [CrossRef]
- Hu, X.; Wu, R.; Shehadeh, L.A.; Zhou, Q.; Jiang, C.; Huang, X.; Zhang, L.; Gao, F.; Liu, X.-B.; Yu, H.; et al. Severe hypoxia exerts parallel and cell-specific regulation of gene expression and alternative splicing in human mesenchymal stem cells. BMC Genom. 2014, 15, 303. [Google Scholar] [CrossRef]
- Hu, X.; Xu, Y.; Zhong, Z.; Wu, Y.; Zhao, J.; Wang, Y.; Cheng, H.; Kong, M.; Zhang, F.; Chen, Q.; et al. A large-scale investigation of hypoxia-preconditioned allogeneic mesenchymal stem cells for myocardial repair in nonhuman primatesnovelty and significance. Circ. Res. 2016, 118, 970–983. [Google Scholar] [CrossRef]
- Bian, S.; Zhang, L.; Duan, L.; Wang, X.; Min, Y.; Yu, H. Extracellular vesicles derived from human bone marrow mesenchymal stem cells promote angiogenesis in a rat myocardial infarction model. J. Mol. Med. 2013, 92, 387–397. [Google Scholar] [CrossRef]
- Zhu, L.-P.; Tian, T.; Wang, J.-Y.; He, J.-N.; Chen, T.; Pan, M.; Xu, L.; Zhang, H.-X.; Qiu, X.-T.; Li, C.-C.; et al. Hypoxia-elicited mesenchymal stem cell-derived exosomes facilitates cardiac repair through miR-125b-mediated prevention of cell death in myocardial infarction. Theranostics 2018, 8, 6163–6177. [Google Scholar] [CrossRef]
- Zhu, J.; Lu, K.; Zhang, N.; Zhao, Y.; Ma, Q.; Shen, J.; Lin, Y.; Xiang, P.; Tang, Y.; Hu, X.; et al. Myocardial reparative functions of exosomes from mesenchymal stem cells are enhanced by hypoxia treatment of the cells via transferring microRNA-210 in an nSMase2-dependent way. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1659–1670. [Google Scholar] [PubMed]
- Han, C.; Zhou, J.; Liang, C.; Liu, B.; Pan, X.; Zhang, Y.; Wang, Y.; Yan, B.; Xie, W.; Liu, F.; et al. Human umbilical cord mesenchymal stem cell derived exosomes encapsulated in functional peptide hydrogels promote cardiac repair. Biomater. Sci. 2019, 7, 2920–2933. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, L.; Gao, Z.; Chen, G.; Zhang, C. Bone marrow stromal/stem cell-derived extracellular vesicles regulate osteoblast activity and differentiation in vitro and promote bone regeneration in vivo. Sci. Rep. 2016, 6, 21961. [Google Scholar] [CrossRef]
- Qi, X.; Zhang, J.; Yuan, H.; Xu, Z.; Li, Q.; Niu, X.; Hu, B.; Wang, Y.; Li, X.-L. Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells repair critical-sized bone defects through enhanced angiogenesis and osteogenesis in osteoporotic rats. Int. J. Boil. Sci. 2016, 12, 836–849. [Google Scholar] [CrossRef]
- Liang, B.; Liang, J.-M.; Ding, J.-N.; Xu, J.; Xu, J.-G.; Chai, Y. Dimethyloxaloylglycine-stimulated human bone marrow mesenchymal stem cell-derived exosomes enhance bone regeneration through angiogenesis by targeting the AKT/mTOR pathway. Stem Cell Res. Ther. 2019, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Chen, Y.; Dunstan, C.; Roohani-Esfahani, S.-I.; Zreiqat, H. Priming adipose stem cells with tumor necrosis factor-alpha preconditioning potentiates their exosome efficacy for bone regeneration. Tissue Eng. Part A 2017, 23, 1212–1220. [Google Scholar] [CrossRef] [PubMed]
- Furuta, T.; Miyaki, S.; Ishitobi, H.; Ogura, T.; Kato, Y.; Kamei, N.; Miyado, K.; Higashi, Y.; Ochi, M. Mesenchymal stem cell-derived exosomes promote fracture healing in a mouse model. STEM CELLS Transl. Med. 2016, 5, 1620–1630. [Google Scholar] [CrossRef]
- Huang, C.-C.; Narayanan, R.; Alapati, S.; Ravindran, S. Exosomes as biomimetic tools for stem cell differentiation: Applications in dental pulp tissue regeneration. Biomaterial 2016, 111, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Chew, J.R.J.; Chuah, S.J.; Teo, K.Y.W.; Zhang, S.; Lai, R.C.; Fu, J.H.; Lim, L.P.; Lim, S.K.; Toh, W.S. Mesenchymal stem cell exosomes enhance periodontal ligament cell functions and promote periodontal regeneration. Acta Biomater. 2019, 89, 252–264. [Google Scholar] [CrossRef]
- Li, W.; Liu, Y.; Zhang, P.; Tang, Y.; Zhou, M.; Jiang, W.; Zhang, X.; Wu, G.; Zhou, Y. Tissue-engineered bone immobilized with human adipose stem cells-derived exosomes promotes bone regeneration. ACS Appl. Mater. Interfaces 2018, 10, 5240–5254. [Google Scholar] [CrossRef]
- Chen, L.; Mou, S.; Li, F.; Zeng, Y.; Sun, Y.; Horch, R.E.; Wei, W.; Wang, Z.; Sun, J. Self-assembled human adipose-derived stem cell-derived extracellular vesicle-functionalized biotin-doped polypyrrole titanium with long-term stability and potential osteoinductive ability. ACS Appl. Mater. Interfaces 2019, 11, 46183–46196. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, X.; Li, H.; Chen, C.-Y.; Hu, B.; Niu, X.; Li, Q.; Zhao, B.; Xie, Z.; Wang, Y. Exosomes/tricalcium phosphate combination scaffolds can enhance bone regeneration by activating the PI3K/Akt signaling pathway. Stem Cell Res. Ther. 2016, 7, 136. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Wang, Z.; Zhang, L.; Lei, Q.; Zhao, A.; Wang, H.; Li, Q.; Cao, Y.; Zhang, W.J.; Chen, Z.-C. Extracellular vesicle-functionalized decalcified bone matrix scaffolds with enhanced pro-angiogenic and pro-bone regeneration activities. Sci. Rep. 2017, 7, 45622. [Google Scholar] [CrossRef]
- Diomede, F.; Gugliandolo, A.; Cardelli, P.; Merciaro, I.; Ettorre, V.; Traini, T.; Bedini, R.; Scionti, D.; Bramanti, A.; Nanci, A.; et al. Three-dimensional printed PLA scaffold and human gingival stem cell-derived extracellular vesicles: a new tool for bone defect repair. Stem Cell Res. Ther. 2018, 9, 104. [Google Scholar] [CrossRef]
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M.B. Osteoarthritis: A disease of the joint as an organ. Arthritis Rheum. 2012, 64, 1697–1707. [Google Scholar] [CrossRef]
- Zavatti, M.; Beretti, F.; Casciaro, F.; Bertucci, E.; Maraldi, T. Comparison of the therapeutic effect of amniotic fluid stem cells and their exosomes on monoiodoacetate-induced animal model of osteoarthritis. BioFactors 2019, 46, 106–117. [Google Scholar] [CrossRef]
- Vonk, L.A.; Van Dooremalen, S.F.J.; Liv, N.; Klumperman, J.; Coffer, P.J.; Saris, D.B.; Lorenowicz, M.J. Mesenchymal stromal/stem cell-derived extracellular vesicles promote human cartilage regeneration in vitro. Theranostics 2018, 8, 906–920. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, S.; Huang, J.; Guo, W.; Chen, J.; Zhang, L.; Zhao, B.; Peng, J.; Wang, A.; Wang, Y.; et al. The ECM-cell interaction of cartilage extracellular matrix on chondrocytes. BioMed Res. Int. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Hosseini-Farahabadi, S.; Geetha-Loganathan, P.; Fu, K.; Nimmagadda, S.; Yang, H.J.; Richman, J.M. Dual functions for WNT5A during cartilage development and in disease. Matrix Boil. 2013, 32, 252–264. [Google Scholar] [CrossRef]
- Mao, G.; Zhang, Z.; Hu, S.; Zhang, Z.; Chang, Z.; Huang, Z.; Liao, W.; Kang, Y. Exosomes derived from miR-92a-3p-overexpressing human mesenchymal stem cells enhance chondrogenesis and suppress cartilage degradation via targeting WNT5A. Stem Cell Res. Ther. 2018, 9, 247. [Google Scholar] [CrossRef]
- Wang, R.; Xu, B.; Xu, H. TGF-beta1 promoted chondrocyte proliferation by regulating Sp1 through MSC-exosomes derived miR-135b. Cell Cycle 2018, 17. [Google Scholar] [CrossRef]
- Zhang, S.; Chuah, S.J.; Lai, R.C.; Hui, J.H.P.; Lim, S.K.; Toh, W.S. MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterial 2018, 156, 16–27. [Google Scholar] [CrossRef]
- Zhang, S.; Chu, W.C.; Lai, R.C.; Lim, S.K.; Hui, J.H.P.; Toh, W.S. Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration. Osteoarthr. Cartil. 2016, 24, 2135–2140. [Google Scholar] [CrossRef]
- Zhang, S.; Teo, K.Y.W.; Chuah, S.J.; Lai, R.C.; Lim, S.K.; Toh, W.S. MSC exosomes alleviate temporomandibular joint osteoarthritis by attenuating inflammation and restoring matrix homeostasis. Biomaterial 2019, 200, 35–47. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, D.; Liu, Z.; Zhou, F.; Dai, J.; Wu, B.; Zhou, J.; Heng, B.C.; Zou, X.H.; Ouyang, H.; et al. Exosomes from embryonic mesenchymal stem cells alleviate osteoarthritis through balancing synthesis and degradation of cartilage extracellular matrix. Stem Cell Res. Ther. 2017, 8, 189. [Google Scholar] [CrossRef]
- Yan, L.; Wu, X. Exosomes produced from 3D cultures of umbilical cord mesenchymal stem cells in a hollow-fiber bioreactor show improved osteochondral regeneration activity. Cell Boil. Toxicol. 2019, 1–14. [Google Scholar] [CrossRef]
- Liu, X.; Yang, Y.; Li, Y.; Niu, X.; Zhao, B.; Wang, Y.; Bao, C.; Xie, Z.; Lin, Q.; Zhu, L. Integration of stem cell-derived exosomes with in situ hydrogel glue as a promising tissue patch for articular cartilage regeneration. Nanoscale 2017, 9, 4430–4438. [Google Scholar] [CrossRef]
- Bishop, E.; Mostafa, S.; Pakvasa, M.; Luu, H.H.; Lee, M.J.; Wolf, J.M.; Ameer, G.A.; He, T.-C.; Reid, R.R. 3-D bioprinting technologies in tissue engineering and regenerative medicine: Current and future trends. Genes Dis. 2017, 4, 185–195. [Google Scholar] [CrossRef]
- Chen, P.; Zheng, L.; Wang, Y.; Tao, M.; Xie, Z.; Xia, C.; Gu, C.; Chen, J.; Qiu, P.; Mei, S.; et al. Desktop-stereolithography 3D printing of a radially oriented extracellular matrix/mesenchymal stem cell exosome bioink for osteochondral defect regeneration. Theranostics 2019, 9, 2439–2459. [Google Scholar] [CrossRef]
- Bruno, S.; Grange, C.; Deregibus, M.C.; Calogero, R.A.; Saviozzi, S.; Collino, F.; Morando, L.; Busca, A.; Falda, M.; Bussolati, B.; et al. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J. Am. Soc. Nephrol. 2009, 20, 1053–1067. [Google Scholar] [CrossRef]
- Zhao, L.; Li, L.; Zhang, P.; Jiang, H.; Chen, J. Genetic communication by extracellular vesicles is an important mechanism underlying stem cell-based therapy-mediated protection against acute kidney injury. Stem Cell Res. Ther. 2019, 10, 119. [Google Scholar] [CrossRef]
- Wang, S.-Y.; Hong, Q.; Zhang, C.-Y.; Yang, Y.-J.; Cai, G.; Chen, X.-M. miRNAs in stem cell-derived extracellular vesicles for acute kidney injury treatment: comprehensive review of preclinical studies. Stem Cell Res. Ther. 2019, 10, 281–287. [Google Scholar] [CrossRef]
- Tomasoni, S.; Longaretti, L.; Rota, C.; Morigi, M.; Conti, S.; Gotti, E.; Capelli, C.; Introna, M.; Remuzzi, G.; Benigni, A. Transfer of growth factor receptor mRNA via exosomes unravels the regenerative effect of mesenchymal stem cells. Stem Cells Dev. 2012, 22, 772–780. [Google Scholar] [CrossRef]
- Gatti, S.; Bruno, S.; Deregibus, M.C.; Sordi, A.; Cantaluppi, V.; Tetta, C.; Camussi, G. Microvesicles derived from human adult mesenchymal stem cells protect against ischaemia-reperfusion-induced acute and chronic kidney injury. Nephrol. Dial. Transplant. 2011, 26, 1474–1483. [Google Scholar] [CrossRef]
- Shen, B.; Liu, J.; Zhang, F.; Wang, Y.; Qin, Y.; Zhou, Z.; Qiu, J.; Fan, Y. CCR2 Positive Exosome released by mesenchymal stem cells suppresses macrophage functions and alleviates ischemia/reperfusion-induced renal injury. Stem Cells Int. 2016, 2016, 1–9. [Google Scholar] [CrossRef]
- Bruno, S.; Grange, C.; Collino, F.; Deregibus, M.C.; Cantaluppi, V.; Biancone, L.; Tetta, C.; Camussi, G. Microvesicles derived from mesenchymal stem cells enhance survival in a lethal model of acute kidney injury. PLoS ONE 2012, 7, e33115. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, S.; Zhao, M.; Wang, C.; Li, L.; Yuan, Y.; Li, L.; Liao, G.; Bresette, W.; Zhang, J.; et al. Injectable extracellular vesicle-released self-assembling peptide nanofiber hydrogel as an enhanced cell-free therapy for tissue regeneration. J. Control. Release 2019, 316, 93–104. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, H.; Xu, W.; Wang, B.; Wu, H.; Tao, Y.; Zhang, B.; Wang, M.; Mao, F.; Yan, Y.; et al. Exosomes released by human umbilical cord mesenchymal stem cells protect against cisplatin-induced renal oxidative stress and apoptosis in vivo and in vitro. Stem Cell Res. Ther. 2013, 4, 34. [Google Scholar] [CrossRef]
- Ju, G.-Q.; Cheng, J.; Zhong, L.; Wu, S.; Zou, X.-Y.; Zhang, G.-Y.; Gu, D.; Miao, S.; Zhu, Y.-J.; Sun, J.; et al. Microvesicles derived from human umbilical cord mesenchymal stem cells facilitate tubular epithelial cell dedifferentiation and growth via hepatocyte growth factor induction. PLoS ONE 2015, 10, e0121534. [Google Scholar] [CrossRef]
- Gu, D.; Zou, X.; Ju, G.; Zhang, G.; Bao, E.; Zhu, Y. Mesenchymal stromal cells derived extracellular vesicles ameliorate acute renal ischemia reperfusion injury by inhibition of mitochondrial fission through miR-30. Stem Cells Int. 2016, 2016, 1–12. [Google Scholar] [CrossRef]
- Zou, X.; Zhang, G.; Cheng, Z.; Yin, D.; Du, T.; Ju, G.; Miao, S.; Liu, G.-H.; Lu, M.; Zhu, Y. Microvesicles derived from human Wharton’s Jelly mesenchymal stromal cells ameliorate renal ischemia-reperfusion injury in rats by suppressing CX3CL1. Stem Cell Res. Ther. 2014, 5, 40. [Google Scholar] [CrossRef]
- Ranghino, A.; Bruno, S.; Bussolati, B.; Moggio, A.; DiMuccio, V.; Tapparo, M.; Biancone, L.; Gontero, P.; Frea, B.; Camussi, G. The effects of glomerular and tubular renal progenitors and derived extracellular vesicles on recovery from acute kidney injury. Stem Cell Res. Ther. 2017, 8, 24. [Google Scholar] [CrossRef]
- Sanchez, M.B.H.; Bruno, S.; Grange, C.; Tapparo, M.; Cantaluppi, V.; Tetta, C.; Camussi, G. Human liver stem cells and derived extracellular vesicles improve recovery in a murine model of acute kidney injury. Stem Cell Res. Ther. 2014, 5, 124. [Google Scholar] [CrossRef]
- Piccoli, G.B.; Grassi, G.; Cabiddu, G.; Nazha, M.; Roggero, S.; Capizzi, I.; De Pascale, A.; Priola, A.M.; Di Vico, C.; Maxia, S.; et al. Diabetic kidney disease: a syndrome rather than a single disease. Rev. Diabet. Stud. 2015, 12, 87–109. [Google Scholar] [CrossRef]
- Jiang, Z.-Z.; Liu, Y.-M.; Niu, X.; Yin, J.-Y.; Hu, B.; Guo, S.-C.; Fan, Y.; Wang, Y.; Wang, N. Exosomes secreted by human urine-derived stem cells could prevent kidney complications from type I diabetes in rats. Stem Cell Res. Ther. 2016, 7, 24. [Google Scholar] [CrossRef]
- Nagaishi, K.; Mizue, Y.; Chikenji, T.S.; Otani, M.; Nakano, M.; Konari, N.; Fujimiya, M. Mesenchymal stem cell therapy ameliorates diabetic nephropathy via the paracrine effect of renal trophic factors including exosomes. Sci. Rep. 2016, 6, 34842. [Google Scholar] [CrossRef]
- Grange, C.; Tritta, S.; Tapparo, M.; Cedrino, M.; Tetta, C.; Camussi, G.; Brizzi, M.F. Stem cell-derived extracellular vesicles inhibit and revert fibrosis progression in a mouse model of diabetic nephropathy. Sci. Rep. 2019, 9, 4468. [Google Scholar] [CrossRef]
- Kholia, S.; Sanchez, M.B.H.; Cedrino, M.; Papadimitriou, E.; Tapparo, M.; Deregibus, M.C.; Brizzi, M.F.; Tetta, C.; Camussi, G. Human liver stem cell-derived extracellular vesicles prevent aristolochic acid-induced kidney fibrosis. Front. Immunol. 2018, 9, 9. [Google Scholar] [CrossRef]
- He, J.; Wang, Y.; Sun, S.; Yu, M.; Wang, C.; Pei, X.; Zhu, B.; Wu, J.; Zhao, W. Bone marrow stem cells-derived microvesicles protect against renal injury in the mouse remnant kidney model. Nephrology 2012, 17, 493–500. [Google Scholar] [CrossRef]
- He, J.; Wang, Y.; Lu, X.; Zhu, B.; Pei, X.; Wu, J.; Zhao, W. Micro-vesicles derived from bone marrow stem cells protect the kidney both in vivo and in vitro by microRNA-dependent repairing. Nephrology 2015, 20, 591–600. [Google Scholar] [CrossRef]
- Matsukura, T.; Inaba, C.; Weygant, E.A.; Kitamura, D.; Janknecht, R.; Matsumoto, H.; Hyink, D.P.; Kashiwada, S.; Obara, T. Extracellular vesicles from human bone marrow mesenchymal stem cells repair organ damage caused by cadmium poisoning in a medaka model. Physiol. Rep. 2019, 7, e14172. [Google Scholar] [CrossRef] [PubMed]
- Nassar, W.; El-Ansary, M.; Sabry, D.; Fayad, T.; Kotb, E.; Temraz, M.; Saad, A.-N.; Essa, W.; Adel, H.; A Mostafa, M. Umbilical cord mesenchymal stem cells derived extracellular vesicles can safely ameliorate the progression of chronic kidney diseases. Biomater. Res. 2016, 20, 21. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.Y.; Lai, R.C.; Wong, W.; Dan, Y.Y.; Lim, S.K.; Ho, H.K. Mesenchymal stem cell-derived exosomes promote hepatic regeneration in drug-induced liver injury models. Stem Cell Res. Ther. 2014, 5, 76. [Google Scholar] [CrossRef] [PubMed]
- Nong, K.; Wang, W.; Niu, X.; Hu, B.; Ma, C.; Bai, Y.; Wu, B.; Wang, Y.; Ai, K. Hepatoprotective effect of exosomes from human-induced pluripotent stem cell–derived mesenchymal stromal cells against hepatic ischemia-reperfusion injury in rats. Cytotherapy 2016, 18, 1548–1559. [Google Scholar] [CrossRef]
- Du, Y.; Li, D.; Han, C.; Wu, H.; Xu, L.; Zhang, M.; Zhang, J.; Chen, X. Exosomes from human-induced pluripotent stem cell–derived mesenchymal stromal cells (hiPSC-MSCs) protect liver against hepatic ischemia/ reperfusion injury via activating sphingosine kinase and sphingosine-1-phosphate signaling pathway. Cell. Physiol. Biochem. 2017, 43, 611–625. [Google Scholar] [CrossRef]
- Cannavo, A.; Liccardo, D.; Komici, K.; Corbi, G.; De Lucia, C.; Femminella, G.D.; Elia, A.; Bencivenga, L.; Ferrara, N.; Koch, W.J.; et al. Sphingosine kinases and sphingosine 1-phosphate receptors: signaling and actions in the cardiovascular system. Front. Pharmacol. 2017, 8. [Google Scholar] [CrossRef]
- Blaho, V.A.; Hla, T. Regulation of mammalian physiology, development, and disease by the sphingosine 1-phosphate and lysophosphatidic acid receptors. Chem. Rev. 2011, 111, 6299–6320. [Google Scholar] [CrossRef]
- Ng, M.L.; Yarla, N.S.; Menschikowski, M.; Sukocheva, O. Regulatory role of sphingosine kinase and sphingosine-1-phosphate receptor signaling in progenitor/stem cells. World J. Stem Cells 2018, 10, 119–133. [Google Scholar] [CrossRef]
- Yao, J.; Zheng, J.; Cai, J.; Zeng, K.; Zhou, C.; Zhang, J.; Li, S.; Li, H.; Chen, L.; He, L.; et al. Extracellular vesicles derived from human umbilical cord mesenchymal stem cells alleviate rat hepatic ischemia-reperfusion injury by suppressing oxidative stress and neutrophil inflammatory response. FASEB J. 2018, 33, 1695–1710. [Google Scholar] [CrossRef]
- Mardpour, S.; Ghanian, M.H.; Abandansari, H.S.; Mardpour, S.; Nazari, A.; Shekari, F.; Baharvand, H. Hydrogel-mediated sustained systemic delivery of mesenchymal stem cell-derived extracellular vesicles improves hepatic regeneration in chronic liver failure. ACS Appl. Mater. Interfaces 2019, 11, 37421–37433. [Google Scholar] [CrossRef]
- Nakamura, Y.; Miyaki, S.; Ishitobi, H.; Matsuyama, S.; Nakasa, T.; Kamei, N.; Akimoto, T.; Higashi, Y.; Ochi, M. Mesenchymal-stem-cell-derived exosomes accelerate skeletal muscle regeneration. FEBS Lett. 2015, 589, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- Mellows, B.; Mitchell, R.; Antonioli, M.; Kretz, O.; Chambers, D.; Zeuner, M.-T.; Denecke, B.; Musante, L.; Ramachandra, D.L.; Debacq-Chainiaux, F.; et al. Protein and molecular characterization of a clinically compliant amniotic fluid stem cell-derived extracellular vesicle fraction capable of accelerating muscle regeneration through enhancement of angiogenesis. Stem Cells Dev. 2017, 26, 1316–1333. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, R.; Mellows, B.; Sheard, J.; Antonioli, M.; Kretz, O.; Chambers, D.; Zeuner, M.-T.; Tomkins, J.E.; Denecke, B.; Musante, L.; et al. Secretome of adipose-derived mesenchymal stem cells promotes skeletal muscle regeneration through synergistic action of extracellular vesicle cargo and soluble proteins. Stem Cell Res. Ther. 2019, 10, 116. [Google Scholar] [CrossRef]
- Wang, C.; Song, W.; Chen, B.; Liu, X.; He, Y. Exosomes isolated from adipose-derived stem cells: a new cell-free approach to prevent the muscle degeneration associated with torn rotator cuffs. Am. J. Sports Med. 2019, 47, 3247–3255. [Google Scholar] [CrossRef]
- Wu, R.; Huang, C.; Wu, Q.; Jia, X.; Liu, M.; Xue, Z.; Qiu, Y.; Niu, X.; Wang, Y. Exosomes secreted by urine-derived stem cells improve stress urinary incontinence by promoting repair of pubococcygeus muscle injury in rats. Stem Cell Res. Ther. 2019, 10, 80. [Google Scholar] [CrossRef]
- Figliolini, F.; Ranghino, A.; Grange, C.; Cedrino, M.; Tapparo, M.; Cavallari, C.; Rossi, A.; Togliatto, G.; Femminò, S.; Gugliuzza, M.V.; et al. Extracellular vesicles from adipose stem cells prevent muscle damage and inflammation in a mouse model of hind limb ischemia: role of Neuregulin-1. Arter. Thromb. Vasc. Boil. 2019, 40, 239–254. [Google Scholar] [CrossRef]
- Velnar, T.; Bailey, T.; Smrkolj, V. The wound healing process: an overview of the cellular and molecular mechanisms. J. Int. Med Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef]
- Zhao, R.; Liang, H.; Clarke, E.; Jackson, C.; Xue, M. Inflammation in Chronic Wounds. Int. J. Mol. Sci. 2016, 17, 2085. [Google Scholar] [CrossRef]
- Shabbir, A.; Cox, A.; Rodriguez-Menocal, L.; Salgado, M.; Van Badiavas, E. Mesenchymal stem cell exosomes induce proliferation and migration of normal and chronic wound fibroblasts, and enhance angiogenesis in vitro. Stem Cells Dev. 2015, 24, 1635–1647. [Google Scholar] [CrossRef]
- Zhang, J.; Guan, J.; Niu, X.; Hu, G.-W.; Guo, S.-C.; Li, Q.; Xie, Z.; Zhang, C.; Wang, Y. Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis. J. Transl. Med. 2015, 13, 49. [Google Scholar] [CrossRef]
- Choi, J.S.; Cho, W.L.; Choi, Y.J.; Kim, J.D.; Park, H.-A.; Kim, S.Y.; Park, J.H.; Jo, D.-G.; Cho, Y.W. Functional recovery in photo-damaged human dermal fibroblasts by human adipose-derived stem cell extracellular vesicles. J. Extracell. Vesicles 2019, 8, 1565885. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Wang, J.; Zhou, X.; Xiong, Z.; Zhao, J.; Yu, R.; Huang, F.; Zhang, H.; Chen, L. Exosomes derived from human adipose mensenchymal stem cells accelerates cutaneous wound healing via optimizing the characteristics of fibroblasts. Sci. Rep. 2016, 6, 32993. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.D.F.; Cunha, P.D.S.; Carregal, V.; Silva, P.D.C.D.; De Miranda, M.C.; Kunrath-Lima, M.; De Melo, M.I.A.; Faraco, C.C.F.; Barbosa, J.L.; Frézard, F.; et al. Extracellular vesicles from adipose-derived mesenchymal stem/stromal cells accelerate migration and activate akt pathway in human keratinocytes and fibroblasts independently of miR-205 activity. Stem Cells Int. 2017, 2017, 9841035. [Google Scholar] [CrossRef] [PubMed]
- Cooper, D.; Wang, C.; Patel, R.; Trujillo, A.; Patel, N.A.; Prather, J.; Gould, L.J.; Wu, M.H. Human adipose-derived stem cell conditioned media and exosomes containing MALAT1 promote human dermal fibroblast migration and ischemic wound healing. Adv. Wound Care 2018, 7, 299–308. [Google Scholar] [CrossRef]
- Pelizzo, G.; Avanzini, M.; Cornaglia, A.I.; Silvestri, A.; Mantelli, M.; Travaglino, P.; Croce, S.; Romano, P.; Avolio, L.; Iacob, G.; et al. Extracellular vesicles derived from mesenchymal cells: perspective treatment for cutaneous wound healing in pediatrics. Regen. Med. 2018, 13, 385–394. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, M.; Gong, A.; Zhang, X.; Wu, X.; Zhu, Y.; Shi, H.; Wu, L.; Zhu, W.; Qian, H.; et al. HucMSC-exosome mediated-Wnt4 signaling is required for cutaneous wound healing. STEM CELLS 2015, 33, 2158–2168. [Google Scholar] [CrossRef]
- Zhang, B.; Wu, X.; Zhang, X.; Sun, Y.; Yan, Y.; Shi, H.; Zhu, Y.; Wu, L.; Pan, Z.; Zhu, W.; et al. Human umbilical cord mesenchymal stem cell exosomes enhance angiogenesis through the Wnt4/beta-catenin pathway. Stem Cells Transl. Med. 2015, 4, 513–522. [Google Scholar] [CrossRef]
- Zhang, B.; Shi, Y.; Gong, A.; Pan, Z.; Shi, H.; Yang, H.; Fu, H.; Yan, Y.; Zhang, X.; Wang, M.; et al. HucMSC exosome-delivered 14-3-3zeta orchestrates self-control of the Wnt response via modulation of YAP during cutaneous regeneration. Stem Cells 2016, 34, 2485–2500. [Google Scholar] [CrossRef]
- Fang, S.; Xu, C.; Zhang, Y.; Xue, C.; Yang, C.; Bi, H.; Qian, X.; Wu, M.; Ji, K.; Zhao, Y.; et al. Umbilical cord-derived mesenchymal stem cell-derived exosomal microRNAs suppress myofibroblast differentiation by inhibiting the transforming growth factor-beta/SMAD2 pathway during wound healing. Stem Cells Transl. Med. 2016, 5, 1425–1439. [Google Scholar] [CrossRef]
- Kim, S.Y.; Joglekar, M.V.; Hardikar, A.A.; Phan, T.H.; Khanal, D.; Tharkar, P.; Limantoro, C.; Johnson, J.; Kalionis, B.; Chrzanowski, W. Placenta stem/stromal cell-derived extracellular vesicles for potential use in lung repair. Proteom. 2019, 19, e1800166. [Google Scholar] [CrossRef]
- Khatri, M.; Richardson, L.A.; Meulia, T. Mesenchymal stem cell-derived extracellular vesicles attenuate influenza virus-induced acute lung injury in a pig model. Stem Cell Res. Ther. 2018, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Potter, D.R.; Miyazawa, B.Y.; Gibb, S.L.; Deng, X.; Togaratti, P.P.; Croze, R.H.; Srivastava, A.K.; Trivedi, A.; Matthay, M.; Holcomb, J.B.; et al. Mesenchymal stem cell-derived extracellular vesicles attenuate pulmonary vascular permeability and lung injury induced by hemorrhagic shock and trauma. J. Trauma Acute Care Surg. 2018, 84, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Wu, Y.; Zhao, R.; Zhang, K.; Midgley, A.C.; Kong, D.; Li, Z.; Zhao, Q. MSC-derived sEVs enhance patency and inhibit calcification of synthetic vascular grafts by immunomodulation in a rat model of hyperlipidemia. Biomaterial 2019, 204, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Almeria, C.; Weiss, R.; Roy, M.; Tripisciano, C.; Kasper, C.; Weber, V.; Egger, D. Hypoxia conditioned mesenchymal stem cell-derived extracellular vesicles induce increased vascular tube formation in vitro. Front. Bioeng. Biotechnol. 2019, 7, 292. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Li, Q.; Niu, X.; Zhang, G.; Ling, X.; Zhang, J.; Wang, Y.; Deng, Z. Extracellular vesicles secreted by human urine-derived stem cells promote ischemia repair in a mouse model of hind-limb ischemia. Cell. Physiol. Biochem. 2018, 47, 1181–1192. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.K.A.; Perretta, S.; Perrod, G.; Pidial, L.; Lindner, V.; Carn, F.; Lemieux, S.; Alloyeau, D.; Boucenna, I.; Menasché, P.; et al. Thermoresponsive gel embedded with adipose stem-cell-derived extracellular vesicles promotes esophageal fistula healing in a thermo-actuated delivery strategy. ACS Nano 2018, 12, 9800–9814. [Google Scholar] [CrossRef]
- Cao, L.; Xu, H.; Wang, G.; Liu, M.; Tian, D.; Yuan, Z. Extracellular vesicles derived from bone marrow mesenchymal stem cells attenuate dextran sodium sulfate-induced ulcerative colitis by promoting M2 macrophage polarization. Int. Immunopharmacol. 2019, 72, 264–274. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).