Infectious Diseases and the Lymphoid Extracellular Matrix Remodeling: A Focus on Conduit System
Abstract
1. Introduction
2. Extracellular Matrix Composition in Lymph Node and Spleen
3. Structure and Function of the Conduits System in Secondary Lymphoid Organs
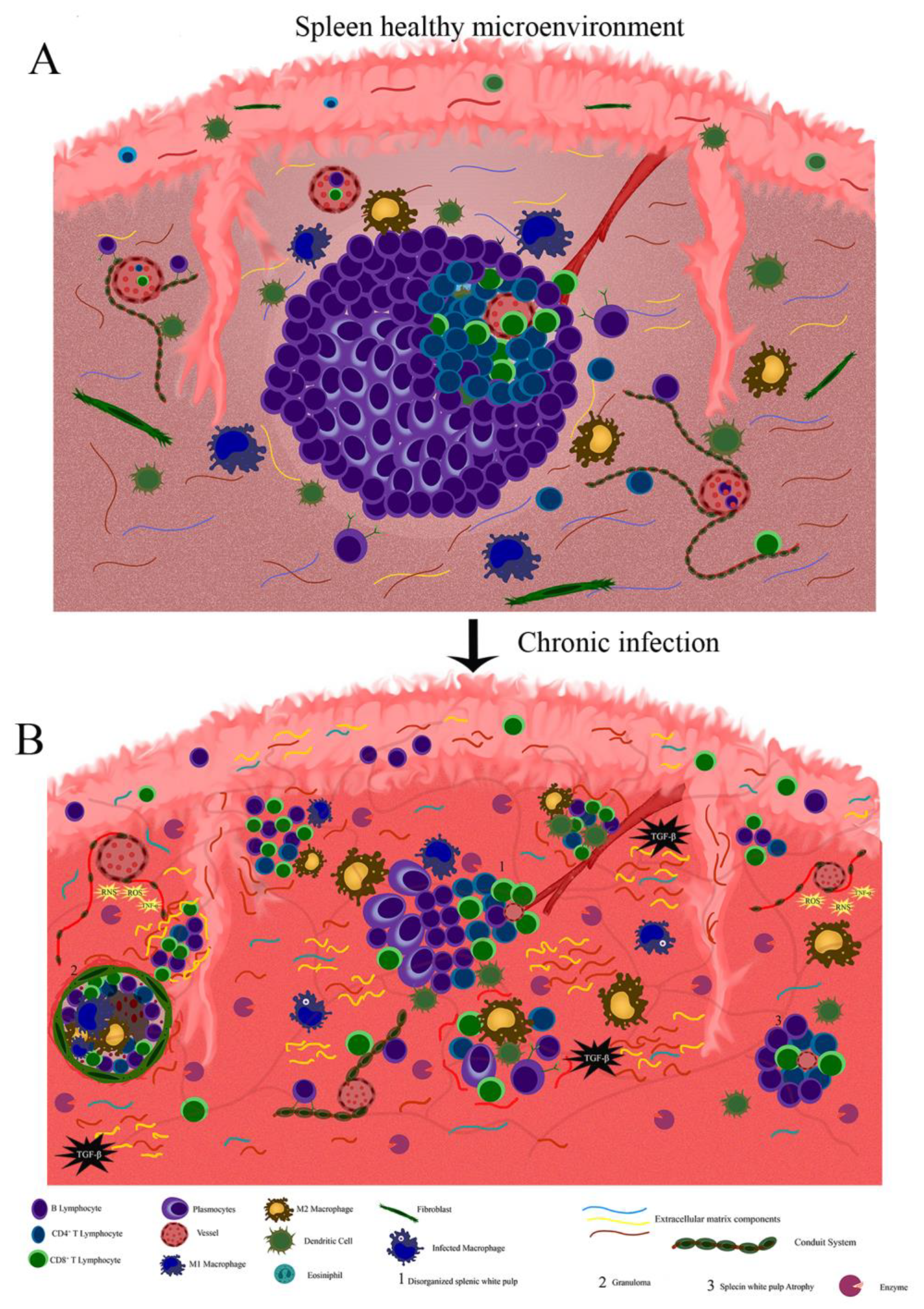
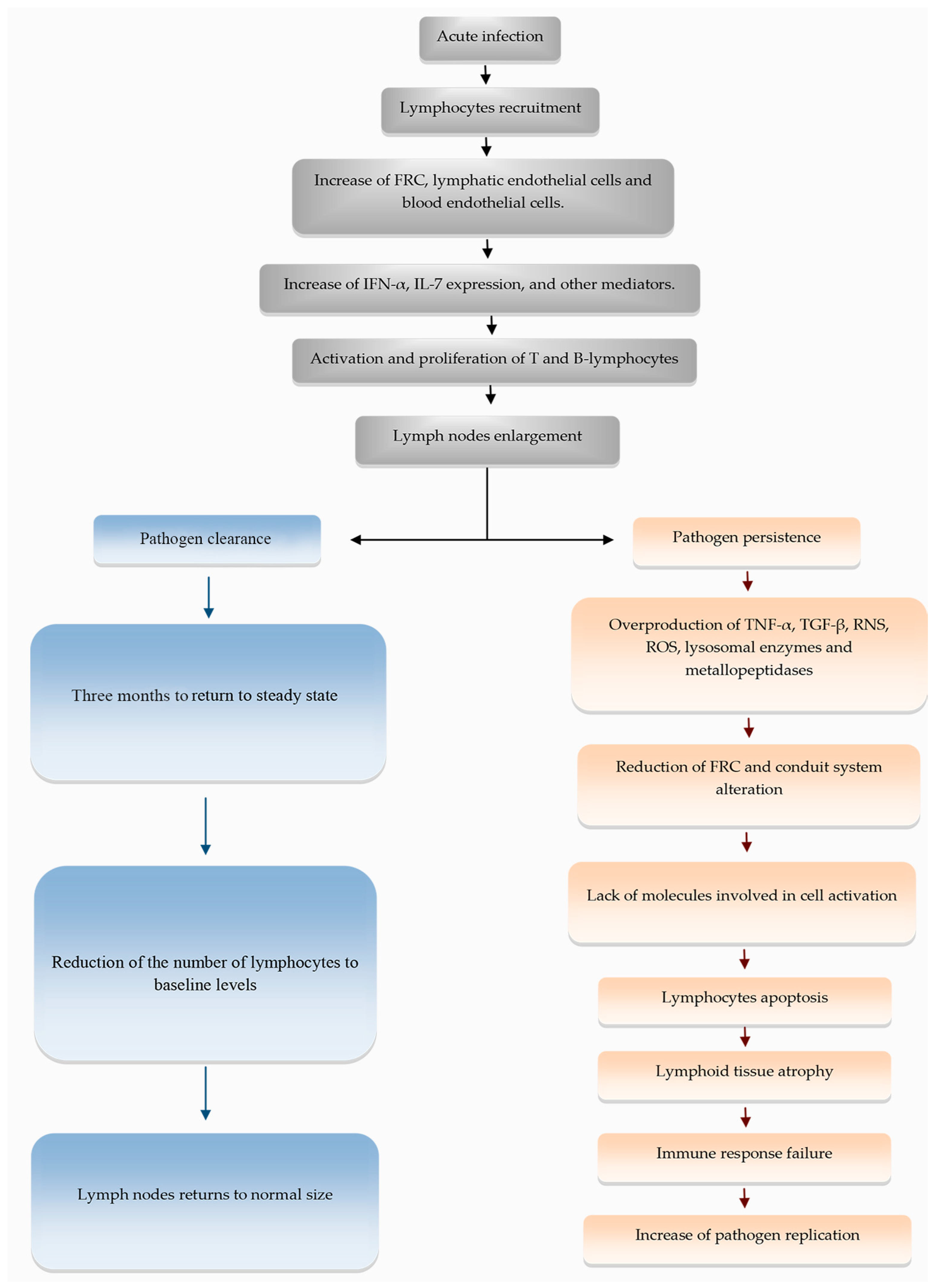
4. Extracellular Matrix and Conduit System Remodeling in Chronic and Infectious Diseases
5. Final Considerations
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bryant-Greenwood, G.D. The extracellular matrix of the human fetal membranes: Structure and function. Placenta 1998, 19, 1–11. [Google Scholar] [CrossRef]
- Loganathan, R.; Rongish, B.J.; Smith, C.M.; Filla, M.B.; Czirok, A.; Bénazéraf, B.; Little, C.D. Extracellular matrix motion and early morphogenesis. Development 2016, 143, 2056–2065. [Google Scholar] [CrossRef] [PubMed]
- Rozario, T.; DeSimone, D.W. The extracellular matrix in development and morphogenesis: A dynamic view. Dev. Biol. 2010, 341, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Strauss, J.F. Extracellular matrix dynamics and fetal membrane rupture. Reprod. Sci. 2013, 20, 140–153. [Google Scholar] [CrossRef] [PubMed]
- Birch, H.L.; Thorpe, C.T.; Rumian, A.P. Specialisation of extracellular matrix for function in tendons and ligaments. Muscles Ligaments Tendons J. 2013, 3, 12–22. [Google Scholar] [CrossRef] [PubMed]
- d’Amaro, R.; Scheidegger, R.; Blumer, S.; Pazera, P.; Katsaros, C.; Graf, D.; Chiquet, M. Putative functions of extracellular matrix glycoproteins in secondary palate morphogenesis. Front. Physiol. 2012, 3, 377. [Google Scholar] [CrossRef]
- Valiente-Alandi, I.; Schafer, A.E.; Blaxall, B.C. Extracellular matrix-mediated cellular communication in the heart. J. Mol. Cell Cardiol. 2016, 91, 228–237. [Google Scholar] [CrossRef]
- Filla, M.S.; Dimeo, K.D.; Tong, T.; Peters, D.M. Disruption of fibronectin matrix affects type IV collagen, fibrillin and laminin deposition into extracellular matrix of human trabecular meshwork (HTM) cells. Exp. Eye Res. 2017, 165, 7–19. [Google Scholar] [CrossRef]
- Halper, J.; Kjaer, M. Basic components of connective tissues and extracellular matrix: Elastin, fibrillin, fibulins, fibrinogen, fibronectin, laminin, tenascins and thrombospondins. Adv. Exp. Med. Biol. 2014, 802, 31–47. [Google Scholar]
- Llacua, L.A.; de Haan, B.J.; de Vos, P. Laminin and collagen IV inclusion in immunoisolating microcapsules reduces cytokine-mediated cell death in human pancreatic islets. J. Tissue Eng. Regen. Med. 2018, 12, 460–467. [Google Scholar] [CrossRef]
- Moreno-Layseca, P.; Icha, J.; Hamidi, H.; Ivaska, J. Integrin trafficking in cells and tissues. Nat. Cell Biol. 2019, 21, 122–132. [Google Scholar] [CrossRef]
- Mouw, J.K.; Ou, G.; Weaver, V.M. Extracellular matrix assembly: A multiscale deconstruction. Nat. Rev. Mol. Cell Biol. 2014, 15, 771–785. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, A.; Medzhitov, R. Control of adaptive immunity by the innate immune system. Nat. Immunol. 2015, 16, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Girard, J.-P.; Moussion, C.; Förster, R. HEVs, lymphatics and homeostatic immune cell trafficking in lymph nodes. Nat. Rev. Immunol. 2012, 12, 762–773. [Google Scholar] [CrossRef] [PubMed]
- Bovay, E.; Sabine, A.; Prat-Luri, B.; Kim, S.; Son, K.; Willrodt, A.-H.; Olsson, C.; Halin, C.; Kiefer, F.; Betsholtz, C.; et al. Multiple roles of lymphatic vessels in peripheral lymph node development. J. Exp. Med. 2018, 215, 2760–2777. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.C.; D’Alessandro, A.; Clement, C.C.; Santambrogio, L. Lymph formation, composition and circulation: A proteomics perspective. Int. Immunol. 2015, 27, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Badylak, S.F.; Freytes, D.O.; Gilbert, T.W. Extracellular matrix as a biological scaffold material: Structure and function. Acta Biomater. 2009, 5, 1–13. [Google Scholar] [CrossRef]
- D’Alessandro, A.; Dzieciatkowska, M.; Peltz, E.D.; Moore, E.E.; Jordan, J.R.; Silliman, C.C.; Banerjee, A.; Hansen, K.C. Dynamic changes in rat mesenteric lymph proteins following trauma using label-free mass spectrometry. Shock 2014, 42, 509–517. [Google Scholar] [CrossRef]
- Hons, M.; Sixt, M. The lymph node filter revealed. Nat. Immunol. 2015, 16, 338–340. [Google Scholar] [CrossRef]
- Sobocinski, G.P.; Toy, K.; Bobrowski, W.F.; Shaw, S.; Anderson, A.O.; Kaldjian, E.P. Ultrastructural localization of extracellular matrix proteins of the lymph node cortex: Evidence supporting the reticular network as a pathway for lymphocyte migration. BMC Immunol. 2010, 11, 42. [Google Scholar] [CrossRef]
- Song, J.; Lokmic, Z.; Lämmermann, T.; Rolf, J.; Wu, C.; Zhang, X.; Hallmann, R.; Hannocks, M.J.; Horn, N.; Ruegg, M.A.; et al. Extracellular matrix of secondary lymphoid organs impacts on B cell fate and survival. Proc. Natl. Acad. Sci. USA 2013, 110, E2915–E2924. [Google Scholar] [CrossRef] [PubMed]
- Kaldjian, E.P.; Gretz, J.E.; Anderson, A.O.; Shi, Y.; Shaw, S. Spatial and molecular organization of lymph node T cell cortex: A labyrinthine cavity bounded by an epithelium-like monolayer of fibroblastic reticular cells anchored to basement membrane-like extracellular matrix. Int. Immunol. 2001, 13, 1243–1253. [Google Scholar] [CrossRef] [PubMed]
- Kurd, N.; Robey, E.A. T cell selection in the thymus: A spatial and temporal perspective. Immunol Rev. 2016, 271, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Bajénoff, M.; Egen, J.G.; Koo, L.Y.; Laugier, J.P.; Brau, F.; Glaichenhaus, N.; Germain, R.N. Stromal cell networks regulate lymphocyte entry, migration, and territoriality in lymph nodes. Immunity 2006, 25, 989–1001. [Google Scholar] [CrossRef] [PubMed]
- Bajénoff, M.; Germain, R.N. B cell follicle development remodels the conduit system and allows soluble antigen delivery to follicular dendritic cells. Blood 2009, 114, 4989–4997. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Louie, D.; Ganguly, A.; Wu, D.; Huang, P.; Liao, S. Elastin Shapes Small Molecule Distribution in Lymph Node Conduits. J. Immunol. 2018, 200, 3142–3150. [Google Scholar] [CrossRef]
- Bronte, V.; Pittet, M.J. The spleen in local and systemic regulation of immunity. Immunity 2013, 39, 806–818. [Google Scholar] [CrossRef]
- den Haan, J.M.; Mebius, R.E.; Kraal, G. Stromal cells of the mouse spleen. Front. Immunol. 2012, 3, 201. [Google Scholar]
- Mebius, R.E.; Kraal, G. Structure and function of the spleen. Nat. Rev. Immunol. 2005, 5, 606–616. [Google Scholar] [CrossRef]
- Lokmic, Z.; Lämmermann, T.; Sixt, M.; Cardell, S.; Hallmann, R.; Sorokin, L. The extracellular matrix of the spleen as a potential organizer of immune cell compartments. Semin. Immunol. 2008, 20, 4–13. [Google Scholar] [CrossRef]
- Lewis, S.M.; Williams, A.; Eisenbarth, S.C. Structure and function of the immune system in the spleen. Sci. Immunol. 2019, 4. [Google Scholar] [CrossRef] [PubMed]
- Theocharis, A.D.; Skandalis, S.S.; Gialeli, C.; Karamanos, N.K. Extracellular matrix structure. Adv. Drug Deliv. Rev. 2016, 97, 4–27. [Google Scholar] [CrossRef] [PubMed]
- Moe, R.E. Electron microscopic appearance of the parenchyma of lymph nodes. Am. J. Anat. 1964, 114, 341–369. [Google Scholar] [CrossRef] [PubMed]
- Gretz, J.E.; Anderson, A.O.; Shaw, S. Cords, channels, corridors and conduits: Critical architectural elements facilitating cell interactions in the lymph node cortex. Immunol. Rev. 1997, 156, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Karttunen, T.; Sormunen, R.; Risteli, L.; Risteli, J.; Autio-Harmainen, H. Immunoelectron microscopic localization of laminin, type IV collagen, and type III pN-collagen in reticular fibers of human lymph nodes. J. Histochem. Cytochem. 1989, 37, 279–286. [Google Scholar] [CrossRef]
- Sixt, M.; Kanazawa, N.; Selg, M.; Samson, T.; Roos, G.; Reinhardt, D.P.; Pabst, R.; Lutz, M.B.; Sorokin, L. The conduit system transports soluble antigens from the afferent lymph to resident dendritic cells in the T cell area of the lymph node. Immunity 2005, 22, 19–29. [Google Scholar] [CrossRef]
- Gretz, J.E.; Norbury, C.C.; Anderson, A.O.; Proudfoot, A.E.; Shaw, S. Lymph-borne chemokines and other low molecular weight molecules reach high endothelial venules via specialized conduits while a functional barrier limits access to the lymphocyte microenvironments in lymph node cortex. J. Exp. Med. 2000, 192, 1425–1440. [Google Scholar] [CrossRef]
- Thierry, G.R.; Kuka, M.; De Giovanni, M.; Mondor, I.; Brouilly, N.; Iannacone, M.; Bajénoff, M. The conduit system exports locally secreted IgM from lymph nodes. J. Exp. Med. 2018, 215, 2972–2983. [Google Scholar] [CrossRef]
- Sixt, M.; Engelhardt, B.; Pausch, F.; Hallmann, R.; Wendler, O.; Sorokin, L.M. Endothelial cell laminin isoforms, laminins 8 and 10, play decisive roles in T cell recruitment across the blood-brain barrier in experimental autoimmune encephalomyelitis. J. Cell Biol. 2001, 153, 933–946. [Google Scholar] [CrossRef]
- Sakai, L.Y.; Keene, D.R.; Engvall, E. Fibrillin, a new 350-kD glycoprotein, is a component of extracellular microfibrils. J. Cell Biol. 1986, 103, 2499–2509. [Google Scholar] [CrossRef]
- Zhang, H.; Apfelroth, S.D.; Hu, W.; Davis, E.C.; Sanguineti, C.; Bonadio, J.; Mecham, R.P.; Ramirez, F. Structure and expression of fibrillin-2, a novel microfibrillar component preferentially located in elastic matrices. J. Cell Biol. 1994, 124, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Bajénoff, M.; Granjeaud, S.; Guerder, S. The strategy of T cell antigen-presenting cell encounter in antigen-draining lymph nodes revealed by imaging of initial T cell activation. J. Exp. Med. 2003, 198, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Nolte, M.A.; Beliën, J.A.M.; Schadee-Eestermans, I.; Jansen, W.; Unger, W.W.J.; van Rooijen, N.; Kraal, G.; Mebius, R.E. A conduit system distributes chemokines and small blood-borne molecules through the splenic white pulp. J. Exp. Med. 2003, 198, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Gregory, J.L.; Walter, A.; Alexandre, Y.O.; Hor, J.L.; Liu, R.; Ma, J.Z.; Devi, S.; Tokuda, N.; Owada, Y.; Mackay, L.K.; et al. Infection Programs Sustained Lymphoid Stromal Cell Responses and Shapes Lymph Node Remodeling upon Secondary Challenge. Cell Rep. 2017, 18, 406–418. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-Y.; Vogt, T.K.; Favre, S.; Scarpellino, L.; Huang, H.-Y.; Tacchini-Cottier, F.; Luther, S.A. Trapping of naive lymphocytes triggers rapid growth and remodeling of the fibroblast network in reactive murine lymph nodes. Proc. Natl. Acad. Sci. USA 2014, 111, E109–E118. [Google Scholar] [CrossRef] [PubMed]
- Onder, L.; Narang, P.; Scandella, E.; Chai, Q.; Iolyeva, M.; Hoorweg, K.; Halin, C.; Richie, E.; Kaye, P.; Westermann, J.; et al. IL-7-producing stromal cells are critical for lymph node remodeling. Blood 2012, 120, 4675–4683. [Google Scholar] [CrossRef] [PubMed]
- Nyström, A.; Bornert, O.; Kühl, T.; Gretzmeier, C.; Thriene, K.; Dengjel, J.; Pfister-Wartha, A.; Kiritsi, D.; Bruckner-Tuderman, L. Impaired lymphoid extracellular matrix impedes antibacterial immunity in epidermolysis bullosa. Proc. Natl. Acad. Sci. USA 2018, 115, E705–E714. [Google Scholar] [CrossRef]
- Py, B.F.; Gonzalez, S.F.; Long, K.; Kim, M.-S.; Kim, Y.-A.; Zhu, H.; Yao, J.; Degauque, N.; Villet, R.; Ymele-Leki, P.; et al. Cochlin produced by follicular dendritic cells promotes antibacterial innate immunity. Immunity 2013, 38, 1063–1072. [Google Scholar] [CrossRef]
- Furler, R.L.; Newcombe, K.L.; Del Rio Estrada, P.M.; Reyes-Terán, G.; Uittenbogaart, C.H.; Nixon, D.F. Histoarchitectural Deterioration of Lymphoid Tissues in HIV-1 Infection and in Aging. AIDS Res. Hum. Retrovir. 2019, 35, 1148–1159. [Google Scholar] [CrossRef]
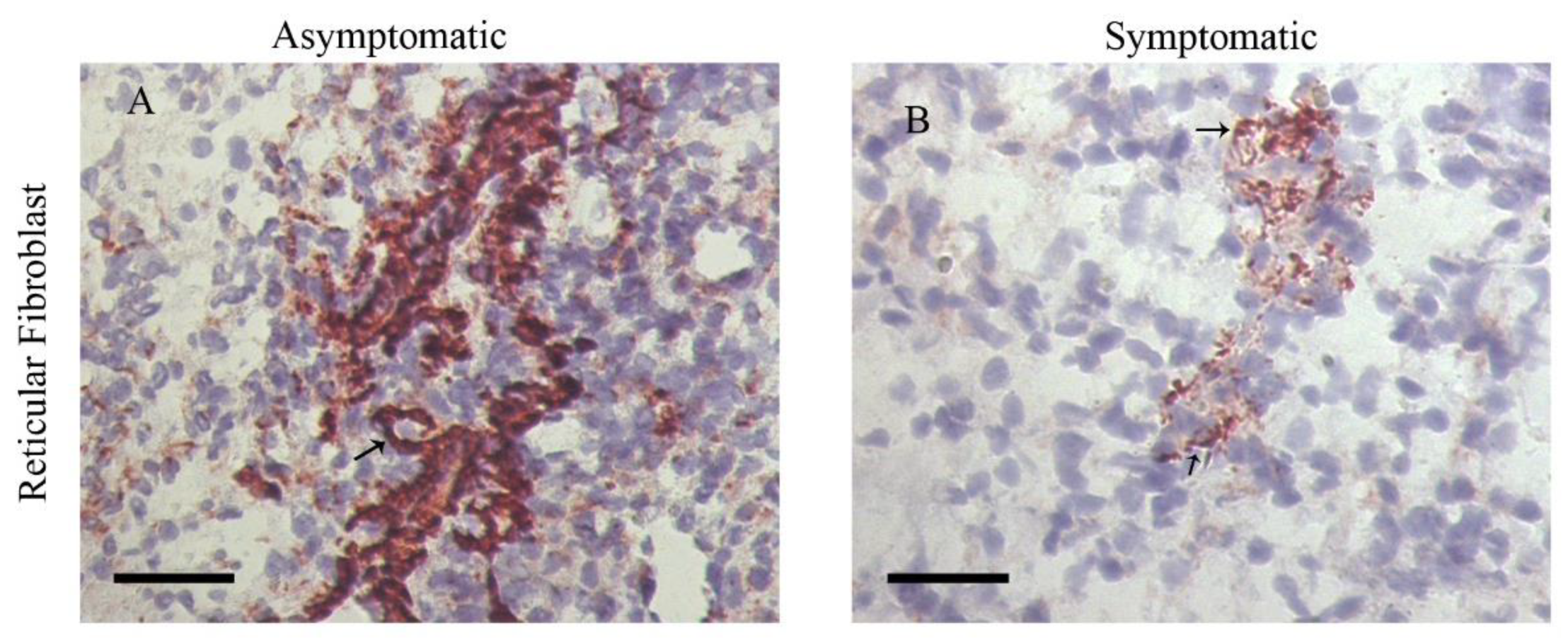
- da Silva, A.V.A.; Figueiredo, F.B.; Menezes, R.C.; Mendes-Junior, A.A.; de Miranda, L.H.M.; Cupolillo, E.; Porrozzi, R.; Morgado, F.N. Morphophysiological changes in the splenic extracellular matrix of Leishmania infantum-naturally infected dogs is associated with alterations in lymphoid niches and the CD4+ T cell frequency in spleens. PLoS Negl. Trop. Dis. 2018, 12, e0006445. [Google Scholar] [CrossRef]
- Silva, L.C.; Castro, R.S.; Figueiredo, M.M.; Michalick, M.S.M.; Tafuri, W.L.; Tafuri, W.L. Canine visceral leishmaniasis as a systemic fibrotic disease. Int. J. Exp. Pathol. 2013, 94, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.S.; Andrade, A.C.; Santana, C.C.; Santos, L.Q.; de Oliveira, C.I.; Veras, P.S.T.; Vassallo, J.; dos-Santos, W.L. Low CXCL13 expression, splenic lymphoid tissue atrophy and germinal center disruption in severe canine visceral leishmaniasis. PLoS ONE 2012, 7, e29103. [Google Scholar] [CrossRef] [PubMed]
- Santana, C.C.; Vassallo, J.; de Freitas, L.A.R.; Oliveira, G.G.S.; Pontes-de-Carvalho, L.C.; dos-Santos, W.L.C. Inflammation and structural changes of splenic lymphoid tissue in visceral leishmaniasis: A study on naturally infected dogs. Parasite Immunol. 2008, 30, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Tellier, M.C.; Greco, G.; Klotman, M.; Mosoian, A.; Cara, A.; Arap, W.; Ruoslahti, E.; Pasqualini, R.; Schnapp, L.M. Superfibronectin, a multimeric form of fibronectin, increases HIV infection of primary CD4+ T lymphocytes. J. Immunol. 2000, 164, 3236–3245. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Gu, H.; Hu, J.; Hu, S.; Wang, X.; Liu, X.; Jiao, X.; Liu, X. Quantitative proteomics identify an association between extracellular matrix degradation and immunopathology of genotype VII Newcastle disease virus in the spleen in chickens. J. Proteom. 2018, 181, 201–212. [Google Scholar] [CrossRef]
- Bíró, E.; Kocsis, K.; Nagy, N.; Molnár, D.; Kabell, S.; Palya, V.; Oláh, I. Origin of the chicken splenic reticular cells influences the effect of the infectious bursal disease virus on the extracellular matrix. Avian Pathol. 2011, 40, 199–206. [Google Scholar] [CrossRef]
- Estes, J.D. Pathobiology of HIV/SIV-associated changes in secondary lymphoid tissues. Immunol. Rev. 2013, 254, 65–77. [Google Scholar] [CrossRef]
- Mueller, S.N.; Matloubian, M.; Clemens, D.M.; Sharpe, A.H.; Freeman, G.J.; Gangappa, S.; Larsen, C.P.; Ahmed, R. Viral targeting of fibroblastic reticular cells contributes to immunosuppression and persistence during chronic infection. Proc. Natl. Acad. Sci. USA 2007, 104, 15430–15435. [Google Scholar] [CrossRef]
- Ibrahim, M.K.; Barnes, J.L.; Anstead, G.M.; Jimenez, F.; Travi, B.L.; Peniche, A.G.; Osorio, E.Y.; Ahuja, S.S.; Melby, P.C. The malnutrition-related increase in early visceralization of Leishmania donovani is associated with a reduced number of lymph node phagocytes and altered conduit system flow. PLoS Negl. Trop. Dis. 2013, 7, e2329. [Google Scholar] [CrossRef]
- Fütterer, A.; Mink, K.; Luz, A.; Kosco-Vilbois, M.H.; Pfeffer, K. The Lymphotoxin β Receptor Controls Organogenesis and Affinity Maturation in Peripheral Lymphoid Tissues. Immunity 1998, 9, 59–70. [Google Scholar] [CrossRef]
- Moseman, E.A.; Iannacone, M.; Bosurgi, L.; Tonti, E.; Chevrier, N.; Tumanov, A.; Fu, Y.X.; Hacohen, N.; von Andrian, U.H. B cell maintenance of subcapsular sinus macrophages protects against a fatal viral infection independent of adaptive immunity. Immunity 2012, 36, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Shaabani, N.; Khairnar, V.; Duhan, V.; Zhou, F.; Tur, R.F.; Häussinger, D.; Recher, M.; Tumanov, A.V.; Hardt, C.; Pinschewer, D.; et al. Two separate mechanisms of enforced viral replication balance innate and adaptive immune activation. J. Autoimmun. 2016, 67, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Teijaro, J.R.; Ng, C.; Lee, A.M.; Sullivan, B.M.; Sheehan, K.C.F.; Welch, M.; Schreiber, R.D.; de la Torre, J.C.; Oldstone, M.B. Persistent LCMV Infection Is Controlled by Blockade of Type I Interferon Signaling. Science 2013, 340, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Wilson, E.B.; Yamada, D.H.; Elsaesser, H.; Herskovitz, J.; Deng, J.; Cheng, G.; Aronow, B.J.; Karp, C.L.; Brooks, D.G. Blockade of Chronic Type I Interferon Signaling to Control Persistent LCMV Infection. Science 2013, 340, 202–207. [Google Scholar] [CrossRef]
- Reynoso, G.V.; Weisberg, A.S.; Shannon, J.P.; McManus, D.T.; Shores, L.; Americo, J.L.; Stan, R.V.; Yewdell, J.W.; Hickman, H.D. Lymph node conduits transport virions for rapid T cell activation. Nat. Immunol. 2019, 20, 602–612. [Google Scholar] [CrossRef]
- Michel, B.; Meyerett-Reid, C.; Johnson, T.; Ferguson, A.; Wyckoff, C.; Pulford, B.; Bender, H.; Avery, A.; Telling, G.; Dow, S.; et al. Incunabular immunological events in prion trafficking. Sci. Rep. 2012, 2, 440. [Google Scholar] [CrossRef]
- Lin, Y.; Leung, G.; Louie, D.; Bogoslowski, A.; Ross, J.; Kubes, P.; von der Weid, P.Y.; Liao, S. Perinodal Adipose Tissue Participates in Immune Protection through a Lymphatic Vessel-Independent Route. J. Immunol. 2018, 201, 296–305. [Google Scholar] [CrossRef]
- Stranford, S.; Ruddle, N.H. Follicular dendritic cells, conduits, lymphatic vessels, and high endothelial venules in tertiary lymphoid organs: Parallels with lymph node stroma. Front. Immunol. 2012, 3, 350. [Google Scholar] [CrossRef]
- Link, A.; Hardie, D.L.; Favre, S.; Britschgi, M.R.; Adams, D.H.; Sixt, M.; Cyster, J.G.; Buckley, C.D.; Luther, S.A. Association of T-zone reticular networks and conduits with ectopic lymphoid tissues in mice and humans. Am. J. Pathol. 2011, 178, 1662–1675. [Google Scholar] [CrossRef]
- Vilar-Pereira, G.; Resende Pereira, I.; de Souza Ruivo, L.A.; Cruz Moreira, O.; da Silva, A.A.; Britto, C.; Lannes-Vieira, J. Combination Chemotherapy with Suboptimal Doses of Benznidazole and Pentoxifylline Sustains Partial Reversion of Experimental Chagas’ Heart Disease. Antimicrob. Agents Chemother. 2016, 60, 4297–4309. [Google Scholar] [CrossRef]
- Silva, A.A.; Silva, R.R.; Gibaldi, D.; Mariante, R.M.; Dos Santos, J.B.; Pereira, I.R.; Moreira, O.C.; Lannes-Vieira, J. Priming astrocytes with TNF enhances their susceptibility to Trypanosoma cruzi infection and creates a self-sustaining inflammatory milieu. J. Neuroinflammation 2017, 14, 182. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morgado, F.N.; da Silva, A.V.A.; Porrozzi, R. Infectious Diseases and the Lymphoid Extracellular Matrix Remodeling: A Focus on Conduit System. Cells 2020, 9, 725. https://doi.org/10.3390/cells9030725
Morgado FN, da Silva AVA, Porrozzi R. Infectious Diseases and the Lymphoid Extracellular Matrix Remodeling: A Focus on Conduit System. Cells. 2020; 9(3):725. https://doi.org/10.3390/cells9030725
Chicago/Turabian StyleMorgado, Fernanda N., Aurea Virgínia A. da Silva, and Renato Porrozzi. 2020. "Infectious Diseases and the Lymphoid Extracellular Matrix Remodeling: A Focus on Conduit System" Cells 9, no. 3: 725. https://doi.org/10.3390/cells9030725
APA StyleMorgado, F. N., da Silva, A. V. A., & Porrozzi, R. (2020). Infectious Diseases and the Lymphoid Extracellular Matrix Remodeling: A Focus on Conduit System. Cells, 9(3), 725. https://doi.org/10.3390/cells9030725






