Macrophage-Derived Extracellular Vesicle Promotes Hair Growth
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Isolation of Dermal Papilla Cells
2.3. Isolation of Extracellular Vesicles and Condition Media for Macrophages
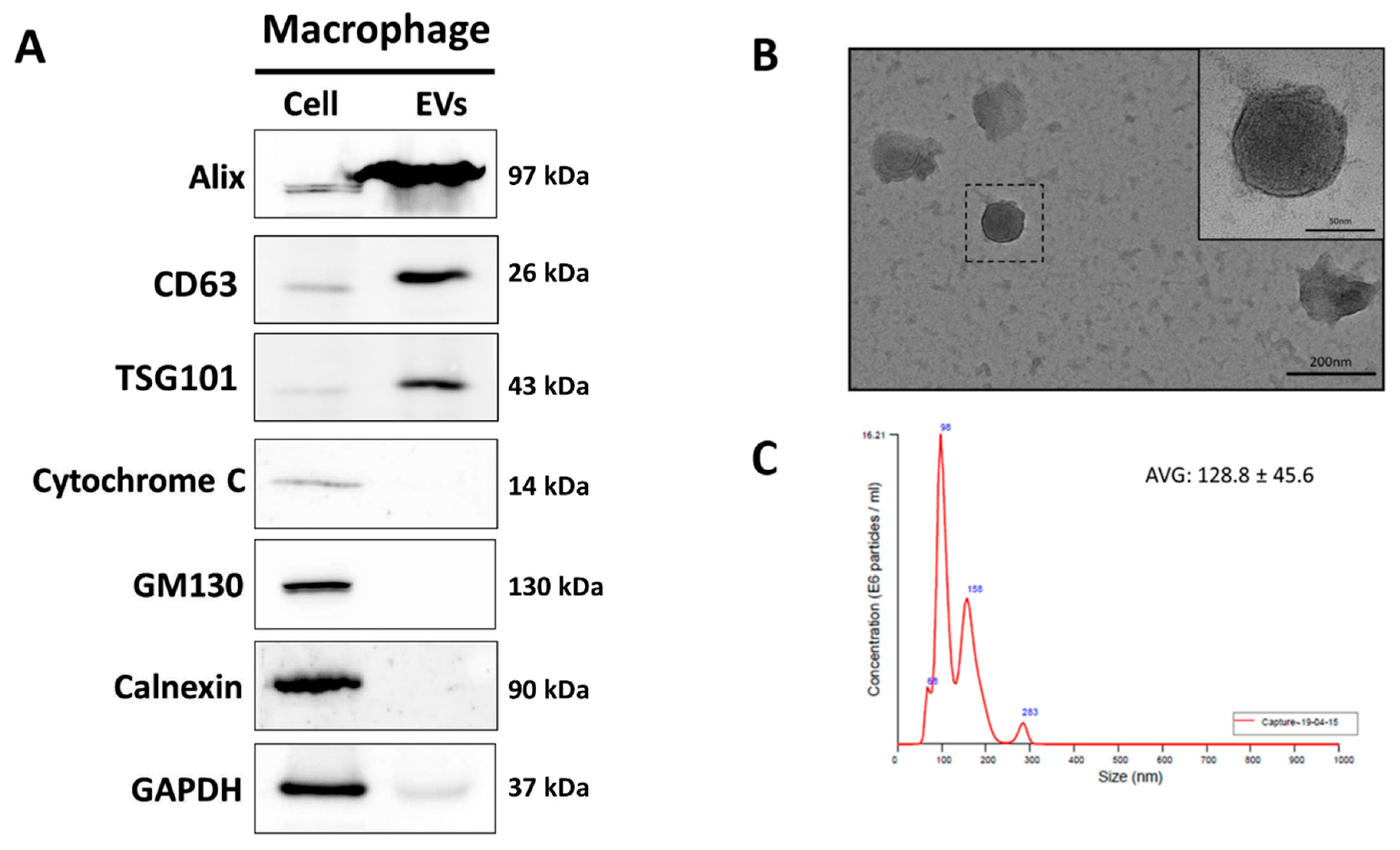
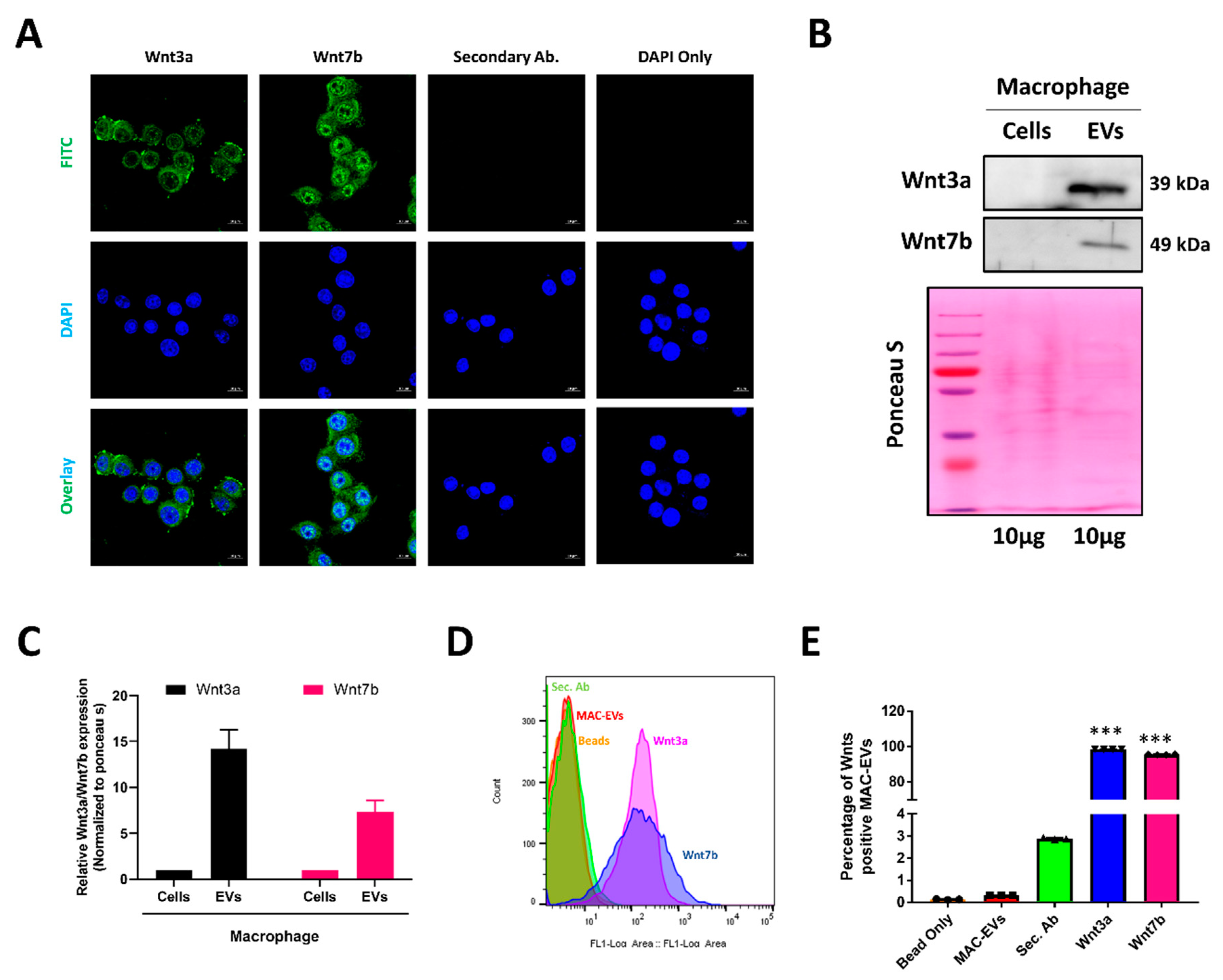
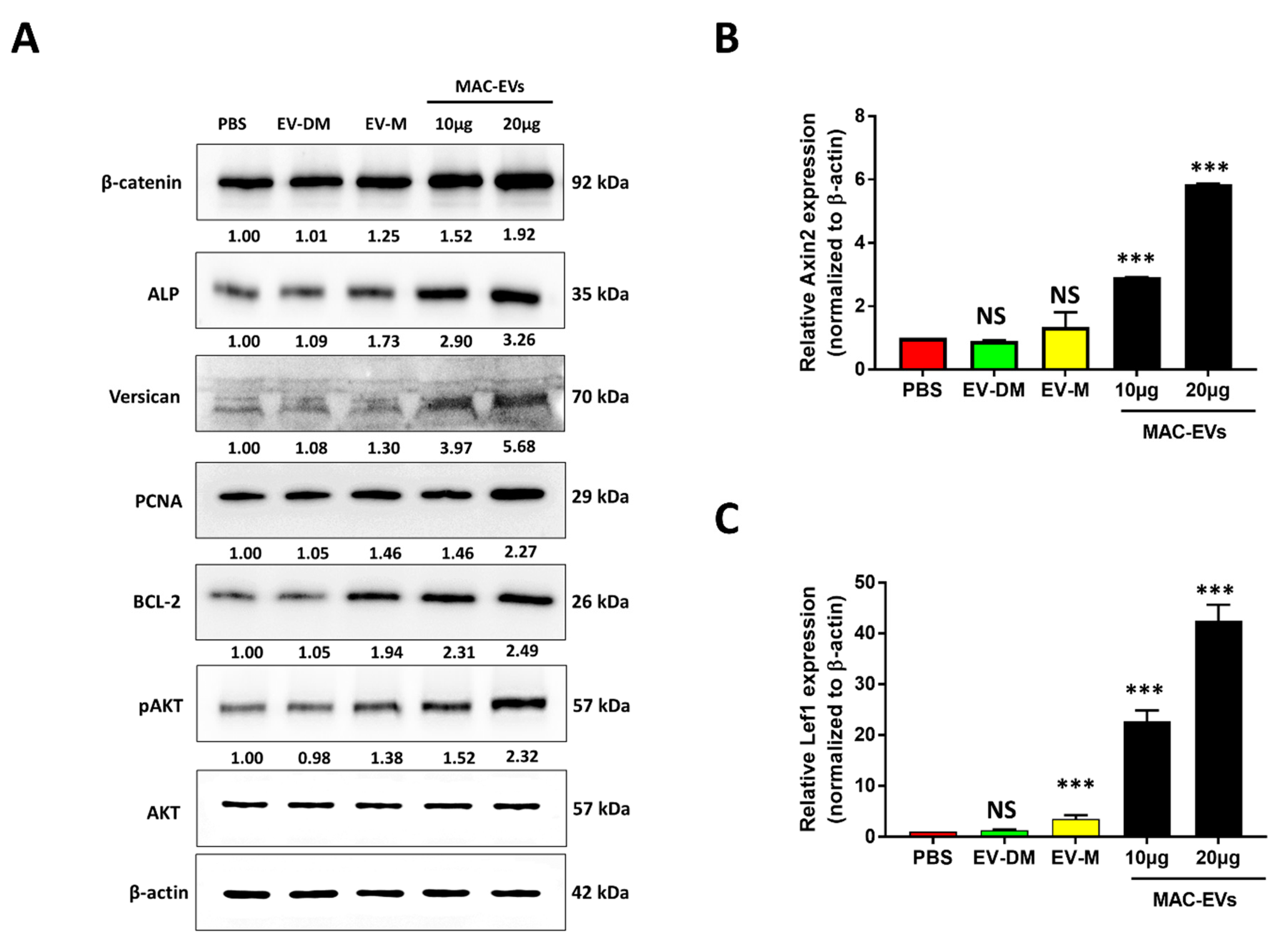
2.4. Western Blot Analysis
2.5. Transmission Electron Microscopy (TEM)
2.6. Nanoparticle Tracking Analysis (NTA)
2.7. Flow Cytometry
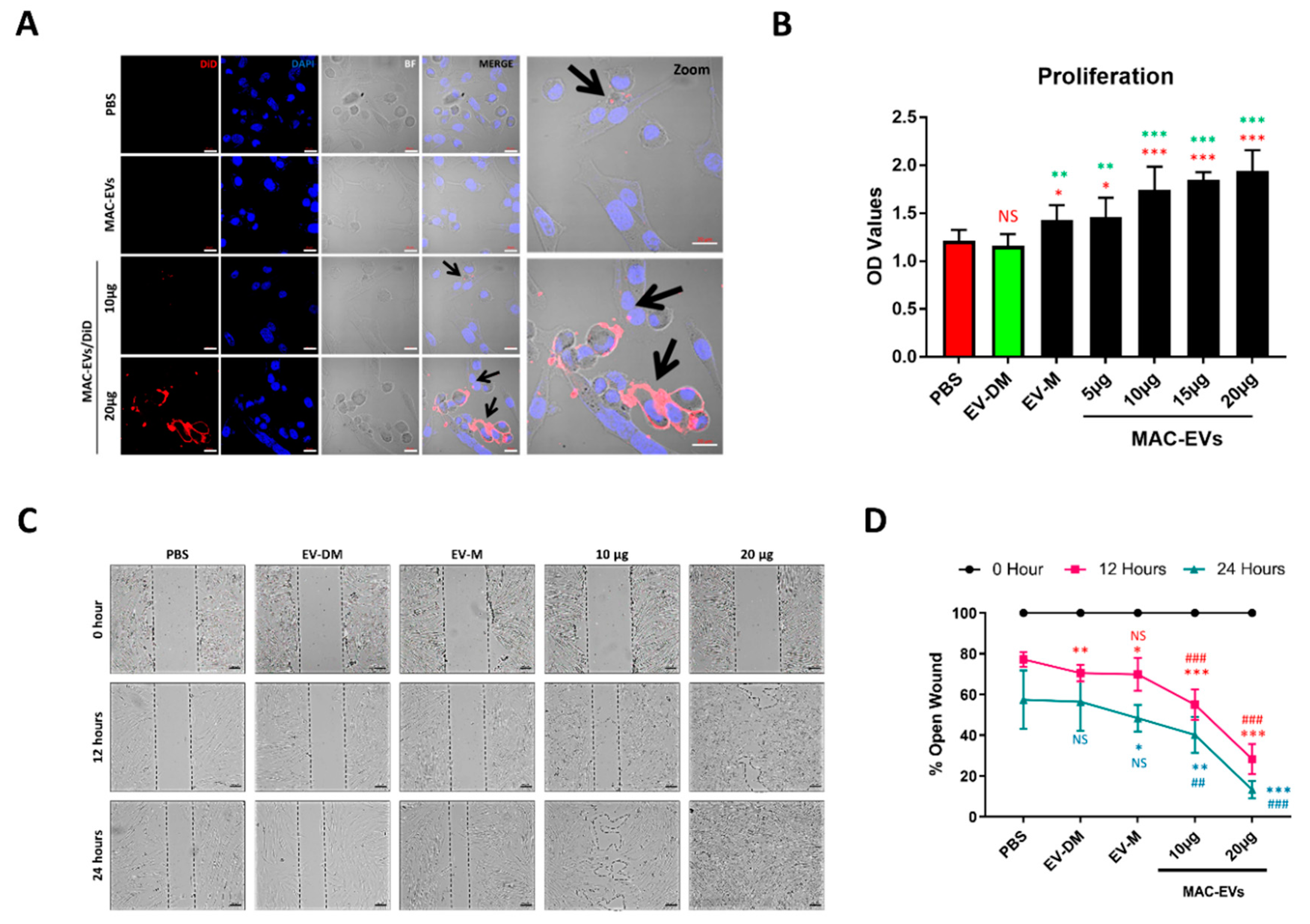
2.8. EV Interaction and Internalization Assay
2.9. In Vitro Cell Proliferation Assay
2.10. In Vitro Cell Migration Assay
2.11. RNA Extraction and Reverse Transcriptase Polymerase chain reaction (RT-PCR)
2.12. Real-Time Polymerase Chain Reaction (Real-Time PCR)
2.13. Immunofluorescence (IF) Assay
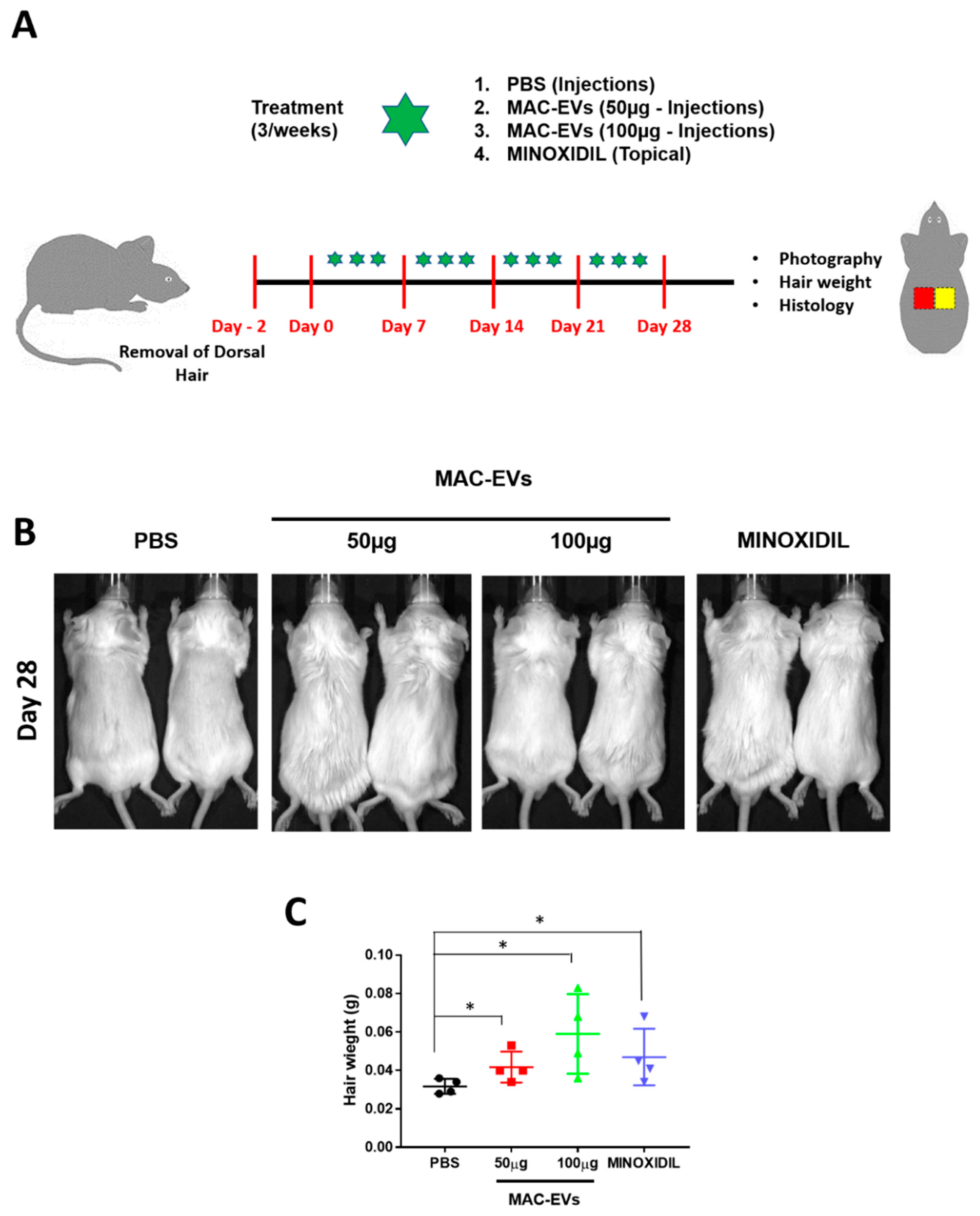
2.14. In Vivo Experiments and HFs Weight Measurement
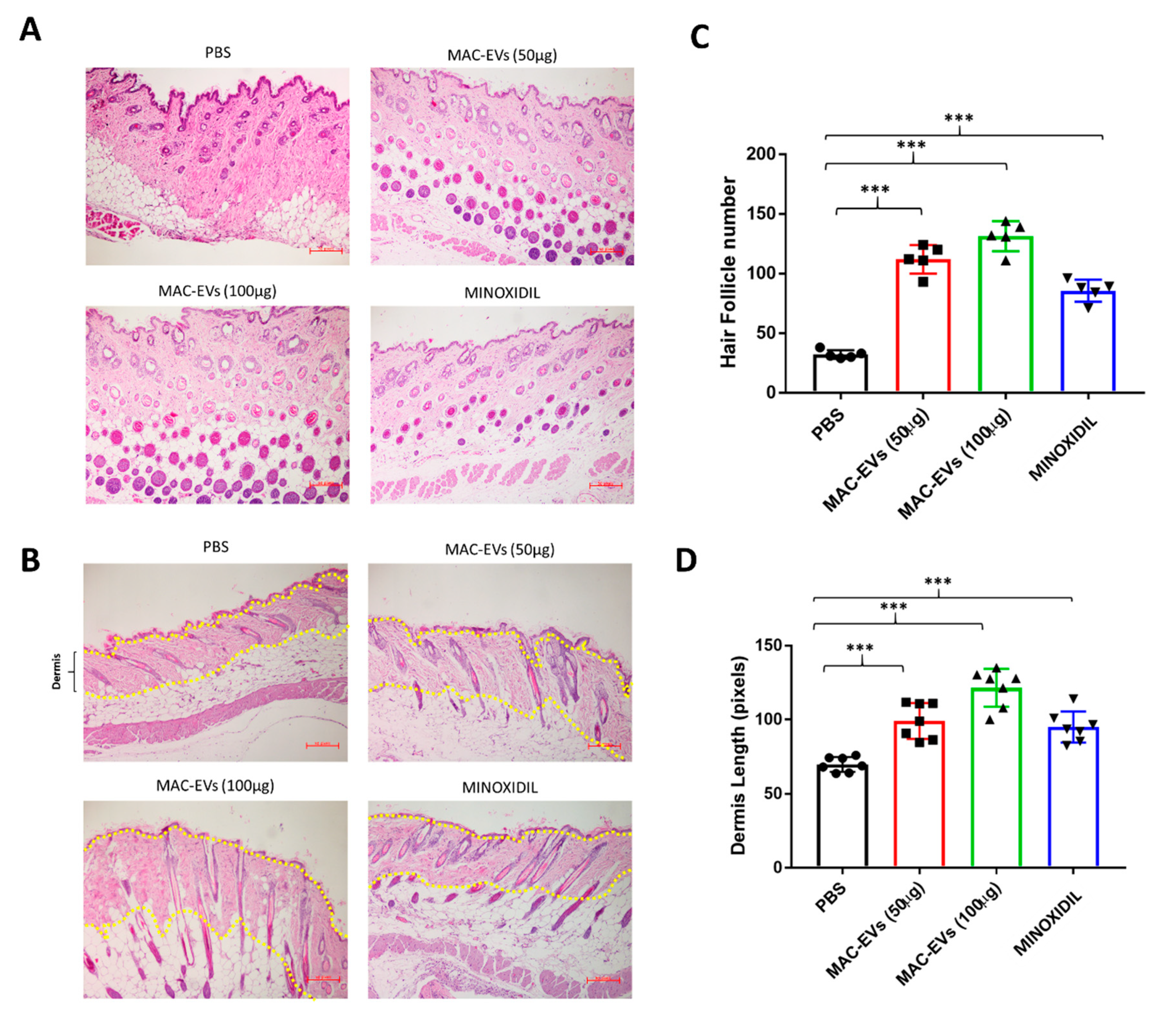
2.15. Histological Analysis
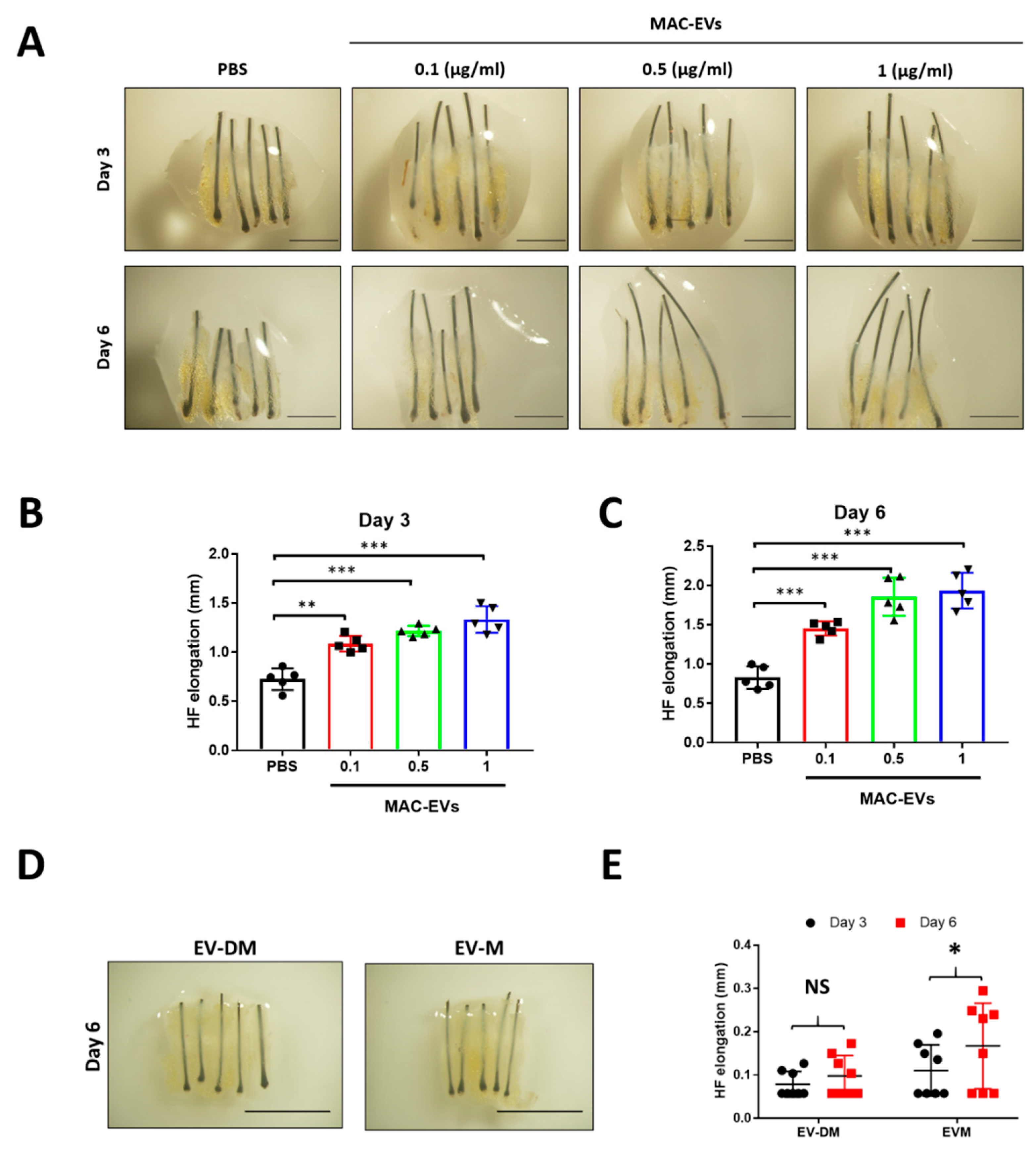
2.16. Human Hair Shaft Elongation
2.17. Statistical Analysis
3. Results
3.1. Characterization of MAC-EVs
3.2. Identification of Hair Growth Inducing Wnt Proteins in EVs and Its Membrane
3.3. The MAC-EVs Attach to DP Cell Membrane and are Internalized
3.4. MAC-EV Treatment Increases Cell Proliferation and Migration of DP Cells
3.5. MAC-EVs Increase the Levels of Marker Proteins, Survival- and Proliferation-Markers and Activate the Wnt/β-Catenin Signaling Pathway in DP Cells
3.6. Treatment with MAC-EV Upregulates Expression of Hair Inducing Growth Factors in DP Cells
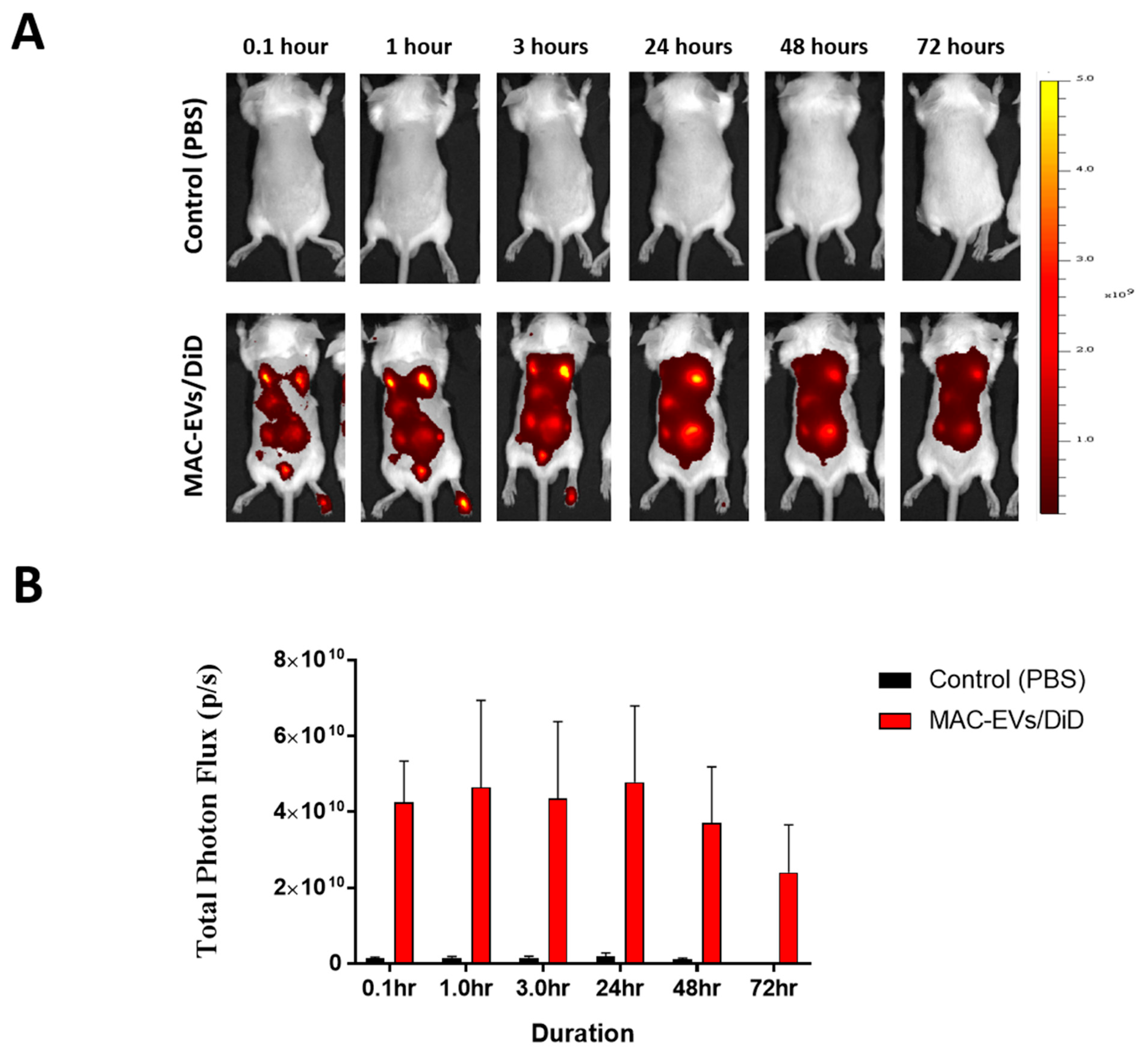
3.7. Determination of MAC-EVs Treatment Intervals in Balb/c Mice
3.8. Hair Growth Effects of MAC-EVs in Balb/c Mice
3.9. MAC-EVs Promote the HF Number and Dermis Thickness in Mice
3.10. MAC-EVs Elongates the Hair Shaft of Human HFs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Dong, L.; Hao, H.; Xia, L.; Liu, J.; Ti, D.; Tong, C.; Hou, Q.; Han, Q.; Zhao, Y.; Liu, H.; et al. Treatment of MSCs with Wnt1a-conditioned medium activates DP cells and promotes hair follicle regrowth. Sci. Rep. 2014, 4, 5432. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, R.L.; Gangadaran, P.; Bak, S.S.; Oh, J.M.; Kalimuthu, S.; Lee, H.W.; Baek, S.H.; Zhu, L.; Sung, Y.K.; Jeong, S.Y.; et al. Extracellular vesicles derived from MSCs activates dermal papilla cell in vitro and promotes hair follicle conversion from telogen to anagen in mice. Sci. Rep. 2017, 7, 15560. [Google Scholar] [CrossRef] [PubMed]
- Plikus, M.V.; Chuong, C.-M. Complex Hair Cycle Domain Patterns and Regenerative Hair Waves in Living Rodents. J. Investig. Dermatol. 2008, 128, 1071–1080. [Google Scholar] [CrossRef]
- Toyoshima, K.-E.; Asakawa, K.; Ishibashi, N.; Toki, H.; Ogawa, M.; Hasegawa, T.; Irié, T.; Tachikawa, T.; Sato, A.; Takeda, A.; et al. Fully functional hair follicle regeneration through the rearrangement of stem cells and their niches. Nat. Commun. 2012, 3, 784. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.-E.; Miao, Y.; Wang, J.; Jiang, W.; Fan, Z.-X.; Liu, X.-M.; Hu, Z.-Q. As a carrier-transporter for hair follicle reconstitution, platelet-rich plasma promotes proliferation and induction of mouse dermal papilla cells. Sci. Rep. 2017, 7, 1125. [Google Scholar] [CrossRef] [PubMed]
- Gangadaran, P.; Rajendran, R.L.; Lee, H.W.; Kalimuthu, S.; Hong, C.M.; Jeong, S.Y.; Lee, S.-W.; Lee, J.; Ahn, B.-C. Extracellular vesicles from mesenchymal stem cells activates VEGF receptors and accelerates recovery of hindlimb ischemia. J. Control. Release 2017, 264, 112–126. [Google Scholar] [CrossRef] [PubMed]
- Gangadaran, P.; Li, X.J.; Lee, H.W.; Oh, J.M.; Kalimuthu, S.; Rajendran, R.L.; Son, S.H.; Baek, S.H.; Singh, T.D.; Zhu, L.; et al. A new bioluminescent reporter system to study the biodistribution of systematically injected tumor-derived bioluminescent extracellular vesicles in mice. Oncotarget 2017, 8, 109894–109914. [Google Scholar] [CrossRef]
- Gangadaran, P.; Hong, C.M.; Oh, J.M.; Rajendran, R.L.; Kalimuthu, S.; Son, S.H.; Gopal, A.; Zhu, L.; Baek, S.H.; Jeong, S.Y.; et al. In vivo Non-invasive Imaging of Radio-Labeled Exosome-Mimetics Derived From Red Blood Cells in Mice. Front. Pharmacol. 2018, 9, 817. [Google Scholar] [CrossRef]
- Son, S.H.; Gangadaran, P.; Ahn, B.-C. A novel strategy of transferring NIS protein to cells using extracellular vesicles leads to increase in iodine uptake and cytotoxicity. Int. J. Nanomed. 2019, 14, 1779–1787. [Google Scholar] [CrossRef]
- Kwack, M.H.; Seo, C.H.; Gangadaran, P.; Ahn, B.; Kim, M.K.; Kim, J.C.; Sung, Y.K. Exosomes derived from human dermal papilla cells promote hair growth in cultured human hair follicles and augment the hair-inductive capacity of cultured dermal papilla spheres. Exp. Dermatol. 2019, 28, 854–857. [Google Scholar] [CrossRef]
- Gangadaran, P.; Hong, C.M.; Ahn, B.-C. An Update on in Vivo Imaging of Extracellular Vesicles as Drug Delivery Vehicles. Front. Pharmacol. 2018, 9, 169. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Contreras, M.; Shah, S.H.; Tamayo, A.; Robbins, P.D.; Golberg, R.B.; Mendez, A.J.; Ricordi, C. Plasma-derived exosome characterization reveals a distinct microRNA signature in long duration Type 1 diabetes. Sci. Rep. 2017, 7, 5998. [Google Scholar] [CrossRef]
- Yim, N.; Ryu, S.-W.; Choi, K.; Lee, K.R.; Lee, S.; Choi, H.; Kim, J.; Shaker, M.R.; Sun, W.; Park, J.-H.; et al. Exosome engineering for efficient intracellular delivery of soluble proteins using optically reversible protein–protein interaction module. Nat. Commun. 2016, 7, 12277. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Kalimuthu, S.; Gangadaran, P.; Oh, J.M.; Lee, H.W.; Baek, S.H.; Jeong, S.Y.; Lee, S.-W.; Lee, J.; Ahn, B.-C. Exosomes Derived From Natural Killer Cells Exert Therapeutic Effect in Melanoma. Theranostics 2017, 7, 2732–2745. [Google Scholar] [CrossRef] [PubMed]
- Greening, D.; Gopal, S.K.; Xu, R.; Simpson, R.; Chen, W. Exosomes and their roles in immune regulation and cancer. Semin. Cell Dev. Boil. 2015, 40, 72–81. [Google Scholar] [CrossRef]
- Pitt, J.M.; Charrier, M.; Viaud, S.; André, F.; Besse, B.; Chaput, N.; Zitvogel, L. Dendritic Cell–Derived Exosomes as Immunotherapies in the Fight against Cancer. J. Immunol. 2014, 193, 1006–1011. [Google Scholar] [CrossRef]
- Yuan, D.; Zhao, Y.; Banks, W.A.; Bullock, K.M.; Haney, M.; Batrakova, E.; Kabanov, A.V. Macrophage exosomes as natural nanocarriers for protein delivery to inflamed brain. Biomater. 2017, 142, 1–12. [Google Scholar] [CrossRef]
- Castellana, D.; Paus, R.; Perez-Moreno, M. Macrophages contribute to the cyclic activation of adult hair follicle stem cells. PLoS Boil. 2014, 12, e1002002. [Google Scholar] [CrossRef]
- McElwee, K.J.; Huth, A.; Kissling, S.; Hoffmann, R. Macrophage-Stimulating Protein Promotes Hair Growth Ex Vivo and Induces Anagen from Telogen Stage Hair Follicles In Vivo. J. Investig. Dermatol. 2004, 123, 34–40. [Google Scholar] [CrossRef]
- Wang, X.; Chen, H.; Tian, R.; Zhang, Y.; Drutskaya, M.S.; Wang, C.; Ge, J.; Fan, Z.; Kong, D.; Wang, X.; et al. Macrophages induce AKT/β-catenin-dependent Lgr5+ stem cell activation and hair follicle regeneration through TNF. Nat. Commun. 2017, 8, 14091. [Google Scholar] [CrossRef]
- Hardman, J.A.; Muneeb, F.; Pople, J.; Bhogal, R.; Shahmalak, A.; Paus, R. Human Perifollicular Macrophages Undergo Apoptosis, Express Wnt Ligands, and Switch their Polarization during Catagen. J. Investig. Dermatol. 2019, 139, 2543–2546.e9. [Google Scholar] [CrossRef] [PubMed]
- Bak, S.-S.; Sung, Y.K.; Kim, S.-K. 7-Phloroeckol promotes hair growth on human follicles in vitro. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2014, 387, 789–793. [Google Scholar] [CrossRef] [PubMed]
- Alam, H.; Sehgal, L.; Kundu, S.T.; Dalal, S.N.; Vaidya, M.M. Novel function of keratins 5 and 14 in proliferation and differentiation of stratified epithelial cells. Mol. Boil. Cell 2011, 22, 4068–4078. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.I.; Al-Reza, S.M.; Kang, S.C. Hair growth promoting effect of Zizyphus jujuba essential oil. Food Chem. Toxicol. 2010, 48, 1350–1354. [Google Scholar] [CrossRef] [PubMed]
- Dmello, C.; Sawant, S.; Alam, H.; Gangadaran, P.; Mogre, S.; Tiwari, R.; D’Souza, Z.; Narkar, M.; Thorat, R.; Patil, K.; et al. Vimentin regulates differentiation switch via modulation of keratin 14 levels and their expression together correlates with poor prognosis in oral cancer patients. PLoS ONE 2017, 12, e0172559. [Google Scholar] [CrossRef]
- Kwack, M.H.; Lee, J.H.; Seo, C.H.; Kim, J.C.; Kim, M.K.; Sung, Y.K. Dickkopf-1 is involved in dexamethasone-mediated hair follicle regression. Exp. Dermatol. 2017, 26, 952–954. [Google Scholar] [CrossRef]
- Kishimoto, J.; Burgeson, R.E.; Morgan, B.A. Wnt signaling maintains the hair-inducing activity of the dermal papilla. Genome Res. 2000, 14, 1181–1185. [Google Scholar]
- Kwack, M.H.; Kim, M.K.; Kim, J.C.; Sung, Y.K. Wnt5a attenuates Wnt/β-catenin signalling in human dermal papilla cells. Exp. Dermatol. 2013, 22, 229–231. [Google Scholar] [CrossRef]
- Kandyba, E.; Kobielak, K. Wnt7b is an important intrinsic regulator of hair follicle stem cell homeostasis and hair follicle cycling. Stem Cells 2014, 32, 886–901. [Google Scholar] [CrossRef]
- Enshell-Seijffers, D.; Lindon, C.; Kashiwagi, M.; Morgan, B.A. β-catenin Activity in the Dermal Papilla Regulates Morphogenesis and Regeneration of Hair. Dev. Cell 2010, 18, 633–642. [Google Scholar] [CrossRef]
- Kishimoto, J.; Ehama, R.; Wu, L.; Jiang, S.; Jiang, N.; Burgeson, R.E. Selective activation of the versican promoter by epithelial– mesenchymal interactions during hair follicle development. Proc. Natl. Acad. Sci. USA 1999, 96, 7336–7341. [Google Scholar] [CrossRef] [PubMed]
- Iida, M.; Ihara, S.; Matsuzaki, T. Hair cycle-dependent changes of alkaline phosphatase activity in the mesenchyme and epithelium in mouse vibrissal follicles. Dev. Growth Differ. 2007, 49, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Balañá, M.E.; Charreau, H.E.; Leirós, G.J. Epidermal stem cells and skin tissue engineering in hair follicle regeneration. World J. Stem Cells 2015, 7, 711–727. [Google Scholar] [CrossRef] [PubMed]
- Rezza, A.; Wang, Z.; Sennett, R.; Qiao, W.; Wang, N.; Heitman, N.; Mok, K.W.; Clavel, C.; Yi, R.; Zandstra, P.; et al. Signaling Networks among Stem Cell Precursors, Transit-Amplifying Progenitors, and their Niche in Developing Hair Follicles. Cell Rep. 2016, 14, 3001–3018. [Google Scholar] [CrossRef]
- Kalimuthu, S.; Gangadaran, P.; Li, X.J.; Oh, J.M.; Lee, H.W.; Jeong, S.Y.; Lee, S.-W.; Lee, J.; Ahn, B.-C. In Vivo therapeutic potential of mesenchymal stem cell-derived extracellular vesicles with optical imaging reporter in tumor mice model. Sci. Rep. 2016, 6, 30418. [Google Scholar] [CrossRef]
- Takada, S.; Satomi, Y.; Kurata, T.; Ueno, N.; Norioka, S.; Kondoh, H.; Takao, T.; Takada, S. Monounsaturated Fatty Acid Modification of Wnt Protein: Its Role in Wnt Secretion. Dev. Cell 2006, 11, 791–801. [Google Scholar] [CrossRef]
- Willert, K.; Brown, J.D.; Danenberg, E.; Duncan, A.W.; Weissman, I.L.; Reya, T.; Yates, J.R.; Nusse, R. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature 2003, 423, 448–452. [Google Scholar] [CrossRef]
- Huelsken, J.; Vogel, R.; Erdmann, B.; Cotsarelis, G.; Birchmeier, W. β-Catenin Controls Hair Follicle Morphogenesis and Stem Cell Differentiation in the Skin. Cell 2001, 105, 533–545. [Google Scholar] [CrossRef]
- Dong, L.; Hao, H.; Liu, J.; Ti, D.; Tong, C.; Hou, Q.; Li, M.; Zheng, J.; Liu, G.; Fu, X.; et al. A Conditioned Medium of Umbilical Cord Mesenchymal Stem Cells Overexpressing Wnt7a Promotes Wound Repair and Regeneration of Hair Follicles in Mice. Stem Cells Int. 2017, 2017, 1–13. [Google Scholar] [CrossRef]
- Sahoo, S.; Klychko, E.; Thorne, T.; Misener, S.; Schultz, K.M.; Millay, M.; Ito, A.; Liu, T.; Kamide, C.; Agrawal, H.; et al. Exosomes from human CD34(+) stem cells mediate their proangiogenic paracrine activity. Circ. Res. 2011, 109, 724–728. [Google Scholar] [CrossRef]
- Gross, J.C.; Chaudhary, V.; Bartscherer, K.; Boutros, M. Active Wnt proteins are secreted on exosomes. Nature 2012, 14, 1036–1045. [Google Scholar] [CrossRef] [PubMed]
- Mc Gough, I.; Vincent, J.-P. Exosomes in developmental signalling. Development 2016, 143, 2482–2493. [Google Scholar] [CrossRef] [PubMed]
- Le Riche, A.; Aberdam, E.; Marchand, L.; Frank, E.; Jahoda, C.; Petit, I.; Bordes, S.; Closs, B.; Aberdam, D. Extracellular Vesicles from Activated Dermal Fibroblasts Stimulate Hair Follicle Growth Through Dermal Papilla-Secreted Norrin. Stem Cells 2019, 37, 1166–1175. [Google Scholar] [CrossRef]
- Prada, I.; Meldolesi, J. Binding and Fusion of Extracellular Vesicles to the Plasma Membrane of Their Cell Targets. Int. J. Mol. Sci. 2016, 17, 1296. [Google Scholar] [CrossRef]
- Feng, Q.; Zhang, C.; Lum, D.; Druso, J.E.; Blank, B.; Wilson, K.F.; Welm, A.; Antonyak, M.A.; Cerione, R.A. A class of extracellular vesicles from breast cancer cells activates VEGF receptors and tumour angiogenesis. Nat. Commun. 2017, 8, 14450. [Google Scholar] [CrossRef]
- Bahta, A.W.; Farjo, N.; Farjo, B.; Philpott, M. Premature Senescence of Balding Dermal Papilla Cells In Vitro Is Associated with p16INK4a Expression. J. Investig. Dermatol. 2008, 128, 1088–1094. [Google Scholar] [CrossRef]
- Zheng, M.; Jang, Y.-J.; Choi, N.; Kim, D.-Y.; Han, T.W.; Yeo, J.H.; Lee, J.; Sung, J.-H. Hypoxia improves hair inductivity of dermal papilla cells via nuclear NADPH oxidase 4-mediated reactive oxygen species generation’. Br. J. Dermatol. 2019, 181, 523–534. [Google Scholar] [CrossRef]
- Qiao, J.; Zawadzka, A.; Philips, E.; Turetsky, A.; Batchelor, S.; Peacock, J.; Durrant, S.; Garlick, D.; Kemp, P.; Teumer, J. Hair follicle neogenesis induced by cultured human scalp dermal papilla cells. Regen. Med. 2009, 4, 667–676. [Google Scholar] [CrossRef]
- McElwee, K.J.; Kissling, S.; Wenzel, E.; Huth, A.; Hoffmann, R. Cultured Peribulbar Dermal Sheath Cells Can Induce Hair Follicle Development and Contribute to the Dermal Sheath and Dermal Papilla. J. Investig. Dermatol. 2003, 121, 1267–1275. [Google Scholar] [CrossRef]
- Cadigan, K.; I Liu, Y. Wnt signaling: Complexity at the surface. J. Cell Sci. 2006, 119, 395–402. [Google Scholar] [CrossRef]
- Gordon, M.; Nusse, R. Wnt Signaling: Multiple Pathways, Multiple Receptors, and Multiple Transcription Factors. J. Boil. Chem. 2006, 281, 22429–22433. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Shin, Y.; Cheng, R.; Park, K.; Hu, Y.; McBride, J.; He, X.; Takahashi, Y.; Ma, J.-X. Receptor heterodimerization as a novel mechanism for the regulation of Wnt/β-catenin signaling. J. Cell Sci. 2014, 127, 4857–4869. [Google Scholar] [CrossRef] [PubMed]
- E Oro, A.; Scott, M.P. Splitting Hairs. Cell 1998, 95, 575–578. [Google Scholar] [CrossRef]
- Tong, T.; Kim, N.; Park, T. Topical Application of Oleuropein Induces Anagen Hair Growth in Telogen Mouse Skin. PLoS ONE 2015, 10, e0129578. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kwack, M.H.; Kang, B.M.; Kim, M.-K.; Kim, J.C.; Sung, Y.K. Minoxidil activates β-catenin pathway in human dermal papilla cells: A possible explanation for its anagen prolongation effect. J. Dermatol. Sci. 2011, 62, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Driskell, R.R.; Clavel, C.; Rendl, M.; Watt, F.E. Hair follicle dermal papilla cells at a glance. J. Cell Sci. 2011, 124, 1179–1182. [Google Scholar] [CrossRef]
- Zhou, L.; Xu, M.; Yang, Y.; Yang, K.; Wickett, R.R.; Andl, T.; Millar, S.E.; Zhang, Y. Activation of β-Catenin Signaling in CD133-Positive Dermal Papilla Cells Drives Postnatal Hair Growth. PLoS ONE 2016, 11, e0160425. [Google Scholar] [CrossRef]
- Shim, J.H. Hair growth-promoting effect of human dermal stem/progenitor cell-derived conditioned medium. Tissue Eng. Regen. Med. 2015, 12, 268–275. [Google Scholar] [CrossRef]
- Ahmad, S.; Singh, N.; I Glazer, R. Role of AKT1 in 17beta-estradiol- and insulin-like growth factor I (IGF-I)-dependent proliferation and prevention of apoptosis in MCF-7 breast carcinoma cells. Biochem. Pharmacol. 1999, 58, 425–430. [Google Scholar] [CrossRef]
- Cory, S.; Huang, P.D.C.S.; Adams, J.M. The Bcl-2 family: Roles in cell survival and oncogenesis. Oncogene 2003, 22, 8590–8607. [Google Scholar] [CrossRef]
- Van De Wetering, M.; Cavallo, R.; Dooijes, D.; Van Beest, M.; Van Es, J.; Loureiro, J.; Ypma, A.; Hursh, D.; Jones, T.; Bejsovec, A.; et al. Armadillo Coactivates Transcription Driven by the Product of the Drosophila Segment Polarity Gene dTCF. Cell 1997, 88, 789–799. [Google Scholar] [CrossRef]
- Zhang, X.; Gaspard, J.P.; Chung, D.C. Regulation of vascular endothelial growth factor by the Wnt and K-ras pathways in colonic neoplasia. Cancer Res. 2001, 61, 6050–6054. [Google Scholar] [PubMed]
- Lindner, G.; Menrad, A.; Gherardi, E.; Merlino, G.; Welker, P.; Handjiski, B.; Roloff, B.; Paus, R. Involvement of hepatocyte growth factor/scatter factor and Met receptor signaling in hair follicle morphogenesis and cycling. FASEB J. 2000, 14, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Peus, D.; Pittelkow, M.R. GROWTH FACTORS IN HAIR ORGAN DEVELOPMENT AND THE HAIR GROWTH CYCLE. Dermatol. Clin. 1996, 14, 559–572. [Google Scholar] [CrossRef]
- Pierce, G.F.; Yanagihara, D.; Klopchin, K.; Danilenko, D.; Hsu, E.; Kenney, W.C.; Morris, C.F. Stimulation of all epithelial elements during skin regeneration by keratinocyte growth factor. J. Exp. Med. 1994, 179, 831–840. [Google Scholar] [CrossRef]
- Stenn, K.S.; Combates, N.J.; Eilertsen, K.J.; Gordon, J.S.; Pardinas, J.R.; Parimoo, S.; Prouty, S.M. HAIR FOLLICLE GROWTH CONTROLS. Dermatol. Clin. 1996, 14, 543–558. [Google Scholar] [CrossRef]
- Diaz, S.F.; Torres, S.M.F.; Nogueira, S.A.F.; Gilbert, S.; Jessen, C.R. The impact of body site, topical melatonin and brushing on hair regrowth after clipping normal Siberian Husky dogs. Veter- Dermatol. 2006, 17, 45–50. [Google Scholar] [CrossRef]
- Bassukas, I.D.; Hornstein, O.P. Effects of plucking on the anatomy of the anagen hair bulb. A light microscopic study. Arch Dermatol Res 1989, 281, 188–192. [Google Scholar] [CrossRef]
- Kim, J.H.; Herrmann, F.; Sulzberger, M.B. The effect of plucking and of clipping on the growth of hair in guinea-pigs. J. Investig. Dermatol. 1962, 38, 351–356. [Google Scholar] [CrossRef]
- Xie, Y.; McElwee, K.J.; Owen, G.R.; Häkkinen, L.; Larjava, H.; H, L. Integrin β6-Deficient Mice Show Enhanced Keratinocyte Proliferation and Retarded Hair Follicle Regression after Depilation. J. Investig. Dermatol. 2012, 132, 547–555. [Google Scholar] [CrossRef]
- Yano, K.; Brown, L.F.; Detmar, M. Control of hair growth and follicle size by VEGF-mediated angiogenesis. J. Clin. Investig. 2001, 107, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Aktaş, H.; Alan, S.; Türkoğlu, E.B.; Sevik, Ö. Could Topical Minoxidil Cause Non-Arteritic Anterior Ischemic Optic Neuropathy? J. Clin. Diagn. Res. 2016, 10, WD01–WD02. [Google Scholar]
- Rossi, A.; Cantisani, C.; Melis, L.; Iorio, A.; Scali, E.; Calvieri, S. Minoxidil use in dermatology, side effects and recent patents. Recent Patents Inflamm. Allergy Drug Discov. 2012, 6, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.S.; Kwack, M.H.; Kim, M.K.; Kim, J.C.; Sung, Y.K. Attenuation of Dickkopf 1-Induced Hair Growth Inhibition in Cultured Human Hair Follicles by Tianeptine. Ann. Dermatol. 2017, 29, 102–105. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajendran, R.L.; Gangadaran, P.; Seo, C.H.; Kwack, M.H.; Oh, J.M.; Lee, H.W.; Gopal, A.; Sung, Y.K.; Jeong, S.Y.; Lee, S.-W.; et al. Macrophage-Derived Extracellular Vesicle Promotes Hair Growth. Cells 2020, 9, 856. https://doi.org/10.3390/cells9040856
Rajendran RL, Gangadaran P, Seo CH, Kwack MH, Oh JM, Lee HW, Gopal A, Sung YK, Jeong SY, Lee S-W, et al. Macrophage-Derived Extracellular Vesicle Promotes Hair Growth. Cells. 2020; 9(4):856. https://doi.org/10.3390/cells9040856
Chicago/Turabian StyleRajendran, Ramya Lakshmi, Prakash Gangadaran, Chang Hoon Seo, Mi Hee Kwack, Ji Min Oh, Ho Won Lee, Arunnehru Gopal, Young Kwan Sung, Shin Young Jeong, Sang-Woo Lee, and et al. 2020. "Macrophage-Derived Extracellular Vesicle Promotes Hair Growth" Cells 9, no. 4: 856. https://doi.org/10.3390/cells9040856
APA StyleRajendran, R. L., Gangadaran, P., Seo, C. H., Kwack, M. H., Oh, J. M., Lee, H. W., Gopal, A., Sung, Y. K., Jeong, S. Y., Lee, S.-W., Lee, J., & Ahn, B.-C. (2020). Macrophage-Derived Extracellular Vesicle Promotes Hair Growth. Cells, 9(4), 856. https://doi.org/10.3390/cells9040856







