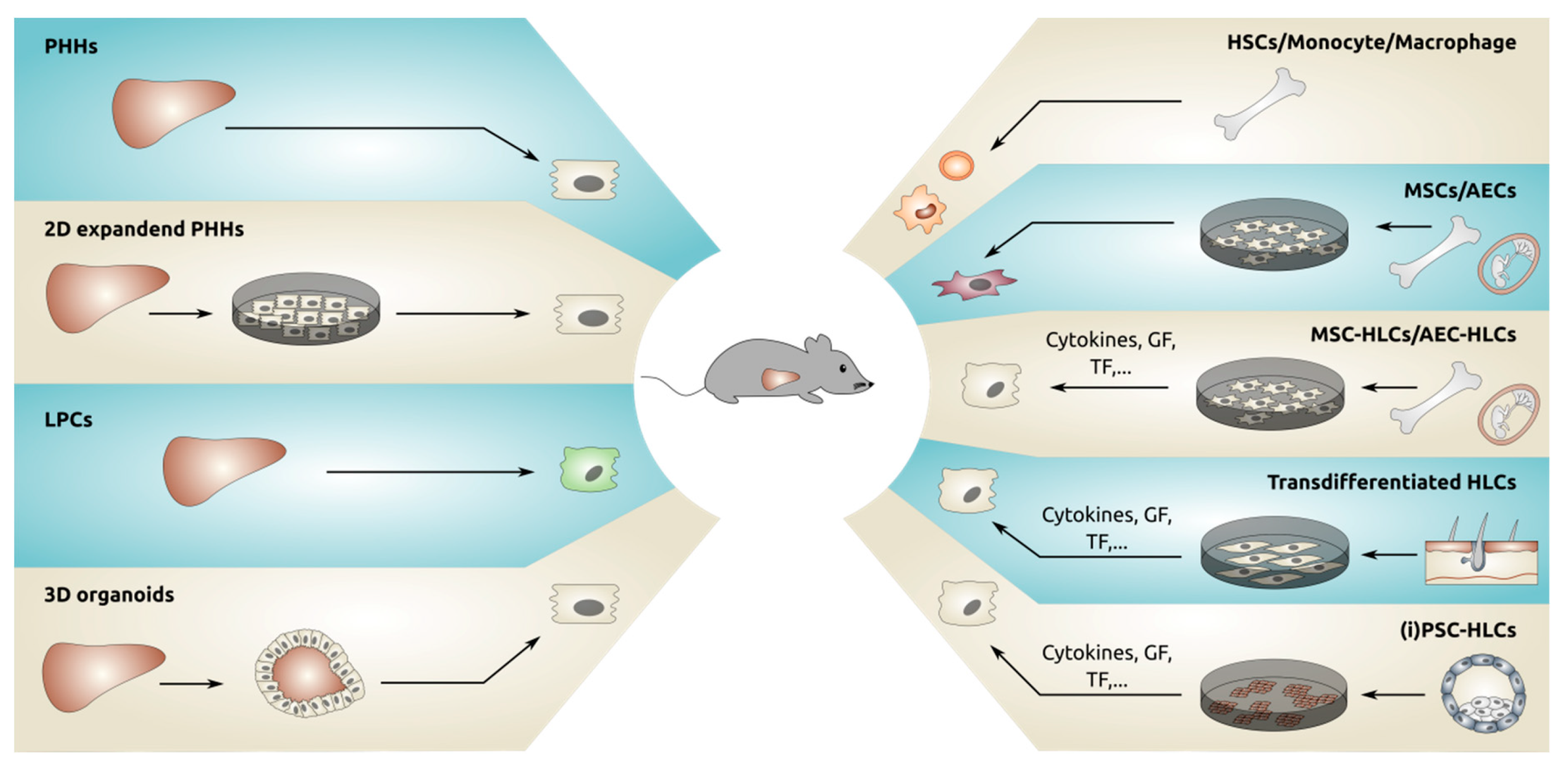
Alternative Cell Sources for Liver Parenchyma Repopulation: Where Do We Stand?
Abstract
1. Introduction
2. Animal Models Used to Investigate Hepatocyte Transplantation in Liver
2.1. Animal Models
2.1.1. Partial Hepatectomy
2.1.2. Chemical Toxin Induced Hepatocyte Loss
2.1.3. Transgene-Induced Liver Damage Mouse Models
2.1.4. Additional Methods to Improve Engraftment Efficiency
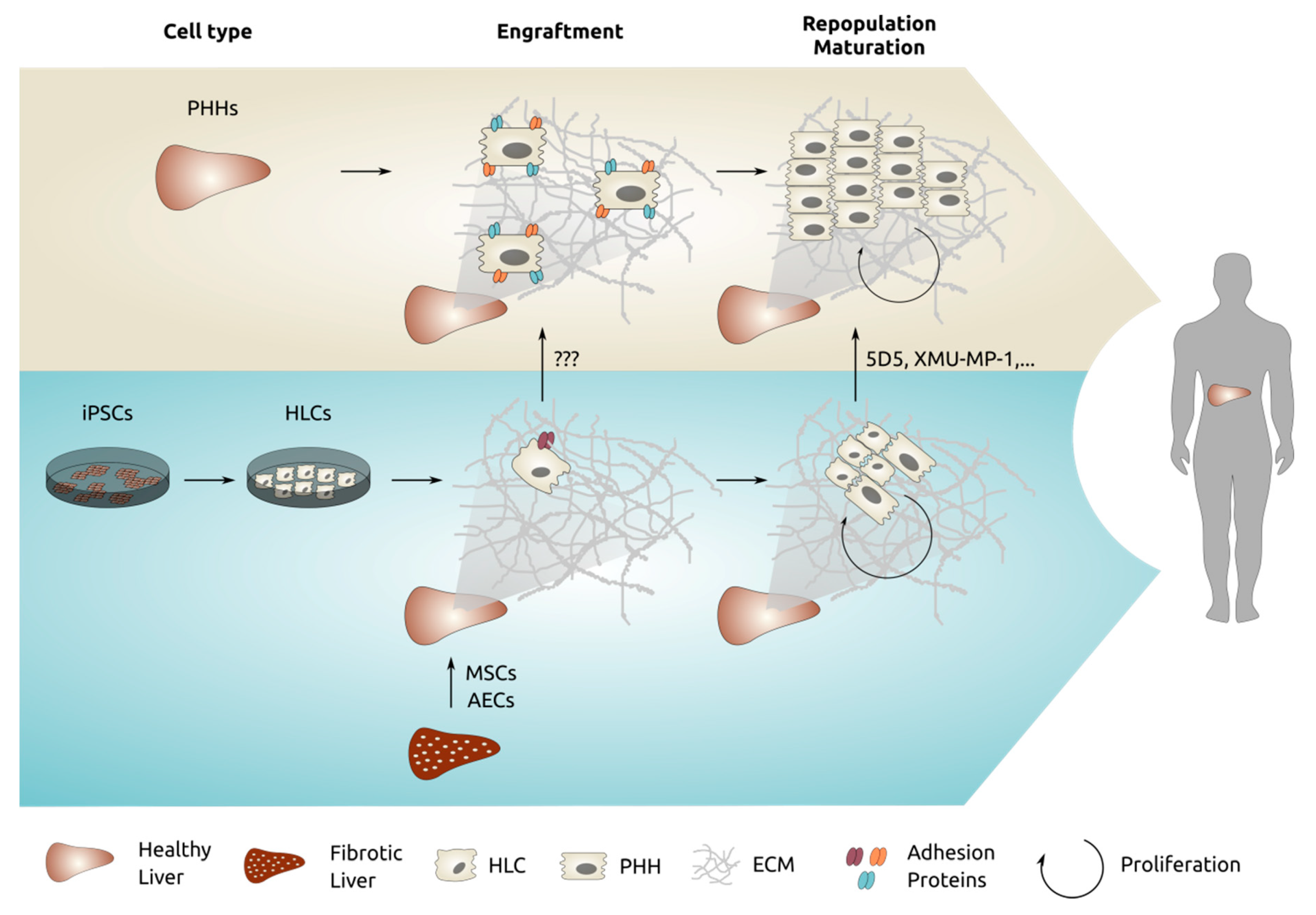
2.2. Read-Out of Engraftment and Repopulation
3. Grafting of Gold-Standard Control, Freshly Isolated Primary Human Hepatocytes
4. Strategies to Create Larger Numbers of Hepatocytes from Primary Liver Tissue
4.1. 2D Cultured Hepatocytes
4.2. Liver Progenitor Cells
4.3. Liver Organoids
5. Generating Hepatocytes from Tissues Other Than Liver
5.1. Hematopoietic Stem Cells
5.2. Mesenchymal Stromal Cells
5.2.1. Undifferentiated Mesenchymal Stromal Cells
5.2.2. Hepatic Differentiated Mesenchymal Stromal Cells
5.3. Amniotic Epithelial Cells
5.4. Transdifferentiation of Fibroblasts Towards Hepatocytes
5.4.1. Direct Transdifferentiation of Fibroblasts Towards Hepatocyte-Like Cells
5.4.2. Transdifferentiation of Somatic Cells Towards Hepatocyte-Like Cells Via an Intermediate Progenitor Stage
5.5. Pluripotent Stem Cell Derived Hepatocytes
5.5.1. Differentiation of Pluripotent Stem Cells Towards Hepatocytes Via Embryoid Body Formation
5.5.2. Directed Differentiation of Pluripotent Stem Cells Towards Hepatocytes
6. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Ko, S.; Russell, J.O.; Molina, L.M.; Monga, S.P. Liver Progenitors and Adult Cell Plasticity in Hepatic Injury and Repair: Knowns and Unknowns. Annu. Rev. Pathol. Mech. Dis. 2020, 15, 23–50. [Google Scholar] [CrossRef] [PubMed]
- Najimi, M.; Defresne, F.; Sokal, E.M. Concise Review: Updated Advances and Current Challenges in Cell Therapy for Inborn Liver Metabolic Defects. Stem Cells Transl. Med. 2016, 5, 1117–1125. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Willenbring, H.; Akkari, Y.; Torimaru, Y.; Foster, M.; Al-Dhalimy, M.; Lagasse, E.; Finegold, M.; Olson, S.; Grompe, M. Cell fusion is the principal source of bone-marrow-derived hepatocytes. Nature 2003, 422, 897–901. [Google Scholar] [CrossRef] [PubMed]
- Fujino, H.; Hiramatsu, H.; Tsuchiya, A.; Niwa, A.; Noma, H.; Shiota, M.; Umeda, K.; Yoshimoto, M.; Ito, M.; Heike, T.; et al. Human cord blood CD34+ cells develop into hepatocytes in the livers of NOD/SCID/γcnull mice through cell fusion. FASEB J. 2007, 21, 3499–3510. [Google Scholar] [CrossRef] [PubMed]
- Woo, D.H.; Kim, S.E.S.K.; Lim, H.J.; Heo, J.; Park, H.S.; Kang, G.Y.; Kim, S.E.S.K.; You, H.J.; Hoeppner, D.J.; Kim, Y.; et al. Direct and indirect contribution of human embryonic stem cellderived hepatocyte-like cells to liver repair in mice. Gastroenterology 2012, 142, 602–611. [Google Scholar] [CrossRef]
- Li, T.; Yan, Y.; Wang, B.; Qian, H.; Zhang, X.; Shen, L.; Wang, M.; Zhou, Y.; Zhu, W.; Li, W.; et al. Exosomes Derived from Human Umbilical Cord Mesenchymal Stem Cells Alleviate Liver Fibrosis. Stem Cells Dev. 2012, 22, 845–854. [Google Scholar] [CrossRef]
- Dhawan, A.; Puppi, J.; Hughes, R.D.; Mitry, R.R. Human hepatocyte transplantation: Current experience and future challenges. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 288–298. [Google Scholar] [CrossRef]
- Overturf, K.; Al-Dhalimy, M.; Ou, C.N.; Finegold, M.; Grompe, M. Serial transplantation reveals the stem-cell-like regenerative potential of adult mouse hepatocytes. Am. J. Pathol. 1997, 151, 1273–1280. [Google Scholar]
- Shi, D.; Zhang, J.; Zhou, Q.; Xin, J.; Jiang, J.; Jiang, L.; Wu, T.; Li, J.; Ding, W.; Li, J.; et al. Quantitative evaluation of human bone mesenchymal stem cells rescuing fulminant hepatic failure in pigs. Gut 2017, 66, 955–964. [Google Scholar] [CrossRef]
- Michalopoulos, G.K. Liver regeneration after partial hepatectomy: Critical analysis of mechanistic dilemmas. Am. J. Pathol. 2010, 176, 2–13. [Google Scholar] [CrossRef]
- Villa-trevio, S.; Leavert, D.D. Effects of the Hepatotoxic Agents Retrorsine and Aflatoxin B1 on Hepatic Protein Synthesis in Rat. Biochem. J. 1968, 109, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Rhim, J.A.; Sandgren, E.P.; Degen, J.L.; Palmiter, R.D.; Brinster, R.L. Replacement of diseased mouse liver by hepatic cell transplantation. Science 1994, 263, 1149–1152. [Google Scholar] [CrossRef] [PubMed]
- Tateno, C.; Kawase, Y.; Tobita, Y.; Hamamura, S.; Ohshita, H.; Yokomichi, H.; Sanada, H.; Kakuni, M.; Shiota, A.; Kojima, Y.; et al. Generation of novel Chimeric mice with humanized livers by using hemizygous cDNA-uPA/SCID mice. PLoS ONE 2015, 10, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Petersen, J.; Dandri, M.; Gupta, S.; Rogler, C.E. Liver repopulation with xenogenic hepatocytes in B and T cell-deficient mice leads to chronic hepadnavirus infection and clonal growth of hepatocellular carcinoma. Proc. Natl. Acad. Sci. USA 1998, 95, 310–315. [Google Scholar] [CrossRef]
- Dandri, M.; Burda, M.R.; Török, E.; Polok, J.M.; Iwanska, A.; Sommer, G.; Rogiers, X.; Rogler, C.E.; Gupta, S.; Will, H.; et al. Repopulation of mouse liver with human hepatocytes and in vivo infection with hepatitis B virus. Hepatology 2001, 33, 981–988. [Google Scholar] [CrossRef]
- Haridass, D.; Yuan, Q.; Becker, P.D.; Cantz, T.; Iken, M.; Rothe, M.; Narain, N.; Bock, M.; Nörder, M.; Legrand, N.; et al. Repopulation efficiencies of adult hepatocytes, fetal liver progenitor cells, and embryonic stem cell-derived hepatic cells in albumin-promoter- enhancer urokinase-type plasminogen activator mice. Am. J. Pathol. 2009, 175, 1483–1492. [Google Scholar] [CrossRef]
- Azuma, H.; Paulk, N.; Ranade, A.; Dorrell, C.; Al-Dhalimy, M.; Ellis, E.; Strom, S.; Kay, M.A.; Finegold, M.; Grompe, M. Robust expansion of human hepatocytes in Fah-/-/Rag2-/-/Il2rg-/- mice. Nat. Biotechnol. 2007, 25, 903–910. [Google Scholar] [CrossRef]
- Bissig, K.-D.; Le, T.T.; Woods, N.-B.; Verma, I.M. Repopulation of adult and neonatal mice with human hepatocytes: A chimeric animal model. Proc. Natl. Acad. Sci. USA 2007, 104, 20507–20511. [Google Scholar] [CrossRef]
- Wilson, E.M.; Bial, J.; Tarlow, B.; Bial, G.; Jensen, B.; Greiner, D.L.; Brehm, M.A.; Grompe, M. Extensive double humanization of both liver and hematopoiesis in FRGN mice. Stem Cell Res. 2014, 13, 404–412. [Google Scholar] [CrossRef]
- Yuan, L.; Liu, X.; Zhang, L.; Li, X.; Zhang, Y.; Wu, K.; Chen, Y.; Cao, J.; Hou, W.; Zhang, J.; et al. A chimeric humanized mouse model by engrafting the human induced pluripotent stem cell-derived hepatocyte-like cell for the chronic hepatitis B virus infection. Front. Microbiol. 2018, 9, 1–14. [Google Scholar] [CrossRef]
- Yuan, L.; Zhang, Y.; Liu, X.; Chen, Y.; Zhang, L.; Cao, J.; Li, X.; Wang, M.; Wu, K.; Zhang, J.; et al. Agonist c-Met Monoclonal Antibody Augments the Proliferation of hiPSC-derived Hepatocyte-Like Cells and Improves Cell Transplantation Therapy for Liver Failure in Mice. Theranostics 2019, 9, 2115–2128. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, M.; Kawai, K.; Mitsui, T.; Taniguchi, K.; Monnai, M.; Wakui, M.; Ito, M.; Suematsu, M.; Peltz, G.; Nakamura, M.; et al. The reconstituted “humanized liver” in TK-NOG mice is mature and functional. Biochem. Biophys. Res. Commun. 2011, 405, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Kosaka, K.; Hiraga, N.; Imamura, M.; Yoshimi, S.; Murakami, E.; Nakahara, T.; Honda, Y.; Ono, A.; Kawaoka, T.; Tsuge, M.; et al. A novel TK-NOG based humanized mouse model for the study of HBV and HCV infections. Biochem. Biophys. Res. Commun. 2013, 441, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Washburn, M.L.; Bility, M.T.; Zhang, L.; Kovalev, G.I.; Frelinger, J.A.; Barry, W.; Ploss, A.; Rice, C.M.; Su, L. A humanized mouse model to study hepatitis C virus infection, immune response, and liver disease. Gastroenterology 2011, 140, 1334–1344. [Google Scholar] [CrossRef] [PubMed]
- Bility, M.T.; Zhang, L.; Washburn, M.L.; Curtis, T.A.; Kovalev, G.I.; Su, L. Generation of a humanized mouse model with both human immune system and liver cells to model hepatitis C virus infection and liver immunopathogenesis. Nat. Protoc. 2012, 7, 1608–1617. [Google Scholar] [CrossRef] [PubMed]
- Tateno, C.; Yoshizane, Y.; Saito, N.; Kataoka, M.; Utoh, R.; Yamasaki, C.; Tachibana, A.; Soeno, Y.; Asahina, K.; Hino, H.; et al. Near completely humanized liver in mice shows human-type metabolic responses to drugs. Am. J. Pathol. 2004, 165, 901–912. [Google Scholar] [CrossRef]
- Huch, M.; Gehart, H.; Van Boxtel, R.; Hamer, K.; Blokzijl, F.; Verstegen, M.M.A.A.; Ellis, E.; Van Wenum, M.; Fuchs, S.A.; De Ligt, J.; et al. Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell 2015, 160, 299–312. [Google Scholar] [CrossRef]
- Berry, M.N.; Friend, D.S. High-yield preparation of isolated rat liver parenchymal cells: A biochemical and fine structural study. J. Cell Biol. 1969, 43, 506–520. [Google Scholar] [CrossRef]
- Groth, C.G.; Arborgh, B.; Björkén, C.; Sundberg, B.; Lundgren, G. Correction of hyperbilirubinemia in the glucuronyltransferase-deficient rat by intraportal hepatocyte transplantation. Transplant. Proc. 1977, 9, 313–316. [Google Scholar]
- Guguen-Guillouzo, C.; Campion, J.P.; Brissot, P.; Glaise, D.; Launois, B.; Bourel, M.; Guillouzo, A. High yield preparation of isolated human adult hepatocytes by enzymatic perfusion of the liver. Cell Biol. Int. Rep. 1982, 6, 625–628. [Google Scholar] [CrossRef]
- Meuleman, P.; Libbrecht, L.; De Vos, R.; De Hemptinne, B.; Gevaert, K.; Vandekerckhove, J.; Roskams, T.; Leroux-Roels, G. Morphological and biochemical characterization of a human liver in a uPA-SCID mouse chimera. Hepatology 2005, 41, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Mercer, D.F.; Schiller, D.E.; Elliott, J.F.; Douglas, D.N.; Hao, C.; Rinfret, A.; Addison, W.R.; Fischer, K.P.; Churchill, T.A.; Lakey, J.R.T.; et al. Hepatitis C virus replication in mice with chimeric human livers. Nat. Med. 2001, 7, 927–933. [Google Scholar] [CrossRef] [PubMed]
- Meuleman, P.; Steyaert, S.; Libbrecht, L.; Couvent, S.; Van Houtte, F.; Clinckspoor, F.; De Hemptinne, B.; Roskams, T.; Vanlandschoot, P.; Leroux-Roels, G. Human hepatocytes secrete soluble CD14, a process not directly influenced by HBV and HCV infection. Clin. Chim. Acta 2006, 366, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Meuleman, P.; Catanese, M.T.; Verhoye, L.; Desombere, I.; Jones, C.T.; Sheahan, T.; Grzyb, K.; Rice, C.M.; Leroux-roels, G.; Nicosia, A.; et al. A human monoclonal antibody targeting SR-BI precludes hepatitis C virus infection and viral spread in vitro and in vivo. Hepatology 2012, 55, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Bissig, K.D.; Wieland, S.F.; Tran, P.; Isogawa, M.; Le, T.T.; Chisari, F.V.; Verma, I.M. Human liver chimeric mice provide a model for hepatitis B and C virus infection and treatment. J. Clin. Invest. 2010, 120, 924–930. [Google Scholar] [CrossRef] [PubMed]
- Grompe, M.; Strom, S. Mice with human livers. Gastroenterology 2013, 145, 1209–1214. [Google Scholar] [CrossRef]
- Ostrowska, A.; Bode, D.C.; Pruss, J.; Bilir, B.; Smith, G.D.; Zeisloft, S. Investigation of functional and morphological integrity of freshly isolated and cryopreserved human hepatocytes. Cell Tissue Bank. 2000, 1, 55–68. [Google Scholar] [CrossRef]
- Terry, C.; Mitry, R.R.; Lehec, S.C.; Muiesan, P.; Rela, M.; Heaton, N.D.; Hughes, R.D.; Dhawan, A. The effects of cryopreservation on human hepatocytes obtained from different sources of liver tissue. Cell Transplant. 2005, 14, 585–594. [Google Scholar] [CrossRef]
- Iansante, V.; Mitry, R.R.; Filippi, C.; Fitzpatrick, E.; Dhawan, A. Human hepatocyte transplantation for liver disease: Current status and future perspectives. Pediatr. Res. 2018, 83, 232–240. [Google Scholar] [CrossRef]
- Mito, M.; Kusano, M. Hepatocyte Transplantation in Man. Cell Transplant. 1993, 2, 65–74. [Google Scholar] [CrossRef]
- Fisher, R.A.; Strom, S.C. Human hepatocyte transplantation: Worldwide results. Transplantation 2006, 82, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Gehart, H.; Artegiani, B.; Löpez-Iglesias, C.; Dekkers, F.; Basak, O.; van Es, J.; Chuva de Sousa Lopes, S.M.; Begthel, H.; Korving, J.; et al. Long-Term Expansion of Functional Mouse and Human Hepatocytes as 3D Organoids. Cell 2018, 175, 1591–1606.e19. [Google Scholar] [CrossRef] [PubMed]
- El Filali, E.; Duijst, S.; Hiralall, J.K.; Legrand, N.; van Gulik, T.; Hoekstra, R.; Seppen, J. Human fetal liver cells for regulated ex vivo erythropoietin gene therapy. Mol. Ther. Methods Clin. Dev. 2014, 1, 14003. [Google Scholar] [CrossRef]
- He, Z.; Zhang, H.; Zhang, X.; Xie, D.; Chen, Y.; Wangensteen, K.J.; Ekker, S.C.; Firpo, M.; Liu, C.; Xiang, D.; et al. Liver xeno-repopulation with human hepatocytes in Fah -/-Rag2-/- mice after pharmacological immunosuppression. Am. J. Pathol. 2010, 177, 1311–1319. [Google Scholar] [CrossRef] [PubMed]
- Billerbeck, E.; Mommersteeg, M.C.; Shlomai, A.; Xiao, J.W.; Andrus, L.; Bhatta, A.; Vercauteren, K.; Michailidis, E.; Dorner, M.; Krishnan, A.; et al. Humanized mice efficiently engrafted with fetal hepatoblasts and syngeneic immune cells develop human monocytes and NK cells. J. Hepatol. 2016, 65, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Unzu, C.; Planet, E.; Brandenberg, N.; Fusil, F.; Cassano, M.; Perez-Vargas, J.; Friedli, M.; Cosset, F.-L.; Lutolf, M.P.; Wildhaber, B.E.; et al. Pharmacological Induction of a Progenitor State for the Efficient Expansion of Primary Human Hepatocytes. Hepatology 2019, 69, 2214–2231. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kang, K.; Lee, S.B.; Seo, D.; Yoon, S.; Kim, S.J.; Jang, K.; Jung, Y.K.; Lee, K.G.; Factor, V.M.; et al. Small molecule-mediated reprogramming of human hepatocytes into bipotent progenitor cells. J. Hepatol. 2019, 70, 97–107. [Google Scholar] [CrossRef]
- Fu, G.B.; Huang, W.J.; Zeng, M.; Zhou, X.; Wu, H.P.; Liu, C.C.; Wu, H.; Weng, J.; Zhang, H.D.; Cai, Y.C.; et al. Expansion and differentiation of human hepatocyte-derived liver progenitor-like cells and their use for the study of hepatotropic pathogens. Cell Res. 2019, 29, 8–22. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, L.; Liu, W.; Ma, X.; Cen, J.; Sun, Z.; Wang, C.; Feng, S.; Zhang, Z.; Yue, L.; et al. In Vitro Expansion of Primary Human Hepatocytes with Efficient Liver Repopulation Capacity. Cell Stem Cell 2018, 23, 806–819. [Google Scholar] [CrossRef]
- Katsuda, T.; Matsuzaki, J.; Yamaguchi, T.; Yamada, Y.; Prieto-Vila, M.; Hosaka, K.; Takeuchi, A.; Saito, Y.; Ochiya, T. Generation of human hepatic progenitor cells with regenerative and metabolic capacities from primary hepatocytes. eLife 2019, 8, e47313. [Google Scholar] [CrossRef]
- Irudayaswamy, A.; Muthiah, M.; Zhou, L.; Hung, H.; Jumat, N.H.B.; Haque, J.; Teoh, N.; Farrell, G.; Riehle, K.J.; Lin, J.S.; et al. Long-Term Fate of Human Fetal Liver Progenitor Cells Transplanted in Injured Mouse Livers. Stem Cells 2018, 36, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Sokal, E.M.; Stéphenne, X.; Ottolenghi, C.; Jazouli, N.; Clapuyt, P.; Lacaille, F.; Najimi, M.; de Lonlay, P.; Smets, F. Liver Engraftment and Repopulation by In Vitro Expanded Adult Derived Human Liver Stem Cells in a Child with Ornithine Carbamoyltransferase Deficiency. JIMD Rep. 2013, 13, 65–72. [Google Scholar]
- Smets, F.; Dobbelaere, D.; McKiernan, P.; Dionisi-Vici, C.; Broue, P.; Jacquemin, E.; Lopes, A.I.; Goncalves, I.; Mandel, H.; Pawlowska, J.; et al. Phase I/II Trial of Liver-derived Mesenchymal Stem Cells in Pediatric Liver-based Metabolic Disorders: A Prospective, Open Label, Multicenter, Partially Randomized, Safety Study of One Cycle of Heterologous Human Adult Liver-derived Progenitor Cells (HepaStem) in Urea Cycle Disorders and Crigler-Najjar Syndrome Patients. Transplantation 2019, 103, 1903–1915. [Google Scholar] [PubMed]
- Clevers, H. Modeling Development and Disease with Organoids. Cell 2016, 165, 1586–1597. [Google Scholar] [CrossRef] [PubMed]
- Lombard, C.A.; Prigent, J.; Sokal, E.M. Human Liver Progenitor Cells for Liver Repair. Cell Med. 2013, 5, 1–16. [Google Scholar] [CrossRef]
- Garnier, D.; Li, R.; Delbos, F.; Fourrier, A.; Collet, C.; Guguen-Guillouzo, C.; Chesné, C.; Nguyen, T.H. Expansion of human primary hepatocytes in vitro through their amplification as liver progenitors in a 3D organoid system. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Petersen, B.E.; Bowen, W.C.; Patrene, K.D.; Mars, W.M.; Sullivan, A.K.; Murase, N.; Boggs, S.S.; Greenberger, J.S.; Goff, J.P. Bone marrow as a potential source of hepatic oval cells. Science 1999, 284, 1168–1170. [Google Scholar] [CrossRef]
- Alison, M.R.; Poulsom, R.; Jeffery, R.; Dhillon, A.P.; Quaglia, A.; Jacob, J.; Novelli, M.; Prentice, G.; Williamson, J.; Wright, N.A. Hepatocytes from non-hepatic adult stem cells. Nature 2000, 406, 257. [Google Scholar] [CrossRef]
- Lagasse, E.; Connors, H.; Al-Dhalimy, M.; Reitsma, M.; Dohse, M.; Osborne, L.; Wang, X.; Finegold, M.; Weissman, I.L.; Grompe, M. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat. Med. 2000, 6, 1229–1234. [Google Scholar] [CrossRef]
- Willenbring, H.; Bailey, A.S.; Foster, M.; Akkari, Y.; Dorrell, C.; Olson, S.; Finegold, M.; Fleming, W.H.; Grompe, M. Myelomonocytic cells are sufficient for therapeutic cell fusion in liver. Nat. Med. 2004, 10, 744–748. [Google Scholar] [CrossRef]
- Vassilopoulos, G.; Wang, P.R.; Russell, D.W. Transplanted bone marrow regenerates liver by cell fusion. Nature 2003, 422, 901–904. [Google Scholar] [CrossRef] [PubMed]
- Myerson, D.; Parkin, R.K. Donor-derived hepatocytes in human hematopoietic cell transplant recipients: Evidence of fusion. Virchows Arch. 2019, 474, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Montini, E.; Al-Dhalimy, M.; Lagasse, E.; Finegold, M.; Grompe, M. Kinetics of liver repopulation after bone marrow transplantation. Am. J. Pathol. 2002, 161, 565–574. [Google Scholar] [CrossRef]
- Jang, Y.-Y.; Collector, M.I.; Baylin, S.B.; Diehl, A.M.; Sharkis, S.J. Hematopoietic stem cells convert into liver cells within days without fusion. Nat. Cell Biol. 2004, 6, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Campard, D.; Lysy, P.A.; Najimi, M.; Sokal, E.M. Native Umbilical Cord Matrix Stem Cells Express Hepatic Markers and Differentiate Into Hepatocyte-like Cells. Gastroenterology 2008, 134, 833–848. [Google Scholar] [CrossRef]
- Yang, J.F.; Cao, H.C.; Pan, Q.L.; Yu, J.; Li, J.; Li, L.J. Mesenchymal stem cells from the human umbilical cord ameliorate fulminant hepatic failure and increase survival in mice. Hepatob. Pancreat. Dis. Int. 2015, 14, 186–193. [Google Scholar] [CrossRef]
- Zhang, D.G.; Jiang, M.Y.; Miao, D. Transplanted human amniotic membrane-derived mesenchymal stem cells ameliorate carbon tetrachloride-induced liver cirrhosis in mouse. PLoS ONE 2011, 6, e16789. [Google Scholar] [CrossRef]
- Cargnoni, A.; Farigu, S.; Cotti Piccinelli, E.; Bonassi Signoroni, P.; Romele, P.; Vanosi, G.; Toschi, I.; Cesari, V.; Barros Sant’Anna, L.; Magatti, M.; et al. Effect of human amniotic epithelial cells on pro-fibrogenic resident hepatic cells in a rat model of liver fibrosis. J. Cell. Mol. Med. 2018, 22, 1202–1213. [Google Scholar] [CrossRef]
- Yuan, L.; Jiang, J.; Liu, X.; Zhang, Y.; Zhang, L.; Xin, J.; Wu, K.; Li, X.; Cao, J.; Guo, X.; et al. HBV infection-induced liver cirrhosis development in dual-humanised mice with human bone mesenchymal stem cell transplantation. Gut 2019, 68, 2044–2056. [Google Scholar] [CrossRef]
- Meier, R.P.H.; Mahou, R.; Morel, P.; Meyer, J.; Montanari, E.; Muller, Y.D.; Christofilopoulos, P.; Wandrey, C.; Gonelle-Gispert, C.; Bühler, L.H. Microencapsulated human mesenchymal stem cells decrease liver fibrosis in mice. J. Hepatol. 2015, 62, 634–641. [Google Scholar] [CrossRef]
- Rengasamy, M.; Singh, G.; Fakharuzi, N.A.; Siddikuzzaman; Balasubramanian, S.; Swamynathan, P.; Thej, C.; Sasidharan, G.; Gupta, P.K.; Das, A.K.; et al. Transplantation of human bone marrow mesenchymal stromal cells reduces liver fibrosis more effectively than Wharton’s jelly mesenchymal stromal cells. Stem Cell Res. Ther. 2017, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Khuu, D.N.; Nyabi, O.; Maerckx, C.; Sokal, E.; Najimi, M. Adult Human Liver Mesenchymal Stem/Progenitor Cells Participate in Mouse Liver Regeneration after Hepatectomy. Cell Transplant. 2013, 22, 1369–1380. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Zhang, Z.; Xu, R.; Lin, H.; Fu, J.; Zou, Z.; Zhang, A.; Shi, J.; Chen, L.; Lv, S.; et al. Human Mesenchymal Stem Cell Transfusion Is Safe and Improves Liver Function in Acute-on-Chronic Liver Failure Patients. Stem Cells Transl. Med. 2012, 1, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lin, H.; Shi, M.; Xu, R.; Fu, J.; Lv, J.; Chen, L.; Lv, S.; Li, Y.; Yu, S.; et al. Human umbilical cord mesenchymal stem cells improve liver function and ascites in decompensated liver cirrhosis patients. J. Gastroenterol. Hepatol. 2012, 27, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Suk, K.T.; Yoon, J.-H.; Kim, M.Y.; Kim, C.W.; Kim, J.K.; Park, H.; Hwang, S.G.; Kim, D.J.; Lee, B.S.; Lee, S.H.; et al. Transplantation With Autologous Bone Marrow-Derived Mesenchymal Stem Cells for Alcoholic Cirrhosis: Phase 2 Trial. Hepatology 2016, 64, 2185–2197. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Zhao, L.; Duan, J.; Li, L. Strategies to improve the efficiency of mesenchymal stem cell transplantation for reversal of liver fibrosis. J. Cell. Mol. Med. 2019, 23, 1657–1670. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, Y.; Li, Q.; Liu, K.; Hou, J.; Shao, C.; Wang, Y. Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat. Rev. Nephrol. 2018, 14, 493–507. [Google Scholar] [CrossRef]
- Wang, J.; Cen, P.; Chen, J.; Fan, L.; Li, J.; Cao, H.; Li, L. Role of mesenchymal stem cells, their derived factors, and extracellular vesicles in liver failure. Stem Cell Res. Ther. 2017, 8, 137. [Google Scholar] [CrossRef]
- Freeman, B.T.; Kouris, N.A.; Ogle, B.M. Tracking Fusion of Human Mesenchymal Stem Cells After Transplantation to the Heart. Stem Cells Transl. Med. 2015, 4, 685–694. [Google Scholar] [CrossRef]
- Haga, H.; Yan, I.K.; Takahashi, K.; Matsuda, A.; Patel, T. Extracellular vesicles from bone marrow-derived mesenchymal stem cells improve survival from lethal hepatic failure in mice. Stem Cells Transl. Med. 2017, 6, 1262–1272. [Google Scholar] [CrossRef]
- Baertschiger, R.M.; Serre-Beinier, V.; Morel, P.; Bosco, D.; Peyrou, M.; Clément, S.; Sgroi, A.; Kaelin, A.; Buhler, L.H.; Gonelle-Gispert, C. Fibrogenic potential of human multipotent mesenchymal stromal cells in injured liver. PLoS ONE 2009, 4, e6657. [Google Scholar] [CrossRef]
- Berglund, A.K.; Fortier, L.A.; Antczak, D.F.; Schnabel, L. V Immunoprivileged no more: Measuring the immunogenicity of allogeneic adult mesenchymal stem cells. Stem Cell Res. Ther. 2017, 8, 288. [Google Scholar] [CrossRef] [PubMed]
- Magatti, M.; Vertua, E.; Cargnoni, A.; Silini, A.; Parolini, O. The Immunomodulatory Properties of Amniotic Cells: The Two Sides of the Coin. Cell Transplant. 2018, 27, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Russo, F.P.; Alison, M.R.; Bigger, B.W.; Amofah, E.; Florou, A.; Amin, F.; Bou-Gharios, G.; Jeffery, R.; Iredale, J.P.; Forbes, S.J. The Bone Marrow Functionally Contributes to Liver Fibrosis. Gastroenterology 2006, 130, 1807–1821. [Google Scholar] [CrossRef] [PubMed]
- Aurich, H.; Sgodda, M.; Kaltwaßer, P.; Vetter, M.; Weise, A.; Liehr, T.; Brulport, M.; Hengstler, J.G.; Dollinger, M.M.; Fleig, W.E.; et al. Hepatocyte differentiation of mesenchymal stem cells from human adipose tissue in vitro promotes hepatic integration in vivo. Gut 2009, 58, 570–581. [Google Scholar] [CrossRef]
- Banas, A.; Teratani, T.; Yamamoto, Y.; Tokuhara, M.; Takeshita, F.; Quinn, G.; Okochi, H.; Ochiya, T. Adipose tissue-derived mesenchymal stem cells as a source of human hepatocytes. Hepatology 2007, 46, 219–228. [Google Scholar] [CrossRef]
- Sargiacomo, C.; El-Kehdy, H.; Dallmeier, K.; De Kock, J.; Hernandez-Kelly, C.; Rogiers, V.; Ortega, A.; Neyts, J.; Sokal, E.; Najimi, M. Upregulation of sodium taurocholate cotransporter polypeptide during hepatogenic differentiation of umbilical cord matrix mesenchymal stem cells facilitates hepatitis B entry. Stem Cell Res. Ther. 2017, 8, 1–11. [Google Scholar] [CrossRef]
- Ong, S.Y.; Dai, H.; Leong, K.W. Inducing hepatic differentiation of human mesenchymal stem cells in pellet culture. Biomaterials 2006, 27, 4087–4097. [Google Scholar] [CrossRef]
- Zhao, Q.; Ren, H.; Li, X.; Chen, Z.; Zhang, X.; Gong, W.; Liu, Y.; Pang, T.; Han, Z.C. Differentiation of human umbilical cord mesenchymal stromal cells into low immunogenic hepatocyte-like cells. Cytotherapy 2009, 11, 414–426. [Google Scholar] [CrossRef]
- Ling, L.; Ni, Y.; Wang, Q.; Wang, H.; Hao, S.; Hu, Y.; Jiang, W.; Hou, Y. Transdifferentiation of mesenchymal stem cells derived from human fetal lung to hepatocyte-like cells. Cell Biol. Int. 2008, 32, 1091–1098. [Google Scholar] [CrossRef]
- Lue, J.; Lin, G.; Ning, H.; Xiong, A.; Lin, C.S.; Glenn, J.S. Transdifferentiation of adipose-derived stem cells into hepatocytes: A new approach. Liver Int. 2010, 30, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.Q.; Zang, W.J.; Bao, L.J.; Li, D.L.; Song, T.S.; Xu, X.L.; Yu, X.J. Fibroblast growth factor-4 and hepatocyte growth factoc induce differentiation of human umbilical cord blood-derived mesenchymal stem cells into hepatocytes. World J. Gastroenterol. 2005, 11, 7461–7465. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, R.E.; Reyes, M.; Koodie, L.; Jiang, Y.; Blackstad, M.; Lund, T.; Lenvik, T.; Johnson, S.; Hu, W.S.; Verfaillie, C.M. Multipotent adult progenitor cells from bone marrow differentiate into functional hepatocyte-like cells. J. Clin. Invest. 2002, 109, 1291–1302. [Google Scholar] [CrossRef] [PubMed]
- Snykers, S.; Vanhaecke, T.; Papeleu, P.; Luttun, A.; Jiang, Y.; Vander Heyden, Y.; Verfaillie, C.; Rogiers, V. Sequential exposure to cytokines reflecting embryogenesis: The key for in vitro differentiation of adult bone marrow stem cells into functional hepatocyte-like cells. Toxicol. Sci. 2006, 94, 330–341. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yu, Y.B.; Song, Y.; Chen, Y.; Zhang, F.; Qi, F.Z. Differentiation of umbilical cord mesenchymal stem cells into hepatocytes in comparison with bone marrow mesenchymal stem cells. Mol. Med. Rep. 2018, 18, 2009–2016. [Google Scholar] [CrossRef]
- An, S.Y.; Han, J.; Lim, H.J.; Park, S.Y.; Kim, J.H.; Do, B.R.; Kim, J.H. Valproic acid promotes differentiation of hepatocyte-like cells from whole human umbilical cord-derived mesenchymal stem cells. Tissue Cell 2014, 46, 127–135. [Google Scholar] [CrossRef]
- Zhou, X.; Cui, L.; Zhou, X.; Yang, Q.; Wang, L.; Guo, G.; Hou, Y.; Cai, W.; Han, Z.; Shi, Y.; et al. Induction of hepatocyte-like cells from human umbilical cord-derived mesenchymal stem cells by defined microRNAs. J. Cell. Mol. Med. 2017, 21, 881–893. [Google Scholar] [CrossRef]
- Cui, L.; Shi, Y.; Zhou, X.; Wang, X.; Wang, J.; Lan, Y.; Wang, M.; Zheng, L.; Li, H.; Wu, Q.; et al. A set of microRNAs mediate direct conversion of human umbilical cord lining-derived mesenchymal stem cells into hepatocytes. Cell Death Dis. 2013, 4, 1–9. [Google Scholar] [CrossRef]
- Khosravi, M.; Azarpira, N.; Shamdani, S.; Hojjat-Assari, S.; Naserian, S.; Karimi, M.H. Differentiation of umbilical cord derived mesenchymal stem cells to hepatocyte cells by transfection of miR-106a, miR-574-3p, and miR-451. Gene 2018, 667, 1–9. [Google Scholar] [CrossRef]
- Ji, R.; Zhang, N.; You, N.; Li, Q.; Liu, W.; Jiang, N.; Liu, J.; Zhang, H.; Wang, D.; Tao, K.; et al. The differentiation of MSCs into functional hepatocyte-like cells in a liver biomatrix scaffold and their transplantation into liver-fibrotic mice. Biomaterials 2012, 33, 8995–9008. [Google Scholar] [CrossRef]
- Hashemi, S.M.; Soleimani, M.; Zargarian, S.S.; Haddadi-Asl, V.; Ahmadbeigi, N.; Soudi, S.; Gheisari, Y.; Hajarizadeh, A.; Mohammadi, Y. In vitro differentiation of human cord blood-derived unrestricted somatic stem cells into hepatocyte-like cells on poly(ε-caprolactone) nanofiber scaffolds. Cells Tissues Organs 2009, 190, 135–149. [Google Scholar] [CrossRef]
- Hang, H.; Yu, Y.; Wu, N.; Huang, Q.; Xia, Q.; Bian, J. Induction of highly functional hepatocytes from human umbilical cord mesenchymal stem cells by HNF4α transduction. PLoS ONE 2014, 9, e104133. [Google Scholar] [CrossRef]
- Stock, P.; Staege, M.S.; Müller, L.P.; Sgodda, M.; Völker, A.; Volkmer, I.; Lützkendorf, J.; Christ, B. Hepatocytes Derived From Adult Stem Cells. Transplant. Proc. 2008, 40, 620–623. [Google Scholar] [CrossRef]
- Liu, Q.-W.; Liu, Q.-Y.; Li, J.-Y.; Wei, L.; Ren, K.-K.; Zhang, X.-C.; Ding, T.; Xiao, L.; Zhang, W.-J.; Wu, H.-Y.; et al. Therapeutic efficiency of human amniotic epithelial stem cell-derived functional hepatocyte-like cells in mice with acute hepatic failure. Stem Cell Res. Ther. 2018, 9, 321. [Google Scholar] [CrossRef]
- El-Kehdy, H.; Pourcher, G.; Zhang, W.; Hamidouche, Z.; Goulinet-Mainot, S.; Sokal, E.; Charbord, P.; Najimi, M.; Dubart-Kupperschmitt, A. Hepatocytic Differentiation Potential of Human Fetal Liver Mesenchymal Stem Cells: In Vitro and in Vivo Evaluation. Stem Cells Int. 2016, 2016, 1–12. [Google Scholar] [CrossRef]
- Magatti, M.; Stefani, F.R.; Papait, A.; Cargnoni, A.; Masserdotti, A.; Silini, A.R.; Parolini, O. Perinatal Mesenchymal Stromal Cells and Their Possible Contribution to Fetal-Maternal Tolerance. Cells 2019, 8, 1401. [Google Scholar] [CrossRef]
- Tahan, A.C.; Tahan, V. Placental amniotic epithelial cells and their therapeutic potential in liver diseases. Front. Med. 2014, 1, 48. [Google Scholar] [CrossRef]
- Alhomrani, M.; Correia, J.; Zavou, M.; Leaw, B.; Kuk, N.; Xu, R.; Saad, M.I.; Hodge, A.; Greening, D.W.; Lim, R.; et al. The Human Amnion Epithelial Cell Secretome Decreases Hepatic Fibrosis in Mice with Chronic Liver Fibrosis. Front. Pharmacol. 2017, 8, 748. [Google Scholar] [CrossRef]
- Manuelpillai, U.; Lourensz, D.; Vaghjiani, V.; Tchongue, J.; Lacey, D.; Tee, J.-Y.; Murthi, P.; Chan, J.; Hodge, A.; Sievert, W. Human amniotic epithelial cell transplantation induces markers of alternative macrophage activation and reduces established hepatic fibrosis. PLoS ONE 2012, 7, e38631. [Google Scholar] [CrossRef]
- Rodriguez, N.S.; Yanuaria, L.; Parducho, K.M.R.; Garcia, I.M.; Varghese, B.A.; Grubbs, B.H.; Miki, T. Liver-Directed Human Amniotic Epithelial Cell Transplantation Improves Systemic Disease Phenotype in Hurler Syndrome Mouse Model. Stem Cells Transl. Med. 2017, 6, 1583–1594. [Google Scholar] [CrossRef][Green Version]
- Skvorak, K.J.; Dorko, K.; Marongiu, F.; Tahan, V.; Hansel, M.C.; Gramignoli, R.; Gibson, K.M.; Strom, S.C. Placental stem cell correction of murine intermediate maple syrup urine disease. Hepatology 2013, 57, 1017–1023. [Google Scholar] [CrossRef]
- Hong, S.-B.; Seo, M.-S.; Park, S.-B.; Seo, Y.-J.; Kim, J.-S.; Kang, K.-S. Therapeutic effects of human amniotic epithelial stem cells in Niemann-Pick type C1 mice. Cytotherapy 2012, 14, 630–638. [Google Scholar] [CrossRef]
- Marongiu, F.; Gramignoli, R.; Dorko, K.; Miki, T.; Ranade, A.R.; Paola Serra, M.; Doratiotto, S.; Sini, M.; Sharma, S.; Mitamura, K.; et al. Hepatic differentiation of amniotic epithelial cells. Hepatology 2011, 53, 1719–1729. [Google Scholar] [CrossRef]
- Coronado, R.E.; Somaraki-Cormier, M.; Ong, J.L.; Halff, G.A. Hepatocyte-like cells derived from human amniotic epithelial, bone marrow, and adipose stromal cells display enhanced functionality when cultured on decellularized liver substrate. Stem Cell Res. 2019, 38, 101471. [Google Scholar] [CrossRef]
- Chen, J.; Jones, P.A. Potentiation of MyoD1 activity by 5-aza-2’-deoxycytidine. Cell Growth Differ. 1990, 1, 383–392. [Google Scholar]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef]
- Ni, X.; Gao, Y.; Wu, Z.; Ma, L.; Chen, C.; Wang, L.; Lin, Y.; Hui, L.; Pan, G. Functional human induced hepatocytes (hiHeps) with bile acid synthesis and transport capacities: A novel in vitro cholestatic model. Sci. Rep. 2016, 6, 1–16. [Google Scholar] [CrossRef]
- Huang, P.; Zhang, L.; Gao, Y.; He, Z.; Yao, D.; Wu, Z.; Cen, J.; Chen, X.; Liu, C.; Hu, Y.; et al. Direct reprogramming of human fibroblasts to functional and expandable hepatocytes. Cell Stem Cell 2014, 14, 370–384. [Google Scholar] [CrossRef]
- Kogiso, T.; Nagahara, H.; Otsuka, M.; Shiratori, K.; Dowdy, S.F. Transdifferentiation of human fibroblasts into hepatocyte-like cells by defined transcriptional factors. Hepatol. Int. 2013, 7, 937–944. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, H.; Ding, S. Reprogramming fibroblasts toward cardiomyocytes, neural stem cells and hepatocytes by cell activation and signaling-directed lineage conversion. Nat. Protoc. 2015, 10, 959–973. [Google Scholar] [CrossRef]
- Pournasr, B.; Asghari-Vostikolaee, M.H.; Baharvand, H. Transcription factor-mediated reprograming of fibroblasts to hepatocyte-like cells. Eur. J. Cell Biol. 2015, 94, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Rezvani, M.; Harbell, J.; Mattis, A.N.; Wolfe, A.R.; Benet, L.Z.; Willenbring, H.; Ding, S. Mouse liver repopulation with hepatocytes generated from human fibroblasts. Nature 2014, 508, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Wang, J.; Jia, J.; Song, N.; Xiang, C.; Xu, J.; Hou, Z.; Su, X.; Liu, B.; Jiang, T.; et al. Human hepatocytes with drug metabolic function induced from fibroblasts by lineage reprogramming. Cell Stem Cell 2014, 14, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Pacher, M.; Balakrishnan, A.; Yuan, Q.; Tsay, H.-C.; Yang, D.; Reetz, J.; Brandes, S.; Dai, Z.; Putzer, B.M.; et al. Direct Reprogramming of Hepatic Myofibroblasts into Hepatocytes In Vivo Attenuates Liver Fibrosis. Cell Stem Cell 2016, 18, 797–808. [Google Scholar] [CrossRef]
- Weissman, I.L. Stem cells: Units of development, units of regeneration, and units in evolution. Cell 2000, 100, 157–168. [Google Scholar] [CrossRef]
- Verfaillie, C. Pluripotent stem cells. Transfus. Clin. Biol. 2009, 16, 65–69. [Google Scholar] [CrossRef]
- Mitalipov, S.; Wolf, D. Totipotency, pluripotency and nuclear reprogramming. Adv. Biochem. Eng. Biotechnol. 2009, 114, 185–199. [Google Scholar]
- Thomson, J.A.; Itskovitz-Eldor, J.; Shapiro, S.S.; Waknitz, M.A.; Swiergiel, J.J.; Marshall, V.S.; Jones, J.M. Embryonic stem cell lines derived from human blastocysts. Science 1998, 282, 1145–1147. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Basma, H.; Soto-Gutiérrez, A.; Yannam, G.R.; Liu, L.; Ito, R.; Yamamoto, T.; Ellis, E.; Carson, S.D.; Sato, S.; Chen, Y.; et al. Differentiation and Transplantation of Human Embryonic Stem Cell-Derived Hepatocytes. Gastroenterology 2009, 136, 990–999.e4. [Google Scholar] [CrossRef]
- Li, H.Y.; Chien, Y.; Chen, Y.J.; Chen, S.F.; Chang, Y.L.; Chiang, C.H.; Jeng, S.Y.; Chang, C.M.; Wang, M.L.; Chen, L.K.; et al. Reprogramming induced pluripotent stem cells in the absence of c-Myc for differentiation into hepatocyte-like cells. Biomaterials 2011, 32, 5994–6005. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Catana, A.; Meng, Y.; Yamamoto, N.; He, S.; Gupta, S.; Gambhir, S.S.; Zern, M.A. Differentiation and Enrichment of Hepatocyte-Like Cells from Human Embryonic Stem Cells In Vitro and In Vivo. Stem Cells 2007, 25, 3058–3068. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, R.; Pettinato, G.; Beeston, J.T.; Lee, D.D.; Wen, X.; Mangino, M.J.; Fisher, R.A. Transplantation of human stem cell-derived hepatocytes in an animal model of acute liver failure. Surgery 2015, 158, 349–359. [Google Scholar] [CrossRef]
- Schwartz, R.E.; Linehan, J.L.; Painschab, M.S.; Hu, W.-S.; Verfaillie, C.M.; Kaufman, D.S. Defined conditions for development of functional hepatic cells from human embryonic stem cells. Stem Cells Dev. 2005, 14, 643–655. [Google Scholar] [CrossRef]
- Carpentier, A.; Tesfaye, A.; Chu, V.; Nimgaonkar, I.; Zhang, F.; Lee, S.B.; Thorgeirsson, S.S.; Feinstone, S.M.; Liang, T.J. Engrafted human stem cell-derived hepatocytes establish an infectious HCV murine model. J. Clin. Invest. 2014, 124, 4953–4964. [Google Scholar] [CrossRef]
- Asgari, S.; Moslem, M.; Bagheri-Lankarani, K.; Pournasr, B.; Miryounesi, M.; Baharvand, H. Differentiation and Transplantation of Human Induced Pluripotent Stem Cell-derived Hepatocyte-like Cells. Stem Cell Rev. Rep. 2013, 9, 493–504. [Google Scholar] [CrossRef]
- Liu, H.; Kim, Y.; Sharkis, S.; Marchionni, L.; Jang, Y.Y. In vivo liver regeneration potential of human induced pluripotent stem cells from diverse origins. Sci. Transl. Med. 2011, 3, 82ra39. [Google Scholar] [CrossRef]
- Yan, F.; Wang, Y.; Zhang, W.; Chang, M.; He, Z.; Xu, J.; Shang, C.; Chen, T.; Liu, J.; Wang, X.; et al. Human embryonic stem cell–derived hepatoblasts are an optimal lineage stage for hepatitis C virus infection. Hepatology 2017, 66, 717–735. [Google Scholar] [CrossRef]
- Helsen, N.; Debing, Y.; Paeshuyse, J.; Dallmeier, K.; Boon, R.; Coll, M.; Sancho-Bru, P.; Claes, C.; Neyts, J.; Verfaillie, C.M. Stem cell-derived hepatocytes: A novel model for hepatitis E virus replication. J. Hepatol. 2016, 64, 565–573. [Google Scholar] [CrossRef]
- Nakamori, D.; Takayama, K.; Nagamoto, Y.; Mitani, S.; Sakurai, F.; Tachibana, M.; Mizuguchi, H. Hepatic maturation of human iPS cell-derived hepatocyte-like cells by ATF5, c/EBPα, and PROX1 transduction. Biochem. Biophys. Res. Commun. 2015, 5–10. [Google Scholar] [CrossRef]
- Nagamoto, Y.; Takayama, K.; Ohashi, K.; Okamoto, R.; Sakurai, F.; Tachibana, M.; Kawabata, K.; Mizuguchi, H. Transplantation of a human iPSC-derived hepatocyte sheet increases survival in mice with acute liver failure. J. Hepatol. 2016, 64, 1068–1075. [Google Scholar] [CrossRef] [PubMed]
- Nagamoto, Y.; Takayama, K.; Tashiro, K.; Tateno, C.; Sakurai, F.; Tachibana, M.; Kawabata, K.; Ikeda, K.; Tanaka, Y.; Mizuguchi, H. Efficient Engraftment of Human Induced Pluripotent Stem Cell-Derived Hepatocyte-Like Cells in uPA/SCID Mice by Overexpression of FNK, a Bcl-xLMutant Gene. Cell Transplant. 2015, 24, 1127–1138. [Google Scholar] [CrossRef] [PubMed]
- Möbus, S.; Yang, D.; Yuan, Q.; Lüdtke, T.H.-W.H.W.; Balakrishnan, A.; Sgodda, M.; Rani, B.; Kispert, A.; Araúzo-Bravo, M.J.; Vogel, A.; et al. MicroRNA-199a-5p inhibition enhances the liver repopulation ability of human embryonic stem cell-derived hepatic cells. J. Hepatol. 2015, 62, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Takayama, K.; Kawabata, K.; Nagamoto, Y.; Kishimoto, K.; Tashiro, K.; Sakurai, F.; Tachibana, M.; Kanda, K.; Hayakawa, T.; Furue, M.K.; et al. 3D spheroid culture of hESC/hiPSC-derived hepatocyte-like cells for drug toxicity testing. Biomaterials 2013, 34, 1781–1789. [Google Scholar] [CrossRef]
- Gieseck, R.L.; Hannan, N.R.F.; Bort, R.; Hanley, N.A.; Drake, R.A.L.; Cameron, G.W.W.; Wynn, T.A.; Vallier, L. Maturation of induced pluripotent stem cell derived hepatocytes by 3D-culture. PLoS ONE 2014, 9, e86372. [Google Scholar] [CrossRef]
- Zabulica, M.; Srinivasan, R.C.; Vosough, M.; Hammarstedt, C.; Wu, T.; Gramignoli, R.; Ellis, E.; Kannisto, K.; Collin De L’Hortet, A.; Takeishi, K.; et al. Guide to the Assessment of Mature Liver Gene Expression in Stem Cell-Derived Hepatocytes. Stem Cells Dev. 2019, 28, 907–919. [Google Scholar] [CrossRef]
- Baxter, M.; Withey, S.; Harrison, S.; Segeritz, C.-P.; Zhang, F.; Atkinson-Dell, R.; Rowe, C.; Gerrard, D.T.; Sison-Young, R.; Jenkins, R.; et al. Phenotypic and functional analyses show stem cell-derived hepatocyte-like cells better mimic fetal rather than adult hepatocytes. J. Hepatol. 2015, 62, 581–589. [Google Scholar] [CrossRef]
- Ulvestad, M.; Nordell, P.; Asplund, A.; Rehnström, M.; Jacobsson, S.; Holmgren, G.; Davidson, L.; Brolén, G.; Edsbagge, J.; Björquist, P.; et al. Drug metabolizing enzyme and transporter protein profiles of hepatocytes derived from human embryonic and induced pluripotent stem cells. Biochem. Pharmacol. 2013, 86, 691–702. [Google Scholar] [CrossRef]
- Godoy, P.; Schmidt-Heck, W.; Natarajan, K.; Lucendo-Villarin, B.; Szkolnicka, D.; Asplund, A.; Bjorquist, P.; Widera, A.; Stoeber, R.; Campos, G.; et al. Gene networks and transcription factor motifs defining the differentiation of stem cells into hepatocyte-like cells. J. Hepatol. 2015, 63, 934–942. [Google Scholar] [CrossRef]
- Hurrell, T.; Segeritz, C.-P.; Vallier, L.; Lilley, K.S.; Cromarty, A.D. A proteomic time course through the differentiation of human induced pluripotent stem cells into hepatocyte-like cells. Sci. Rep. 2019, 9, 3270. [Google Scholar] [CrossRef]
- Godoy, P.; Widera, A.; Schmidt-Heck, W.; Campos, G.; Meyer, C.; Cadenas, C.; Reif, R.; Stöber, R.; Hammad, S.; Pütter, L.; et al. Gene network activity in cultivated primary hepatocytes is highly similar to diseased mammalian liver tissue. Arch. Toxicol. 2016, 90, 2513–2529. [Google Scholar] [CrossRef] [PubMed]
- Heslop, J.A.; Rowe, C.; Walsh, J.; Sison-Young, R.; Jenkins, R.; Kamalian, L.; Kia, R.; Hay, D.; Jones, R.P.; Malik, H.Z.; et al. Mechanistic evaluation of primary human hepatocyte culture using global proteomic analysis reveals a selective dedifferentiation profile. Arch. Toxicol. 2016, 91, 439–452. [Google Scholar] [CrossRef] [PubMed]
- Benten, D.; Kumaran, V.; Joseph, B.; Schattenberg, J.; Popov, Y.; Schuppan, D.; Gupta, S. Hepatocyte transplantation activates hepatic stellate cells with beneficial modulation of cell engraftment in the rat. Hepatology 2005, 42, 1072–1081. [Google Scholar] [CrossRef] [PubMed]
- Joseph, B.; Malhi, H.; Bhargava, K.K.; Palestro, C.J.; McCuskey, R.S.; Gupta, S. Kupffer cells participate in early clearance of syngeneic hepatocytes transplanted in the rat liver. Gastroenterology 2002, 123, 1677–1685. [Google Scholar] [CrossRef] [PubMed]
- Kumaran, V.; Joseph, B.; Benten, D.; Gupta, S. Integrin and extracellular matrix interactions regulate engraftment of transplanted hepatocytes in the rat liver. Gastroenterology 2005, 129, 1643–1653. [Google Scholar] [CrossRef] [PubMed]
- Fagg, W.S.; Liu, N.; Yang, M.-J.; Cheng, K.; Chung, E.; Kim, J.-S.; Wu, G.; Fair, J. Magnetic Targeting of Stem Cell Derivatives Enhances Hepatic Engraftment into Structurally Normal Liver. Cell Transplant. 2017, 26, 1868–1877. [Google Scholar] [CrossRef]
- Bohnen, N.I.; Charron, M.; Reyes, J.; Rubinstein, W.; Strom, S.C.; Swanson, D.; Towbin, R. Use of indium-111-labeled hepatocytes to determine the biodistribution of transplanted hepatocytes through portal vein infusion. Clin. Nucl. Med. 2000, 25, 447–450. [Google Scholar] [CrossRef]
- Dusabineza, A.-C.; Najimi, M.; van Hul, N.; Legry, V.; Khuu, D.N.; van Grunsven, L.A.; Sokal, E.; Leclercq, I.A. Hepatic Stellate Cells Improve Engraftment of Human Primary Hepatocytes: A Preclinical Transplantation Study in an Animal Model. Cell Transplant. 2015, 24, 2557–2571. [Google Scholar] [CrossRef]
- Enami, Y.; Bandi, S.; Kapoor, S.; Krohn, N.; Joseph, B.; Gupta, S. Hepatic stellate cells promote hepatocyte engraftment in rat liver after prostaglandin-endoperoxide synthase inhibition. Gastroenterology 2009, 136, 2356–2364. [Google Scholar] [CrossRef][Green Version]
- Fan, F.; He, Z.; Kong, L.-L.; Yuan, Q.; Xia, N.-S.; Chen, Q.; Wu, X.; Chen, L.; Sun, X.; Liu, H.; et al. Pharmacological targeting of kinases MST1 and MST2 augments tissue repair and regeneration. Sci. Transl. Med. 2016, 8, 352ra108. [Google Scholar] [CrossRef]
| Phenomenon | Definition |
|---|---|
| Liver engraftment | Engraftment is the initial incorporation of transplanted cells into the liver tissue, whether or not the transplanted cells proliferate to replace a significant proportion of the liver mass. |
| Liver repopulation | Repopulation occurs following engraftment and is the result of extensive proliferation of transplanted cells that have hepatocyte functions in the host liver. In this review, presence of at least 5–10% of donor cells in the host liver 2 months post transplantation is considered ‘successful liver repopulation’. This cut-off was defined based on the observation that in many instances, 5–10% of the hepatocytes must be replaced to obtain a therapeutic effect of the transplanted cells [7]. |
| Serial transplantation | Serial transplantation is defined as the isolation of donor hepatocytes from a primary host and the engraftment of these isolated donor hepatocytes into a secondary host, which is possible as hepatocytes can undergo a number of cell divisions [8]. |
| Cell fusion | After transplantation of cells into the liver (best illustrated following transplantation of monocytes), donor cells can fuse with host cells, thereby generating (either mono- or bi-nucleated) polyploid cells. Genes expressed from the donor genome could then compensate for low or absent gene expression from the host genome (e.g., due to inborn genetic disorders). Such events could easily be misinterpreted as transdifferentiation. |
| Characteristics of Cells to Transplant | Repopulation Index (%) | hAlb Blood Levels | Mechanism | Serial Transplantation | References | |||
|---|---|---|---|---|---|---|---|---|
| Early | Late | Early | Late | |||||
| PHHs | Alb+ AFP− | 10%–50% | 20%–95% | 1–5 mg/mL | 5–15 mg/mL | Functional integration in liver parenchyma | Yes | [13,17,26,31,33,34] |
| 2D expanded PHHs | Passage < 4 | ND | 50%–90% | <500 μg/mL | 2–10 mg/mL | Functional integration in liver parenchyma | ND | [49,50] |
| Passage > 6 | ND | 1%–40% | <10 μg/mL | 1–5 mg/mL | ||||
| LPCs | Several markers have been used, but mostly fetal cells | <5% | 0%–10% | 0–1 mg/mL | <100 μg/mL | Functional integration in liver parenchyma | Yes | [44,45,51] |
| 3D organoids | Alb+ AFP+ | ND | ND | <100 μg/mL | ND | Functional integration in liver parenchyma | ND | [42] |
| HCSs/Monocytes/ Macrophages | CD34+/CD14+ | 0% | 10%–30% | ND | ND | Fusion with host hepatocytes | ND | [3,60,61] |
| MSCs | Several markers have been used | 0%–10% | 0%–50% | 0–1 mg/mL | 0–2 mg/mL | Immunomodulation, Paracrine effects, … | ND | [9,69,81] |
| MSC-HLCs | Alb+ AFP+ | 1%–5% | ND | 0–10 ng/mL | ND | Immunomodulation, Paracrine effects, … | ND | [86,89,97,98] |
| AECs/AEC-HLCs | Several markers have been used/Alb+, AFP+ | 0.1%–3.5% | 0.1%–5% | ND | ND | Immunomodulation, Paracrine effects, … | ND | [111,113] |
| Transdifferentiated HLCs | Alb+ AFP+/- | 1%–30% | ~2% | 0–300 μg/mL | <100 μg/mL | Functional integration in liver parenchyma | ND | [118,122,123] |
| (i)PSC-HLCs | Alb+ AFP+ | 1%–20 % | 1%–45% | 0–2 mg/mL | 0–2 mg/mL | Functional integration in liver parenchyma | ND | [20,21,137,141,142] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tricot, T.; De Boeck, J.; Verfaillie, C. Alternative Cell Sources for Liver Parenchyma Repopulation: Where Do We Stand? Cells 2020, 9, 566. https://doi.org/10.3390/cells9030566
Tricot T, De Boeck J, Verfaillie C. Alternative Cell Sources for Liver Parenchyma Repopulation: Where Do We Stand? Cells. 2020; 9(3):566. https://doi.org/10.3390/cells9030566
Chicago/Turabian StyleTricot, Tine, Jolan De Boeck, and Catherine Verfaillie. 2020. "Alternative Cell Sources for Liver Parenchyma Repopulation: Where Do We Stand?" Cells 9, no. 3: 566. https://doi.org/10.3390/cells9030566
APA StyleTricot, T., De Boeck, J., & Verfaillie, C. (2020). Alternative Cell Sources for Liver Parenchyma Repopulation: Where Do We Stand? Cells, 9(3), 566. https://doi.org/10.3390/cells9030566





