AR/ER Ratio Correlates with Expression of Proliferation Markers and with Distinct Subset of Breast Tumors
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Immunohistochemistry (IHC)
2.3. RNA Extraction
2.4. Reverse-Transcription PCR (RT-PCR)
2.5. qPCR Assays
2.6. Validation Cohort
2.7. AR-Ness Signature on Validation Cohort
2.8. Statistical Analyses
3. Results
3.1. Patients and Tumor Characteristics
3.2. Assessment of AR/ER Ratio
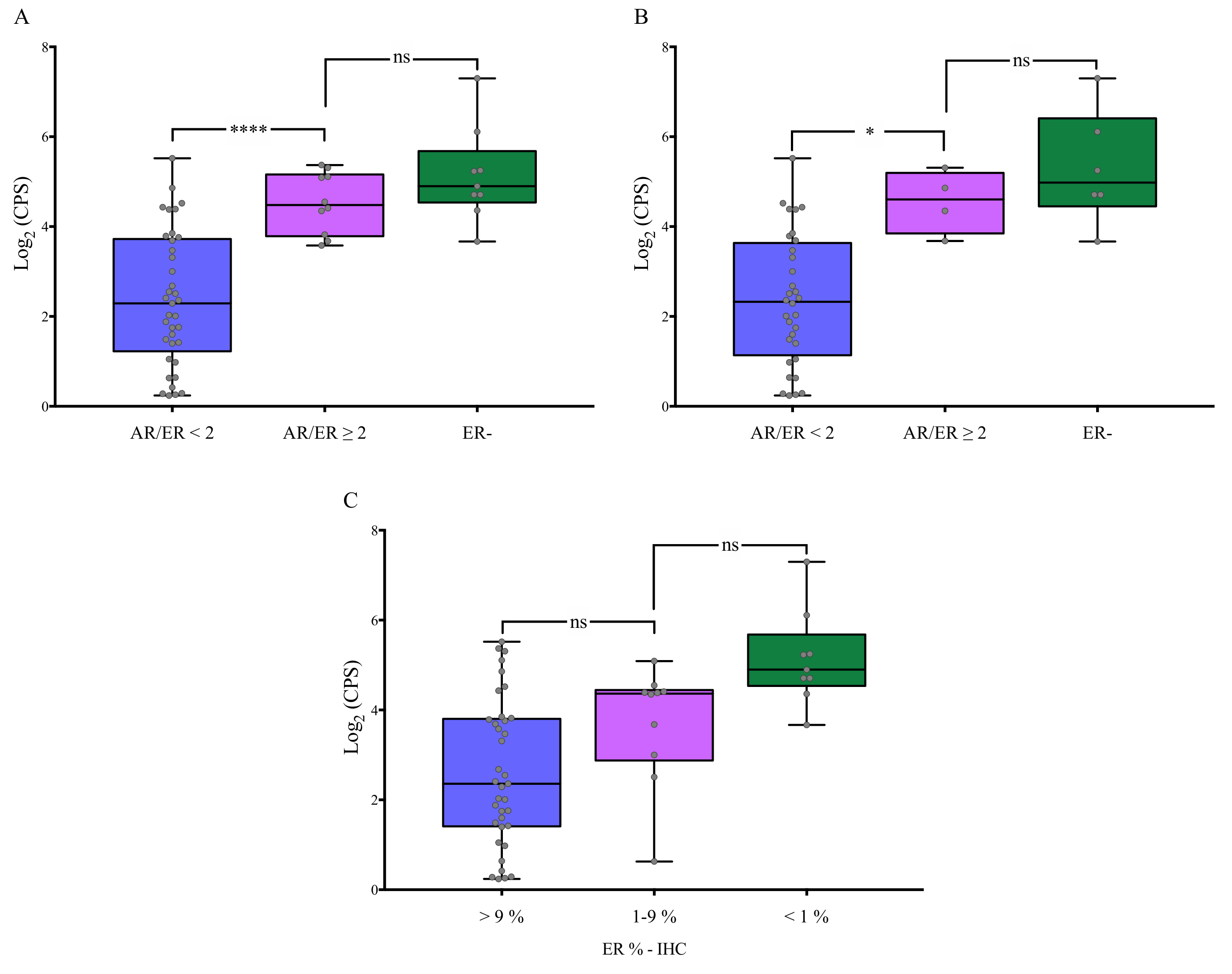
3.3. Association of AR/ER Ratio with Genes Involved in BC Proliferation
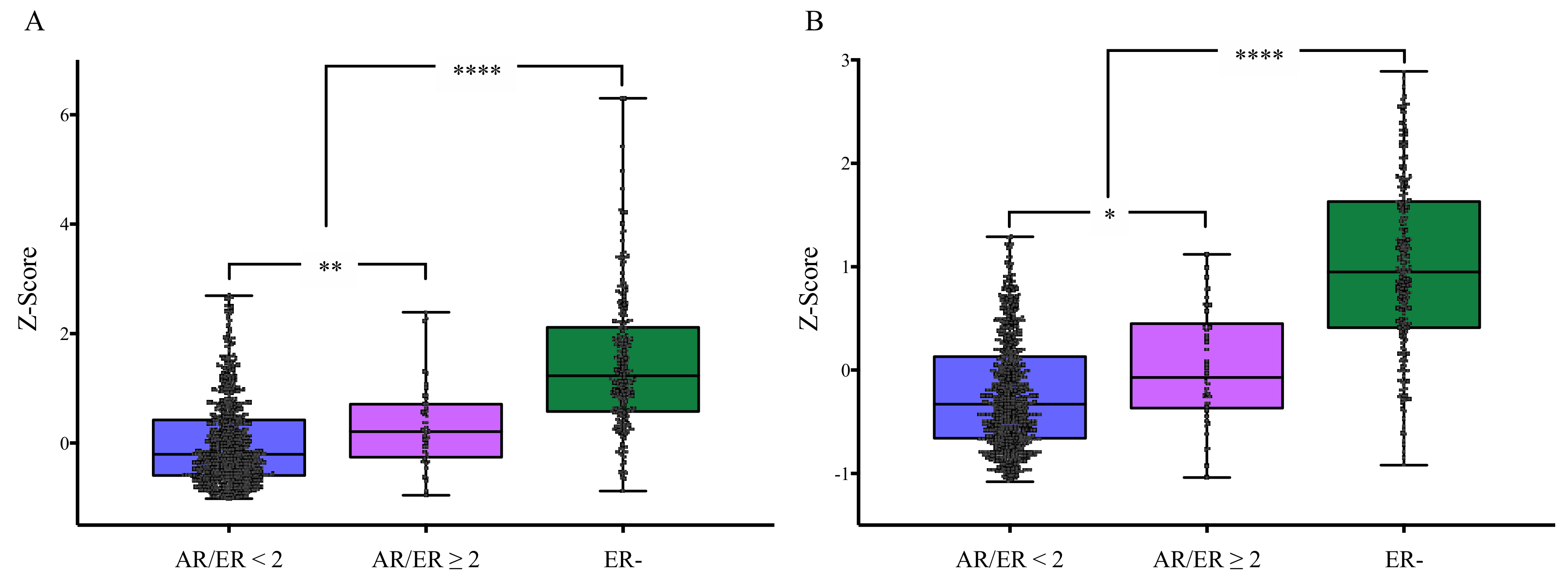
3.4. Association of AR/ER Ratio with Cellular Proliferation in Validation Cohort
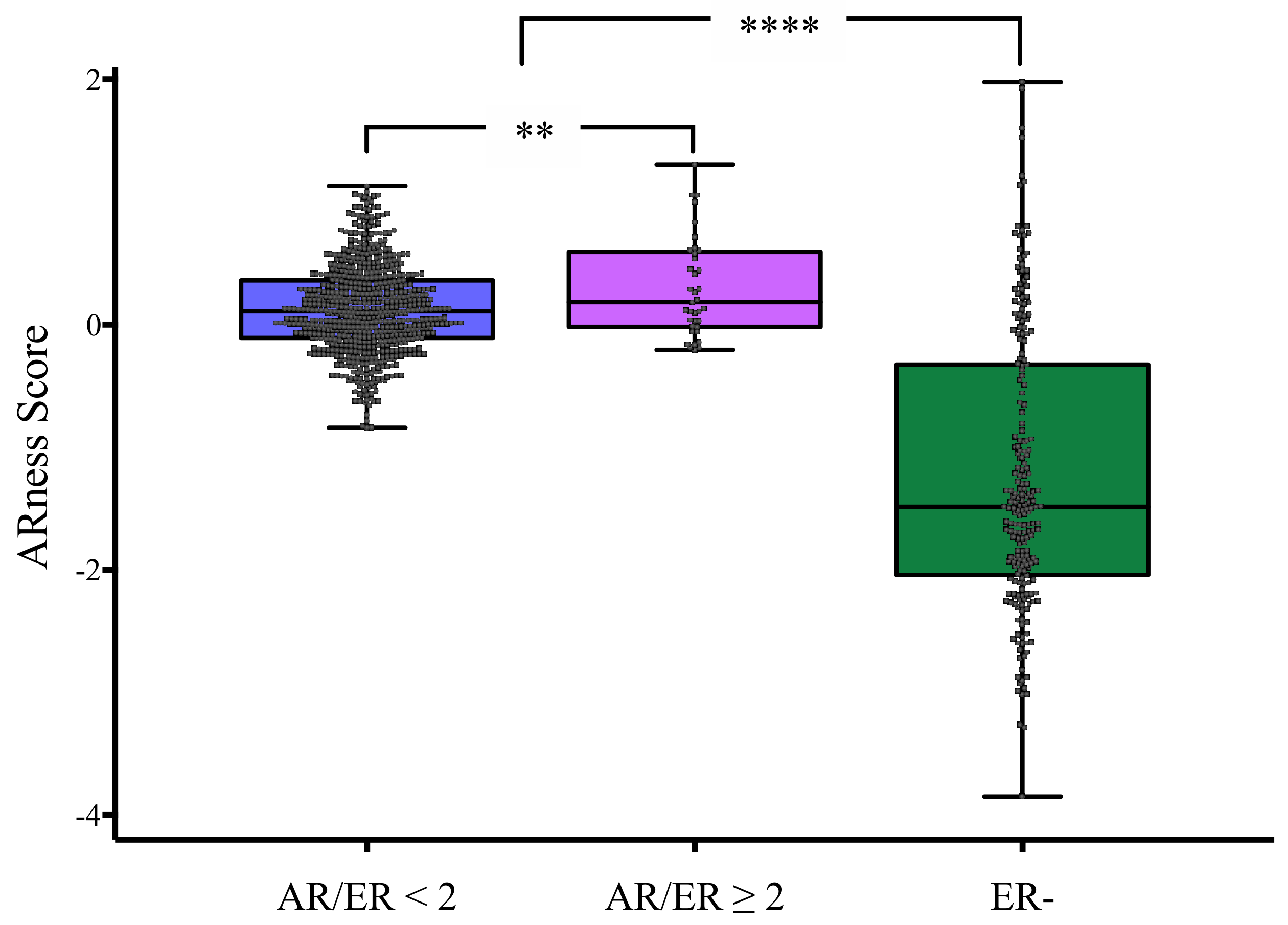
3.5. AR-Ness Signature Evaluation in Validation Cohort
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McNamara, K.M.; Moore, N.L.; Hickey, T.E.; Sasano, H.; Tilley, W.D. Complexities of androgen receptor signalling in breast cancer. Endocr. Relat. Cancer 2014, 21, T161–T181. [Google Scholar] [CrossRef] [PubMed]
- Aleskandarany, M.A.; Abduljabbar, R.; Ashankyty, I.; Elmouna, A.; Jerjees, D.; Ali, S.; Buluwela, L.; Diez-Rodriguez, M.; Caldas, C.; Green, A.R.; et al. Prognostic significance of androgen receptor expression in invasive breast cancer: Transcriptomic and protein expression analysis. Breast Cancer Res. Treat. 2016, 159, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Castellano, I.; Allia, E.; Accortanzo, V.; Vandone, A.M.; Chiusa, L.; Arisio, R.; Durando, A.; Donadio, M.; Bussolati, G.; Coates, A.S.; et al. Androgen receptor expression is a significant prognostic factor in estrogen receptor positive breast cancers. Breast Cancer Res. Treat. 2010, 124, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.S.; Kuang, X.Y.; Sun, W.L.; Xu, Y.; Zheng, Y.Z.; Liu, Y.R.; Lang, G.T.; Qiao, F.; Hu, X.; Shao, Z.M. Androgen receptor expression predicts different clinical outcomes for breast cancer patients stratified by hormone receptor status. Oncotarget 2016, 7, 41285–41293. [Google Scholar] [CrossRef] [PubMed]
- Vera-Badillo, F.E.; Templeton, A.J.; de Gouveia, P.; Diaz-Padilla, I.; Bedard, P.L.; Al-Mubarak, M.; Seruga, B.; Tannock, I.F.; Ocana, A.; Amir, E. Androgen receptor expression and outcomes in early breast cancer: A systematic review and meta-analysis. J. Natl Cancer Inst. 2014, 106, djt319. [Google Scholar] [CrossRef]
- Ringner, M.; Fredlund, E.; Hakkinen, J.; Borg, A.; Staaf, J. GOBO: Gene expression-based outcome for breast cancer online. PLoS ONE 2011, 6, e17911. [Google Scholar] [CrossRef]
- Collins, L.C.; Cole, K.S.; Marotti, J.D.; Hu, R.; Schnitt, S.J.; Tamimi, R.M. Androgen receptor expression in breast cancer in relation to molecular phenotype: Results from the Nurses’ Health Study. Mod. Pathol. 2011, 24, 924–931. [Google Scholar] [CrossRef]
- Buchanan, G.; Birrell, S.N.; Peters, A.A.; Bianco-Miotto, T.; Ramsay, K.; Cops, E.J.; Yang, M.; Harris, J.M.; Simila, H.A.; Moore, N.L.; et al. Decreased androgen receptor levels and receptor function in breast cancer contribute to the failure of response to medroxyprogesterone acetate. Cancer Res. 2005, 65, 8487–8496. [Google Scholar] [CrossRef]
- Peters, A.A.; Buchanan, G.; Ricciardelli, C.; Bianco-Miotto, T.; Centenera, M.M.; Harris, J.M.; Jindal, S.; Segara, D.; Jia, L.; Moore, N.L.; et al. Androgen receptor inhibits estrogen receptor-alpha activity and is prognostic in breast cancer. Cancer Res. 2009, 69, 6131–6140. [Google Scholar] [CrossRef]
- Doane, A.S.; Danso, M.; Lal, P.; Donaton, M.; Zhang, L.; Hudis, C.; Gerald, W.L. An estrogen receptor-negative breast cancer subset characterized by a hormonally regulated transcriptional program and response to androgen. Oncogene 2006, 25, 3994–4008. [Google Scholar] [CrossRef]
- Cochrane, D.R.; Bernales, S.; Jacobsen, B.M.; Cittelly, D.M.; Howe, E.N.; D’Amato, N.C.; Spoelstra, N.S.; Edgerton, S.M.; Jean, A.; Guerrero, J.; et al. Role of the androgen receptor in breast cancer and preclinical analysis of enzalutamide. Breast Cancer Res. 2014, 16, R7. [Google Scholar] [CrossRef] [PubMed]
- Rangel, N.; Rondon-Lagos, M.; Annaratone, L.; Osella-Abate, S.; Metovic, J.; Mano, M.P.; Bertero, L.; Cassoni, P.; Sapino, A.; Castellano, I. The role of the AR/ER ratio in ER-positive breast cancer patients. Endocr. Relat. Cancer 2018, 25, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Gerratana, L.; Basile, D.; Buono, G.; De Placido, S.; Giuliano, M.; Minichillo, S.; Coinu, A.; Martorana, F.; De Santo, I.; Del Mastro, L.; et al. Androgen receptor in triple negative breast cancer: A potential target for the targetless subtype. Cancer Treat. Rev. 2018, 68, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.J.; Jain, R.K.; Leung, S.; Choo, J.; Nielsen, T.; Huntsman, D.; Nakshatri, H.; Badve, S. FOXA1 is an independent prognostic marker for ER-positive breast cancer. Breast Cancer Res. Treat. 2012, 131, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Gingras, I.; Desmedt, C.; Ignatiadis, M.; Sotiriou, C. CCR 20th Anniversary Commentary: Gene-Expression Signature in Breast Cancer--Where Did It Start and Where Are We Now? Clin. Cancer Res. 2015, 21, 4743–4746. [Google Scholar] [CrossRef]
- Stover, D.G.; Coloff, J.L.; Barry, W.T.; Brugge, J.S.; Winer, E.P.; Selfors, L.M. The Role of Proliferation in Determining Response to Neoadjuvant Chemotherapy in Breast Cancer: A Gene Expression-Based Meta-Analysis. Clin. Cancer Res. 2016, 22, 6039–6050. [Google Scholar] [CrossRef]
- Dedic Plavetic, N.; Jakic-Razumovic, J.; Kulic, A.; Vrbanec, D. Prognostic value of proliferation markers expression in breast cancer. Med. Oncol. 2013, 30, 523. [Google Scholar] [CrossRef]
- Inoue, K.; Fry, E.A. Novel Molecular Markers for Breast Cancer. Biomark. Cancer 2016, 8, 25–42. [Google Scholar] [CrossRef]
- Dai, X.; Li, T.; Bai, Z.; Yang, Y.; Liu, X.; Zhan, J.; Shi, B. Breast cancer intrinsic subtype classification, clinical use and future trends. Am. J. Cancer Res. 2015, 5, 2929–2943. [Google Scholar]
- Parker, J.S.; Mullins, M.; Cheang, M.C.; Leung, S.; Voduc, D.; Vickery, T.; Davies, S.; Fauron, C.; He, X.; Hu, Z.; et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J. Clin. Oncol. 2009, 27, 1160–1167. [Google Scholar] [CrossRef]
- Solin, L.J.; Gray, R.; Baehner, F.L.; Butler, S.M.; Hughes, L.L.; Yoshizawa, C.; Cherbavaz, D.B.; Shak, S.; Page, D.L.; Sledge, G.W., Jr.; et al. A multigene expression assay to predict local recurrence risk for ductal carcinoma in situ of the breast. J. Natl. Cancer Inst. 2013, 105, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Annaratone, L.; Marchio, C.; Sapino, A. Tissues under-vacuum to overcome suboptimal preservation. N. Biotechnol. 2019, 52, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Goldhirsch, A.; Winer, E.P.; Coates, A.S.; Gelber, R.D.; Piccart-Gebhart, M.; Thurlimann, B.; Senn, H.J.; Panel, M. Personalizing the treatment of women with early breast cancer: Highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann. Oncol. 2013, 24, 2206–2223. [Google Scholar] [CrossRef]
- Hammond, M.E.; Hayes, D.F.; Dowsett, M.; Allred, D.C.; Hagerty, K.L.; Badve, S.; Fitzgibbons, P.L.; Francis, G.; Goldstein, N.S.; Hayes, M.; et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J. Clin. Oncol. 2010, 28, 2784–2795. [Google Scholar] [CrossRef] [PubMed]
- Marchio, C.; Lambros, M.B.; Gugliotta, P.; Di Cantogno, L.V.; Botta, C.; Pasini, B.; Tan, D.S.; Mackay, A.; Fenwick, K.; Tamber, N.; et al. Does chromosome 17 centromere copy number predict polysomy in breast cancer? A fluorescence in situ hybridization and microarray-based CGH analysis. J. Pathol. 2009, 219, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Wolff, A.C.; Hammond, M.E.H.; Allison, K.H.; Harvey, B.E.; McShane, L.M.; Dowsett, M. HER2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update Summary. J. Oncol. Pract. 2018, 14, 437–441. [Google Scholar] [CrossRef]
- Bustreo, S.; Osella-Abate, S.; Cassoni, P.; Donadio, M.; Airoldi, M.; Pedani, F.; Papotti, M.; Sapino, A.; Castellano, I. Optimal Ki67 cut-off for luminal breast cancer prognostic evaluation: A large case series study with a long-term follow-up. Breast Cancer Res. Treat. 2016, 157, 363–371. [Google Scholar] [CrossRef]
- Korbie, D.J.; Mattick, J.S. Touchdown PCR for increased specificity and sensitivity in PCR amplification. Nat. Protoc. 2008, 3, 1452–1456. [Google Scholar] [CrossRef]
- Elloumi, F.; Hu, Z.; Li, Y.; Parker, J.S.; Gulley, M.L.; Amos, K.D.; Troester, M.A. Systematic bias in genomic classification due to contaminating non-neoplastic tissue in breast tumor samples. BMC Med. Genomics 2011, 4, 54. [Google Scholar] [CrossRef]
- Van ‘t Veer, L.J.; Dai, H.; van de Vijver, M.J.; He, Y.D.; Hart, A.A.; Mao, M.; Peterse, H.L.; van der Kooy, K.; Marton, M.J.; Witteveen, A.T.; et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature 2002, 415, 530–536. [Google Scholar] [CrossRef]
- Colaprico, A.; Silva, T.C.; Olsen, C.; Garofano, L.; Cava, C.; Garolini, D.; Sabedot, T.S.; Malta, T.M.; Pagnotta, S.M.; Castiglioni, I.; et al. TCGAbiolinks: An R/Bioconductor package for integrative analysis of TCGA data. Nucleic Acids Res. 2016, 44, e71. [Google Scholar] [CrossRef] [PubMed]
- Daemen, A.; Manning, G. HER2 is not a cancer subtype but rather a pan-cancer event and is highly enriched in AR-driven breast tumors. Breast Cancer Res. 2018, 20, 8. [Google Scholar] [CrossRef] [PubMed]
- Sotiriou, C.; Pusztai, L. Gene-expression signatures in breast cancer. N. Engl. J. Med. 2009, 360, 790–800. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Xia, X.; Liu, N.; Cai, J.; Guo, Z.; Li, Y.; Jiang, L.; Dou, Q.P.; Tang, D.; Huang, H.; et al. Growth arrest and apoptosis induction in androgen receptor-positive human breast cancer cells by inhibition of USP14-mediated androgen receptor deubiquitination. Oncogene 2018, 37, 1896–1910. [Google Scholar] [CrossRef] [PubMed]
- Barton, V.N.; D’Amato, N.C.; Gordon, M.A.; Christenson, J.L.; Elias, A.; Richer, J.K. Androgen Receptor Biology in Triple Negative Breast Cancer: A Case for Classification as AR+ or Quadruple Negative Disease. Horm. Cancer 2015, 6, 206–213. [Google Scholar] [CrossRef]
- Lehmann, B.D.; Bauer, J.A.; Chen, X.; Sanders, M.E.; Chakravarthy, A.B.; Shyr, Y.; Pietenpol, J.A. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J. Clin. Investig. 2011, 121, 2750–2767. [Google Scholar] [CrossRef]
- Ando, S.; De Amicis, F.; Rago, V.; Carpino, A.; Maggiolini, M.; Panno, M.L.; Lanzino, M. Breast cancer: From estrogen to androgen receptor. Mol. Cell Endocrinol. 2002, 193, 121–128. [Google Scholar] [CrossRef]
- Cops, E.J.; Bianco-Miotto, T.; Moore, N.L.; Clarke, C.L.; Birrell, S.N.; Butler, L.M.; Tilley, W.D. Antiproliferative actions of the synthetic androgen, mibolerone, in breast cancer cells are mediated by both androgen and progesterone receptors. J. Steroid Biochem. Mol. Biol. 2008, 110, 236–243. [Google Scholar] [CrossRef]
- Greeve, M.A.; Allan, R.K.; Harvey, J.M.; Bentel, J.M. Inhibition of MCF-7 breast cancer cell proliferation by 5alpha-dihydrotestosterone; a role for p21(Cip1/Waf1). J. Mol. Endocrinol. 2004, 32, 793–810. [Google Scholar] [CrossRef]
- Ni, M.; Chen, Y.; Lim, E.; Wimberly, H.; Bailey, S.T.; Imai, Y.; Rimm, D.L.; Liu, X.S.; Brown, M. Targeting androgen receptor in estrogen receptor-negative breast cancer. Cancer Cell 2011, 20, 119–131. [Google Scholar] [CrossRef]
- Robinson, J.L.; Macarthur, S.; Ross-Innes, C.S.; Tilley, W.D.; Neal, D.E.; Mills, I.G.; Carroll, J.S. Androgen receptor driven transcription in molecular apocrine breast cancer is mediated by FoxA1. EMBO J. 2011, 30, 3019–3027. [Google Scholar] [CrossRef]
- Itoh, M.; Iwamoto, T.; Matsuoka, J.; Nogami, T.; Motoki, T.; Shien, T.; Taira, N.; Niikura, N.; Hayashi, N.; Ohtani, S.; et al. Estrogen receptor (ER) mRNA expression and molecular subtype distribution in ER-negative/progesterone receptor-positive breast cancers. Breast Cancer Res. Treat. 2014, 143, 403–409. [Google Scholar] [CrossRef]
- Iwamoto, T.; Booser, D.; Valero, V.; Murray, J.L.; Koenig, K.; Esteva, F.J.; Ueno, N.T.; Zhang, J.; Shi, W.; Qi, Y.; et al. Estrogen receptor (ER) mRNA and ER-related gene expression in breast cancers that are 1% to 10% ER-positive by immunohistochemistry. J. Clin. Oncol. 2012, 30, 729–734. [Google Scholar] [CrossRef] [PubMed]
- De Amicis, F.; Thirugnansampanthan, J.; Cui, Y.; Selever, J.; Beyer, A.; Parra, I.; Weigel, N.L.; Herynk, M.H.; Tsimelzon, A.; Lewis, M.T.; et al. Androgen receptor overexpression induces tamoxifen resistance in human breast cancer cells. Breast Cancer Res. Treat. 2010, 121, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Azariadis, K.; Kiagiadaki, F.; Pelekanou, V.; Bempi, V.; Alexakis, K.; Kampa, M.; Tsapis, A.; Castanas, E.; Notas, G. Androgen Triggers the Pro-Migratory CXCL12/CXCR4 Axis in AR-Positive Breast Cancer Cell Lines: Underlying Mechanism and Possible Implications for the Use of Aromatase Inhibitors in Breast Cancer. Cell Physiol. Biochem. 2017, 44, 66–84. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Li, L.; Zhang, N.; Liu, J.; Zhang, L.; Gao, H.; Wang, G.; Li, Y.; Zhang, Y.; Li, X.; et al. Androgen and AR contribute to breast cancer development and metastasis: An insight of mechanisms. Oncogene 2016. [Google Scholar] [CrossRef] [PubMed]
- Ades, F.; Zardavas, D.; Bozovic-Spasojevic, I.; Pugliano, L.; Fumagalli, D.; de Azambuja, E.; Viale, G.; Sotiriou, C.; Piccart, M. Luminal B breast cancer: Molecular characterization, clinical management, and future perspectives. J. Clin. Oncol. 2014, 32, 2794–2803. [Google Scholar] [CrossRef] [PubMed]
- Naderi, A.; Hughes-Davies, L. A functionally significant cross-talk between androgen receptor and ErbB2 pathways in estrogen receptor negative breast cancer. Neoplasia 2008, 10, 542–548. [Google Scholar] [CrossRef]
- Migliaccio, A.; Di Domenico, M.; Castoria, G.; Nanayakkara, M.; Lombardi, M.; de Falco, A.; Bilancio, A.; Varricchio, L.; Ciociola, A.; Auricchio, F. Steroid receptor regulation of epidermal growth factor signaling through Src in breast and prostate cancer cells: Steroid antagonist action. Cancer Res. 2005, 65, 10585–10593. [Google Scholar] [CrossRef]
- Thrane, S.; Lykkesfeldt, A.E.; Larsen, M.S.; Sorensen, B.S.; Yde, C.W. Estrogen receptor alpha is the major driving factor for growth in tamoxifen-resistant breast cancer and supported by HER/ERK signaling. Breast Cancer Res. Treat. 2013, 139, 71–80. [Google Scholar] [CrossRef]

| Characteristics | ER + n (%) | ER − n (%) | P Value (Fisher Test) | |
|---|---|---|---|---|
| Total number of patients | 47 | 9 | - | |
| Median age (Interval) | 62 (35–82) | 60 (43–78) | 0.591 * | |
| Tumor Size (missing 1 case) | <20 mm | 17 (36.2) | - | 0.028 |
| ≥20 mm | 30 (63.8) | 9 (100) | ||
| Metastatic Lymph nodes | pN0 | 22 (46.8) | 7 (77.8) | 0.230 |
| pN1–3 | 14 (29.8) | 1 (11.1) | ||
| pN > 3 | 11 (23.4) | 1 (11.1) | ||
| Grading | 1 | 4 (8.5) | - | 0.085 |
| 2 | 20 (42.6) | 1 (11.1) | ||
| 3 | 23 (48.9) | 8 (88.9) | ||
| Histotype | IDC-NST | 36 (76.6) | 5 (55.6) | 0.245 |
| ILC | 2 (4.3) | - | ||
| Mixed type | 5 (10.6) | - | ||
| others | 4 (8.5) | 4 (44.4) | ||
| Vascular invasion | No | 7 (14.9) | 3 (33.3) | 0.192 |
| Yes | 40 (85.1) | 6 (66.7) | ||
| PgR | 0 | 17 (36.2) | 9 (100) | <0.0001 |
| ≥1 | 30 (63.8) | - | ||
| Ki67 | <20% | 14 (29.8) | 1 (11.1) | 0.235 |
| ≥20% | 33 (70.2) | 8 (88.9) | ||
| HER2 | Negative | 36 (76.6) | 6 (66.7) | 0.399 |
| Positive | 11 (23.4) | 3 (33.3) | ||
| AR | 0 | 6 (12.8) | 3 (33.3) | 0.148 |
| ≥1% | 41 (87.2) | 6 (66.7) | ||
| IHC – Surrogate Subtype | Luminal A-Like | 12 (25.5) | - | <0.0001 |
| Luminal B-Like (HER2-) | 24 (51.1) | - | ||
| Luminal B-Like (HER2+) | 11 (23.4) | - | ||
| HER2+/ER- | - | 3 (33.3) | ||
| TNBC | - | 6 (66.7) | ||
| Characteristics | AR/ER < 2 n (%) | AR/ER > 2 n (%) | P Value (Fisher Test) | |
|---|---|---|---|---|
| Total number of patients | 37 (78.7) | 10(21.3) | - | |
| Median Age (Interval) | 62 (35–79) | 65 (47–82) | 0.309 * | |
| Grading | 1 | 3 (8.1) | 1 (10) | 0.215 |
| 2 | 18 (48.6) | 2 (20) | ||
| 3 | 43 (16.1) | 7 (70) | ||
| Tumor size | <20 mm | 12 (33.3) | 4 (40) | 0.485 |
| ≥20 mm | 24 (66.7) | 6 (60) | ||
| Metastatic Lymph nodes | 0 | 20 (54.1) | 2 (20) | 0.108 |
| 1–3 | 9 (24.3) | 5 (50) | ||
| >3 | 8 (21.6) | 3 (30) | ||
| Vascular invasion | No | 4 (10.8) | 3 (30) | 0.155 |
| Yes | 33 (89.2) | 7 (70) | ||
| PgR | <20% | 26 (70.3) | 4 (40) | 0.136 |
| ≥20% | 11 (29.7) | 6 (60) | ||
| Ki-67 | <20% | 12 (32.4) | 2 (20) | 0.366 |
| ≥20% | 25 (67.6) | 8 (80) | ||
| HER2 Status | Negative | 32 (86.5) | 4 (40) | 0.01 |
| Positive | 5 (13.5) | 6 (60) | ||
| ER% | Mean (interval) | 78 (2–99) | 21 (2–50) | <0.001 * |
| AR% | Mean (interval) | 70 (0–99) | 83 (50–100) | 0.319 * |
| IHC – Surrogate Subtype | Luminal A | 12 (32.4) | 0 (0) | 0.004 |
| Luminal B-Like (HER2-) | 20 (54.1) | 4 (40) | ||
| Luminal B-Like (HER2+) | 5 (13.5) | 6 (60) | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rangel, N.; Rondon-Lagos, M.; Annaratone, L.; Aristizábal-Pachon, A.F.; Cassoni, P.; Sapino, A.; Castellano, I. AR/ER Ratio Correlates with Expression of Proliferation Markers and with Distinct Subset of Breast Tumors. Cells 2020, 9, 1064. https://doi.org/10.3390/cells9041064
Rangel N, Rondon-Lagos M, Annaratone L, Aristizábal-Pachon AF, Cassoni P, Sapino A, Castellano I. AR/ER Ratio Correlates with Expression of Proliferation Markers and with Distinct Subset of Breast Tumors. Cells. 2020; 9(4):1064. https://doi.org/10.3390/cells9041064
Chicago/Turabian StyleRangel, Nelson, Milena Rondon-Lagos, Laura Annaratone, Andrés Felipe Aristizábal-Pachon, Paola Cassoni, Anna Sapino, and Isabella Castellano. 2020. "AR/ER Ratio Correlates with Expression of Proliferation Markers and with Distinct Subset of Breast Tumors" Cells 9, no. 4: 1064. https://doi.org/10.3390/cells9041064
APA StyleRangel, N., Rondon-Lagos, M., Annaratone, L., Aristizábal-Pachon, A. F., Cassoni, P., Sapino, A., & Castellano, I. (2020). AR/ER Ratio Correlates with Expression of Proliferation Markers and with Distinct Subset of Breast Tumors. Cells, 9(4), 1064. https://doi.org/10.3390/cells9041064








