Bone Regeneration: A Novel Osteoinductive Function of Spongostan by the Interplay between Its Nano- and Microtopography
Abstract
1. Introduction
2. Materials and Methods
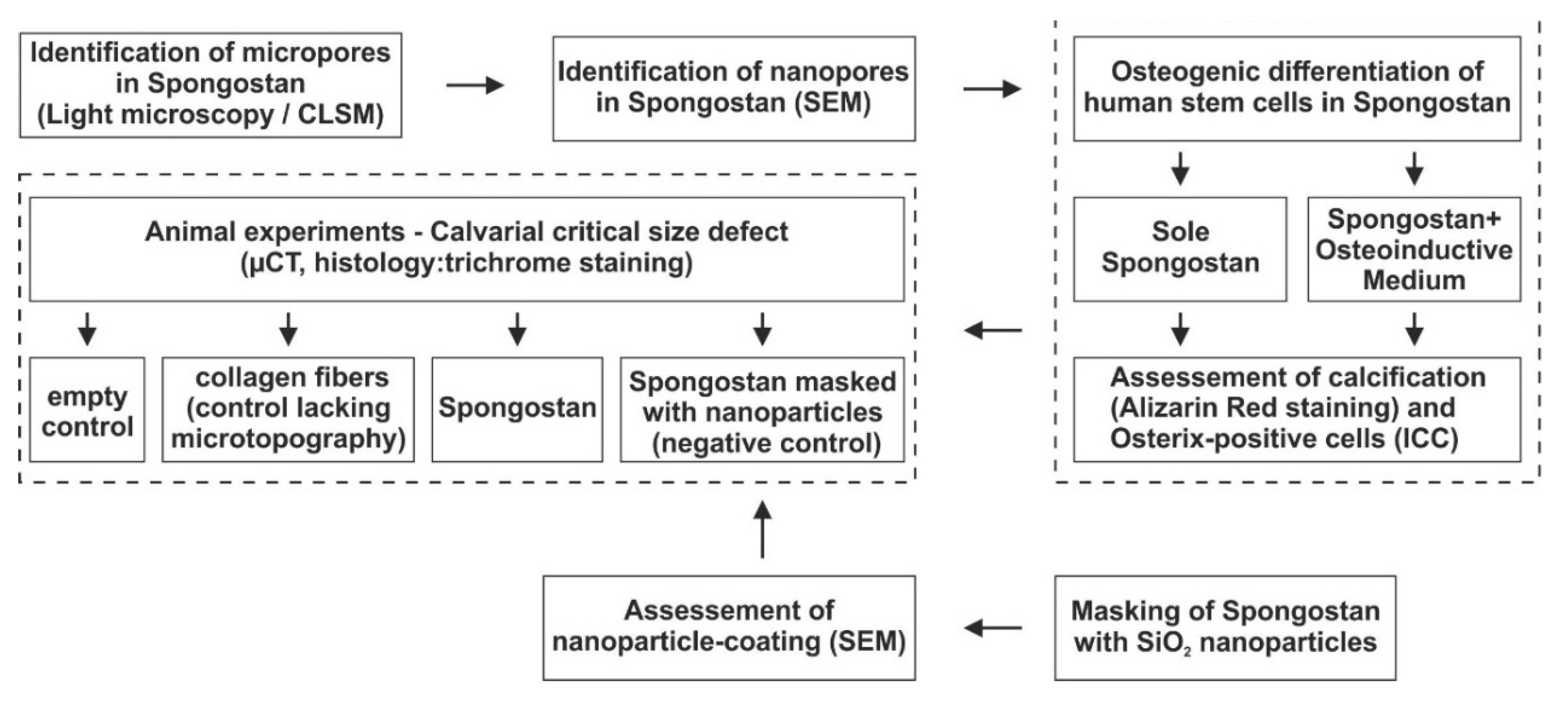
2.1. Study Design
2.2. Spongostan
2.3. Collagen Fibrils
2.4. SiO2 Nanoparticles and Coating of Spongostan
2.5. Light Microscopy
2.6. Scanning Electron Microscopy
2.7. Osteogenic Differentiation of Human Stem Cells in Spongostan and Detection of Calcium Deposition
2.8. Immunocytochemistry
2.9. Animals
2.10. Animal Surgery
2.11. Postoperative Care
2.12. Euthanasia and Sample Extraction
2.13. Micro-CT
2.14. Statistics
2.15. Histology
3. Results
3.1. Identification of Micropores of 60.66 ± 24.48 µm Diameter in the Clinically Approved Collagen Sponge Spongostan
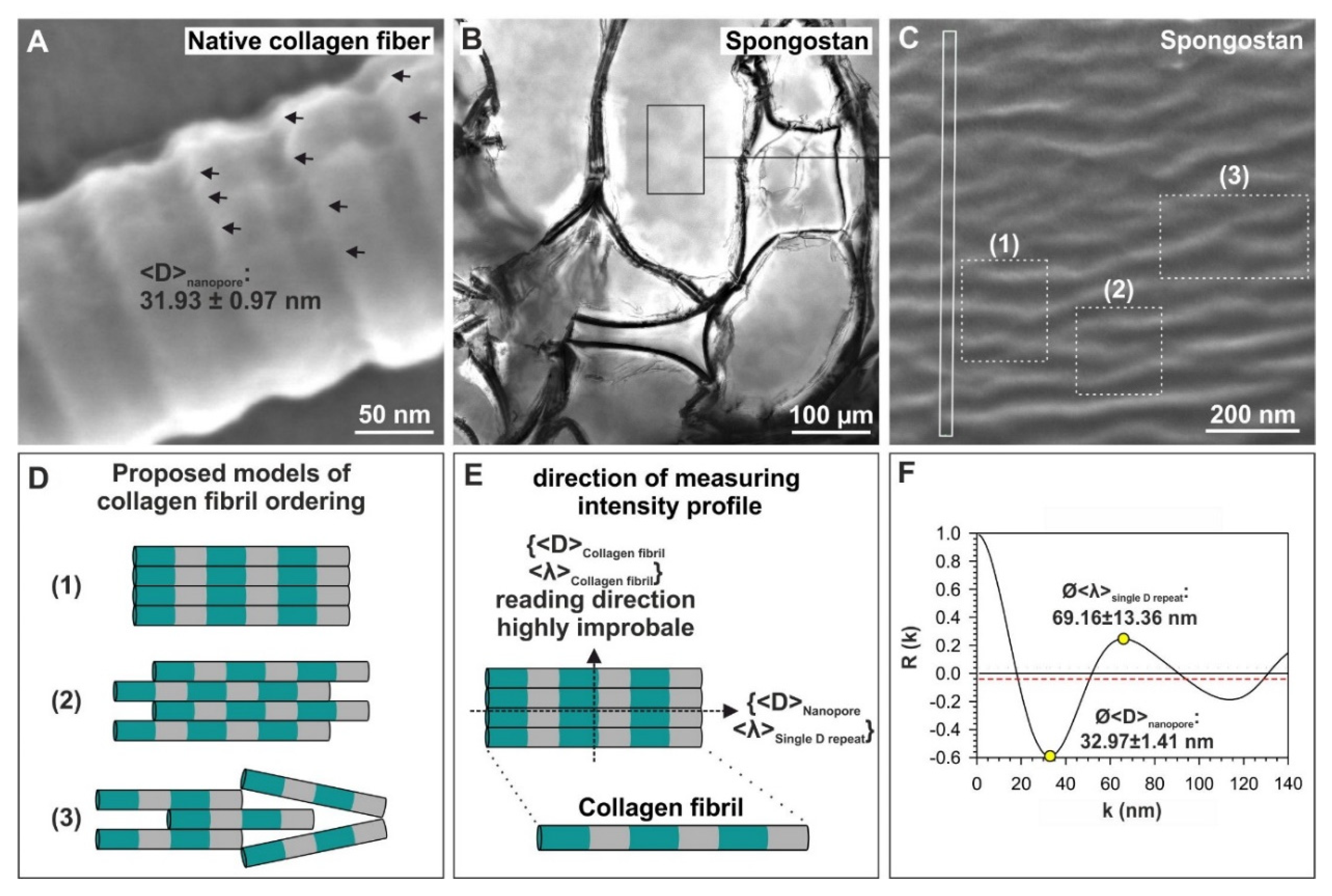
3.2. Spongostan Reveals a Distinct Nanotopography of 32.97 ± 1.41 nm Pores
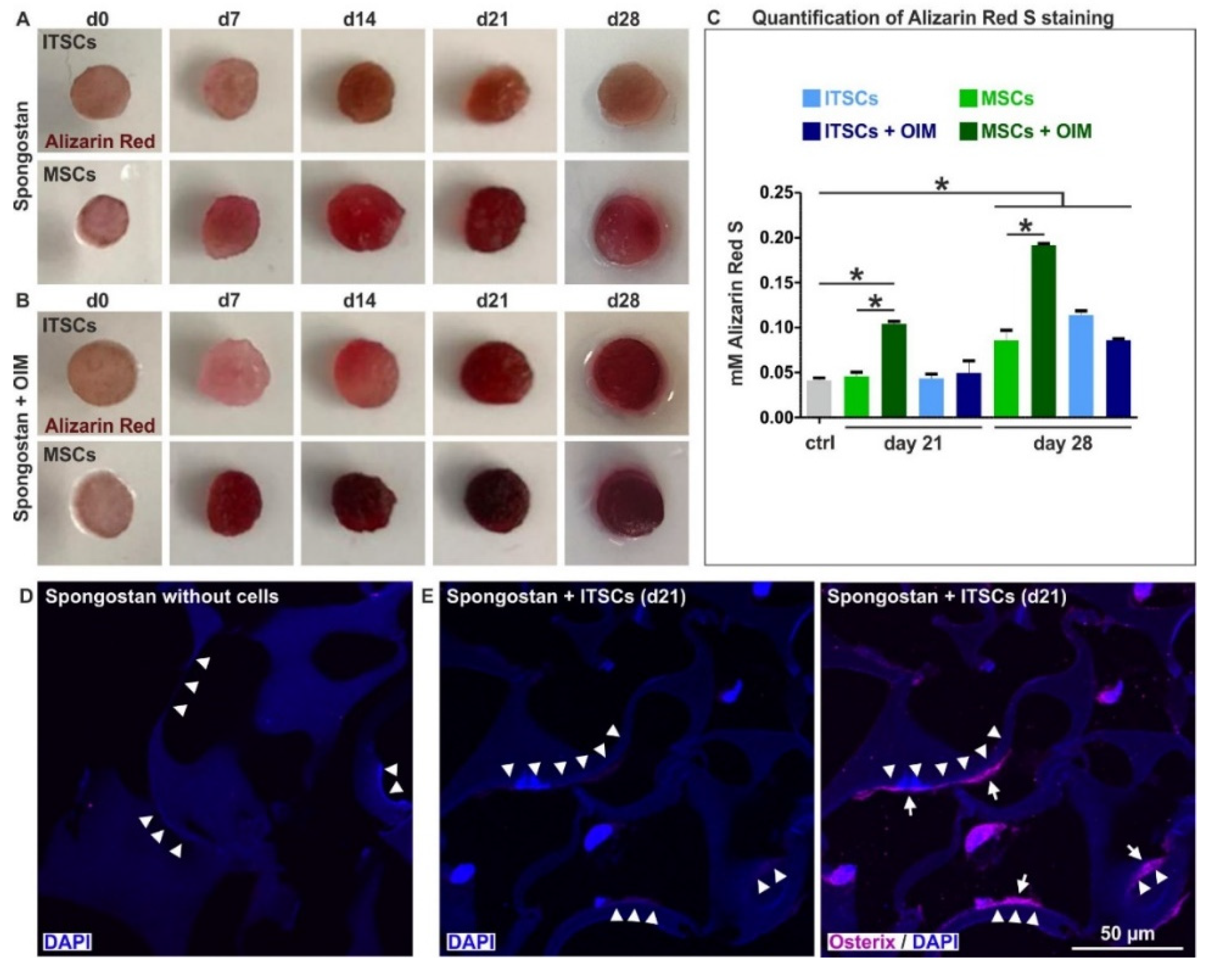
3.3. Adult Human Stem Cells Efficiently Undergo Osteogenic Differentiation by the Micro- and Nanotopography Present on Spongostan
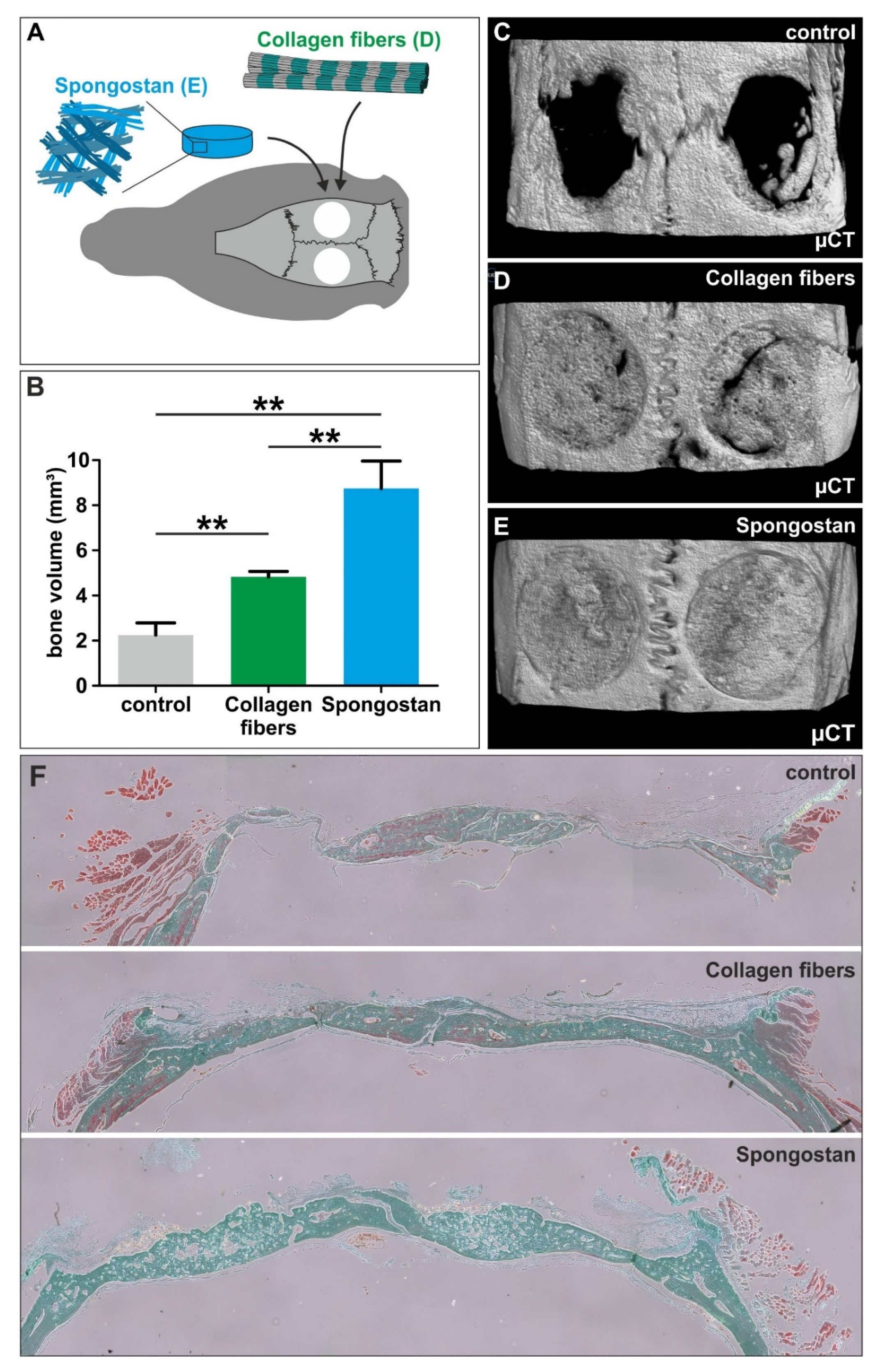
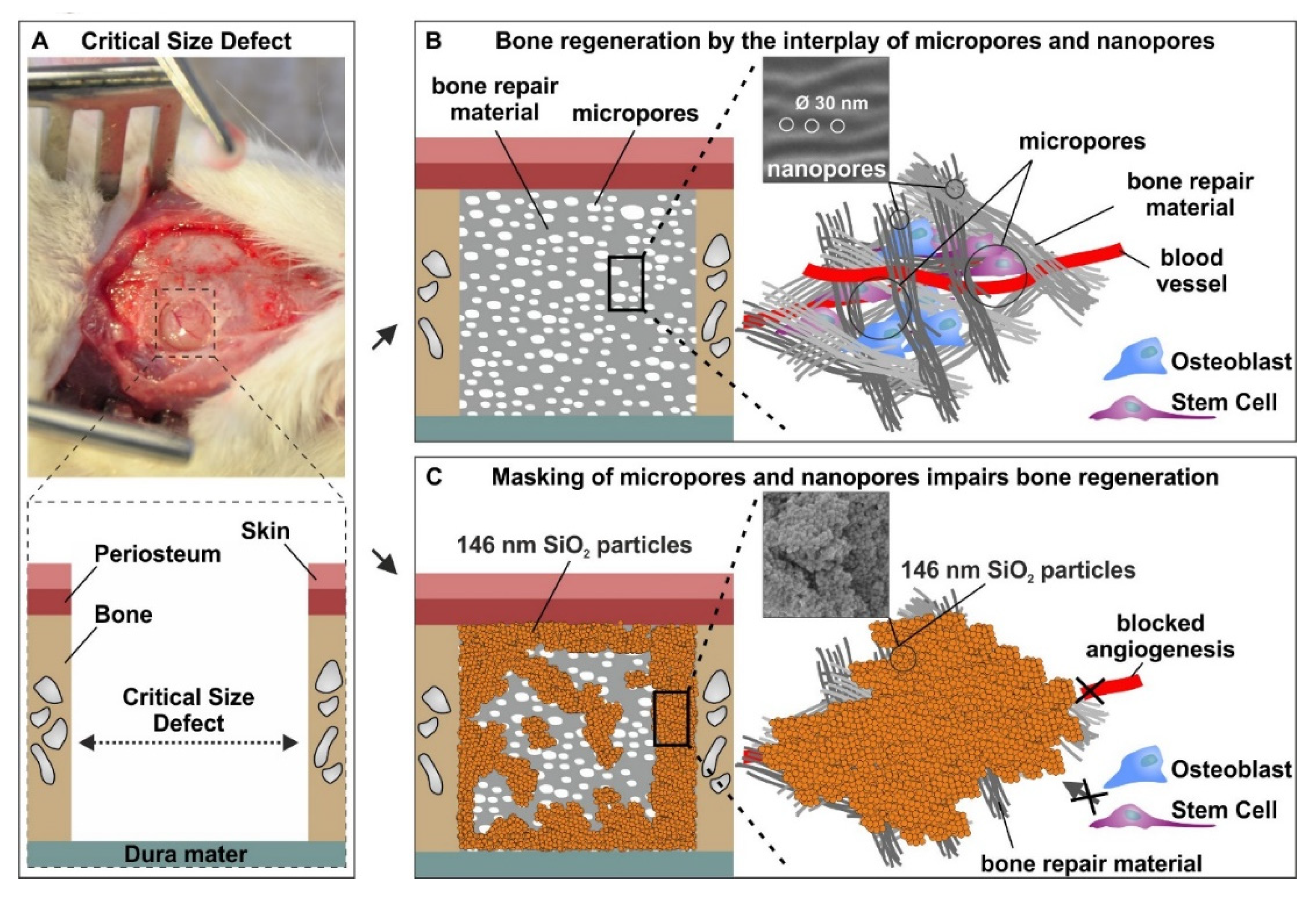
3.4. The Micro- and Nanotopography of Spongostan is Solely Sufficient to Completely Regenerate a Critical-size Calvarial Rat Bone Defect
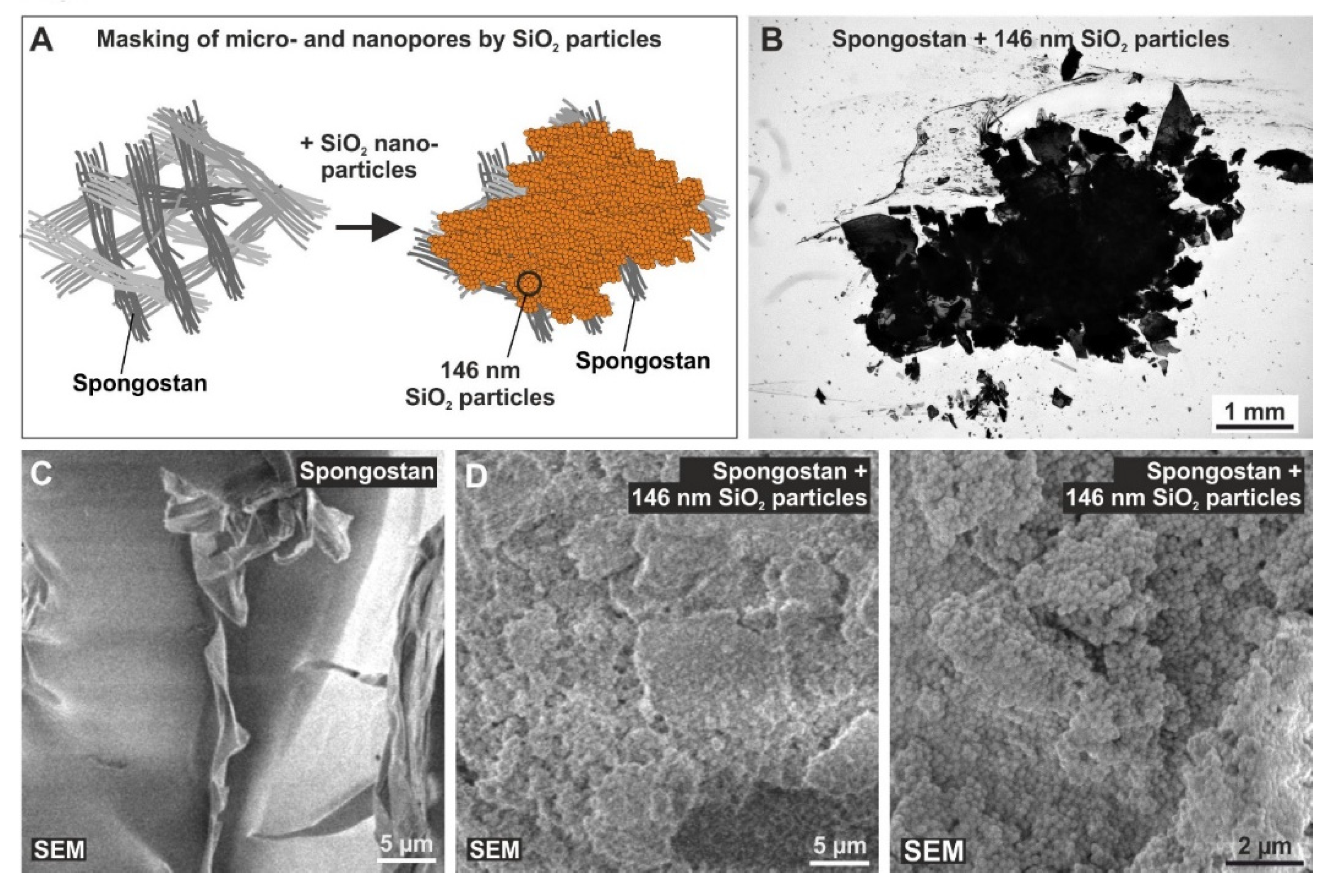
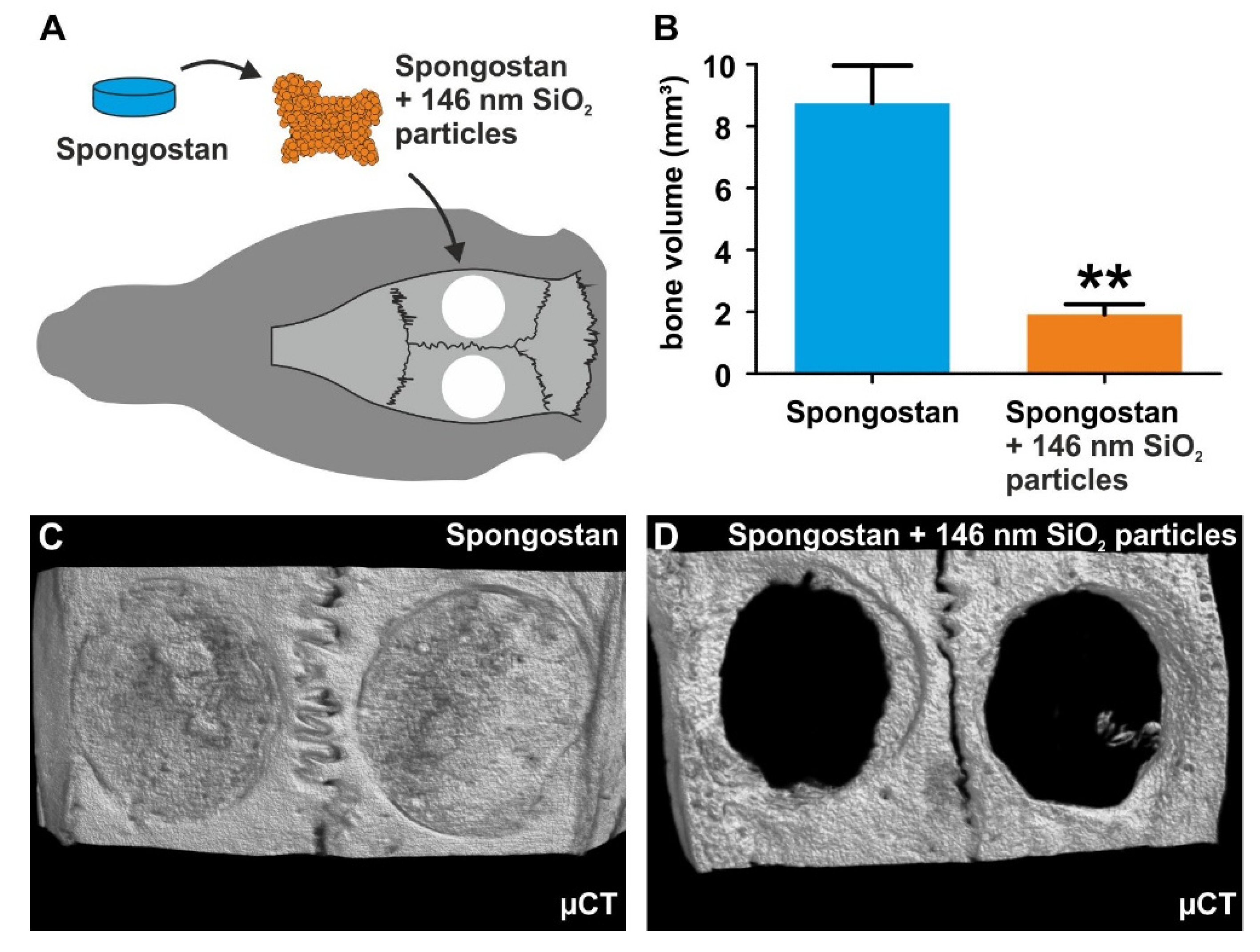
3.5. Successful Masking of Micro- and Nanopores Using SiO2 Nanoparticles Impairs the Osteoinductive Properties of Spongostan In Vivo
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Antonova, E.; Le, T.K.; Burge, R.; Mershon, J. Tibia shaft fractures: Costly burden of nonunions. BMC Musculoskelet Disord. 2013, 14, 42. [Google Scholar] [CrossRef] [PubMed]
- Bell, A.; Templeman, D.; Weinlein, J.C. Nonunion of the Femur and Tibia: An Update. Orthop Clin. North. Am. 2016, 47, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Kolk, A.; Handschel, J.; Drescher, W.; Rothamel, D.; Kloss, F.; Blessmann, M.; Heiland, M.; Wolff, K.D.; Smeets, R. Current trends and future perspectives of bone substitute materials-from space holders to innovative biomaterials. J. Craniomaxillofac. Surg. 2012, 40, 706–718. [Google Scholar] [CrossRef] [PubMed]
- Campana, V.; Milano, G.; Pagano, E.; Barba, M.; Cicione, C.; Salonna, G.; Lattanzi, W.; Logroscino, G. Bone substitutes in orthopaedic surgery: From basic science to clinical practice. J. Mater. Sci. Mater. Med. 2014, 25, 2445–2461. [Google Scholar] [CrossRef]
- Rodriguez, A.E.; Nowzari, H. The long-term risks and complications of bovine-derived xenografts: A case series. J. Indian Soc. Periodontol. 2019, 23, 487–492. [Google Scholar] [CrossRef]
- Gong, T.; Xie, J.; Liao, J.; Zhang, T.; Lin, S.; Lin, Y. Nanomaterials and bone regeneration. Bone Res. 2015, 3, 15029. [Google Scholar] [CrossRef]
- Hodge, A.J.; Petruska, J.A. Aspects of Protein Structure; Ramachandran, G.N., Ed.; Academic Press: New York, NY, USA, 1963; pp. 289–300. [Google Scholar]
- Fang, M.; Holl, M.M. Variation in type I collagen fibril nanomorphology: The significance and origin. Bonekey Rep. 2013, 2, 394. [Google Scholar] [CrossRef]
- Alexander, B.; Daulton, T.L.; Genin, G.M.; Lipner, J.; Pasteris, J.D.; Wopenka, B.; Thomopoulos, S. The nanometre-scale physiology of bone: Steric modelling and scanning transmission electron microscopy of collagen-mineral structure. J. R. Soc. Interface 2012, 9, 1774–1786. [Google Scholar] [CrossRef]
- Mould, A.P.; Hulmes, D.J.S.; Holmes, D.F.; Cummings, C.; Sear, C.H.J.; Chapman, J.A. D-periodic assemblies of type I procollagen. J. Mol. Biol. 1990, 211, 581–594. [Google Scholar] [CrossRef]
- Hochstrat, E.; Muller, M.; Frank, A.; Michel, P.; Hansen, U.; Raschke, M.J.; Kronenberg, D.; Stange, R. Cryopreservation of tendon tissue using dimethyl sulfoxide combines conserved cell vitality with maintained biomechanical features. PLoS ONE 2019, 14, e0215595. [Google Scholar] [CrossRef]
- Kim, D.; Lee, B.; Thomopoulos, S.; Jun, Y.-S. The role of confined collagen geometry in decreasing nucleation energy barriers to intrafibrillar mineralization. Nat. Commun. 2018, 9, 962. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.N.; Jee, S.E.; Jiao, K.; Tonggu, L.; Li, M.; Wang, L.; Yang, Y.D.; Bian, J.H.; Breschi, L.; Jang, S.S.; et al. Collagen intrafibrillar mineralization as a result of the balance between osmotic equilibrium and electroneutrality. Nat. Mater. 2017, 16, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Greiner, J.F.; Gottschalk, M.; Fokin, N.; Buker, B.; Kaltschmidt, B.P.; Dreyer, A.; Vordemvenne, T.; Kaltschmidt, C.; Hutten, A.; Kaltschmidt, B. Natural and synthetic nanopores directing osteogenic differentiation of human stem cells. Nanomedicine. 2019, 17, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Bauer, S.; von der Mark, K.; Schmuki, P. Nanosize and vitality: TiO2 nanotube diameter directs cell fate. Nano Lett. 2007, 7, 1686–1691. [Google Scholar] [CrossRef]
- Teo, B.K.; Wong, S.T.; Lim, C.K.; Kung, T.Y.; Yap, C.H.; Ramagopal, Y.; Romer, L.H.; Yim, E.K. Nanotopography modulates mechanotransduction of stem cells and induces differentiation through focal adhesion kinase. ACS Nano 2013, 7, 4785–4798. [Google Scholar] [CrossRef] [PubMed]
- Dalby, M.J.; Gadegaard, N.; Tare, R.; Andar, A.; Riehle, M.O.; Herzyk, P.; Wilkinson, C.D.; Oreffo, R.O. The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nat. Mater. 2007, 6, 997–1003. [Google Scholar] [CrossRef]
- Petersen, A.; Princ, A.; Korus, G.; Ellinghaus, A.; Leemhuis, H.; Herrera, A.; Klaumünzer, A.; Schreivogel, S.; Woloszyk, A.; Schmidt-Bleek, K.; et al. A biomaterial with a channel-like pore architecture induces endochondral healing of bone defects. Nat. Commun. 2018, 9, 4430. [Google Scholar] [CrossRef]
- Bing, J. Experimental observations on the use of a Danish gelatine sponge preparation (spongostan) as an absorbable haemostatic agent. Acta Pharm. Toxicol (Copenh) 1947, 3, 364–372. [Google Scholar] [CrossRef]
- Cegielski, M.; Izykowska, I.; Podhorska-Okolow, M.; Zabel, M.; Dziegiel, P. Development of foreign body giant cells in response to implantation of Spongostan as a scaffold for cartilage tissue engineering. In Vivo 2008, 22, 203–206. [Google Scholar]
- Arias-Gallo, J.; Chamorro-Pons, M.; Avendano, C.; Gimenez-Gallego, G. Influence of acidic fibroblast growth factor on bone regeneration in experimental cranial defects using spongostan and Bio-Oss as protein carriers. J. Craniofac. Surg. 2013, 24, 1507–1514. [Google Scholar] [CrossRef]
- Lin, C.Y.; Chang, Y.H.; Li, K.C.; Lu, C.H.; Sung, L.Y.; Yeh, C.L.; Lin, K.J.; Huang, S.F.; Yen, T.C.; Hu, Y.C. The use of ASCs engineered to express BMP2 or TGF-beta3 within scaffold constructs to promote calvarial bone repair. Biomaterials 2013, 34, 9401–9412. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Z.K.; Lai, P.L.; Toh, E.K.; Weng, C.H.; Tseng, H.W.; Chang, P.Z.; Chen, C.C.; Cheng, C.M. Osteogenic differentiation of preosteoblasts on a hemostatic gelatin sponge. Sci. Rep. 2016, 6, 32884. [Google Scholar] [CrossRef] [PubMed]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Hauser, S.; Widera, D.; Qunneis, F.; Muller, J.; Zander, C.; Greiner, J.; Strauss, C.; Luningschror, P.; Heimann, P.; Schwarze, H.; et al. Isolation of novel multipotent neural crest-derived stem cells from adult human inferior turbinate. Stem Cells Dev. 2012, 21, 742–756. [Google Scholar] [CrossRef] [PubMed]
- Greiner, J.F.; Hauser, S.; Widera, D.; Muller, J.; Qunneis, F.; Zander, C.; Martin, I.; Mallah, J.; Schuetzmann, D.; Prante, C.; et al. Efficient animal-serum free 3D cultivation method for adult human neural crest-derived stem cell therapeutics. Eur. Cell Mater. 2011, 22, 403–419. [Google Scholar] [CrossRef]
- Gregory, C.A.; Gunn, W.G.; Peister, A.; Prockop, D.J. An Alizarin red-based assay of mineralization by adherent cells in culture: Comparison with cetylpyridinium chloride extraction. Anal. Biochem 2004, 329, 77–84. [Google Scholar] [CrossRef]
- Spicer, P.P.; Kretlow, J.D.; Young, S.; Jansen, J.A.; Kasper, F.K.; Mikos, A.G. Evaluation of bone regeneration using the rat critical-size calvarial defect. Nat. Protoc. 2012, 7, 1918–1929. [Google Scholar] [CrossRef]
- Kadiyala, K.; Jaiswal, N.; Bruder, S.P. Culture-Expanded, Bone Marrow-Derived Mesenchymal Stem Cells Can Regenerate a Critical-Sized Segmental Bone Defect. Tissue Eng. 1997, 3, 173–185. [Google Scholar] [CrossRef]
- Greiner, J.F.; Grunwald, L.M.; Muller, J.; Sudhoff, H.; Widera, D.; Kaltschmidt, C.; Kaltschmidt, B. Culture bag systems for clinical applications of adult human neural crest-derived stem cells. Stem Cell Res. 2014, 5, 34. [Google Scholar] [CrossRef]
- Hofemeier, A.D.; Hachmeister, H.; Pilger, C.; Schurmann, M.; Greiner, J.F.; Nolte, L.; Sudhoff, H.; Kaltschmidt, C.; Huser, T.; Kaltschmidt, B. Label-free nonlinear optical microscopy detects early markers for osteogenic differentiation of human stem cells. Sci. Rep. 2016, 6, 26716. [Google Scholar] [CrossRef]
- Schmitz, J.P.; Hollinger, J.O. The critical-size defect as an experimental model for craniomandibulofacial nonunions. Clin. Orthop Relat Res. 1986, 299–308. [Google Scholar] [CrossRef]
- Traub, W.; Arad, T.; Weiner, S. Three-dimensional ordered distribution of crystals in turkey tendon collagen fibers. Proc. Natl. Acad. Sci. USA 1989, 86, 9822–9826. [Google Scholar] [CrossRef]
- Sisson, K.; Zhang, C.; Farach-Carson, M.C.; Chase, D.B.; Rabolt, J.F. Fiber diameters control osteoblastic cell migration and differentiation in electrospun gelatin. J. Biomed. Mater. Res. 2010, 94, 1312–1320. [Google Scholar] [CrossRef]
- Aquino-Martinez, R.; Rodriguez-Carballo, E.; Gamez, B.; Artigas, N.; Carvalho-Lobato, P.; Manzanares-Cespedes, M.C.; Rosa, J.L.; Ventura, F. Mesenchymal Stem Cells Within Gelatin/CaSO4 Scaffolds Treated Ex Vivo with Low Doses of BMP-2 and Wnt3a Increase Bone Regeneration. Tissue Eng. Part. A 2016, 22, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.K.F.; Gulati, G.S.; Sinha, R.; Tompkins, J.V.; Lopez, M.; Carter, A.C.; Ransom, R.C.; Reinisch, A.; Wearda, T.; Murphy, M.; et al. Identification of the Human Skeletal Stem Cell. Cell 2018, 175, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Cipitria, A.; Lange, C.; Schell, H.; Wagermaier, W.; Reichert, J.C.; Hutmacher, D.W.; Fratzl, P.; Duda, G.N. Porous scaffold architecture guides tissue formation. J. Bone Min. Res. 2012, 27, 1275–1288. [Google Scholar] [CrossRef]
- Lan Levengood, S.K.; Polak, S.J.; Wheeler, M.B.; Maki, A.J.; Clark, S.G.; Jamison, R.D.; Wagoner Johnson, A.J. Multiscale osteointegration as a new paradigm for the design of calcium phosphate scaffolds for bone regeneration. Biomaterials 2010, 31, 3552–3563. [Google Scholar] [CrossRef]
- Hollister, S.J. Porous scaffold design for tissue engineering. Nat. Mater. 2005, 4, 518–524. [Google Scholar] [CrossRef]
- Gauthier, O.; Bouler, J.M.; Aguado, E.; Pilet, P.; Daculsi, G. Macroporous biphasic calcium phosphate ceramics: Influence of macropore diameter and macroporosity percentage on bone ingrowth. Biomaterials 1998, 19, 133–139. [Google Scholar] [CrossRef]
- Payo-Payo, A.; Genovart, M.; Bertolero, A.; Pradel, R.; Oro, D. Consecutive cohort effects driven by density-dependence and climate influence early-life survival in a long-lived bird. Proc. Biol. Sci. 2016, 283. [Google Scholar] [CrossRef]
- Paganelli, C.; Fontana, P.; Porta, F.; Majorana, A.; Pazzaglia, U.E.; Sapelli, P.L. Indications on suitable scaffold as carrier of stem cells in the alveoloplasty of cleft palate. J. Oral Rehabil. 2006, 33, 625–629. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Brammer, K.S.; Li, Y.S.; Teng, D.; Engler, A.J.; Chien, S.; Jin, S. Stem cell fate dictated solely by altered nanotube dimension. Proc. Natl. Acad. Sci. USA 2009, 106, 2130–2135. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Grater, S.V.; Corbellini, F.; Rinck, S.; Bock, E.; Kemkemer, R.; Kessler, H.; Ding, J.; Spatz, J.P. Impact of order and disorder in RGD nanopatterns on cell adhesion. Nano Lett. 2009, 9, 1111–1116. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vordemvenne, T.; Wähnert, D.; Koettnitz, J.; Merten, M.; Fokin, N.; Becker, A.; Büker, B.; Vogel, A.; Kronenberg, D.; Stange, R.; et al. Bone Regeneration: A Novel Osteoinductive Function of Spongostan by the Interplay between Its Nano- and Microtopography. Cells 2020, 9, 654. https://doi.org/10.3390/cells9030654
Vordemvenne T, Wähnert D, Koettnitz J, Merten M, Fokin N, Becker A, Büker B, Vogel A, Kronenberg D, Stange R, et al. Bone Regeneration: A Novel Osteoinductive Function of Spongostan by the Interplay between Its Nano- and Microtopography. Cells. 2020; 9(3):654. https://doi.org/10.3390/cells9030654
Chicago/Turabian StyleVordemvenne, Thomas, Dirk Wähnert, Julian Koettnitz, Madlen Merten, Nadine Fokin, Andreas Becker, Björn Büker, Asaria Vogel, Daniel Kronenberg, Richard Stange, and et al. 2020. "Bone Regeneration: A Novel Osteoinductive Function of Spongostan by the Interplay between Its Nano- and Microtopography" Cells 9, no. 3: 654. https://doi.org/10.3390/cells9030654
APA StyleVordemvenne, T., Wähnert, D., Koettnitz, J., Merten, M., Fokin, N., Becker, A., Büker, B., Vogel, A., Kronenberg, D., Stange, R., Wittenberg, G., Greiner, J. F., Hütten, A., Kaltschmidt, C., & Kaltschmidt, B. (2020). Bone Regeneration: A Novel Osteoinductive Function of Spongostan by the Interplay between Its Nano- and Microtopography. Cells, 9(3), 654. https://doi.org/10.3390/cells9030654







