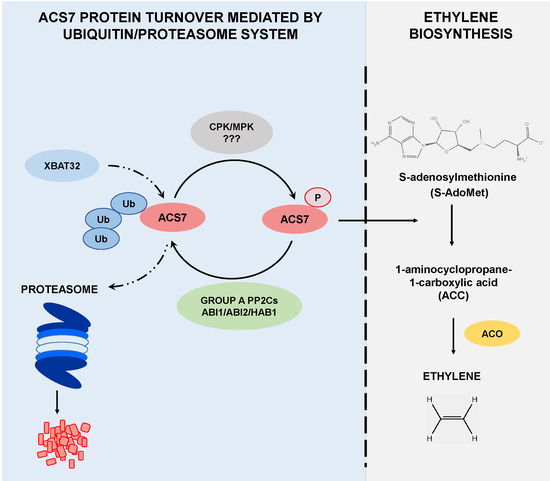
Protein Phosphatases Type 2C Group A Interact with and Regulate the Stability of ACC Synthase 7 in Arabidopsis
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials, Growth Conditions and Treatments
2.2. Plant Transformation
2.3. Vector Constructs
2.4. Microscopy Studies in Arabidopsis Protoplasts
2.5. Ethylene Measurements
2.6. Recombinant Protein Expression and Purification
2.7. Pull-Down Assay
2.8. Cell-Free Degradation Assay
2.9. Immunoblotting
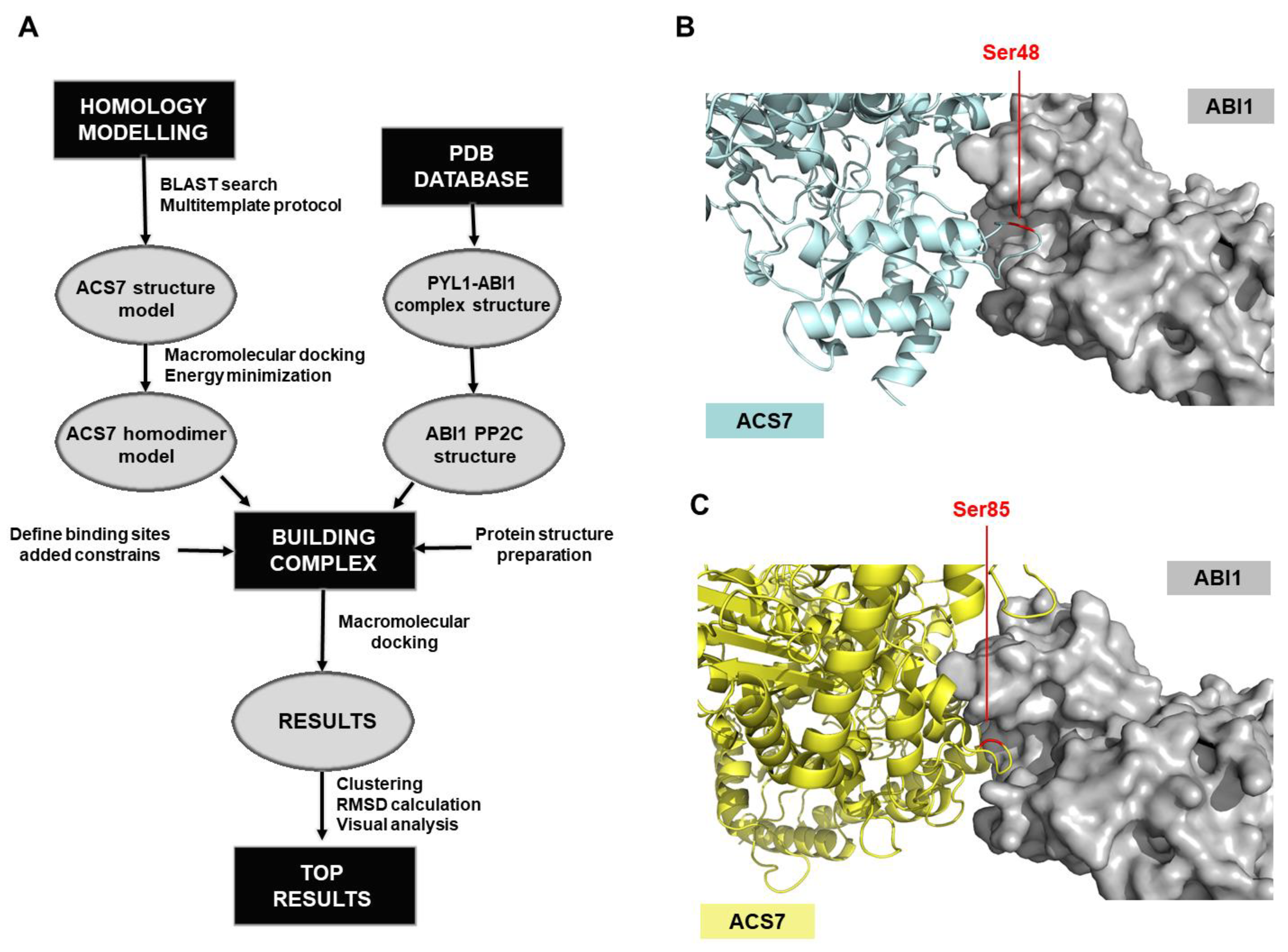
2.10. ACS7-ABI1 Protein Complex Modeling
3. Results
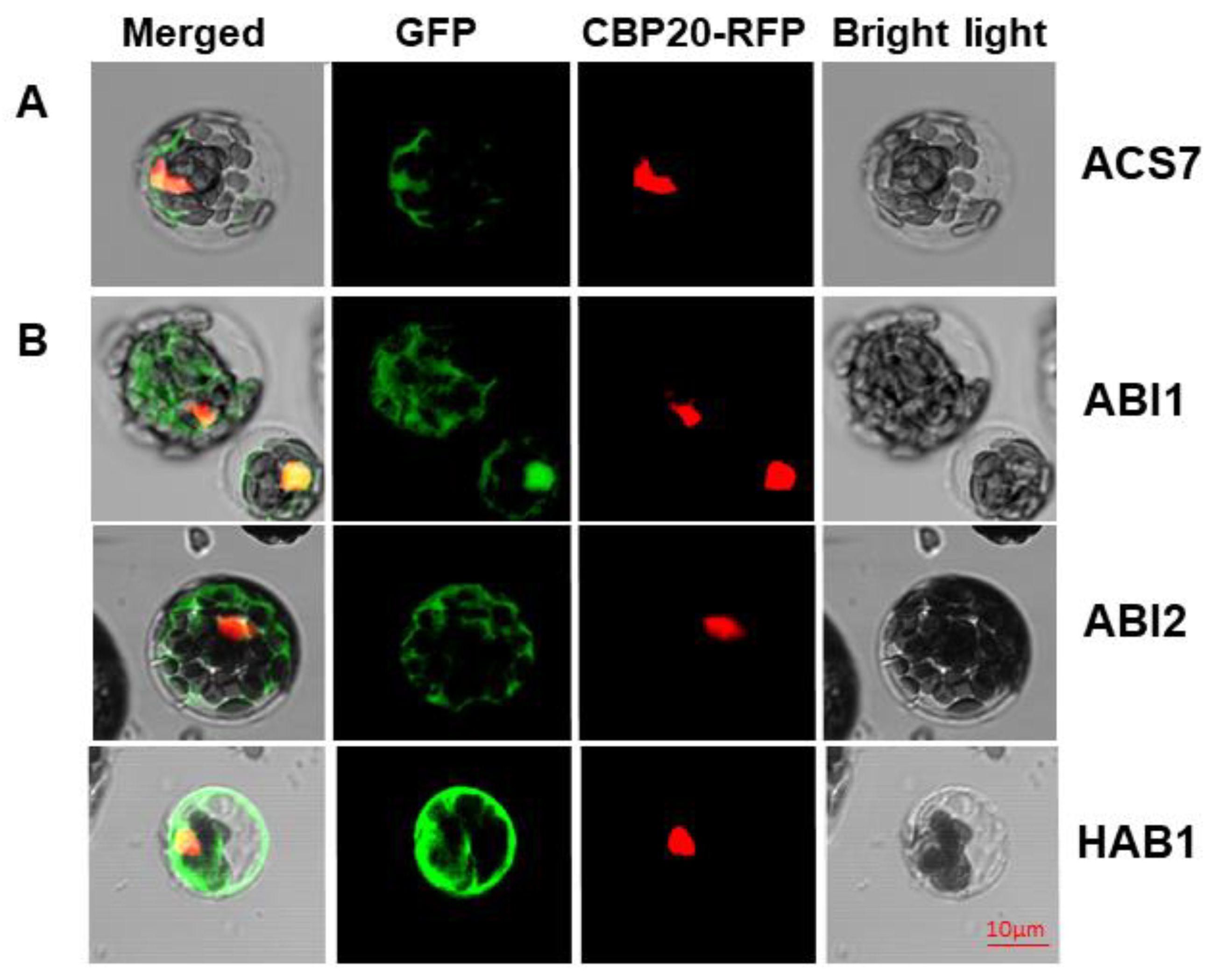
3.1. ACS7 and Group A PP2Cs Are Localized in both Nucleus and Cytoplasm
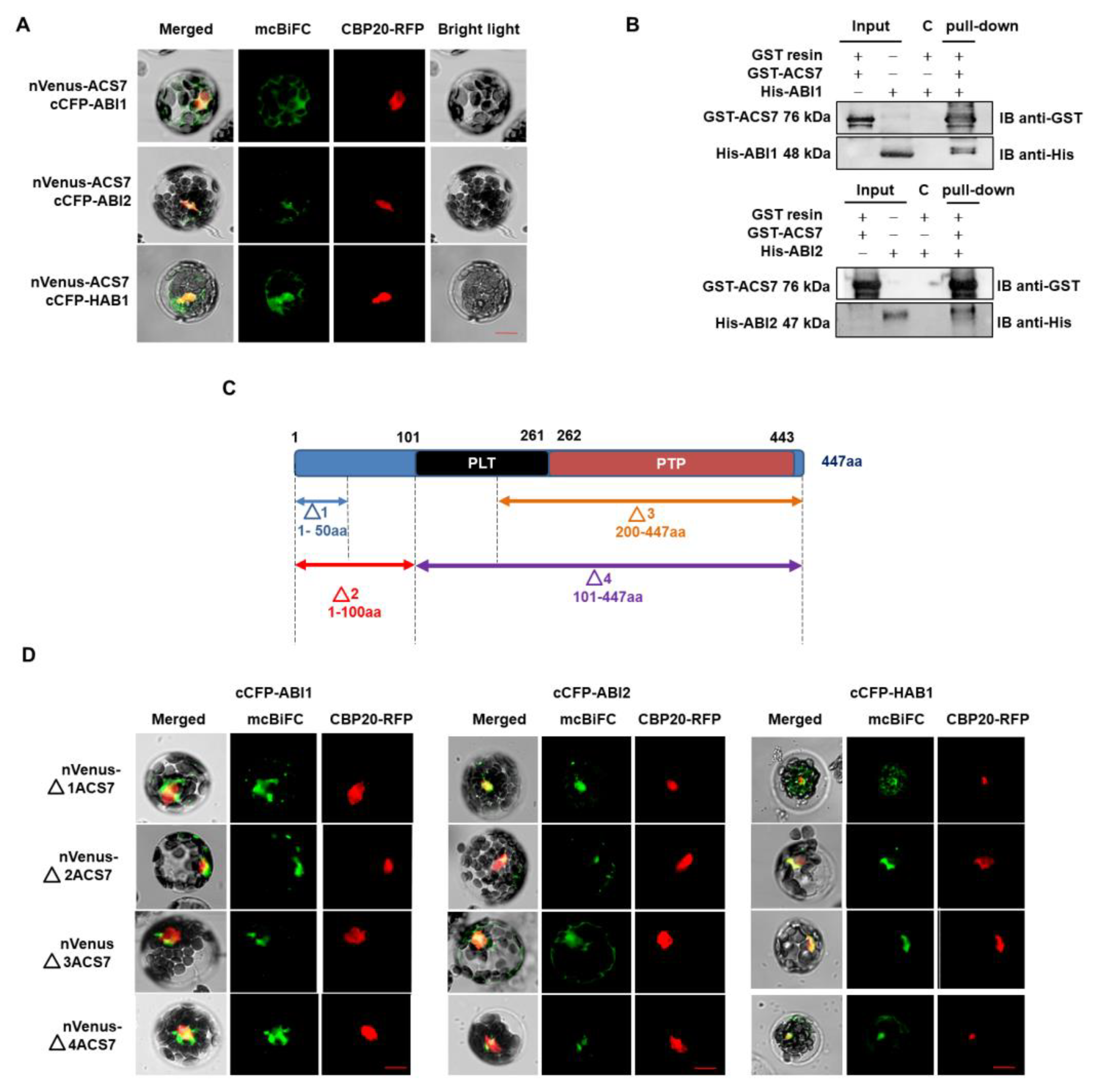
3.2. ACS7 Interacts with Group A Protein Phosphatases Type 2C
3.3. Modeling of the ACS7–ABI1 PP2C Complex
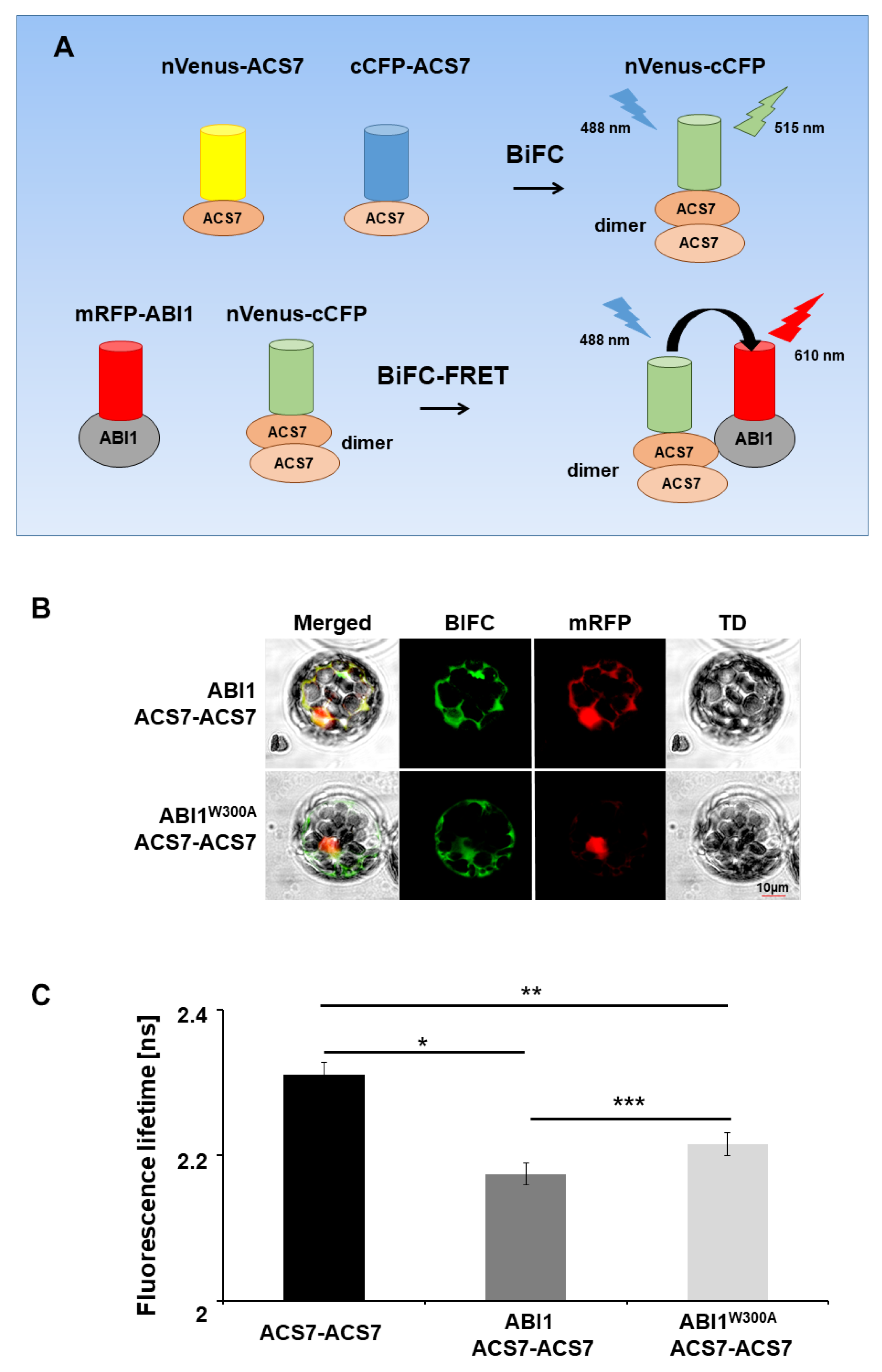
3.4. ABI1 W300 Is Important for Interaction with ACS7
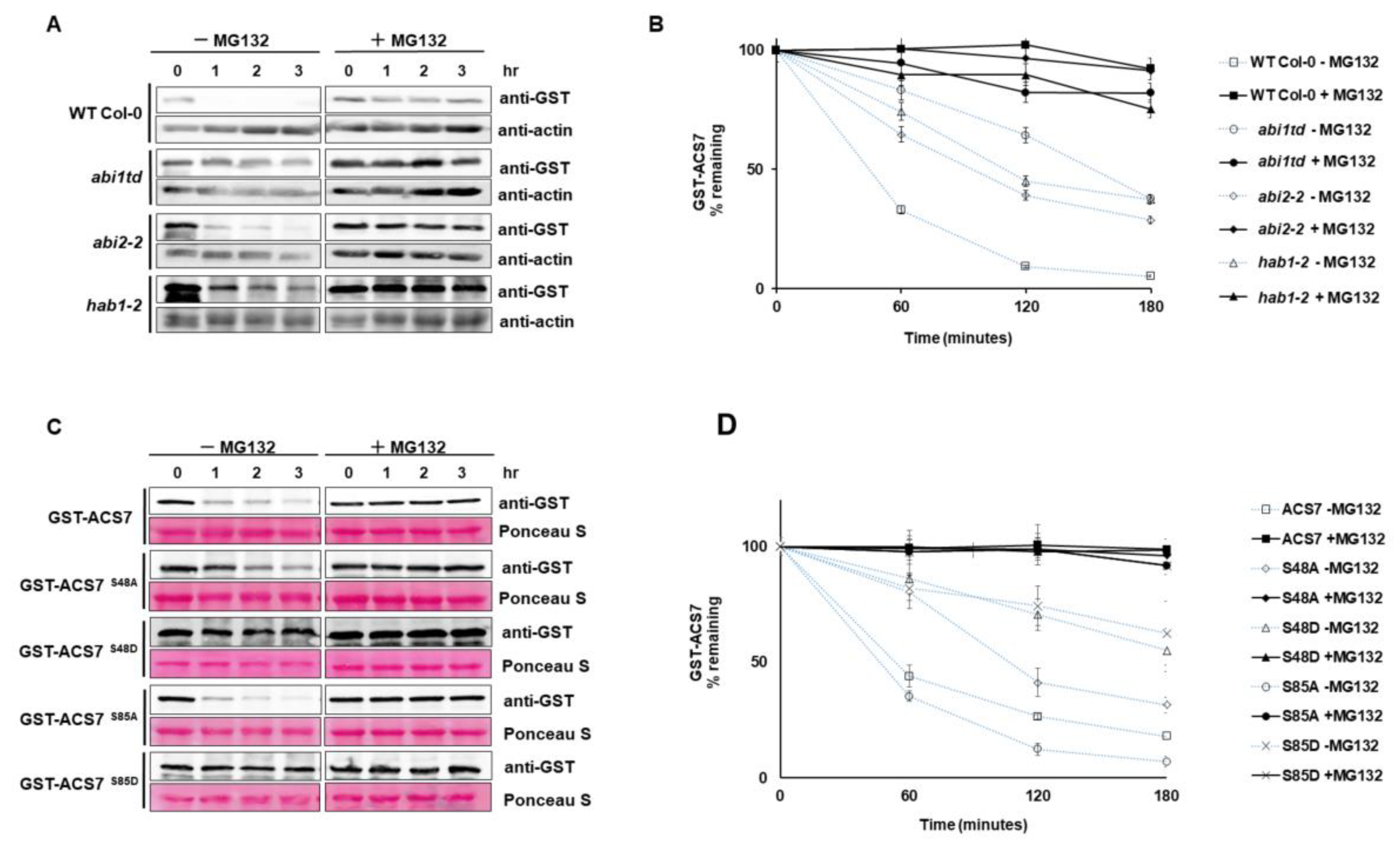
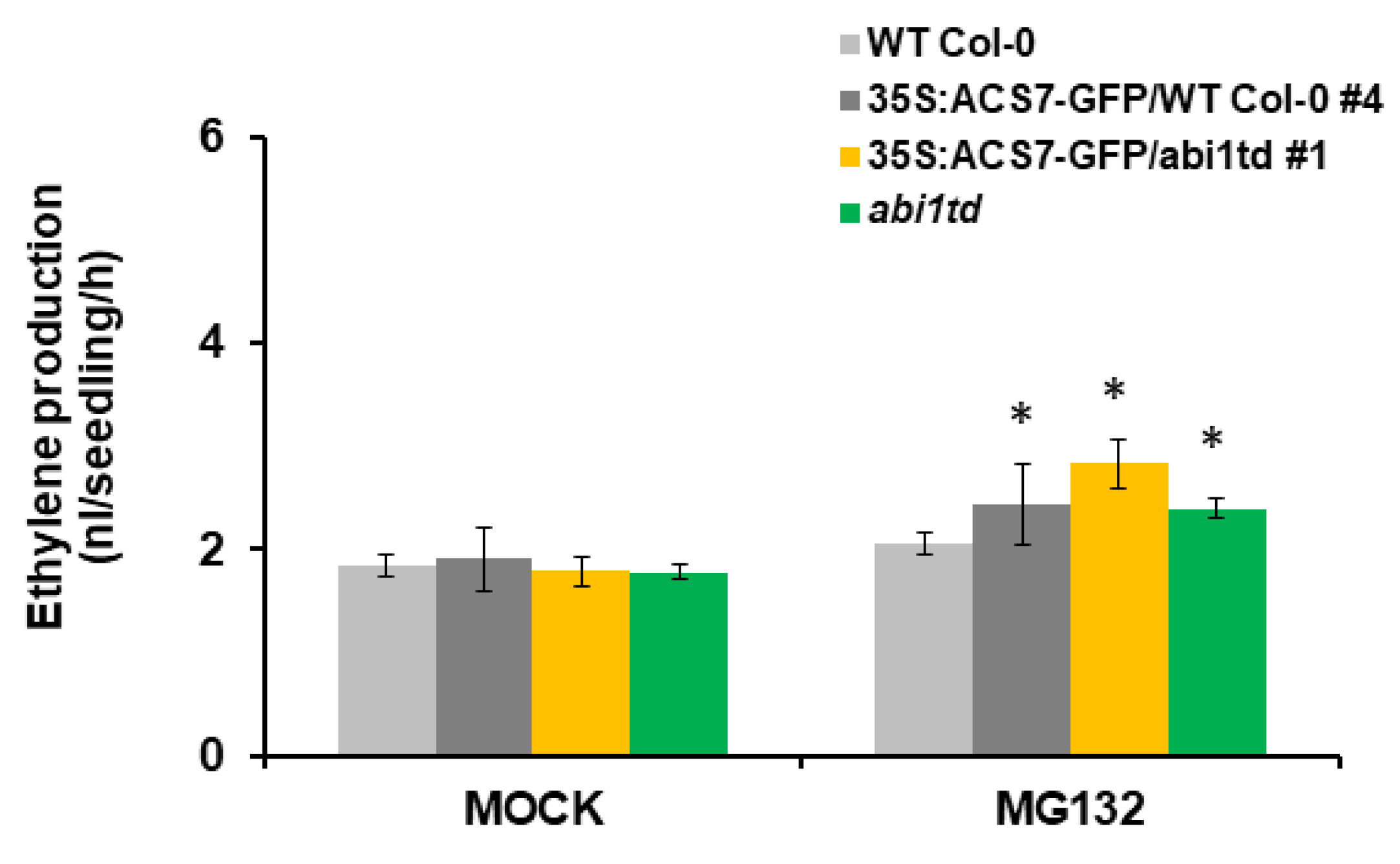
3.5. ABI1 Regulates ACS7 Turnover
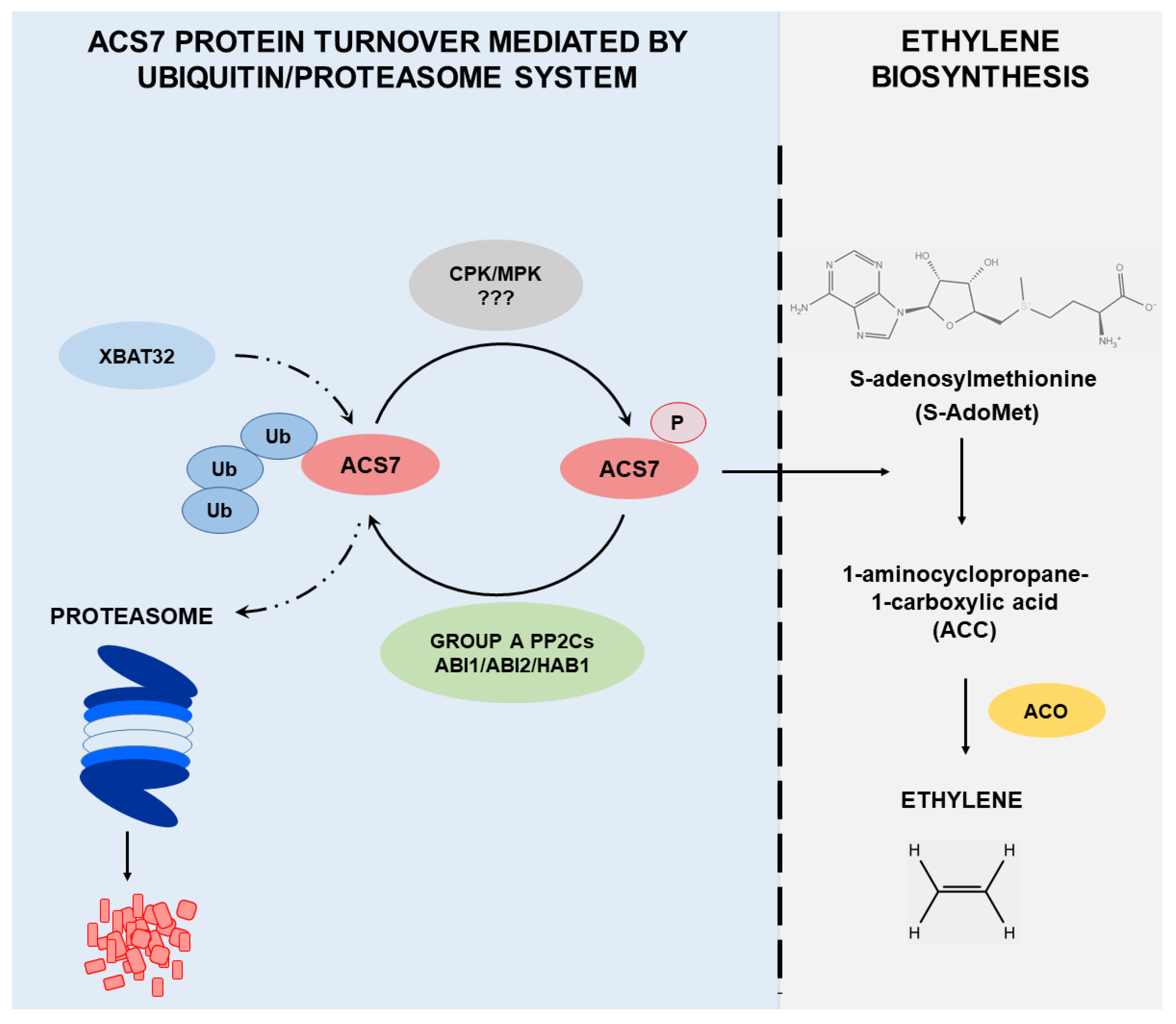
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Van Doorn, W.G. Categories of Petal Senescence and Abscission: A Re-evaluation. Ann. Bot. 2001, 87, 447–456. [Google Scholar] [CrossRef]
- Ruperti, B.; Cattivelli, L.; Pagni, S.; Ramina, A. Ethylene-responsive genes are differentially regulated during abscission, organ senescence and wounding in peach (Prunus persica). J. Exp. Bot. 2002, 53, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Chaves, A.L.S.; Mello-Farias, P.C. de Ethylene and fruit ripening: From illumination gas to the control of gene expression, more than a century of discoveries. Genet. Mol. Biol. 2006, 29, 508–515. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Khan, N.A.; Ferrante, A.; Trivellini, A.; Francini, A.; Khan, M.I.R. Ethylene Role in Plant Growth, Development and Senescence: Interaction with Other Phytohormones. Front. Plant Sci. 2017, 8, 6484. [Google Scholar] [CrossRef]
- Hinz, M.; Wilson, I.; Yang, J.; Buerstenbinder, K.; Llewellyn, D.; Dennis, E.S.; Sauter, M.; Dolferus, R. Arabidopsis RAP2.2: An ethylene response transcription factor that is important for hypoxia survival. Plant Physiol. 2010, 153, 757–772. [Google Scholar] [CrossRef]
- Pesquet, E.; Tuominen, H. Ethylene stimulates tracheary element differentiation in Zinnia elegans cell cultures. New Phytol. 2011, 190, 138–149. [Google Scholar] [CrossRef]
- Zhu, J.-H.; Xu, J.; Chang, W.-J.; Zhang, Z.-L. Isolation and Molecular Characterization of 1-Aminocyclopropane-1-carboxylic Acid Synthase Genes in Hevea brasiliensis. Int. J. Mol. Sci. 2015, 16, 4136–4149. [Google Scholar] [CrossRef]
- Lee, H.Y.; Yoon, G.M. Regulation of Ethylene Biosynthesis by Phytohormones in Etiolated Rice (Oryza sativa L.) Seedlings. Mol. Cells 2018, 41, 311–319. [Google Scholar]
- Yamagami, T.; Tsuchisaka, A.; Yamada, K.; Haddon, W.F.; Harden, L.A.; Theologis, A. Biochemical Diversity among the 1-Amino-cyclopropane-1-Carboxylate Synthase Isozymes Encoded by theArabidopsisGene Family. J. Boil. Chem. 2003, 278, 49102–49112. [Google Scholar] [CrossRef]
- Yoshida, H.; Nagata, M.; Saito, K.; Wang, L.-C.; Ecker, J.R. Arabidopsis ETO1 specifically interacts with and negatively regulates type 2 1-aminocyclopropane-1-carboxylate synthases. BMC Plant Boil. 2005, 5, 14. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, S. Phosphorylation of 1-aminocyclopropane-1-carboxylic acid synthase by MPK6, a stress-responsive mitogen-activated protein kinase, induces ethylene biosynthesis in arabidopsis. Plant Cell 2004, 16, 3386–3399. [Google Scholar] [CrossRef]
- Kamiyoshihara, Y.; Iwata, M.; Fukaya, T.; Tatsuki, M.; Mori, H. Turnover of LeACS2, a wound-inducible 1-aminocyclopropane-1-carboxylic acid synthase in tomato, is regulated by phosphorylation/dephosphorylation. Plant J. 2010, 64, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Prasad, M.E.; Schofield, A.; Lyzenga, W.; Liu, H.; Stone, S.L. Arabidopsis RING E3 ligase XBAT32 regulates lateral root production through its role in ethylene biosynthesis. Plant Physiol. 2010, 153, 1587–1596. [Google Scholar] [CrossRef] [PubMed]
- Lyzenga, W.J.; Booth, J.K.; Stone, S.L. The Arabidopsis RING-type E3 ligase XBAT32 mediates the proteasomal degradation of the ethylene biosynthetic enzyme, 1-aminocyclopropane-1-carboxylate synthase 7. Plant J. 2012, 71, 23–34. [Google Scholar] [CrossRef]
- Nodzon, L.A.; Xu, W.-H.; Wang, Y.; Pi, L.-Y.; Chakrabarty, P.K.; Song, W.-Y. The ubiquitin ligase XBAT32 regulates lateral root development in Arabidopsis. Plant J. 2004, 40, 996–1006. [Google Scholar] [CrossRef] [PubMed]
- Chang, I.-F.; Curran, A.; Woolsey, R.; Quilici, D.; Cushman, J.C.; Mittler, R.; Harmon, A.; Harper, J.F. Proteomic profiling of tandem affinity purified 14-3-3 protein complexes in Arabidopsis thaliana. Proteomics 2009, 9, 2967–2985. [Google Scholar] [CrossRef]
- Su, C.-H.; Zhao, R.; Zhang, F.; Qu, C.; Chen, B.; Feng, Y.-H.; Phan, L.; Chen, J.; Wang, H.; Wang, H.; et al. 14-3-3s Exerts Tumor-Suppressor Activity Mediated by Regulation of COP1 Stability. Mol. Cell. Pathobiol. 2011, 71, 884–894. [Google Scholar] [CrossRef]
- Yoon, G.M.; Kieber, J.J. ACC synthase and its cognate E3 ligase are inversely regulated by light. Plant Signal. Behav. 2013, 8, e26478. [Google Scholar] [CrossRef]
- Huang, S.-J.; Chang, C.-L.; Wang, P.-H.; Tsai, M.-C.; Hsu, P.-H.; Chang, I.-F. A type III ACC synthase, ACS7, is involved in root gravitropism in Arabidopsis thaliana. J. Exp. Bot. 2013, 64, 4343–4360. [Google Scholar] [CrossRef]
- Xiong, L.; Xiao, D.; Xu, X.; Guo, Z.; Wang, N.N. The non-catalytic N-terminal domain of ACS7 is involved in the post-translational regulation of this gene in Arabidopsis. J. Exp. Bot. 2014, 65, 4397–4408. [Google Scholar] [CrossRef]
- Song, J.-D.; Lee, D.-H.; Rhew, T.H.; Lee, C.-H. Effects of light on the expression of 1-aminocyclopropane-1-carboxylic acid synthase and oxidase genes in mung bean hypocotyls. J. Photoscience 2003, 10, 189–193. [Google Scholar]
- Sun, G.; Mei, Y.; Deng, D.; Xiong, L.; Sun, L.; Zhang, X.; Wen, Z.; Liu, S.; You, X.; Wang, D.; et al. N-Terminus-Mediated Degradation of ACS7 Is Negatively Regulated by Senescence Signaling to Allow Optimal Ethylene Production during Leaf Development in Arabidopsis. Front. Plant Sci. 2017, 8, 2066. [Google Scholar] [CrossRef] [PubMed]
- Tsuchisaka, A.; Theologis, A. Unique and Overlapping Expression Patterns among the Arabidopsis 1-Amino-Cyclopropane-1-Carboxylate Synthase Gene Family Members. Plant Physiol. 2004, 136, 2982–3000. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Zhen, Z.; Peng, J.; Chang, L.; Gong, Q.; Wang, N.N. Loss of ACS7 confers abiotic stress tolerance by modulating ABA sensitivity and accumulation in Arabidopsis. J. Exp. Bot. 2011, 62, 4875–4887. [Google Scholar] [CrossRef] [PubMed]
- Ludwików, A.; Cieśla, A.; Kasprowicz-Maluśki, A.; Mitula, F.; Tajdel, M.; Galganski, L.; Ziolkowski, P.; Kubiak, P.; Małecka, A.; Piechalak, A.; et al. Arabidopsis Protein Phosphatase 2C ABI1 Interacts with Type I ACC Synthases and Is Involved in the Regulation of Ozone-Induced Ethylene Biosynthesis. Mol. Plant 2014, 7, 960–976. [Google Scholar] [CrossRef] [PubMed]
- Rubio, S.; Rodrigues, A.; Saez, A.; Dizon, M.B.; Galle, A.; Kim, T.-H.; Santiago, J.; Flexas, J.; Schroeder, J.I.; Rodriguez, P.L. Triple Loss of Function of Protein Phosphatases Type 2C Leads to Partial Constitutive Response to Endogenous Abscisic Acid. Plant Physiol. 2009, 150, 1345–1355. [Google Scholar] [CrossRef]
- Ludwików, A.; Kierzek, D.; Gallois, P.; Zeef, L.; Sadowski, J. Gene expression profiling of ozone-treated Arabidopsis abi1td insertional mutant: Protein phosphatase 2C ABI1 modulates biosynthesis ratio of ABA and ethylene. Planta 2009, 230, 1003–1017. [Google Scholar] [CrossRef]
- Earley, K.W.; Haag, J.R.; Pontes, O.; Opper, K.; Juehne, T.; Song, K.; Pikaard, C. Gateway-compatible vectors for plant functional genomics and proteomics. Plant J. 2006, 45, 616–629. [Google Scholar] [CrossRef]
- Clough, S.J.; Bent, A. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef]
- Chakrabarty, R.; Banerjee, R.; Chung, S.-M.; Farman, M.; Citovsky, V.; Hogenhout, S.A.; Tzfira, T.; Goodin, M. pSITE Vectors for Stable Integration or Transient Expression of Autofluorescent Protein Fusions in Plants: Probing Nicotiana benthamiana- Virus Interactions. Mol. Plant-Microbe Interact. 2007, 20, 740–750. [Google Scholar] [CrossRef]
- Mitula, F.; Tajdel, M.; Cieśla, A.; Kasprowicz-Maluśki, A.; Kulik, A.; Babula-Skowronska, D.; Michalak, M.; Dobrowolska, G.; Sadowski, J.; Ludwików, A. Arabidopsis ABA-Activated Kinase MAPKKK18 is Regulated by Protein Phosphatase 2C ABI1 and the Ubiquitin-Proteasome Pathway. Plant Cell Physiol. 2015, 56, 2351–2367. [Google Scholar] [CrossRef] [PubMed]
- Llères, D.; Swift, S.; Lamond, A.I. Detecting Protein-Protein Interactions in vivo with FRET using Multiphoton Fluorescence Lifetime Imaging Microscopy (FLIM). Curr. Protoc. Cytom. 2007, 42. [Google Scholar] [CrossRef] [PubMed]
- Russinova, E.; Borst, J.-W.; Kwaaitaal, M.; Caño-Delgado, A.; Yin, Y.; Chory, J.; De Vries, S.C. Heterodimerization and Endocytosis of Arabidopsis Brassinosteroid Receptors BRI1 and AtSERK3 (BAK1). Plant Cell 2004, 16, 3216–3229. [Google Scholar] [CrossRef] [PubMed]
- Hagemeier, C.; Bannister, A.J.; Cook, A.; Kouzarides, T. The activation domain of transcription factor PU.1 binds the retinoblastoma (RB) protein and the transcription factor TFIID in vitro: RB shows sequence similarity to TFIID and TFIIB. Proc. Natl. Acad. Sci. USA 1993, 90, 1580–1584. [Google Scholar] [CrossRef] [PubMed]
- Yip, W.-K.; Dong, J.-G.; Yang, S.F. Purification and Characterization of 1-Aminocyclopropane-1-Carboxylate Synthase from Apple Fruits. Plant Physiol. 1991, 95, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Qin, C.; Zhao, J.; Wang, X. Phospholipase Dα1-derived phosphatidic acid interacts with ABI1 phosphatase 2C and regulates abscisic acid signaling. Proc. Natl. Acad. Sci. USA 2004, 101, 9508–9513. [Google Scholar] [CrossRef]
- Moes, D.; Himmelbach, A.; Korte, A.; Haberer, G.; Grill, E. Nuclear localization of the mutant protein phosphatase abi1 is required for insensitivity towards ABA responses in Arabidopsis. Plant J. 2008, 54, 806–819. [Google Scholar] [CrossRef]
- Ma, Y.; Szostkiewicz, I.; Korte, A.; Moes, D.; Yang, Y.; Christmann, A.; Grill, E. Regulators of PP2C Phosphatase Activity Function as Abscisic Acid Sensors. Science 2009, 324, 1064–1068. [Google Scholar] [CrossRef]
- Wang, K.; He, J.; Zhao, Y.; Wu, T.; Zhou, X.; Ding, Y.; Kong, L.; Wang, X.; Wang, Y.; Li, J.; et al. EAR1 Negatively Regulates ABA Signaling by Enhancing 2C Protein Phosphatase Activity. Plant Cell 2018, 30, 815–834. [Google Scholar] [CrossRef]
- Kierzkowski, D.; Kmieciak, M.; Piontek, P.; Wojtaszek, P.; Jarmolowski, A.; Kulinska, Z.S. The Arabidopsis CBP20 targets the cap-binding complex to the nucleus, and is stabilized by CBP80. Plant J. 2009, 59, 814–825. [Google Scholar] [CrossRef]
- Gehl, C.; Waadt, R.; Kudla, J.; Mendel, R.R.; Hänsch, R.; Kudla, J. New GATEWAY vectors for High Throughput Analyses of Protein–Protein Interactions by Bimolecular Fluorescence Complementation. Mol. Plant 2009, 2, 1051–1058. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Yoon, G.M.; Chen, Y.; Kieber, J.J. Regulation of the turnover of ACC synthases by phytohormones and heterodimerization in Arabidopsis. Plant J. 2017, 91, 491–504. [Google Scholar] [CrossRef] [PubMed]
- Yin, P.; Fan, H.; Hao, Q.; Yuan, X.; Wu, D.; Pang, Y.; Yan, C.; Li, W.; Wang, J.; Yan, N. Structural insights into the mechanism of abscisic acid signaling by PYL proteins. Nat. Struct. Mol. Boil. 2009, 16, 1230–1236. [Google Scholar] [CrossRef] [PubMed]
- Joo, S.; Liu, Y.; Lueth, A.; Zhang, S. MAPK phosphorylation-induced stabilization of ACS6 protein is mediated by the non-catalytic C-terminal domain, which also contains the cis-determinant for rapid degradation by the 26S proteasome pathway. Plant J. 2008, 54, 129–140. [Google Scholar] [CrossRef]
- Santiago, J.; Dupeux, F.; Betz, K.; Antoni, R.; Gonzalez-Guzman, M.; Rodriguez, L.; Márquez, J.A.; Rodriguez, P.L. Structural insights into PYR/PYL/RCAR ABA receptors and PP2Cs. Plant Sci. 2012, 182, 3–11. [Google Scholar] [CrossRef]
- Melcher, K.; Ng, L.-M.; Zhou, X.E.; Soon, F.-F.; Xu, Y.; Suino-Powell, K.M.; Park, S.-Y.; Weiner, J.J.; Fujii, H.; Chinnusamy, V.; et al. A gate–latch–lock mechanism for hormone signalling by abscisic acid receptors. Nature 2009, 462, 602–608. [Google Scholar] [CrossRef]
- Miyazono, K.; Miyakawa, T.; Sawano, Y.; Kubota, K.; Kang, H.-J.; Asano, A.; Miyauchi, Y.; Takahashi, M.; Zhi, Y.; Fujita, Y.; et al. Structural basis of abscisic acid signalling. Nature 2009, 462, 609–614. [Google Scholar] [CrossRef]
- Dupeux, F.; Antoni, R.; Betz, K.; Santiago, J.; Gonzalez-Guzman, M.; Rodriguez, L.; Rubio, S.; Park, S.-Y.; Cutler, S.R.; Rodriguez, P.L.; et al. Modulation of Abscisic Acid Signaling in Vivo by an Engineered Receptor-Insensitive Protein Phosphatase Type 2C Allele. Plant Physiol. 2011, 156, 106–116. [Google Scholar] [CrossRef]
- Dupeux, F.; Santiago, J.; Betz, K.; Twycross, J.; Park, S.-Y.; Rodriguez, L.; Gonzalez-Guzman, M.; Jensen, M.R.; Krasnogor, N.; Blackledge, M.; et al. A thermodynamic switch modulates abscisic acid receptor sensitivity. EMBO J. 2011, 30, 4171–4184. [Google Scholar] [CrossRef]
- Shyu, Y.J.; Suarez, C.D.; Hu, C.-D. Visualization of ternary complexes in living cells by using a BiFC-based FRET assay. Nat. Protoc. 2008, 3, 1693–1702. [Google Scholar] [CrossRef]
- Tsuchisaka, A.; Yu, G.; Jin, H.; Alonso, J.M.; Ecker, J.R.; Zhang, X.; Gao, S.; Theologis, A. A Combinatorial Interplay Among the 1-Aminocyclopropane-1-Carboxylate Isoforms Regulates Ethylene Biosynthesis in Arabidopsis thaliana. Genetics 2009, 183, 979–1003. [Google Scholar] [CrossRef] [PubMed]
- Sebastià, C.H.; Hardin, S.C.; Clouse, S.D.; Kieber, J.J.; Huber, S.C. Identification of a new motif for CDPK phosphorylation in vitro that suggests ACC synthase may be a CDPK substrate. Arch. Biochem. Biophys. 2004, 428, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Christians, M.J.; Gingerich, D.; Hansen, M.; Binder, B.; Kieber, J.J.; Vierstra, R.D. The BTB ubiquitin ligases ETO1, EOL1 and EOL2 act collectively to regulate ethylene biosynthesis in Arabidopsis by controlling type-2 ACC synthase levels. Plant J. 2008, 57, 332–345. [Google Scholar] [CrossRef] [PubMed]
- Skottke, K.R.; Yoon, G.M.; Kieber, J.J.; DeLong, A. Protein phosphatase 2A controls ethylene biosynthesis by differentially regulating the turnover of ACC synthase isoforms. PLoS Genet. 2011, 7, e1001370. [Google Scholar] [CrossRef]
- Li, G.; Meng, X.; Wang, R.; Mao, G.; Han, L.; Liu, Y.; Zhang, S. Dual-level regulation of ACC synthase activity by MPK3/MPK6 cascade and its downstream WRKY transcription factor during ethylene induction in arabidopsis. PLoS Genet. 2012, 8, e1002767. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, R.; Umezawa, T.; Mizoguchi, T.; Takahashi, S.; Takahashi, F.; Shinozaki, K. The regulatory domain of SRK2E/OST1/SnRK2.6 interacts with ABI1 and integrates abscisic acid (ABA) and osmotic stress signals controlling stomatal closure in Arabidopsis. J. Biol. Chem. 2006, 281, 5310–5318. [Google Scholar] [CrossRef]
- Leung, J.; Orfanidi, S.; Chefdor, F.; Mészáros, T.; Bolte, S.; Mizoguchi, T.; Shinozaki, K.; Giraudat, J.; Bögre, L. Antagonistic interaction between MAP kinase and protein phosphatase 2C in stress recovery. Plant Sci. 2006, 171, 596–606. [Google Scholar] [CrossRef]
- McLoughlin, F.; Galvan-Ampudia, C.S.; Julkowska, M.; Caarls, L.; Van Der Does, D.; Laurière, C.; Munnik, T.; Haring, M.A.; Testerink, C. The Snf1-related protein kinases SnRK2.4 and SnRK2.10 are involved in maintenance of root system architecture during salt stress. Plant J. 2012, 72, 436–449. [Google Scholar] [CrossRef]
- Xie, T.; Ren, R.; Zhang, Y.-Y.; Pang, Y.; Yan, C.; Gong, X.; He, Y.; Li, W.; Miao, D.; Hao, Q.; et al. Molecular Mechanism for Inhibition of a Critical Component in the Arabidopsis thaliana Abscisic Acid Signal Transduction Pathways, SnRK2.6, by Protein Phosphatase ABI1*. J. Boil. Chem. 2011, 287, 794–802. [Google Scholar] [CrossRef]
- Brandt, B.; Brodsky, D.E.; Xue, S.; Negi, J.; Iba, K.; Kangasjärvi, J.; Ghassemian, M.; Stephan, A.B.; Hu, H.; Schroeder, J.I. Reconstitution of abscisic acid activation of SLAC1 anion channel by CPK6 and OST1 kinases and branched ABI1 PP2C phosphatase action. Proc. Natl. Acad. Sci. USA 2012, 109, 10593–10598. [Google Scholar] [CrossRef]
- Rodrigues, A.; Adamo, M.C.; Crozet, P.; Margalha, L.; Confraria, A.; Martinho, C.; Elias, C.; Rabissi, A.; Lumbreras, V.; Gonzalez-Guzman, M.; et al. ABI1 and PP2CA Phosphatases Are Negative Regulators of Snf1-Related Protein Kinase1 Signaling in Arabidopsis. Plant Cell 2013, 25, 3871–3884. [Google Scholar] [CrossRef] [PubMed]
- Krzywińska, E.; Bucholc, M.; Kulik, A.; Ciesielski, A.; Lichocka, M.; Dębski, J.; Ludwików, A.; Dadlez, M.; Rodriguez, P.L.; Dobrowolska, G. Phosphatase ABI1 and okadaic acid-sensitive phosphoprotein phosphatases inhibit salt stress-activated SnRK2.4 kinase. BMC Plant Boil. 2016, 16, 136. [Google Scholar] [CrossRef] [PubMed]
- Weiner, J.J.; Peterson, F.C.; Volkman, B.F.; Cutler, S.R. Structural and functional insights into core ABA signaling. Curr. Opin. Plant Boil. 2010, 13, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Miyakawa, T.; Fujita, Y.; Yamaguchi-Shinozaki, K.; Tanokura, M. Structure and function of abscisic acid receptors. Trends Plant Sci. 2013, 18, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Miyakawa, T.; Tanokura, M. Structural basis for the regulation of phytohormone receptors. Biosci. Biotechnol. Biochem. 2017, 81, 1261–1273. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Mao, G.; Wang, R.; Liu, Y.; Zhang, S.; And, Y.L.; Li, G.-J.; Yang, K.-Y. Mitogen-activated protein kinase 3 and 6 regulate Botrytis cinerea-induced ethylene production in Arabidopsis. Plant J. 2010, 64, 114–127. [Google Scholar] [CrossRef] [PubMed]
- Vierstra, R.D. The ubiquitin–26S proteasome system at the nexus of plant biology. Nat. Rev. Mol. Cell Boil. 2009, 10, 385–397. [Google Scholar] [CrossRef]
- Santner, A.; Estelle, M. The ubiquitin-proteasome system regulates plant hormone signaling. Plant J. 2010, 61, 1029–1040. [Google Scholar] [CrossRef]
- Jin, Y.; Ye, N.; Zhu, F.; Li, H.; Wang, J.; Jiang, L.; Zhang, J. Calcium-dependent protein kinase CPK28 targets the methionine adenosyltransferases for degradation by the 26S proteasome and affects ethylene biosynthesis and lignin deposition in Arabidopsis. Plant J. 2017, 90, 304–318. [Google Scholar] [CrossRef]
- Yoon, G.M. New Insights into the Protein Turnover Regulation in Ethylene Biosynthesis. Mol. Cells 2015, 38, 597–603. [Google Scholar] [CrossRef]
- Himmelbach, A.; Hoffmann, T.; Leube, M.; Höhener, B.; Grill, E. Homeodomain protein ATHB6 is a target of the protein phosphatase ABI1 and regulates hormone responses in Arabidopsis. EMBO J. 2002, 21, 3029–3038. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Chen, Z.; Gao, J.; Gong, Z. Abscisic acid inhibits root growth in Arabidopsis through ethylene biosynthesis. Plant J. 2014, 79, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Gepstein, S.; Thimann, K.V. The effect of light on the production of ethylene from 1-aminocyclopropane-1-carboxylic acid by leaves. Planta 1980, 149, 196–199. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Shen, X.; Liu, R.; Xue, C.; Wei, N.; Deng, X.W.; Zhong, S. The Red Light Receptor Phytochrome B Directly Enhances Substrate-E3 Ligase Interactions to Attenuate Ethylene Responses. Dev. Cell 2016, 39, 597–610. [Google Scholar] [CrossRef] [PubMed]
- Mattoo, A.K.; Suttle, J.C. The Plant Hormone Ethylene; CRC Press: Boca Raton, FL, USA, 2018; ISBN 9781351084215. [Google Scholar]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marczak, M.; Cieśla, A.; Janicki, M.; Kasprowicz-Maluśki, A.; Kubiak, P.; Ludwików, A. Protein Phosphatases Type 2C Group A Interact with and Regulate the Stability of ACC Synthase 7 in Arabidopsis. Cells 2020, 9, 978. https://doi.org/10.3390/cells9040978
Marczak M, Cieśla A, Janicki M, Kasprowicz-Maluśki A, Kubiak P, Ludwików A. Protein Phosphatases Type 2C Group A Interact with and Regulate the Stability of ACC Synthase 7 in Arabidopsis. Cells. 2020; 9(4):978. https://doi.org/10.3390/cells9040978
Chicago/Turabian StyleMarczak, Małgorzata, Agata Cieśla, Maciej Janicki, Anna Kasprowicz-Maluśki, Piotr Kubiak, and Agnieszka Ludwików. 2020. "Protein Phosphatases Type 2C Group A Interact with and Regulate the Stability of ACC Synthase 7 in Arabidopsis" Cells 9, no. 4: 978. https://doi.org/10.3390/cells9040978
APA StyleMarczak, M., Cieśla, A., Janicki, M., Kasprowicz-Maluśki, A., Kubiak, P., & Ludwików, A. (2020). Protein Phosphatases Type 2C Group A Interact with and Regulate the Stability of ACC Synthase 7 in Arabidopsis. Cells, 9(4), 978. https://doi.org/10.3390/cells9040978