Metabolome Profiling Supports the Key Role of the Spike in Wheat Yield Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Set Up
2.2. Spectral and Thermal Field Measurements
2.3. Leaf and Spike Metabolite Profiling and Isotope Analyses
2.4. Statistical Analysis
3. Results
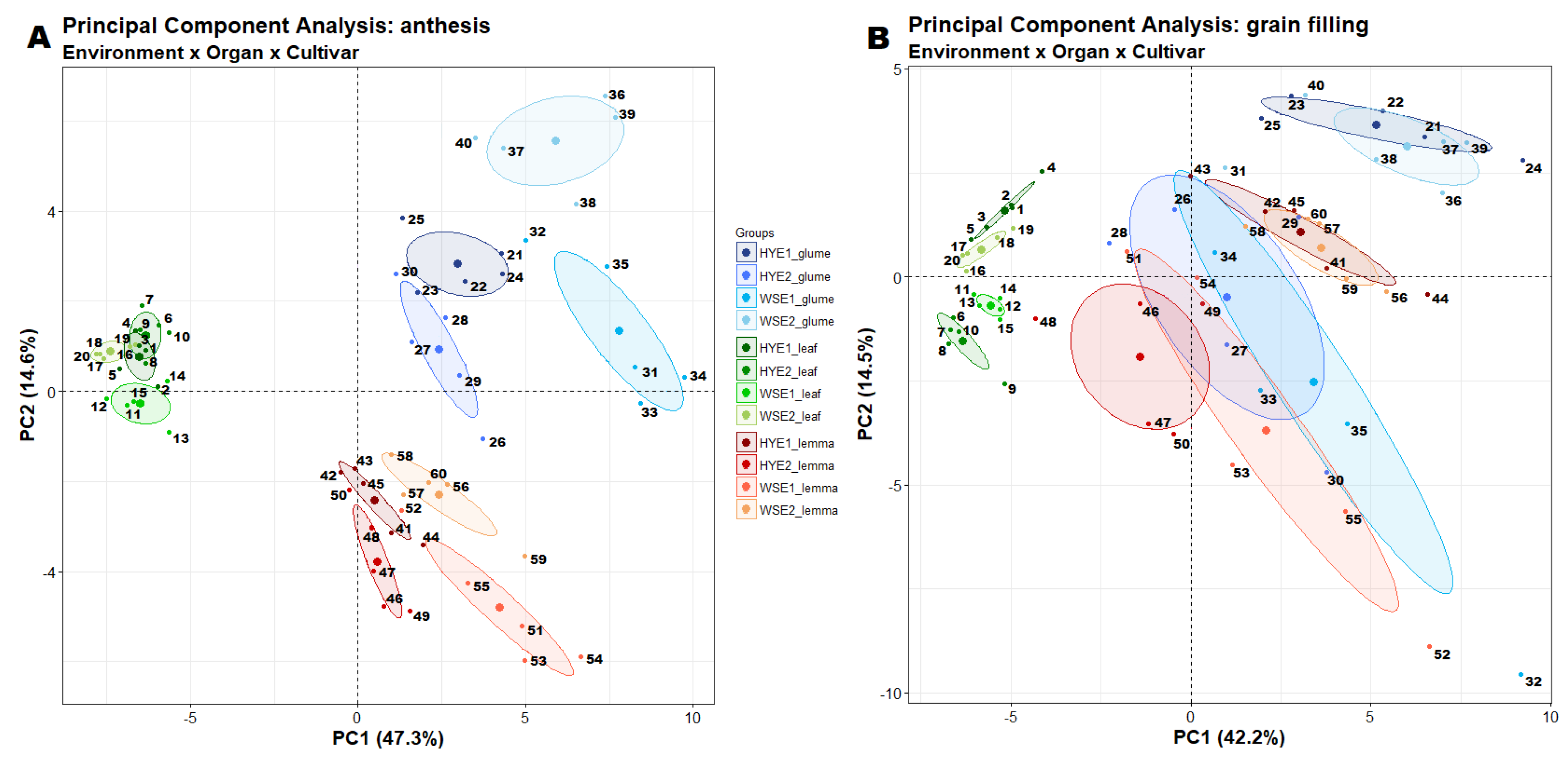
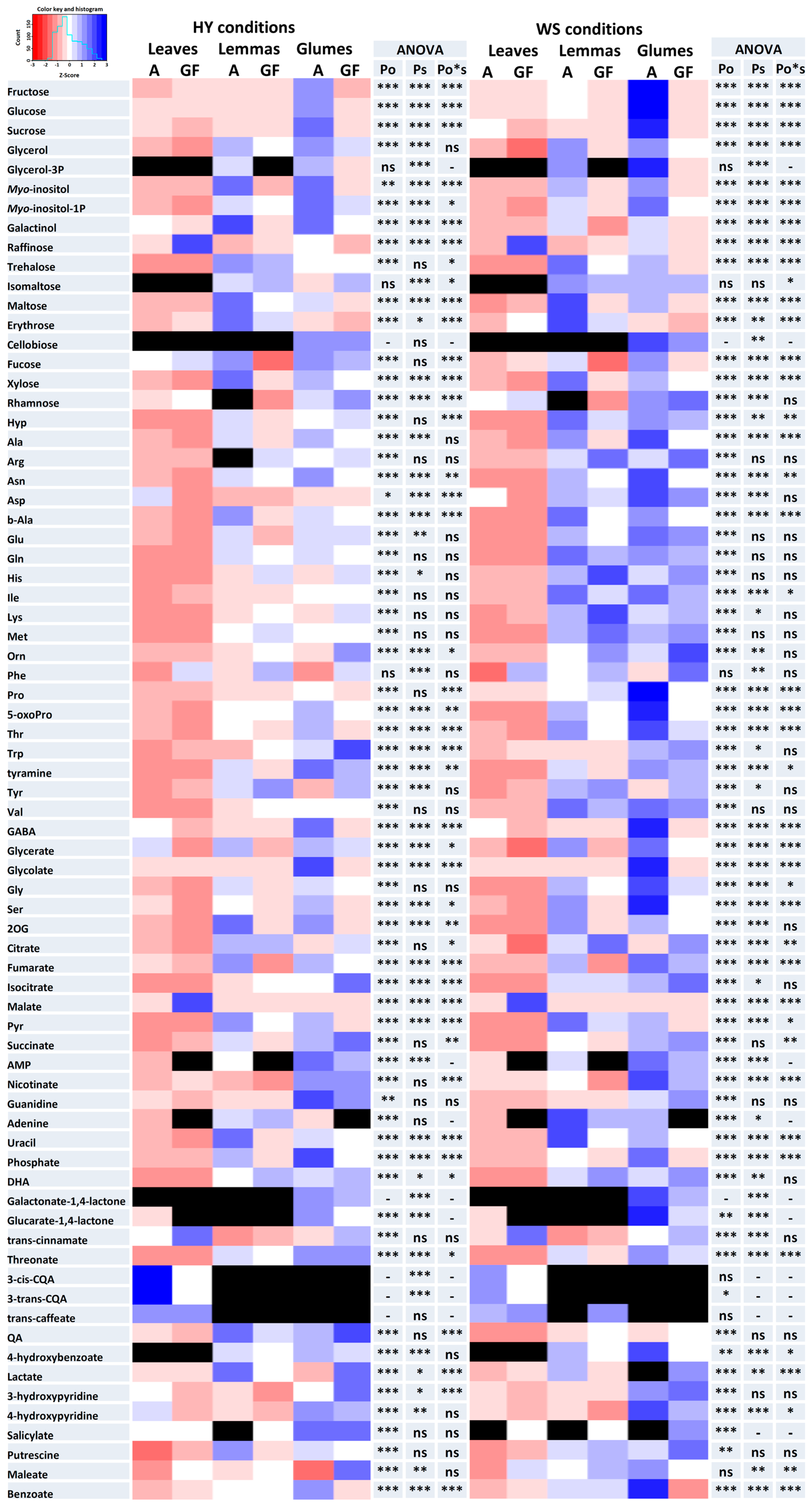
3.1. Metabolome Differences between Organs and Growth Stages
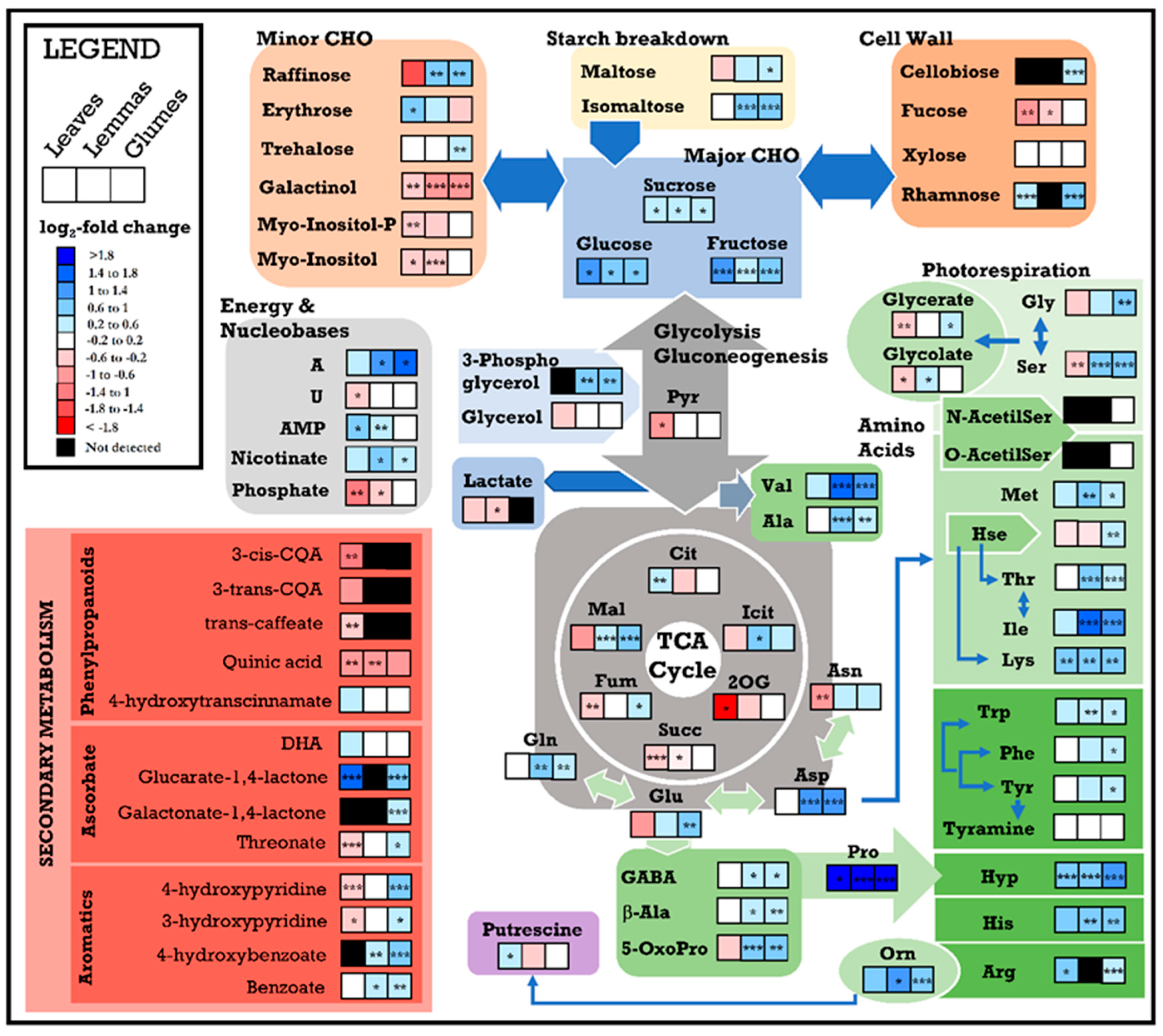
3.2. Changes in the Metabolome Due to Water Stress
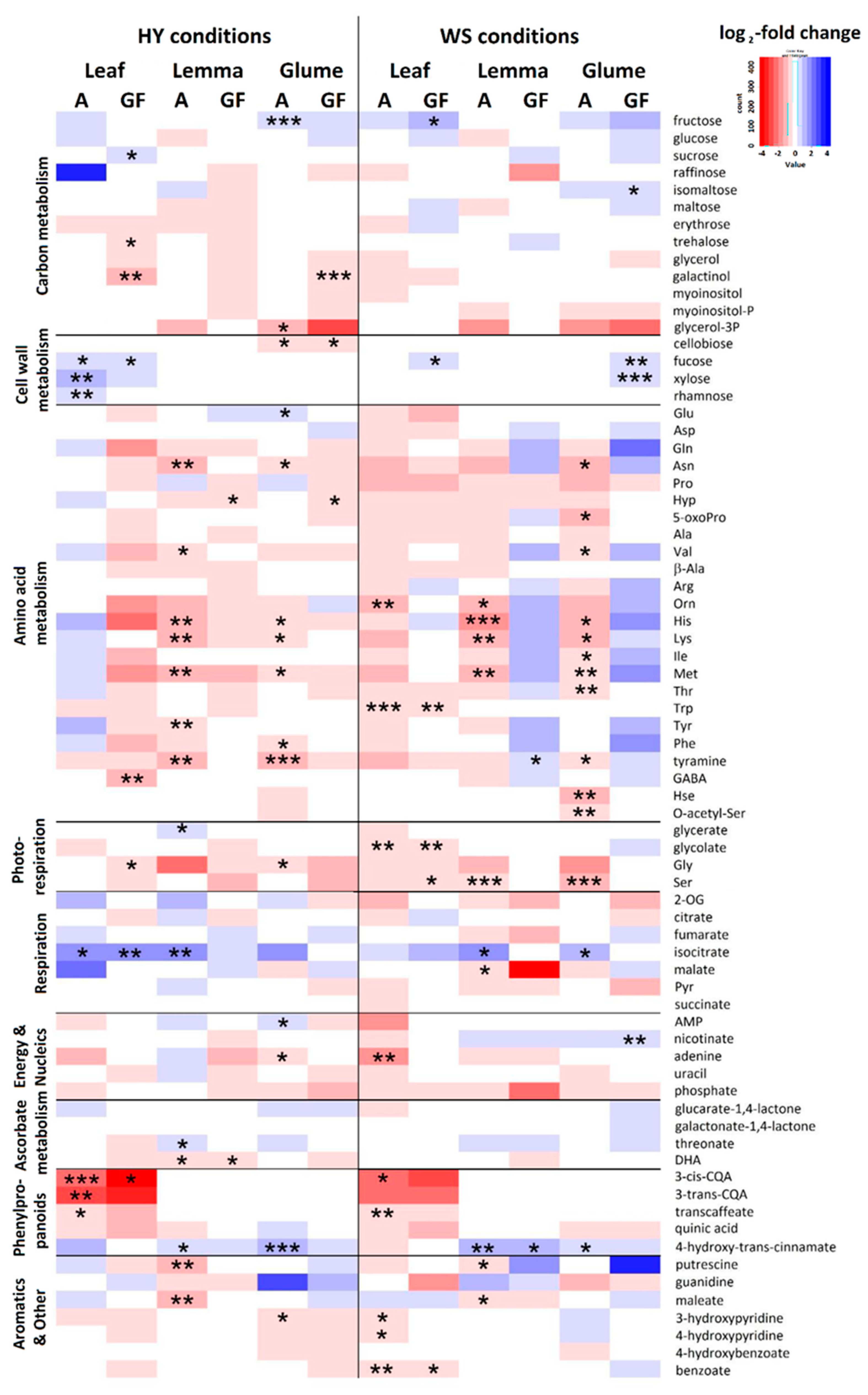
3.3. Metabolic Differences between Genotypes with Contrasting Agronomic Performance
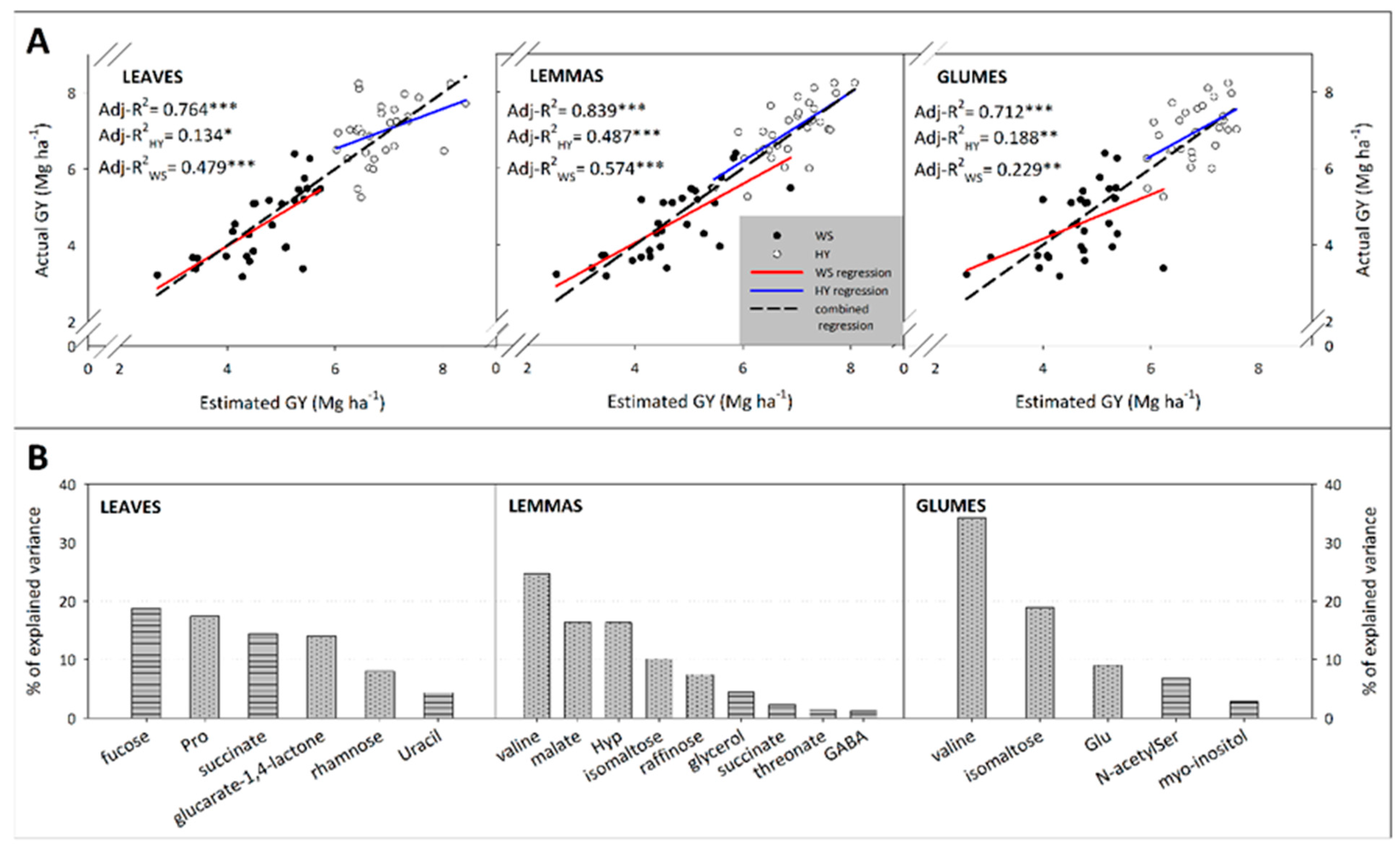
3.4. Predicting Yield from Metabolite Profiles
4. Discussion
4.1. Metabolic Overview of Wheat Flag Leaves and Spike Bracts and Their Phenology-Associated Changes
4.2. Water Stress Effects on Flag Leaf and Spike Metabolomes
4.3. Metabolic Variation between Agronomically Contrasting Genotypes
4.4. Prediction of Yield from Spike Bract and Flag Leaf Metabolomes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Asseng, S.; Ewert, F.; Martre, P.; Rötter, R.; Lobell, D.B.; Cammarano, D.; Kimball, B.A.; Ottman, M.J.; Wall, G.W.; White, J.W.; et al. Rising temperatures reduce global wheat production. Nat. Clim. Chang. 2014, 5, 143–147. [Google Scholar] [CrossRef]
- Climate change 2001: Impacts, adaptation, and vulnerability. Choice Rev. Online 2002, 39, 39. [CrossRef]
- Pfeiffer, W.; Trethowan, R.; Ginkel, M.; Ortiz-Monasterio, I.; Rajaram, S. Breeding for abiotic stress tolerance in wheat. In Abiotic Stresses Plant Resistance through Breeding and Molecular Approaches; No. CIS-4737. CIMMYT; CRC Press: Boca Raton, FL, USA, 2005; pp. 401–489. [Google Scholar]
- Lidon, F.; Almeida, A.S.; Leitão, A.; Silva, M.; Pinheiro, N.; Maçãs, B.; Da Costa, A.R.P. A synoptic overview of durum wheat production in the Mediterranean region and processing following the European Union requirements. Emir. J. Food Agric. 2014, 26, 693. [Google Scholar] [CrossRef]
- Chairi, F.; Diaz, O.V.; Vatter, T.; Aparicio, N.; Nieto-Taladriz, M.T.; Kefauver, S.; Bort, J.; Serret, M.D.; Araus, J.L. Post-green revolution genetic advance in durum wheat: The case of Spain. Field Crop. Res. 2018, 228, 158–169. [Google Scholar] [CrossRef]
- Michaletti, A.; Naghavi, M.R.; Toorchi, M.; Zolla, L.; Rinalducci, S. Metabolomics and proteomics reveal drought-stress responses of leaf tissues from spring-wheat. Sci. Rep. 2018, 8, 5710. [Google Scholar] [CrossRef] [PubMed]
- Vicente, R.; Diaz, O.V.; Medina, S.; Chairi, F.; Kefauver, S.; Bort, J.; Serret, M.D.; Aparicio, N.; Araus, J.L. Durum wheat ears perform better than the flag leaves under water stress: Gene expression and physiological evidence. Environ. Exp. Bot. 2018, 153, 271–285. [Google Scholar] [CrossRef]
- Tezara, W.; Mitchell, V.J.; Driscoll, S.D.; Lawlor, D.W. Water stress inhibits plant photosynthesis by decreasing coupling factor and ATP. Nature 1999, 401, 914–917. [Google Scholar] [CrossRef]
- Ergen, N.Z.; Thimmapuram, J.; Bohnert, H.J.; Budak, H. Transcriptome pathways unique to dehydration tolerant relatives of modern wheat. Funct. Integr. Genom. 2009, 9, 377–396. [Google Scholar] [CrossRef]
- Ullah, N.; Yüce, M.; Gökçe, Z.N. Öztürk; Budak, H. Comparative metabolite profiling of drought stress in roots and leaves of seven Triticeae species. BMC Genom. 2017, 18, 969. [Google Scholar] [CrossRef]
- Xu, Z.; Yu, Z.-W. Nitrogen metabolism in flag leaf and grain of wheat in response to irrigation regimes. J. Plant Nutr. Soil Sci. 2006, 169, 118–126. [Google Scholar] [CrossRef]
- Bown, A.W.; Shelp, B.J. Plant GABA: Not Just a Metabolite. Trends Plant Sci. 2016, 21, 811–813. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, H.A.; Abdellatif, Y.M. Effect of maltose and trehalose on growth, yield and some biochemical components of wheat plant under water stress. Ann. Agric. Sci. 2016, 61, 267–274. [Google Scholar] [CrossRef]
- Lou, L.; Li, X.; Chen, J.; Li, Y.; Tang, Y.; Lv, J. Photosynthetic and ascorbate-glutathione metabolism in the flag leaves as compared to spikes under drought stress of winter wheat (Triticum aestivum L.). PLoS ONE 2018, 13, e0194625. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Bragado, R.; Molero, G.; Reynolds, M.; Araus, J.L. Relative contribution of shoot and ear photosynthesis to grain filling in wheat under good agronomical conditions assessed by differential organ δ13C. J. Exp. Bot. 2014, 65, 5401–5413. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Bragado, R.; ElAzab, A.; Zhou, B.; Serret, M.D.; Bort, J.; Nieto-Taladriz, M.T.; Araus, J.L. Contribution of the ear and the flag leaf to grain filling in durum wheat inferred from the carbon isotope signature: Genotypic and growing conditions effects. J. Integr. Plant Boil. 2014, 56, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Merah, O.; Evon, P.; Monneveux, P. Participation of Green Organs to Grain Filling in Triticum turgidum var durum Grown under Mediterranean Conditions. Int. J. Mol. Sci. 2017, 19, 56. [Google Scholar] [CrossRef]
- Tambussi, E.A.; Bort, J.; Guiamet, J.J.; Nogués, S.; Araus, J.L. The Photosynthetic Role of Ears in C3 Cereals: Metabolism, Water Use Efficiency and Contribution to Grain Yield. Crit. Rev. Plant Sci. 2007, 26, 1–16. [Google Scholar] [CrossRef]
- Jia, S.; Lv, J.; Jiang, S.; Liang, T.; Liu, C.; Jing, Z. Response of wheat ear photosynthesis and photosynthate carbon distribution to water deficit. Photosynth. 2015, 53, 95–109. [Google Scholar] [CrossRef]
- Riedelsheimer, C.; Czedik-Eysenberg, A.; Grieder, C.; Lisec, J.; Technow, F.; Sulpice, R.; Altmann, T.; Stitt, M.; Willmitzer, L.; Melchinger, A.E. Genomic and metabolic prediction of complex heterotic traits in hybrid maize. Nat. Genet. 2012, 44, 217–220. [Google Scholar] [CrossRef]
- Lima, F.D.A.E.; Westhues, M.; Cuadros-Inostroza, Á.; Willmitzer, L.; Melchinger, A.E.; Nikoloski, Z. Metabolic robustness in young roots underpins a predictive model of maize hybrid performance in the field. Plant J. 2017, 90, 319–329. [Google Scholar] [CrossRef]
- Xu, S.; Gong, L.; Zhang, Q. Metabolomic Prediction of Yield in Hybrid Rice. Plant J. 2016, 88, 219–227. [Google Scholar] [CrossRef]
- Riedelsheimer, C.; Lisec, J.; Czedik-Eysenberg, A.; Sulpice, R.; Flis, A.; Grieder, C.; Altmann, T.; Stitt, M.; Willmitzer, L.; Melchinger, A.E. Genome-wide association mapping of leaf metabolic profiles for dissecting complex traits in maize. Proc. Natl. Acad. Sci. USA 2012, 109, 8872–8877. [Google Scholar] [CrossRef]
- Obata, T.; Witt, S.; Lisec, J.; Palacios-Rojas, N.; Florez-Sarasa, I.; Yousfi, S.; Araus, J.L.; Cairns, J.E.; Fernie, A.R. Metabolite Profiles of Maize Leaves in Drought, Heat, and Combined Stress Field Trials Reveal the Relationship between Metabolism and Grain Yield. Plant Physiol. 2015, 169, 2665–2683. [Google Scholar] [CrossRef]
- Medina, S.; Vicente, R.; Nieto-Taladriz, M.T.; Aparicio, N.; Chairi, F.; Vergara-Diaz, O.; Araus, J.L. The Plant-Transpiration Response to Vapor Pressure Deficit (VPD) in Durum Wheat Is Associated with Differential Yield Performance and Specific Expression of Genes Involved in Primary Metabolism and Water Transport. Front. Plant Sci. 2019, 9, 1994. [Google Scholar] [CrossRef] [PubMed]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A decimal code for the growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Gao, B.-C. NDWI—A normalized difference water index for remote sensing of vegetation liquid water from space. Remote. Sens. Environ. 1996, 58, 257–266. [Google Scholar] [CrossRef]
- Babar, M.A.; Reynolds, M.; Van Ginkel, M.; Klatt, A.R.; Raun, W.R.; Stone, M.L. Spectral Reflectance Indices as a Potential Indirect Selection Criteria for Wheat Yield under Irrigation. Crop. Sci. 2006, 46, 578–588. [Google Scholar] [CrossRef]
- Lobos, G.A.; Matus, I.; Rodriguez, A.; Romero-Bravo, S.; Araus, J.L.; Del Pozo, A. Wheat genotypic variability in grain yield and carbon isotope discrimination under Mediterranean conditions assessed by spectral reflectance. J. Integr. Plant Boil. 2014, 56, 470–479. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Sanchez-Bragado, R.; Molero, G.; Reynolds, M.; Araus, J.L. Photosynthetic contribution of the ear to grain filling in wheat: A comparison of different methodologies for evaluation. J. Exp. Bot. 2016, 67, 2787–2798. [Google Scholar] [CrossRef]
- Lisec, J.; Schauer, N.; Kopka, J.; Willmitzer, L.; Fernie, A.R. Gas chromatography mass spectrometry–based metabolite profiling in plants. Nat. Protoc. 2006, 1, 387–396. [Google Scholar] [CrossRef]
- Witt, S.; Galicia, L.; Lisec, J.; Cairns, J.E.; Tiessen, A.; Araus, J.L.; Palacios-Rojas, N.; Fernie, A.R. Metabolic and Phenotypic Responses of Greenhouse-Grown Maize Hybrids to Experimentally Controlled Drought Stress. Mol. Plant 2012, 5, 401–417. [Google Scholar] [CrossRef] [PubMed]
- Lüdemann, A.; Von Malotky, L.; Erban, A.; Kopka, J. TagFinder: Preprocessing Software for the Fingerprinting and the Profiling of Gas Chromatography–Mass Spectrometry Based Metabolome Analyses. In Advanced Structural Safety Studies; Springer Science and Business Media LLC: Berlin, Germany, 2011; Volume 860, pp. 255–286. [Google Scholar]
- Kopka, J.; Schauer, N.; Krueger, S.; Birkemeyer, C.; Usadel, B.; Weckwerth, W.; Gibon, Y.; Stitt, M.; Willmitzer, L.; Fernie, A.R.; et al. GMD@CSB.DB: The Golm Metabolome Database. Bioinformatics 2004, 21, 1635–1638. [Google Scholar] [CrossRef] [PubMed]
- R Development Core Team. R: A Language and Environment for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 1 March 2020).
- Warnes, G.R.; Bolker, B.; Bonebakker, L.; Gentleman, R.; Huber, W.; Liaw, A.; Lumley, T.; Maechler, M.; Magnusson, A.; Moeller, S.; et al. gplots: Various R Programming Tools for Plotting Data. R Package Version. 2009. Available online: https://CRAN.R-project.org/package=gplots (accessed on 1 March 2020).
- January Weiner, M. Package “pca3d”: Three Dimensional PCA Plots; R Package Version 0.8 484. 2015. Available online: https://CRAN.R-project.org/package=pca3d. (accessed on 1 March 2020).
- Epskamp, S.; Cramer, A.O.J.; Waldorp, L.; Schmittmann, V.D.; Borsboom, D. qgraph: Network Visualizations of Relationships in Psychometric Data. J. Stat. Softw. 2012, 48, 1–18. [Google Scholar] [CrossRef]
- Torgo, L. Data Mining with R, Learning with Case Studies, 2nd ed.; Chapman & Hall/CRC Press: Boca Raton, FL, USA, 2017; ISBN 9788578110796. [Google Scholar]
- Draper, N.R.; Smith, H. Applied Regression Analysis; John Wiley & Sons: Hoboken, NJ, USA, 1981; ISBN 0-387-98454-2. [Google Scholar]
- Araus, J.L.; Villegas, D.; Aparicio, N.; del Moral, L.F.G.; El Hani, S.; Rharrabti, Y.; Ferrio, J.P.; Royo, C. Environmental Factors Determining Carbon Isotope Discrimination and Yield in Durum Wheat under Mediterranean Conditions. Crop Sci. 2003, 43, 170. [Google Scholar] [CrossRef]
- Araus, J.L.; Cabrera-Bosquet, L.; Serret, M.D.; Bort, J.; Nieto-Taladriz, M.T. Comparative performance of δ13C, δ18O and δ15N for phenotyping durum wheat adaptation to a dryland environment. Funct. Plant Boil. 2013, 40, 595–608. [Google Scholar] [CrossRef]
- Araus, J.L.; Cairns, J.E. Field high-throughput phenotyping: The new crop breeding frontier. Trends Plant Sci. 2014, 19, 52–61. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Berjon, A.; Miller, J.R. Stress Detection in Crops with Hyperspectral Remote Sensing and Physical Simulation Models. In Proceedings of the 2004 Airborne Imaging Spectroscopy Workshop, Bruges, Belgium, 8 October 2004; pp. 1–5. [Google Scholar]
- Farooq, M.; Hussain, M.; Siddique, K.H.M. Drought Stress in Wheat during Flowering and Grain-filling Periods. Crit. Rev. Plant Sci. 2014, 33, 331–349. [Google Scholar] [CrossRef]
- Bort, J.; Brown, R.H.; Araus, J.L. Lack of C4 photosynthetic metabolism in ears of C3 cereals. Plant Cell Environ. 1995, 18, 697–702. [Google Scholar] [CrossRef]
- Keys, A.J.; Leegood, R.C. Photorespiratory Carbon and Nitrogen Cycling: Evidence from Studies of Mutant and Transgenic Plants. In Chlorophyll a Fluorescence; Springer Science and Business Media LLC: Berlin, Germany, 2002; Volume 12, pp. 115–134. [Google Scholar]
- Alam, M.M.; Nahar, K.; Hasanuzzaman, M.; Fujita, M. Trehalose-induced drought stress tolerance: A comparative study among different Brassica species. Plant Omics 2014, 7, 271. [Google Scholar]
- Martínez-Barajas, E.; Delatte, T.; Schluepmann, H.; De Jong, G.J.; Somsen, G.W.; Nunes, C.; Primavesi, L.; Coello, P.; Mitchell, R.; Paul, M.J. Wheat grain development is characterized by remarkable trehalose 6-phosphate accumulation pregrain filling: Tissue distribution and relationship to SNF1-related protein kinase1 activity. Plant Physiol. 2011, 156, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Ilhan, S.; Ozdemir, F.; Turkan, I. Contribution of trehalose biosynthetic pathway to drought stress tolerance ofCapparis ovataDesf. Plant Boil. 2014, 17, 402–407. [Google Scholar] [CrossRef]
- Ende, W.V.D. Multifunctional fructans and raffinose family oligosaccharides. Front. Plant Sci. 2013, 4, 247. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Motos, J.R.; Ortuño, M.F.; Bernal-Vicente, A.; Díaz-Vivancos, P.; Sánchez-Blanco, M.J.; Hernandez, J.A. Plant Responses to Salt Stress: Adaptive Mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef]
- Cheynier, V.; Comte, G.; Davies, K.M.; Lattanzio, V.; Martens, S. Plant phenolics: Recent advances on their biosynthesis, genetics, and ecophysiology. Plant Physiol. Biochem. 2013, 72, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Yamada, K.; Akiyama, J.; Someya, T.; Odaka, Y.; Takeda, Y.; Tori, M.; Nakashima, K.; Maekawa, T.; Sone, Y. Antioxidation Mechanism Studies of Caffeic Acid: Identification of Antioxidation Products of Methyl Caffeate from Lipid Oxidation. J. Agric. Food Chem. 2008, 56, 5947–5952. [Google Scholar] [CrossRef]
- Hildebrandt, T.M.; Nunes-Nesi, A.; Araújo, W.L.; Braun, H.-P. Amino Acid Catabolism in Plants. Mol. Plant 2015, 8, 1563–1579. [Google Scholar] [CrossRef]
- Obata, T.; Fernie, A.R. The use of metabolomics to dissect plant responses to abiotic stresses. Cell. Mol. Life Sci. 2012, 69, 3225–3243. [Google Scholar] [CrossRef]
- Vicente, R.; Martinez-Carrasco, R.; Pérez, P.; Morcuende, R. New insights into the impacts of elevated CO2, nitrogen, and temperature levels on the regulation of C and N metabolism in durum wheat using network analysis. New Biotechnol. 2018, 40, 192–199. [Google Scholar] [CrossRef]
- Timm, S.; Florian, A.; Wittmiß, M.; Jahnke, K.; Hagemann, M.; Fernie, A.R.; Bauwe, H. Serine Acts as a Metabolic Signal for the Transcriptional Control of Photorespiration-Related Genes in Arabidopsis1 [W]. Plant Physiol. 2013, 162, 379–389. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, J.; Zhao, C.; Zhou, H.; Li, Y.; Zhang, J.; Li, L.; Hu, C.; Li, W.; Peng, X.; et al. A metabolomics study delineating geographical location-associated primary metabolic changes in the leaves of growing tobacco plants by GC-MS and CE-MS. Sci. Rep. 2015, 5, 16346. [Google Scholar] [CrossRef] [PubMed]
- Rachmilevitch, S.; Cousins, A.B.; Bloom, A. Nitrate assimilation in plant shoots depends on photorespiration. Proc. Natl. Acad. Sci. USA 2004, 101, 11506–11510. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Sun, M.; Xie, Y.; Wang, F.; Zhao, Z. Photochemical and antioxidative responses of the glume and flag leaf to seasonal senescence in wheat. Front. Plant Sci. 2015, 6, 358. [Google Scholar] [CrossRef]
- Bowne, J.; Erwin, T.A.; Juttner, J.; Schnurbusch, T.; Langridge, P.; Bacic, A.; Roessner, U. Drought Responses of Leaf Tissues from Wheat Cultivars of Differing Drought Tolerance at the Metabolite Level. Mol. Plant 2012, 5, 418–429. [Google Scholar] [CrossRef] [PubMed]
- Noctor, G.; Mhamdi, A.; Chaouch, S.; Han, Y.; Neukermans, J.; Garcia, B.M.; Queval, G.; Foyer, C.H. Glutathione in plants: An integrated overview. Plant Cell Environ. 2011, 35, 454–484. [Google Scholar] [CrossRef]
- Sukrong, S.; Yun, K.-Y.; Stadler, P.; Kumar, C.; Facciuolo, T.; Moffatt, B.A.; Falcone, D.L.; Lee, W.K.; Cho, M.H. Improved Growth and Stress Tolerance in the Arabidopsis oxt1 Mutant Triggered by Altered Adenine Metabolism. Mol. Plant 2012, 5, 1310–1332. [Google Scholar] [CrossRef]
- Abebe, T.; Melmaiee, K.; Berg, V.; Wise, R. Drought response in the spikes of barley: Gene expression in the lemma, palea, awn, and seed. Funct. Integr. Genom. 2009, 10, 191–205. [Google Scholar] [CrossRef]
- Sanchez-Bragado, R.; Vicente, R.; Molero, G.; Serret, M.D.; Maydup, M.L.; Araus, J.L. New avenues for increasing yield and stability in C3 cereals: Exploring ear photosynthesis. Curr. Opin. Plant Boil. 2020. [Google Scholar] [CrossRef]
- Álvarez, C.; Bermúdez, M.; Ángeles, B.; Romero, L.C.; Gotor, C.; García, I. Cysteine homeostasis plays an essential role in plant immunity. New Phytol. 2011, 193, 165–177. [Google Scholar] [CrossRef]
- Khan, M.N.; Mobin, M.; Abbas, Z.K.; Siddiqui, M.H. Nitric oxide-induced synthesis of hydrogen sulfide alleviates osmotic stress in wheat seedlings through sustaining antioxidant enzymes, osmolyte accumulation and cysteine homeostasis. Nitric Oxide 2017, 68, 91–102. [Google Scholar] [CrossRef]
- Quan, N.T.; Anh, L.H.; Khang, D.T.; Tuyen, P.; Toan, N.P.; Minh, T.N.; Minh, L.T.; Bach, D.T.; Ha, P.T.T.; Elzaawely, A.A.; et al. Involvement of Secondary Metabolites in Response to Drought Stress of Rice (Oryza sativa L.). Agriculture 2016, 6, 23. [Google Scholar] [CrossRef]
- Salvador, V.H.; Lima, R.; Dos Santos, W.D.; Soares, A.R.; Böhm, P.A.F.; Marchiosi, R.; Ferrarese, M.D.L.L.; Ferrarese-Filho, O. Cinnamic Acid Increases Lignin Production and Inhibits Soybean Root Growth. PLoS ONE 2013, 8, e69105. [Google Scholar] [CrossRef] [PubMed]
- Vanholme, R.; Demedts, B.; Morreel, K.; Ralph, J.; Boerjan, W. Lignin Biosynthesis and Structure1. Plant Physiol. 2010, 153, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Bubna, G.A.; Lima, R.; Zanardo, D.Y.L.; Dos Santos, W.D.; Ferrarese, M.D.L.L.; Ferrarese-Filho, O. Exogenous caffeic acid inhibits the growth and enhances the lignification of the roots of soybean (Glycine max). J. Plant Physiol. 2011, 168, 1627–1633. [Google Scholar] [CrossRef] [PubMed]
- Chairi, F.; Sanchez-Bragado, R.; Serret, M.D.; Aparicio, N.; Nieto-Taladriz, M.T.; Araus, J.L. Agronomic and physiological traits related to the genetic advance of semi-dwarf durum wheat: The case of Spain. Plant Sci. 2019, 110210. [Google Scholar] [CrossRef]
- Araújo, W.L.; Martins, A.O.; Fernie, A.R.; Tohge, T. 2-Oxoglutarate: Linking TCA cycle function with amino acid, glucosinolate, flavonoid, alkaloid, and gibberellin biosynthesis. Front. Plant Sci. 2014, 5, 552. [Google Scholar] [CrossRef]
- Popova, T.; De Carvalho, M.Â.A.P.; De Carvalho, M. Ângelo A.P. Citrate and isocitrate in plant metabolism. Biochim. et Biophys. Acta (BBA)—Bioenerg. 1998, 1364, 307–325. [Google Scholar] [CrossRef]
- Bujak, R.; Daghir-Wojtkowiak, E.; Kaliszan, R.; Markuszewski, M. PLS-Based and Regularization-Based Methods for the Selection of Relevant Variables in Non-targeted Metabolomics Data. Front. Mol. Biosci. 2016, 3, 144. [Google Scholar] [CrossRef]
- Saeedipour, S. Relationship of Grain Yield, ABA and Proline Accumulation in Tolerant and Sensitive Wheat Cultivars as Affected by Water Stress. Proc. Natl. Acad. Sci. India Sect. B Boil. Sci. 2013, 83, 311–315. [Google Scholar] [CrossRef]
- Araújo, W.L.; Ishizaki, K.; Nunes-Nesi, A.; Larson, T.; Tohge, T.; Krahnert, I.; Witt, S.; Obata, T.; Schauer, N.; Graham, I.A.; et al. Identification of the 2-hydroxyglutarate and isovaleryl-CoA dehydrogenases as alternative electron donors linking lysine catabolism to the electron transport chain of Arabidopsis mitochondria. Plant Cell 2010, 22, 1549–1563. [Google Scholar] [CrossRef]
- Reiter, W.-D.; Chapple, C.C.S.; Somerville, C.R. Altered Growth and Cell Walls in a Fucose-Deficient Mutant of Arabidopsis. Science 1993, 261, 1032–1035. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Jiang, J.-G. Osmotic adjustment and plant adaptation to environmental changes related to drought and salinity. Environ. Rev. 2010, 18, 309–319. [Google Scholar] [CrossRef]
- Bouché, N.; Fromm, H. GABA in plants: Just a metabolite? Trends Plant Sci. 2004, 9, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Batushansky, A.; Kirma, M.; Grillich, N.; Toubiana, D.; Pham, P.A.; Balbo, I.; Fromm, H.; Galili, G.; Fernie, A.R.; Fait, A. Combined Transcriptomics and Metabolomics of Arabidopsis thaliana Seedlings Exposed to Exogenous GABA Suggest Its Role in Plants Is Predominantly Metabolic. Mol. Plant 2014, 7, 1065–1068. [Google Scholar] [CrossRef]

| Zamadueñas Experimental Station | Colmenar de Oreja Experimental Station | El Majano Experimental Station | |
|---|---|---|---|
| Altitude (mamsl) | 700 | 590 | 20 |
| Coordinates | 41° 42′ N, 4° 42′ W | 40° 04′ N, 3° 31′ W | 37° 14′ N, 6°03′ W |
| Mean Temp.b (°C) | 10.73 | 13.01 | 14.5 |
| Max. mean Temp.b (°C) | 17.45 | 21.45 | 21.6 |
| Min. mean Temp.b (°C) | 4.64 | 5.36 | 8.3 |
| Precipitation b (mm) | 258.4 | 206.8 | 161.8 |
| Sowing date | 24.11.2014 | 21.11.2014 | 11.12.2014 |
| Harvest date | 22.07.2015 | 20.07.2015 | 11.06.2015 |
| Sowing density (seeds m−2) | 250 | 250 | 250 |
| Plot surface (m2) | 10.5 (7 × 1.5) | 10.5 (7 × 1.5) | 10.5 (7 × 1.5) |
| Irrigation provided a (mm) | 125 | - | - |
| Fertilization | |||
| 1st application | 300 kg ha−1 NPK 8:15:15 | 400 kg ha−1 NPK 15:15:15 | 500 kg ha−1 NPK 15:15:15 |
| 2nd application | 300 kg ha−1 CAN 27%N | 150 kg ha−1 Urea 46% | 100 kg ha−1 Urea 46% |
| Soil texture | Loam | Clay-loam | Silty clay loam |
| Soil pH | 8.44 | 8.1 | 7.6 |
| GY (Mg ha−1) | GNY (kg ha−1) | Biomass (Mg ha−1) | HI (%) | TKW (g) | Grains Spike−1 | Grain N (%) | Leaf N (%) | |
|---|---|---|---|---|---|---|---|---|
| Conditions | ||||||||
| HY | 6.98 | 165.3 | 19.53 | 36.04 | 49.48 | 34.62 | 2.39 | 3.92 |
| WS | 4.38 | 120.6 | 14.72 | 31.06 | 40.03 | 31.04 | 2.70 | 3.99 |
| Genotypes | ||||||||
| Pelayo | 6.26b | 152.2 | 18.10 | 34.45ab | 46.11b | 33.05b | 2.52ab | 3.99 |
| Kiko Nick | 5.84ab | 147.9 | 17.66 | 33.03ab | 48.03b | 28.01a | 2.55ab | 4.04 |
| Dorondon | 5.27ab | 128.9 | 15.13 | 36.47b | 38.77a | 40.32c | 2.38a | 3.86 |
| Sula | 6.04b | 138.0 | 17.83 | 33.64ab | 40.74a | 37.31c | 2.50a | 3.82 |
| Don Sebastian | 4.98a | 135.5 | 17.40 | 30.64a | 50.11b | 25.45a | 2.75b | 4.01 |
| Max. | 8.24 | 203.5 | 35.14 | 43.15 | 63.80 | 51.10 | 3.48 | 4.83 |
| Min. | 3.18 | 71.7 | 9.45 | 17.10 | 27.90 | 21.40 | 0.98 | 2.71 |
| CV (%) | 25.9 | 21.6 | 24.3 | 17.9 | 17.3 | 21.8 | 14.5 | 9.4 |
| ANOVA | ||||||||
| PC | 0.000 | 0.000 | 0.000 | 0.002 | 0.000 | 0.002 | 0.000 | 0.375 |
| PG | 0.005 | 0.086 | 0.261 | 0.094 | 0.000 | 0.000 | 0.005 | 0.519 |
| PCxG | 0.063 | 0.517 | 0.716 | 0.935 | 0.918 | 0.166 | 0.104 | 0.788 |
| Anthesis | Grain Filling | Mature Grains | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T | Canopy NDMI | Leaf NDWI | Spike NWI | T | Canopy NDMI | Leaf NDWI | Spike NWI | Grain δ 13C (‰) | Leaf δ 13C (‰) | |
| Conditions | ||||||||||
| HY | 15.71 | −792 | 0.0442 | −0.061 | 26.09 | −695 | 0.0462 | −0.061 | −26.65 | −28.47 |
| WS | 18.22 | −747 | 0.0395 | −0.068 | 33.44 | −607 | 0.0394 | −0.071 | −25.03 | −27.85 |
| Genotypes | ||||||||||
| Pelayo | 17.40 | −0.773 | 0.0402 | −0.065 ab | 29.96 | −654 | 0.0412 | −0.065 | −26.00 | −28.11 |
| Kiko Nick | 17.29 | −0.761 | 0.0413 | −0.068 a | 31.53 | −644 | 0.043 | −0.074 | −26.16 | −28.50 |
| Dorondon | 17.61 | −0.766 | 0.0414 | −0.065 ab | 30.77 | −633 | 0.0401 | −0.061 | −26.04 | −28.16 |
| Sula | 17.32 | −0.782 | 0.0466 | −0.067 a | 31.16 | −651 | 0.0478 | −0.066 | −26.00 | −27.94 |
| Don Sebastian | 17.31 | −0.767 | 0.0398 | −0.058 b | 31.53 | −674 | 0.0422 | −0.065 | −25.49 | −28.12 |
| ANOVA | ||||||||||
| PC | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 | 0.015 | 0.005 | 0.000 | 0.000 |
| PG | 0.952 | 0.683 | 0.058 | 0.007 | 0.952 | 0.885 | 0.145 | 0.150 | 0.699 | 0.196 |
| PCxG | 0.994 | 0.957 | 0.842 | 0.599 | 0.987 | 0.993 | 0.347 | 0.648 | 0.995 | 0.675 |
| Anthesis Stage | Grain Filling Stage | |||||
|---|---|---|---|---|---|---|
| R2 | Adj R2 | RMSE | R2 | Adj R2 | RMSE | |
| Raw intensity | ||||||
| Leaves | ||||||
| Training set | 0.801 | 0.736 | 0.758 | 0.774 | 0.702 | 0.805 |
| Validation set | 0.684 | 0.652 | 0.882 | 0.673 | 0.638 | 0.891 |
| Glumes | ||||||
| Training set | 0.837 | 0.768 | 0.679 | 0.612 | 0.508 | 1.040 |
| Validation set | 0.602 | 0.562 | 0.975 | 0.437 | 0.381 | 1.180 |
| Lemmas | ||||||
| Training set | 0.845 | 0.762 | 0.709 | 0.514 | 0.385 | 1.160 |
| Validation set | 0.651 | 0.616 | 0.925 | 0.252 | 0.178 | 1.370 |
| Log2-transformed intensity | ||||||
| Leaves | ||||||
| Training set | 0.855 | 0.788 | 0.669 | 0.808 | 0.741 | 0.744 |
| Validation set | 0.645 | 0.609 | 0.908 | 0.659 | 0.623 | 0.909 |
| Glumes | ||||||
| Training set | 0.850 | 0.784 | 0.653 | 0.736 | 0.642 | 0.885 |
| Validation set | 0.582 | 0.539 | 0.998 | 0.507 | 0.457 | 1.110 |
| Lemmas | ||||||
| Training set | 0.897 | 0.834 | 0.589 | 0.758 | 0.633 | 0.891 |
| Validation set | 0.687 | 0.655 | 0.878 | 0.417 | 0.358 | 1.210 |
| Leaves | Glumes | Lemmas | ||||||
|---|---|---|---|---|---|---|---|---|
| Metabolite | Effect | DR (%) | Metabolite | Effect | DR (%) | Metabolite | Effect | DR (%) |
| Anthesis | ||||||||
| fucose | + | 100 | Val | − | 93 | Val | − | 99 |
| rhamnose | − | 100 | isomaltose | − | 84 | malate | − | 98 |
| Pro | − | 99 | Glu | − | 83 | Hyp | − | 97 |
| succinate | + | 98 | N-acetylSer | + | 82 | glycerol | + | 96 |
| glucarate-1,4-lactone | − | 77 | myo-inositol | + | 77 | threonate | + | 79 |
| uracil | + | 73 | cellobiose | − | 69 | GABA | + | 75 |
| galactonate | + | 58 | glycerol-3P | − | 67 | succinate | + | 74 |
| Trp | − | 58 | malate | − | 66 | raffinose | − | 71 |
| 3-cis-caffeoylquinic acid | − | 49 | Asn | − | 64 | isomaltose | − | 71 |
| Asp | − | 48 | maltose | − | 63 | Ala | − | 63 |
| Grain filling | ||||||||
| fucose | + | 100 | fucose | + | 100 | trehalose | + | 100 |
| rhamnose | − | 100 | rhamnose | − | 100 | Asp | − | 99 |
| Trp | − | 98 | Trp | + | 99 | Hyp | − | 98 |
| phosphate | + | 93 | Glu | − | 92 | xylose | + | 94 |
| tyramine | − | 88 | Hyp | − | 91 | phosphate | + | 85 |
| Asn | + | 87 | Ala | + | 66 | citrate | − | 84 |
| β-Ala | − | 79 | salicylate | − | 62 | isocitrate | + | 65 |
| maltose | + | 70 | tyramine | + | 53 | 4hydroxypyridine | + | 64 |
| Pro | − | 68 | xylose | + | 49 | succinate | − | 63 |
| erythrose | − | 38 | trehalose | + | 44 | 4-hydroxy-trans-cinnamate | + | 59 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vergara-Diaz, O.; Vatter, T.; Vicente, R.; Obata, T.; Nieto-Taladriz, M.T.; Aparicio, N.; Carlisle Kefauver, S.; Fernie, A.; Araus, J.L. Metabolome Profiling Supports the Key Role of the Spike in Wheat Yield Performance. Cells 2020, 9, 1025. https://doi.org/10.3390/cells9041025
Vergara-Diaz O, Vatter T, Vicente R, Obata T, Nieto-Taladriz MT, Aparicio N, Carlisle Kefauver S, Fernie A, Araus JL. Metabolome Profiling Supports the Key Role of the Spike in Wheat Yield Performance. Cells. 2020; 9(4):1025. https://doi.org/10.3390/cells9041025
Chicago/Turabian StyleVergara-Diaz, Omar, Thomas Vatter, Rubén Vicente, Toshihiro Obata, Maria Teresa Nieto-Taladriz, Nieves Aparicio, Shawn Carlisle Kefauver, Alisdair Fernie, and José Luis Araus. 2020. "Metabolome Profiling Supports the Key Role of the Spike in Wheat Yield Performance" Cells 9, no. 4: 1025. https://doi.org/10.3390/cells9041025
APA StyleVergara-Diaz, O., Vatter, T., Vicente, R., Obata, T., Nieto-Taladriz, M. T., Aparicio, N., Carlisle Kefauver, S., Fernie, A., & Araus, J. L. (2020). Metabolome Profiling Supports the Key Role of the Spike in Wheat Yield Performance. Cells, 9(4), 1025. https://doi.org/10.3390/cells9041025





