Acid Sphingomyelinase Downregulation Enhances Mitochondrial Fusion and Promotes Oxidative Metabolism in a Mouse Model of Melanoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Models
2.2. Animal Handling and Allograft Tumour Model
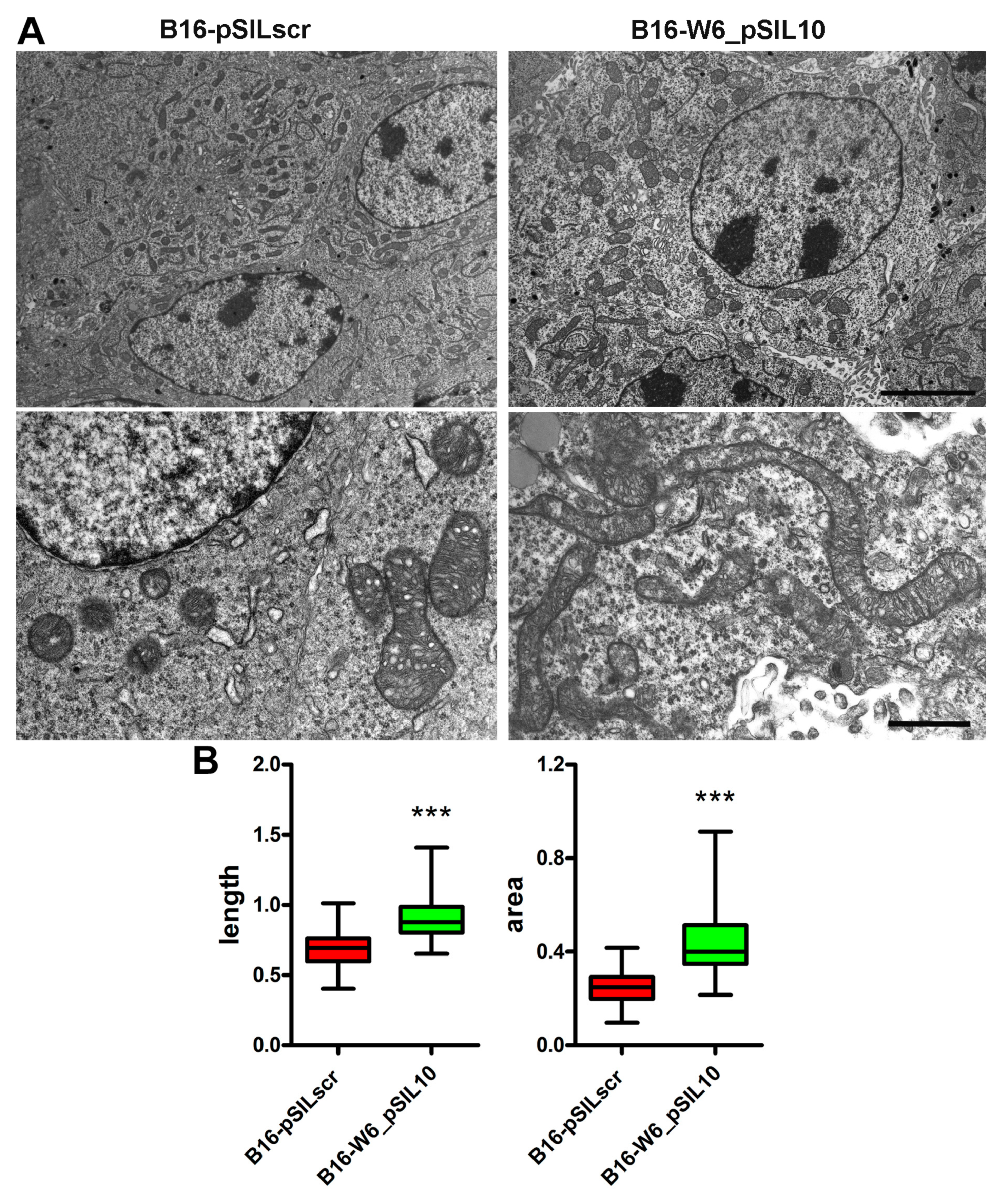
2.3. Transmission Electron Microscopy
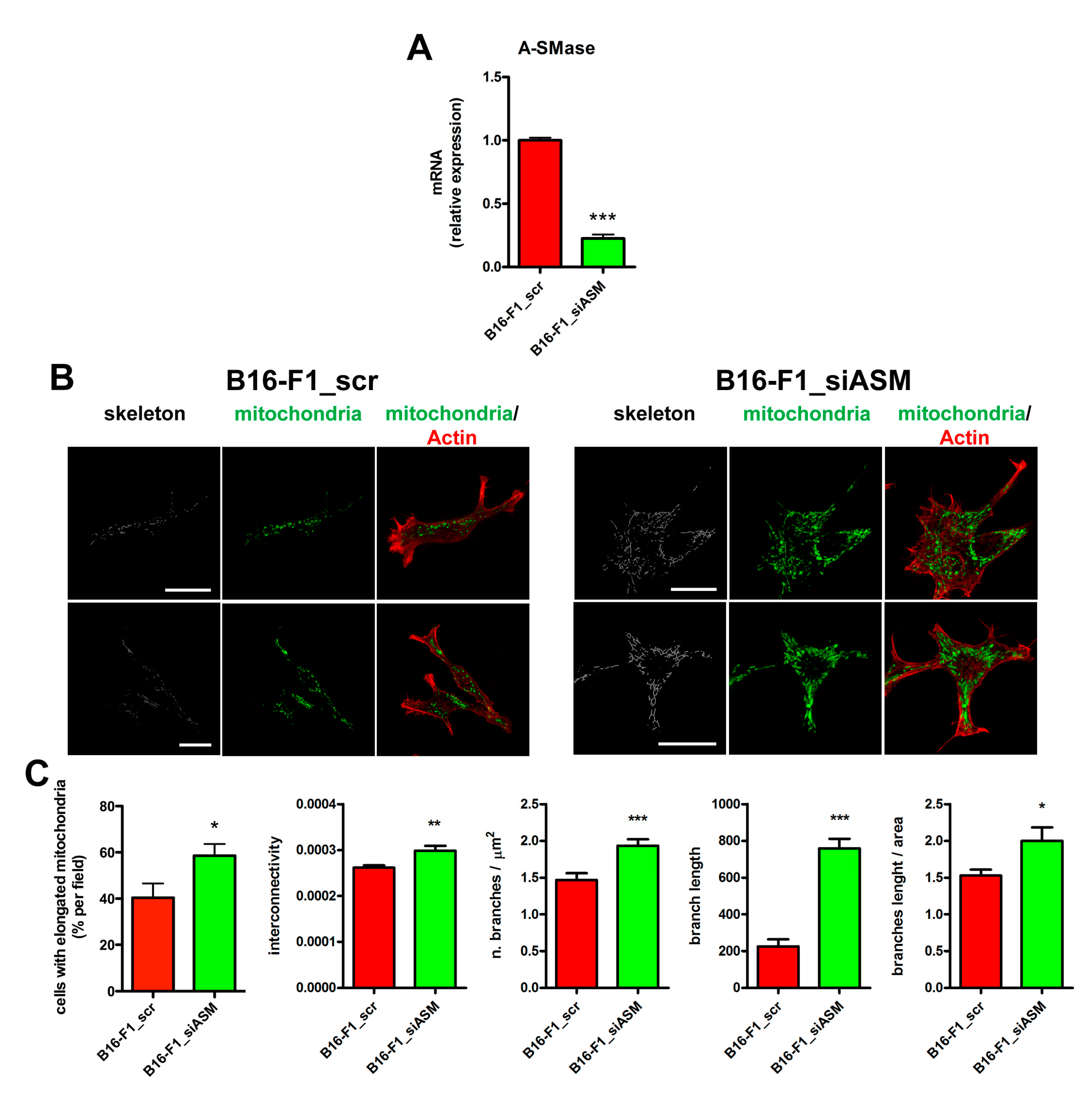
2.4. Immunofluorescence and Mitochondria Morphometric Analysis
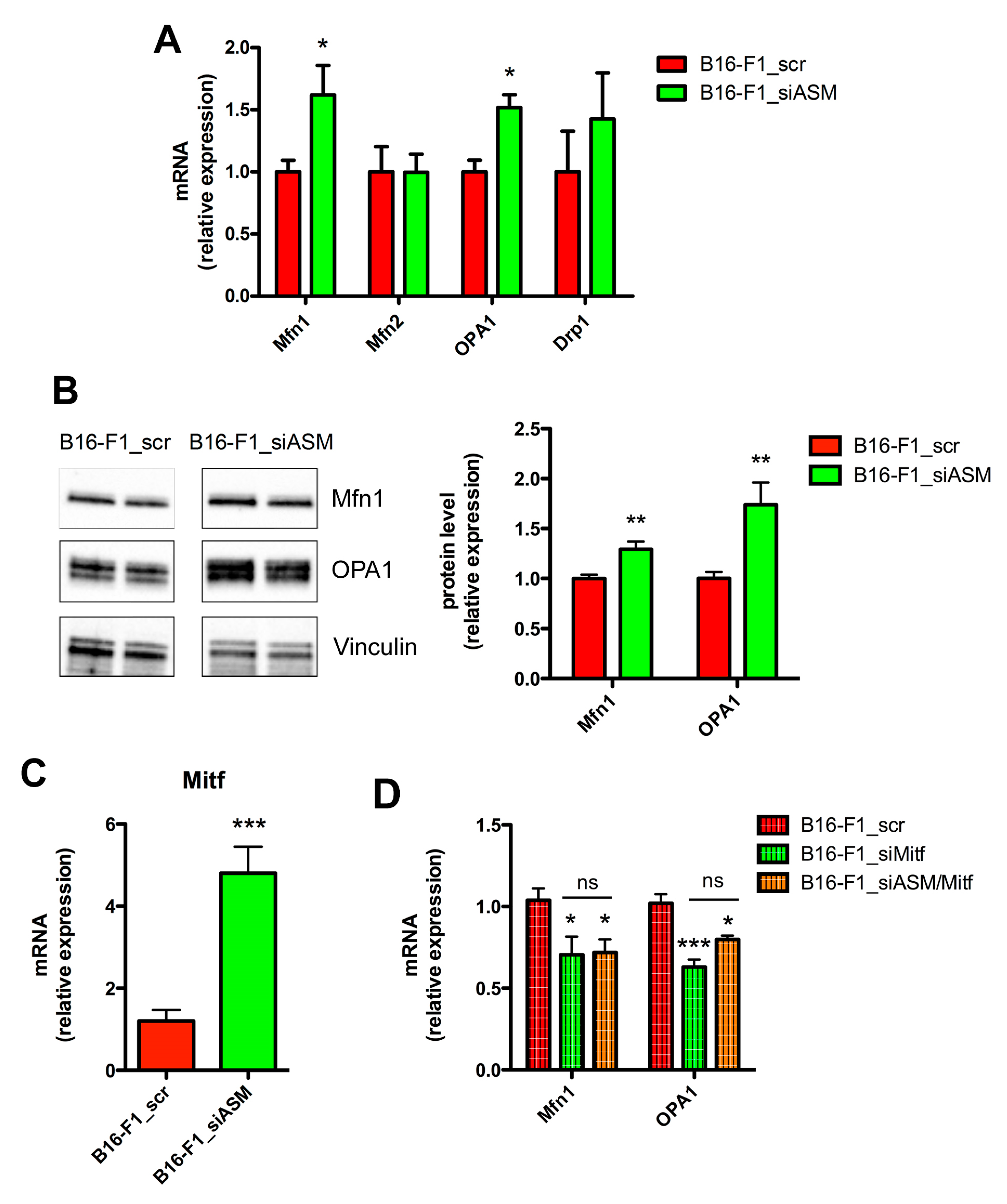
2.5. Quantitative Real Time-PCR (qPCR)
2.6. Protein Isolation and Western Blotting
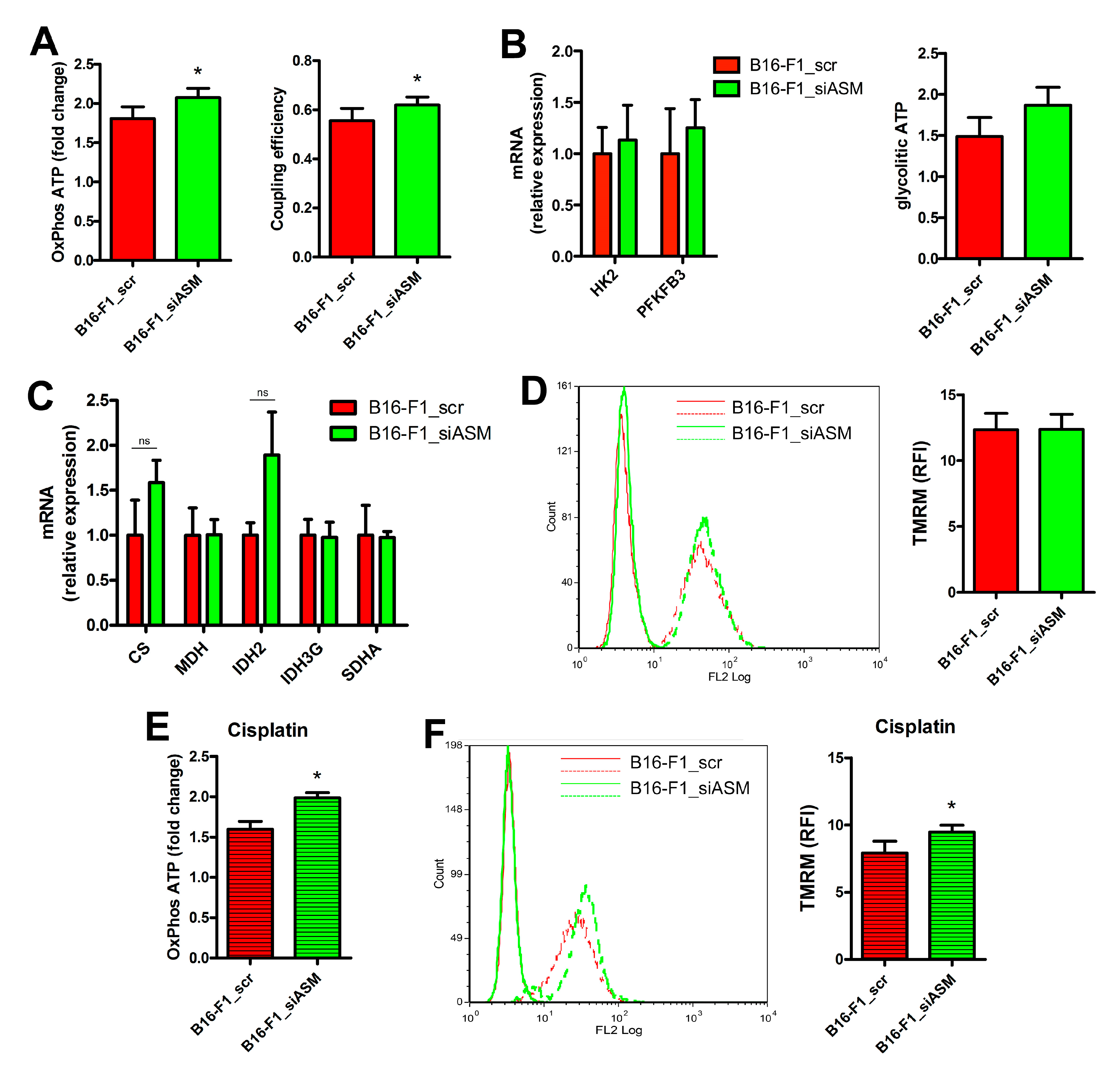
2.7. Mitochondria Respiratory Rate
2.8. ATP Production
2.9. Mitochondrial Membrane Potential Analysis
2.10. Statistical Analysis
3. Results
3.1. A-SMase Expression Determines Mitochondrial Morphology
3.2. A-SMase Expression Regulates Mitochondrial Elongation through Mfn1 and OPA1
3.3. A-SMase Downregulation Improves Mitochondrial Function
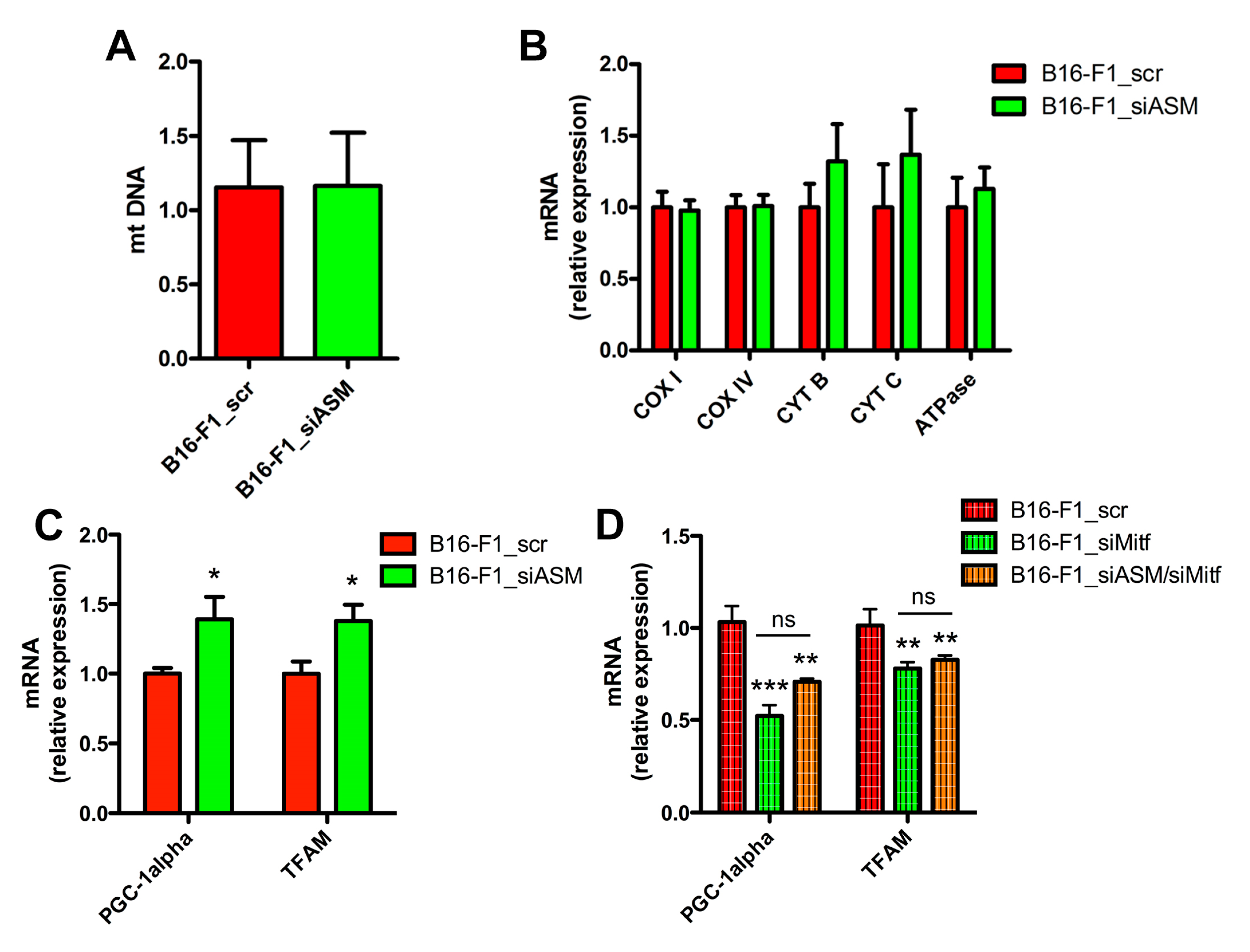
3.4. A-SMase Downregulation Increases Mitochondrial Biogenesis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xu, X.D.; Shao, S.X.; Jiang, H.P.; Cao, Y.W.; Wang, Y.H.; Yang, X.C.; Wang, Y.L.; Wang, X.S.; Niu, H.T. Warburg Effect or Reverse Warburg Effect? A Review of Cancer Metabolism. Oncol. Res. Treat. 2015, 38, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.F.; Obre, E.; de Melo, F.H.M.; Santos, G.C.; Galina, A.; Jasiulionis, M.G.; Rossignol, R.; Rumjanek, F.D.; Amoêdo, N.D. Enhanced OXPHOS, glutaminolysis and β-oxidation constitute the metastatic phenotype of melanoma cells. Biochem. J. 2016, 473, 703–715. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.A.; Richardson, A.D.; Filipp, F.V.; Knutzen, C.A.; Chiang, G.G.; Ronai, Z.A.; Osterman, A.L.; Smith, J.W. Comparative metabolic flux profiling of melanoma cell lines: Beyond the Warburg effect. J. Biol. Chem. 2011, 286, 42626–42634. [Google Scholar] [CrossRef] [PubMed]
- Ruocco, M.R.; Avagliano, A.; Granato, G.; Vigliar, E.; Masone, S.; Montagnani, S.; Arcucci, A. Metabolic flexibility in melanoma: A potential therapeutic target. Semin. Cancer Biol. 2019, 59, 187–207. [Google Scholar] [CrossRef] [PubMed]
- Fischer, G.M.; Vashisht Gopal, Y.N.; McQuade, J.L.; Peng, W.; DeBerardinis, R.J.; Davies, M.A. Metabolic strategies of melanoma cells: Mechanisms, interactions with the tumor microenvironment, and therapeutic implications. Pigment Cell Melanoma Res. 2018, 31, 11–30. [Google Scholar] [CrossRef]
- Gopal, Y.N.V.; Rizos, H.; Chen, G.; Deng, W.; Frederick, D.T.; Cooper, Z.A.; Scolyer, R.A.; Pupo, G.; Komurov, K.; Sehgal, V.; et al. Inhibition of mTORC1/2 Overcomes Resistance to MAPK Pathway Inhibitors Mediated by PGC1 and Oxidative Phosphorylation in Melanoma. Cancer Res. 2014, 74, 7037–7047. [Google Scholar] [CrossRef]
- Vazquez, F.; Lim, J.-H.; Chim, H.; Bhalla, K.; Girnun, G.; Pierce, K.; Clish, C.B.; Granter, S.R.; Widlund, H.R.; Spiegelman, B.M.; et al. PGC1α Expression Defines a Subset of Human Melanoma Tumors with Increased Mitochondrial Capacity and Resistance to Oxidative Stress. Cancer Cell 2013, 23, 287–301. [Google Scholar] [CrossRef]
- Haq, R.; Shoag, J.; Andreu-Perez, P.; Yokoyama, S.; Edelman, H.; Rowe, G.C.; Frederick, D.T.; Hurley, A.D.; Nellore, A.; Kung, A.L.; et al. Oncogenic BRAF Regulates Oxidative Metabolism via PGC1α and MITF. Cancer Cell 2013, 23, 302–315. [Google Scholar] [CrossRef]
- LeBleu, V.S.; O’Connell, J.T.; Gonzalez Herrera, K.N.; Wikman, H.; Pantel, K.; Haigis, M.C.; de Carvalho, F.M.; Damascena, A.; Domingos Chinen, L.T.; Rocha, R.M.; et al. PGC-1α mediates mitochondrial biogenesis and oxidative phosphorylation in cancer cells to promote metastasis. Nat. Cell Biol. 2014, 16, 992–1003. [Google Scholar] [CrossRef]
- Barbi de Moura, M.; Vincent, G.; Fayewicz, S.L.; Bateman, N.W.; Hood, B.L.; Sun, M.; Suhan, J.; Duensing, S.; Yin, Y.; Sander, C.; et al. Mitochondrial Respiration - An Important Therapeutic Target in Melanoma. PLoS ONE 2012, 7, e40690. [Google Scholar] [CrossRef]
- Ho, J.; de Moura, M.; Lin, Y.; Vincent, G.; Thorne, S.; Duncan, L.M.; Hui-Min, L.; Kirkwood, J.M.; Becker, D.; Van Houten, B.; et al. Importance of glycolysis and oxidative phosphorylation in advanced melanoma. Mol. Cancer 2012, 11, 76. [Google Scholar] [CrossRef] [PubMed]
- Jia, D.; Park, J.; Jung, K.; Levine, H.; Kaipparettu, B. Elucidating the Metabolic Plasticity of Cancer: Mitochondrial Reprogramming and Hybrid Metabolic States. Cells 2018, 7, 21. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Frederick, D.T.; Wu, L.; Wei, Z.; Krepler, C.; Srinivasan, S.; Chae, Y.C.; Xu, X.; Choi, H.; Dimwamwa, E.; et al. Targeting mitochondrial biogenesis to overcome drug resistance to MAPK inhibitors. J. Clin. Investig. 2016, 126, 1834–1856. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.C. Fusion and Fission: Interlinked Processes Critical for Mitochondrial Health. Annu. Rev. Genet. 2012, 46, 265–287. [Google Scholar] [CrossRef]
- Archer, S.L. Mitochondrial Dynamics—Mitochondrial Fission and Fusion in Human Diseases. N. Engl. J. Med. 2013, 369, 2236–2251. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.; Chan, D.C. Metabolic regulation of mitochondrial dynamics. J. Cell Biol. 2016, 212, 379–387. [Google Scholar] [CrossRef]
- Senft, D.; Ronai, Z.A. Regulators of mitochondrial dynamics in cancer. Curr. Opin. Cell Biol. 2016, 39, 43–52. [Google Scholar] [CrossRef]
- Dai, W.; Jiang, L. Dysregulated Mitochondrial Dynamics and Metabolism in Obesity, Diabetes, and Cancer. Front. Endocrinol. (Lausanne). 2019, 10, 570. [Google Scholar] [CrossRef]
- Dal Yontem, F.; Kim, S.; Ding, Z.; Grimm, E.; Ekmekcioglu, S.; Akcakaya, H. Mitochondrial dynamic alterations regulate melanoma cell progression. J. Cell. Biochem. 2019, 120, 2098–2108. [Google Scholar] [CrossRef]
- Hosseini, M.; Kasraian, Z.; Rezvani, H.R. Energy metabolism in skin cancers: A therapeutic perspective. Biochim. Biophys. Acta - Bioenerg. 2017, 1858, 712–722. [Google Scholar] [CrossRef]
- Trotta, A.P.; Gelles, J.D.; Serasinghe, M.N.; Loi, P.; Arbiser, J.L.; Chipuk, J.E. Disruption of mitochondrial electron transport chain function potentiates the pro-apoptotic effects of MAPK inhibition. J. Biol. Chem. 2017, 292, 11727–11739. [Google Scholar] [CrossRef] [PubMed]
- Serasinghe, M.N.; Wieder, S.Y.; Renault, T.T.; Elkholi, R.; Asciolla, J.J.; Yao, J.L.; Jabado, O.; Hoehn, K.; Kageyama, Y.; Sesaki, H.; et al. Mitochondrial Division Is Requisite to RAS-Induced Transformation and Targeted by Oncogenic MAPK Pathway Inhibitors. Mol. Cell 2015, 57, 521–536. [Google Scholar] [CrossRef] [PubMed]
- van der Bliek, A.M.; Shen, Q.; Kawajiri, S. Mechanisms of Mitochondrial Fission and Fusion. Cold Spring Harb. Perspect. Biol. 2013, 5, a011072. [Google Scholar] [PubMed]
- Garandeau, D.; Mrad, M.; Levade, T.; Perrotta, C.; Andrieu-Abadie, N.; Diab-Assaf, M. Dysregulation of sphingolipid metabolism in melanoma: Roles in pigmentation, cell survival and tumor progression. In Chemical Probes of Sphingolipid Metabolizing Enzymes; Nieves, I., Sanllehí, P., Abad, J., Fabrias, G., Casas, J., Delgado, A., Fabrias, G., Eds.; Springer International Publishing: Basel, Switzerland, 2015. [Google Scholar]
- Dany, M. Sphingosine metabolism as a therapeutic target in cutaneous melanoma. Transl. Res. 2017, 185, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mullen, T.D.; Obeid, L.M. Ceramide and Apoptosis: Exploring the Enigmatic Connections between Sphingolipid Metabolism and Programmed Cell Death. Anticancer. Agents Med. Chem. 2012, 12, 340–363. [Google Scholar] [CrossRef]
- Garandeau, D.; Noujarede, J.; Leclerc, J.; Imbert, C.; Garcia, V.; Bats, M.L.; Rambow, F.; Gilhodes, J.; Filleron, T.; Meyer, N.; et al. Targeting the sphingosine 1-phosphate axis exerts potent antitumor activity in BRAFI-resistant melanomas. Mol. Cancer Ther. 2019, 18, 289–300. [Google Scholar] [CrossRef]
- Sorli, S.C.; Colié, S.; Albinet, V.; Dubrac, A.; Touriol, C.; Guilbaud, N.; Bedia, C.; Fabrias, G.; Casas, J.; Ségui, B.; et al. The nonlysosomal β-glucosidase GBA2 promotes endoplasmic reticulum stress and impairs tumorigenicity of human melanoma cells. FASEB J. 2013, 27, 489–498. [Google Scholar] [CrossRef]
- Cervia, D.; Assi, E.; De Palma, C.; Giovarelli, M.; Bizzozero, L.; Pambianco, S.; Di Renzo, I.; Zecchini, S.; Moscheni, C.; Vantaggiato, C.; et al. Essential role for acid sphingomyelinase-inhibited autophagy in melanoma response to cisplatin. Oncotarget 2016, 7, 24995–25009. [Google Scholar] [CrossRef]
- Assi, E.; Cervia, D.; Bizzozero, L.; Capobianco, A.; Pambianco, S.; Morisi, F.; De Palma, C.; Moscheni, C.; Pellegrino, P.; Clementi, E.; et al. Modulation of Acid Sphingomyelinase in Melanoma Reprogrammes the Tumour Immune Microenvironment. Mediators Inflamm. 2015, 2015, 370482. [Google Scholar] [CrossRef]
- Bizzozero, L.; Cazzato, D.; Cervia, D.; Assi, E.; Simbari, F.; Pagni, F.; De Palma, C.; Monno, A.; Verdelli, C.; Querini, P.R.; et al. Acid sphingomyelinase determines melanoma progression and metastatic behaviour via the microphtalmia-associated transcription factor signalling pathway. Cell Death Differ. 2014, 21, 507–520. [Google Scholar] [CrossRef]
- Matsumoto, A.; Comatas, K.E.; Liu, L.; Stamler, J.S. Screening for nitric oxide - Dependent protein-protein interactions. Science 2003, 301, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Perrotta, C.; Buonanno, F.; Zecchini, S.; Giavazzi, A.; Proietti Serafini, F.; Catalani, E.; Guerra, L.; Belardinelli, M.C.; Picchietti, S.; Fausto, A.M.; et al. Climacostol reduces tumour progression in a mouse model of melanoma via the p53-dependent intrinsic apoptotic programme. Sci. Rep. 2016, 6, 27281. [Google Scholar] [CrossRef]
- Perrotta, C.; Bizzozero, L.; Falcone, S.; Rovere-Querini, P.; Prinetti, A.; Schuchman, E.H.; Sonnino, S.; Manfredi, A.A.; Clementi, E. Nitric oxide boosts chemoimmunotherapy via inhibition of acid sphingomyelinase in a mouse model of melanoma. Cancer Res. 2007, 67, 7559–7564. [Google Scholar] [CrossRef] [PubMed]
- Perrotta, C.; Cervia, D.; Di Renzo, I.; Moscheni, C.; Bassi, M.T.; Campana, L.; Martelli, C.; Catalani, E.; Giovarelli, M.; Zecchini, S.; et al. Nitric Oxide Generated by Tumor-Associated Macrophages Is Responsible for Cancer Resistance to Cisplatin and Correlated With Syntaxin 4 and Acid Sphingomyelinase Inhibition. Front. Immunol. 2018, 9, 1186. [Google Scholar] [CrossRef] [PubMed]
- Catalani, E.; Proietti Serafini, F.; Zecchini, S.; Picchietti, S.; Fausto, A.M.; Marcantoni, E.; Buonanno, F.; Ortenzi, C.; Perrotta, C.; Cervia, D. Natural products from aquatic eukaryotic microorganisms for cancer therapy: Perspectives on anti-tumour properties of ciliate bioactive molecules. Pharmacol. Res. 2016, 113, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Vantaggiato, C.; Castelli, M.; Giovarelli, M.; Orso, G.; Bassi, M.T.; Clementi, E.; De Palma, C. The Fine Tuning of Drp1-Dependent Mitochondrial Remodeling and Autophagy Controls Neuronal Differentiation. Front. Cell. Neurosci. 2019, 13, 120. [Google Scholar] [CrossRef]
- Wiemerslage, L.; Lee, D. Quantification of mitochondrial morphology in neurites of dopaminergic neurons using multiple parameters. J. Neurosci. Methods 2016, 262, 56–65. [Google Scholar] [CrossRef]
- Cazzato, D.; Assi, E.; Moscheni, C.; Brunelli, S.; De Palma, C.; Cervia, D.; Perrotta, C.; Clementi, E. Nitric oxide drives embryonic myogenesis in chicken through the upregulation of myogenic differentiation factors. Exp. Cell Res. 2014, 320, 269–280. [Google Scholar] [CrossRef]
- Lauria, S.; Perrotta, C.; Casati, S.; Di Renzo, I.; Ottria, R.; Eberini, I.; Palazzolo, L.; Parravicini, C.; Ciuffreda, P. Design, synthesis, molecular modelling and in vitro cytotoxicity analysis of novel carbamate derivatives as inhibitors of Monoacylglycerol lipase. Bioorganic Med. Chem. 2018, 26, 2561–2572. [Google Scholar] [CrossRef]
- De Palma, C.; Morisi, F.; Pambianco, S.; Assi, E.; Touvier, T.; Russo, S.; Perrotta, C.; Romanello, V.; Carnio, S.; Cappello, V.; et al. Deficient nitric oxide signalling impairs skeletal muscle growth and performance: Involvement of mitochondrial dysregulation. Skelet. Muscle 2014, 4, 22. [Google Scholar] [CrossRef]
- Pambianco, S.; Giovarelli, M.; Perrotta, C.; Zecchini, S.; Cervia, D.; Di Renzo, I.; Moscheni, C.; Ripolone, M.; Violano, R.; Moggio, M.; et al. Reversal of Defective Mitochondrial Biogenesis in Limb-Girdle Muscular Dystrophy 2D by Independent Modulation of Histone and PGC-1α Acetylation. Cell Rep. 2016, 17, 3010–3023. [Google Scholar] [CrossRef] [PubMed]
- Zecchini, S.; Giovarelli, M.; Perrotta, C.; Morisi, F.; Touvier, T.; Di Renzo, I.; Moscheni, C.; Bassi, M.T.; Cervia, D.; Sandri, M.; et al. Autophagy controls neonatal myogenesis by regulating the GH-IGF1 system through a NFE2L2- and DDIT3-mediated mechanism. Autophagy 2019, 15, 58–77. [Google Scholar] [CrossRef] [PubMed]
- Catalani, E.; Buonanno, F.; Lupidi, G.; Bongiorni, S.; Belardi, R.; Zecchini, S.; Giovarelli, M.; Coazzoli, M.; De Palma, C.; Perrotta, C.; et al. The natural compound climacostol as a prodrug strategy based on pH activation for efficient delivery of cytotoxic small agents. Front. Chem. 2019, 7, 463. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, D.; Brucoli, M.; Zecchini, S.; Sandoval-Hernandez, A.; Arboleda, G.; Lopez-Vallejo, F.; Delgado, W.; Giovarelli, M.; Coazzoli, M.; Catalani, E.; et al. XIAP as a target of new small organic natural molecules inducing human cancer cell death. Cancers 2019, 11, 1336. [Google Scholar] [CrossRef] [PubMed]
- Amato, R.; Catalani, E.; Dal Monte, M.; Cammalleri, M.; Di Renzo, I.; Perrotta, C.; Cervia, D.; Casini, G. Autophagy-mediated neuroprotection induced by octreotide in an ex vivo model of early diabetic retinopathy. Pharmacol. Res. 2018, 128, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Moscheni, C.; Malucelli, E.; Castiglioni, S.; Procopio, A.; De Palma, C.; Sorrentino, A.; Sartori, P.; Locatelli, L.; Pereiro, E.; Maier, J.A.; et al. 3D Quantitative and Ultrastructural Analysis of Mitochondria in a Model of Doxorubicin Sensitive and Resistant Human Colon Carcinoma Cells. Cancers 2019, 11, 1254. [Google Scholar] [CrossRef]
- Perrotta, C.; Bizzozero, L.; Cazzato, D.; Morlacchi, S.; Assi, E.; Simbari, F.; Zhang, Y.; Gulbins, E.; Bassi, M.T.; Rosa, P.; et al. Syntaxin 4 is required for acid sphingomyelinase activity and apoptotic function. J. Biol. Chem. 2010, 285, 40240–40251. [Google Scholar] [CrossRef]
- Cottone, L.; Capobianco, A.; Gualteroni, C.; Perrotta, C.; Bianchi, M.E.; Rovere-Querini, P.; Manfredi, A.A. 5-Fluorouracil causes leukocytes attraction in the peritoneal cavity by activating autophagy and HMGB1 release in colon carcinoma cells. Int. J. Cancer 2015, 136, 1381–1389. [Google Scholar] [CrossRef]
- Friedman, J.R.; Nunnari, J. Mitochondrial form and function. Nature 2014, 505, 335–343. [Google Scholar] [CrossRef]
- Madeira, V.M.C. Overview of Mitochondrial Bioenergetics. In Methods in molecular biology (Clifton, N.J.); Humana Press, Inc.: Totowa, NJ, USA, 2012; Volume 810, pp. 1–6. [Google Scholar]
- Bost, F.; Kaminski, L. The metabolic modulator PGC-1α in cancer. Am. J. Cancer Res. 2019, 9, 198–211. [Google Scholar]
- Wu, L.W.; Zhang, G.; Herlyn, M. Mitochondrial biogenesis meets chemoresistance in BRAF-mutant melanoma. Mol. Cell. Oncol. 2016, 3, e1179381. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cruz-Bermúdez, A.; Laza-Briviesca, R.; Vicente-Blanco, R.J.; García-Grande, A.; Coronado, M.J.; Laine-Menéndez, S.; Palacios-Zambrano, S.; Moreno-Villa, M.R.; Ruiz-Valdepeñas, A.M.; Lendinez, C.; et al. Cisplatin resistance involves a metabolic reprogramming through ROS and PGC-1α in NSCLC which can be overcome by OXPHOS inhibition. Free Radic. Biol. Med. 2019, 135, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Deschner, B.; Wayne, J.D. Follow-up of the melanoma patient. J. Surg. Oncol. 2019, 119, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Bilal, F.; Montfort, A.; Gilhodes, J.; Garcia, V.; Riond, J.; Carpentier, S.; Filleron, T.; Colacios, C.; Levade, T.; Daher, A.; et al. Sphingomyelin synthase 1 (SMS1) downregulation is associated with sphingolipid reprogramming and a worse prognosis in melanoma. Front. Pharmacol. 2019, 10, 443. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.; La Rocca, V.; Amato, R.; Freer, G.; Pistello, M. Sphingolipid/Ceramide Pathways and Autophagy in the Onset and Progression of Melanoma: Novel Therapeutic Targets and Opportunities. Int. J. Mol. Sci. 2019, 20, 3436. [Google Scholar] [CrossRef]
- Realini, N.; Palese, F.; Pizzirani, D.; Pontis, S.; Basit, A.; Bach, A.; Ganesan, A.; Piomelli, D. Acid ceramidase in melanoma: Expression, localization, and effects of pharmacological inhibition. J. Biol. Chem. 2016, 291, 2422–2434. [Google Scholar] [CrossRef]
- Carpinteiro, A.; Becker, K.A.; Japtok, L.; Hessler, G.; Keitsch, S.; Požgajovà, M.; Schmid, K.W.; Adams, C.; Müller, S.; Kleuser, B.; et al. Regulation of hematogenous tumor metastasis by acid sphingomyelinase. EMBO Mol. Med. 2015, 7, 714–734. [Google Scholar] [CrossRef]
- Becker, K.A.; Beckmann, N.; Adams, C.; Hessler, G.; Kramer, M.; Gulbins, E.; Carpinteiro, A. Melanoma cell metastasis via P-selectin-mediated activation of acid sphingomyelinase in platelets. Clin. Exp. Metastasis 2017, 34, 25–35. [Google Scholar] [CrossRef]
- Ghosh, S.; Jawed, J.J.; Halder, K.; Banerjee, S.; Chowdhury, B.P.; Saha, A.; Juin, S.K.; Majumdar, S.B.; Bose, A.; Baral, R.; et al. TNFα mediated ceramide generation triggers cisplatin induced apoptosis in B16F10 melanoma in a PKCδ independent manner. Oncotarget 2018, 9, 37627–37646. [Google Scholar]
- Stancevic, B.; Varda-Bloom, N.; Cheng, J.; Fuller, J.D.; Rotolo, J.A.; García-Barros, M.; Feldman, R.; Rao, S.; Weichselbaum, R.R.; Harats, D.; et al. Adenoviral Transduction of Human Acid Sphingomyelinase into Neo-Angiogenic Endothelium Radiosensitizes Tumor Cure. PLoS ONE 2013, 8, e69025. [Google Scholar] [CrossRef]
- Smith, E.L.; Schuchman, E.H. Acid sphingomyelinase overexpression enhances the antineoplastic effects of irradiation in vitro and in vivo. Mol. Ther. 2008, 16, 1565–1571. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.; Li, R.; Liu, J.; Zhang, Y.; Zhang, X.; Jin, B.; Liu, Y.; Wang, Z.; Zhong, H.; Wen, S.; et al. Mitofusin-2 over-expresses and leads to dysregulation of cell cycle and cell invasion in lung adenocarcinoma. Med. Oncol. 2015, 32, 132. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Tian, C.; Puszyk, W.M.; Ogunwobi, O.O.; Cao, M.; Wang, T.; Cabrera, R.; Nelson, D.R.; Liu, C. OPA1 downregulation is involved in sorafenib-induced apoptosis in hepatocellular carcinoma. Lab. Investig. 2013, 93, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Youle, R.J.; Karbowski, M. Mitochondrial fission in apoptosis. Nat. Rev. Mol. Cell Biol. 2005, 6, 657–663. [Google Scholar] [CrossRef]
- Tang, Q.; Liu, W.; Zhang, Q.; Huang, J.; Hu, C.; Liu, Y.; Wang, Q.; Zhou, M.; Lai, W.; Sheng, F.; et al. Dynamin-related protein 1-mediated mitochondrial fission contributes to IR -783-induced apoptosis in human breast cancer cells. J. Cell. Mol. Med. 2018, 22, 4474–4485. [Google Scholar] [CrossRef]
- Grandemange, S.; Herzig, S.; Martinou, J.-C. Mitochondrial dynamics and cancer. Semin. Cancer Biol. 2009, 19, 50–56. [Google Scholar] [CrossRef]
- Perrotta, C.; Cervia, D.; De Palma, C.; Assi, E.; Pellegrino, P.; Bassi, M.T.; Clementi, E. The emerging role of Acid Sphingomyelinase in autophagy. Apoptosis 2015, 20, 635–644. [Google Scholar] [CrossRef]
- Dreos, R.; Ambrosini, G.; Périer, R.C.; Bucher, P. The Eukaryotic Promoter Database: Expansion of EPDnew and new promoter analysis tools. Nucleic Acids Res. 2015, 43, D92–D96. [Google Scholar] [CrossRef]
- Vachtenheim, J. The Many Roles of MITF in Melanoma. Single Cell Biol. 2017, 6, 1–4. [Google Scholar] [CrossRef]
- Kawakami, A.; Fisher, D.E. The master role of microphthalmia-associated transcription factor in melanocyte and melanoma biology. Lab. Investig. 2017, 97, 649–656. [Google Scholar] [CrossRef]
- Araujo, L.F.; Siena, A.D.D.; Plaça, J.R.; Brotto, D.B.; Barros, I.I.; Muys, B.R.; Biagi, C.A.O.; Peronni, K.C.; Sousa, J.F.; Molfetta, G.A.; et al. Mitochondrial transcription factor A (TFAM) shapes metabolic and invasion gene signatures in melanoma. Sci. Rep. 2018, 8, 14190. [Google Scholar] [CrossRef] [PubMed]
| Gene Accession Number | Primer Sequence | Amplicon | |
|---|---|---|---|
| A-SMase (smpd1) | NM_011421 | F: 5′-TGGGACTCCTTTGGATGGG-3′ R: 5′-CGGCGCTATGGCACTGAAT-3′ | 134 bp |
| Mfn1 | NM_024200 | F: 5′-CCTACTGCTCCTTCTAACCCA-3′ R: 5′-AGGGACGCCAATCCTGTGA-3′ | 86 bp |
| Mfn2 | NM_133201 | F: 5′-AGAACTGGACCCGGTTACCA-3′ R: 5′-CACTTCGCTGATACCCCTGA-3′ | 82 bp |
| OPA1 | NM_133752 | F: 5′-TGGAAAATGGTTCGAGAGTCAG-3′ R: 5′-CATTCCGTCTCTAGGTTAAAGCG-3′ | 76 bp |
| Drp1 | NM_152816 | F: 5′-GCTGGATCACGGGACAAGTTAA-3′ R: 5′-TGCCTGTTGTTGGTTCCTGAC-3′ | 106 bp |
| Mitf | NM_001113198 NM_008601 NM_001178049 | F: 5′-CCAACAGCCCTATGGCTATGC-3′ R: 5′-CTGGGCACTCACTCTCTGC-3′ | 99 bp |
| HK2 | NM_013820 | F: 5′-TGATCGCCTGCTTATTCACGG-3′ R: 5′-AACCGCCTAGAAATCTCCAGA-3′ | 112 bp |
| PFKFB3 | NM_001177752 | F: 5′-CCCAGAGCCGGGTACAGAA-3′ R: 5′-GGGGAGTTGGTCAGCTTCG-3′ | 88 bp |
| CS | NM_026444 | F: 5′-GGACAATTTTCCAACCAATCTGC-3′ R: 5′-TCGGTTCATTCCCTCTGCATA-3′ | 109 bp |
| MDH | NM_008617 | F: 5′-TTGGGCAACCCCTTTCACTC-3′ R: 5′-GCCTTTCACATTTGCTCTGGTC-3′ | 131 bp |
| IDH2 | NM_173011 | F: 5′-GGAGAAGCCGGTAGTGGAGAT-3′ R: 5′-GGTCTGGTCACGGTTTGGAA-3′ | 139 bp |
| IDH3G | NM_008323 | F: 5′-GGTGCTGCAAAGGCAATGC-3′ R: 5′-TATGCCGCCCACCATACTTAG-3′ | 136 bp |
| SDHA | NM_023281 | F: 5′-GGAACACTCCAAAAACAGACCT-3′ R: 5′-CCACCACTGGGTATTGAGTAGAA-3′ | 106 bp |
| COX I | NC_005089.1 | F: 5′-CCAGTGCTAGCCGCAGGCAT-3′ R: 5′-GCTGGTAGAGAATTGGGTCCCCTCC-3′ | 100 bp |
| COX IV | NM_009941 | F: 5′-TACTTCGGTGTGCCTTCGA-3′ R: 5′-TTAGCATGGACCATTGGATACGG-3′ | 110 bp |
| CYT B | NC_005089.1 | F: 5′-ACGCCATTCTACGCTCAATC -3′ R: 5′-GCTTCGTTGCTTTGAGGTAT-3′ | 110 bp |
| CYT C | NM_007808 | F: 5′-ATAGGGGCATGTCACCTCAAAC-3′ R: 5′-GTGGTTAGCCATGACCTGAAAG-3′ | 172 bp |
| ATPase | NM_016774 | F: 5′-CGTGAGGGCAATGATTTATACCAT-3′ R: 5′-TCCTGGTCTCTGAAGTATTCAGCAA-3′ | 170 bp |
| mtDNA | NC_005089 | F: 5′-CCTATCACCCTTGCCATCAT-3′ R: 5′-GAGGCTGTTGCTTGTGTGAC-3′ | 194 bp |
| RNase P (DNA) | NC_000085 | F: 5′-GAAGGCTCTGCGCGGACTCG-3′ R: 5′-CGAGAGACCGGAATGGGGCCT-3′ | 119 bp |
| PGC-1alpha | NM_008904 | F: 5′-ACTATGAATCAAGCCACTACAGAC-3′ R: 5′-TTCATCCCTCTTGAGCCTTTCG-3′ | 143 bp |
| TFAM | NM_009360 | F: 5′-AAGACCTCGTTCAGCATATAACATT-3′ R: 5′-TTTTCCAAGCCTCATTTACAAGC-3′ | 104 bp |
| 36b4 | NM_007475 | F: 5′-AGGATATGGGATTCGGTCTCTTC-3′ R: 5′-TCATCCTGCTTAAGTGAACAAACT-3′ | 143 bp |
| RPL32 | NM_172086 | F: 5′-TTAAGCGAAACTGGCGGAAAC-3′ R: 5′-TTGTTGCTCCCATAACCGATG-3′ | 100 bp |
| Actin beta | NM_007393 | F: 5′-GGCTGTATTCCCCTCCATCG-3′ R: 5′-CCAGTTGGTAACAATGCCATGT-3′ | 154 bp |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coazzoli, M.; Napoli, A.; Roux-Biejat, P.; De Palma, C.; Moscheni, C.; Catalani, E.; Zecchini, S.; Conte, V.; Giovarelli, M.; Caccia, S.; et al. Acid Sphingomyelinase Downregulation Enhances Mitochondrial Fusion and Promotes Oxidative Metabolism in a Mouse Model of Melanoma. Cells 2020, 9, 848. https://doi.org/10.3390/cells9040848
Coazzoli M, Napoli A, Roux-Biejat P, De Palma C, Moscheni C, Catalani E, Zecchini S, Conte V, Giovarelli M, Caccia S, et al. Acid Sphingomyelinase Downregulation Enhances Mitochondrial Fusion and Promotes Oxidative Metabolism in a Mouse Model of Melanoma. Cells. 2020; 9(4):848. https://doi.org/10.3390/cells9040848
Chicago/Turabian StyleCoazzoli, Marco, Alessandra Napoli, Paulina Roux-Biejat, Clara De Palma, Claudia Moscheni, Elisabetta Catalani, Silvia Zecchini, Vincenzo Conte, Matteo Giovarelli, Sonia Caccia, and et al. 2020. "Acid Sphingomyelinase Downregulation Enhances Mitochondrial Fusion and Promotes Oxidative Metabolism in a Mouse Model of Melanoma" Cells 9, no. 4: 848. https://doi.org/10.3390/cells9040848
APA StyleCoazzoli, M., Napoli, A., Roux-Biejat, P., De Palma, C., Moscheni, C., Catalani, E., Zecchini, S., Conte, V., Giovarelli, M., Caccia, S., Procacci, P., Cervia, D., Clementi, E., & Perrotta, C. (2020). Acid Sphingomyelinase Downregulation Enhances Mitochondrial Fusion and Promotes Oxidative Metabolism in a Mouse Model of Melanoma. Cells, 9(4), 848. https://doi.org/10.3390/cells9040848









